Supplemental guidance on influenza vaccination in adults 65 years of age and older
Download in PDF format
(1.04 MB, 80 pages)
Organization: Public Health Agency of Canada
Date published: 2024-07-25
Cat.: HP40-363/1-2024E-PDF
ISBN: 240048
Pub.: 978-0-660-71464-6
On this page
- Preamble
- Summary of information contained in this NACI statement
- Introduction
- Methods
- Epidemiology
- Vaccine
- Economics
- Discussion
- Recommendations
- Research priorities
- Surveillance issues
- Tables
- List of abbreviations
- Acknowledgements
- Appendix A: Key terminology for understanding economic evaluation evidence
- Appendix B: Methods for quality appraisal and generalizability assessment
- Appendix C: Findings from remaining included economic evaluation studies deemed to have less generalizability to a Canadian setting
- References
Preamble
The National Advisory Committee on Immunization (NACI) is an External Advisory Body that provides the Public Health Agency of Canada (PHAC) with independent, ongoing, and timely medical, scientific, and public health advice in response to questions from PHAC relating to immunization.
In addition to burden of disease and vaccine characteristics, PHAC has expanded the mandate of NACI to include the systematic consideration of programmatic factors in developing evidence based recommendations to facilitate timely decision-making for publicly funded vaccine programs at provincial and territorial levels.
The additional factors to be systematically considered by NACI include: economics, ethics, equity, feasibility, and acceptability. Not all NACI statements will require in-depth analyses of all programmatic factors. While systematic consideration of programmatic factors will be conducted using evidence-informed tools to identify distinct issues that could impact decision-making for recommendation development, only distinct issues identified as being specific to the vaccine or vaccine-preventable disease will be included.
This statement contains NACI's independent advice and recommendations, which are based upon the best current available scientific knowledge. This document is being disseminated for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph. Recommendations for use and other information set out herein may differ from that set out in the product monographs of the Canadian manufacturers of the vaccines. Manufacturer(s) have sought approval of the vaccines and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Summary of information contained in this NACI statement
The following highlights key information for immunization providers. Please refer to the remainder of the statement for details.
What
The following recommendations for influenza vaccination in adults 65 years of age and older supplement the National Advisory Committee on Immunization (NACI)'s overarching recommendations for influenza vaccination, which are available in the NACI Seasonal Influenza Vaccine Statement. NACI recommends that high-dose inactivated influenza vaccine (IIV-HD), adjuvanted inactivated influenza vaccine (IIV-Adj) or recombinant influenza vaccine (RIV) should be offered, when available, over other influenza vaccines for adults 65 years of age and older. If a preferred product is not available, any of the available age-appropriate influenza vaccines should be used.
Who
Adults 65 years of age and older are prioritized to receive influenza vaccines because of the increased risks of severe disease in this population. This supplemental statement provides an evidence summary on the preferential use of 1 or more of the age-appropriate influenza vaccines for adults 65 years of age and older, over other age-appropriate influenza vaccines.
How
Inactivated high-dose, adjuvanted or recombinant influenza vaccines should be offered, when available, over other influenza vaccines for adults 65 years of age and older. If a preferred product is not available, any available age-appropriate influenza vaccines should be used. Influenza vaccination may be given at the same time as, or at any time before or after administration of another vaccine, including COVID-19 vaccine.
Why
Annual influenza vaccination is safe and the best way to prevent influenza and its complications. Adults 65 years of age and older are at higher risk of serious complications from influenza; therefore, NACI undertook a review of evidence to determine whether any age-appropriate influenza vaccines should be preferentially used in this age group. A systematic review of economic literature was also undertaken to inform public health program decision-making. Overall, the evidence supports IIV-HD, IIV-Adj and RIV as having increased benefit as compared to IIV-SD, with no difference in safety.
Introduction
Influenza is a respiratory infection caused primarily by influenza A and B viruses. Older adults are disproportionately affected by serious outcomes from influenza infection and may present with typical or atypical symptoms, as influenza causes respiratory and systemic illness. Prior to the COVID-19 pandemic, influenza was estimated to cause 12,200 hospitalizationsFootnote 1 and 3,500 deathsFootnote 2 annually in Canada, with the majority of deaths occurring in adults 65 years and olderFootnote 3. Considering the burden of influenza disease in this population, the National Advisory Committee on Immunization (NACI) has identified adults 65 years of age and older as 1 of the groups at higher risk of influenza complications and for whom influenza immunization is particularly important (Strong NACI recommendation)Footnote 4.
NACI has conducted several reviews over the years to evaluate the best available scientific and clinical evidence to develop recommendations for the use of influenza vaccines, with a focus on optimizing influenza protection among older adults in CanadaFootnote 5Footnote 6. These recommendations have evolved over time due to the availability of new vaccine products, some of which are designed to enhance immunogenicity in specific age groups, as well as the expansion and accumulation of evidence on influenza vaccines. The most recent NACI literature review update on the efficacy and effectiveness of high-dose (Fluzone® High-Dose) and MF59-adjuvanted (Fluad®) trivalent inactivated influenza vaccines in adults 65 years of age and older was published in May 2018Footnote 7.
The findings of this review supported the conclusions of previous reviews and led to a strengthened NACI recommendation for the use of high-dose egg-based trivalent inactivated influenza vaccine (IIV3-HD) as the preferred vaccine for Canadians 65 years of age and older. Therefore, on an individual level, NACI recommended that for older adults, IIV-HD should be used over standard-dose inactivated influenza vaccines (IIV-SD) given the burden of influenza A(H3N2) disease and the good evidence of IIV3-HD providing better protection compared to IIV3-SD in adults 65 years of age and older.
Other than a recommendation for using IIV-HD over IIV-SD formulations, NACI has not previously made comparative individual-level recommendations on the use of the other available vaccines in this age group. If a preferred product is not available, NACI has recommended that any of the available age-appropriate influenza vaccines should be used. On a public health program level, NACI has recommended that any of the available influenza vaccines authorized in this age group should be used, as there was insufficient evidence on the incremental value of different influenza vaccines to make comparative public health program-level recommendations on the use of the available vaccines.
Evidence on vaccine effectiveness (VE) in adults 65 years of age and older suggests a need for more effective vaccines targeted to this age group. For example, individuals 17 to 59 years of age showed a 2- to 4-fold higher immune response to influenza vaccine as measured by seroconversion and seroprotection rates compared to those 65 years of age and olderFootnote 8. Furthermore, a meta-analysis conducted in adults 65 years of age and older found a lower point estimate of VE against laboratory-confirmed influenza (pooled VE of 49%, 95% CI: 33-62%)Footnote 9 compared to a meta-analysis in healthy adults 18 to 64 years of age (pooled VE of 59%, 95% CI: 51-67%)Footnote 10.
The trigger for this NACI Supplemental Statement on the use of influenza vaccines in adults 65 years of age and older was the expressed desire by provincial and territorial programs for guidance on optimal product choice(s) for older adults. In consideration of the above factors, NACI has undertaken a review of evidence to determine whether any 1 or more of the age-appropriate influenza vaccines for adults 65 years of age and older should be preferentially used over other age-appropriate influenza vaccines. A systematic review of economic literature was also undertaken to inform public health program decision-making.
Guidance objective
The following advisory committee statement on influenza vaccination in adults 65 years of age and older supplements NACI's overarching recommendations for influenza vaccination, which are available in the NACI Seasonal Influenza Vaccine Statement. The objective of this supplemental statement is to provide updated guidance on the use of influenza vaccine in adults 65 years of age and older. This statement describes the disproportionate risk of morbidity and mortality for adults 65 years of age and older who acquire influenza compared to younger age groups; reviews the available evidence on the efficacy, effectiveness and safety of influenza vaccination in adults 65 years of age and older; and explores the economic, ethics, equity, feasibility, and acceptability considerations of immunizing adults 65 years of age and older against influenza.
Methods
In brief, the broad stages in the preparation of a NACI advisory committee statement are:
- Knowledge synthesis: retrieval and summary of literature, assessment of the quality of the evidence (summarized in Table 5: Summary of evidence).
- Synthesis of the body of evidence: benefits (efficacy and effectiveness) and potential harms (safety), considering the quality of the synthesized evidence and, where applicable, the magnitude of effects observed across the studies.
- Use of a published, peer-reviewed framework and evidence-informed tools to ensure that issues related to ethics, equity, feasibility, and acceptability (EEFA) are systematically assessed and integrated into the guidance.
- Use of the evidence to inform recommendations.
Further information on NACI's process and procedures is available elsewhere.
For this supplemental statement, NACI reviewed the key questions for the literature review as proposed by the Influenza Working Group, including such considerations as the burden of influenza illness to be prevented and the target population(s); safety, efficacy, effectiveness, economic evaluations of influenza vaccines; and other aspects of the overall immunization strategy. In preparation for this statement, the GRADE-ADOLOPMENT process was employed to adapt recommendations from the US Advisory Committee on Immunization Practices (ACIP) guideline panel where they assessed the relative benefits and harms of IIV-HD, IIV-Adj, and RIV compared to one another and with IIV-SD in adults 65 years of age and olderFootnote 11.
ACIP applied the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the strength and certainty of the evidence for critical outcomes included in their review. Evidence on the efficacy and cost-effectiveness of influenza vaccines in adults 65 years of age and older was further expanded with 2 additional systematic reviews, both developed in collaboration with the Methods and Applications Group for Indirect Comparisons (MAGIC) through the Drug Safety and Effectiveness Network (DSEN) and supervised by the NACI Influenza Working Group. One (1) review examined the efficacy of influenza vaccines in older adults, while the second review delved into the cost-effectiveness of seasonal influenza vaccines in older adults.
The ACIP conducted a literature search from 1990 through September 7, 2022, to identify eligible studies on the efficacy, effectiveness, and safety of influenza vaccines in older adults. Additionally, DSEN MAGIC performed an initial literature search on influenza vaccine efficacy in older adults, covering the period from inception to March 31, 2022, and subsequently conducted a second updated search on June 20, 2022.
Further details regarding the methodologies employed in both DSEN reviews are available in pre-specified written protocolsFootnote 12Footnote 13.
The evidence and proposed recommendations were presented to NACI for deliberation on September 27, 2023, and approved following a thorough review of the evidence. Relevant considerations, rationale for specific decisions, and knowledge gaps are further described in the following sections.
For a comprehensive description of the methodology and results of the reviews reporting on the safety, efficacy, and effectiveness of influenza vaccines, please refer to Grohskopf et al (2022)Footnote 14 and Veroniki et al (2023)Footnote 15. Data are summarized in sections IV.3 and IV.4 of this statement. For details on the methodology and results on the economic evaluation findings for influenza vaccines in older adults, refer to Section V of this statement.
The overarching policy question addressed in this statement is: Should any age-appropriate influenza vaccine(s) be preferentially used in adults 65 years of age and older? In addition, the following sub-questions were posed:
- Do the relative benefits and harms of IIV-HD, IIV-Adj, IIV-cc and RIV, as compared with one another and with IIV-SD, favour the preferential use of any 1 or more of these vaccines over other age-appropriate influenza vaccines for adults 65 years of age and older?
- Does this recommendation vary by vaccine characteristic (e.g., high dose/standard dose, trivalent/quadrivalent, adjuvanted/unadjuvanted, egg-based/non-egg-based manufacturing?)
- Does this recommendation vary by risk group (e.g., populations with comorbid conditions, sex, previous vaccination, age 80 years and older)?
The literature search and data extraction were conducted according to the following PICO framework (Population, Intervention, Comparators and Outcomes):
P (Population):
- Adults 65 years of age and older
I (Intervention):
- Inactivated influenza vaccine (IIV)-not standard dose (not SD) and recombinant influenza vaccines:
- High-dose inactivated influenza vaccine (IIV-HD)
- MF-59 adjuvanted inactivated influenza vaccine (IIV-Adj)
- Recombinant influenza vaccine (RIV)
- Mammalian cell culture-based vaccine (IIV-cc)
C (Comparator):
- Inactivated standard-dose influenza vaccines (IIV-SD)
- Inactivated influenza vaccine (IIV)-not SD and recombinant influenza vaccines
O (Outcome):
- Vaccine efficacy/effectiveness:
- Lab-confirmed influenza (LCI)
- Influenza-associated outpatient/emergency department (ED) visits (LCI, influenza-like illness [ILI])
- Influenza-associated hospitalization (LCI, ILI)
- Influenza-associated vascular events
- Vaccine safety:
- Any solicited systemic adverse reaction grade ≥3
- Guillain-Barré Syndrome (GBS)
- Any serious adverse events (SAE)
- Any solicited injection site adverse reaction grade ≥3
- Economics:
- Vaccine cost-effectiveness (cost per life year saved, cost per influenza case averted)
- Cost-utility (cost per quality-adjusted life year [QALY])
Note: O (Outcomes) are the critical/important outcomes for decision making.
Abbreviations: ED, emergency department; GBS, Guillain-Barré Syndrome; IIV, inactivated influenza vaccine; IIV-Adj, adjuvanted inactivated influenza vaccine; IIV-cc, mammalian cell culture-based inactivated influenza vaccine; IIV-HD, high-dose inactivated influenza vaccine; IIV-SD, standard dose inactivated influenza vaccine; ILI, influenza-like illness; LCI, laboratory-confirmed influenza; QALY, quality-adjusted life year; RIV, recombinant influenza vaccine; SAE, serious adverse event.
To meet the objective of this statement, supplementary informal literature reviews were conducted as necessary, encompassing:
- The epidemiology and estimated burden of influenza illness among adults 65 years of age and older
- The efficacy, effectiveness, and safety of influenza vaccines in frail older adults 65 years of age and older
- Guidelines and considerations for the use of influenza vaccines in older adults across Canadian provinces, territories and globally
For additional information and NACI's current recommendations on the use of influenza vaccines in adults 65 years of age and older, please refer to the current NACI Statement on seasonal influenza vaccine and to the influenza vaccine chapter in the Canadian Immunization Guide (CIG).
Epidemiology
Estimated burden of influenza among adults 65 years of age and older
Although adults 65 years of age and older only comprise approximately 19% of the Canadian population, this population is over-represented among laboratory-confirmed influenza (LCI) cases, especially in seasons where the A(H3N2) influenza strain predominated (e.g., 2014-2015, 2016-2017, 2017-2018) before the COVID-19 pandemicFootnote 16. Although influenza-associated morbidity and mortality vary each season, in general there is an increased burden of severe disease such as influenza-associated hospitalizations, intensive care unit (ICU) admissions, and deaths in adults 65 years of age and older, especially in seasons when influenza A(H3N2) predominatesFootnote 16. Data derived from Canada's national hospitalization database found that rates of respiratory hospitalizations attributed to influenza were highest among adults 65 years and older at 144.9 per 100,000 compared to 25.8 per 100,000 for adults 45 to 64 years of ageFootnote 17. With regard to influenza-attributable deaths, the annual average mortality rate for adults 65 years and older was estimated to be 108.8 per 100,000, which is substantially higher than the estimated mortality rate of 4.0 per 100,000 for adults 50 to 64 years of ageFootnote 18.
Furthermore, among adults 65 years of age and older, the risk of influenza-related complications is significantly higher with increasing age, the presence and severity of chronic medical conditions, and higher level of frailtyFootnote 19Footnote 20. As with LCI cases, adults 65 years of age and older had higher influenza-related hospitalization rates than younger age groups in most years before the COVID-19 pandemicFootnote 21. During the 2022-2023 influenza season, adults 65 years of age and older had the highest cumulative hospitalization rate (136 per 100,000 population), followed by children under 5 years of age (130 per 100,000 population)Footnote 21. For the relatively brief and unusually late 2021-2022 influenza season, the seasonal hospitalization rate was also highest in adults 65 years ageFootnote 22; and ICU admissions and deaths were most common among adults 65 years of age and older (30% and 59%, respectively)Footnote 22. In the years when A(H3N2) was dominant, over 80% of influenza-associated deaths were in adults 65 years of age and older (e.g., seasons 2014-2015, 2016-2018)Footnote 23.
Influenza vaccination coverage among adults 65 years of age and older
Influenza vaccine coverage among adults 65 years of age and older in Canada is usually relatively high, at approximately 70% in the most recent years. During the 2022-2023 season, influenza vaccination coverage among adults 65 years of age and older was 74%. However, vaccination coverage in this age group still does not meet the national goal of 80% for those at high risk of influenza-related complications, such as older adultsFootnote 24.
Vaccine
Preparation(s) authorized for use in Canada
Five (5) influenza vaccines are authorized and available for use in Canada in adults 65 years of age and older: IIV-Adj, IIV-SD, IIV-HD, RIV and IIV-cc.
Two (2) inactivated influenza vaccines (IIVs) are designed specifically to enhance immunogenicity in adults 65 years of age and older: IIV4-HD, a high-dose quadrivalent inactivated split virion vaccine (Fluzone® High-Dose Quadrivalent, Sanofi Pasteur) and IIV3-Adj, an MF59-adjuvanted trivalent inactivated subunit vaccine (Fluad®, Seqirus).
Fluzone® High-Dose contains 60 µg of haemagglutinin (HA) per strain (compared to 15 µg HA per strain in a standard dose)Footnote 25. Fluzone® High-Dose Quadrivalent, authorized for use in Canada in 2020, is currently the only available high-dose inactivated split virion influenza vaccine in CanadaFootnote 4Footnote 26. A literature review on the efficacy, effectiveness, immunogenicity and safety of high-dose seasonal influenza vaccines, including Fluzone® High-Dose, for adults 65 years of age and older was conducted in 2016Footnote 6 as part of NACI's evidence-based processFootnote 27 to inform the inclusion of Fluzone® High-Dose in the Statement on Seasonal Influenza Vaccine for 2016–2017Footnote 25.
Fluad® is a standard-dose inactivated subunit vaccine containing the adjuvant MF59, which is an oil-in-water emulsion composed of squalene as the oil phase and stabilized with the surfactants polysorbate 80 and sorbitan triolate in citrate bufferFootnote 28. Fluad® and its pediatric formulation (Fluad Pediatric®, Seqirus) are the only seasonal influenza vaccines available for use in Canada with an adjuvant. Evidence on the efficacy, effectiveness, immunogenicity, and safety of Fluad® was first reviewed in 2011Footnote 5 to inform the inclusion of Fluad® in the Statement on Seasonal Influenza for 2011–2012Footnote 29 and subsequently supplemented with additional effectiveness evidence in the Statement on Seasonal Influenza for 2014–2015Footnote 30.
All inactivated influenza vaccines (IIV) available in Canada are produced in eggs, except for Flucelvax Quad (IIV4-cc), which is a mammalian cell culture-based quadrivalent inactivated, subunit influenza vaccine that is prepared from viruses propagated in mammalian cell lines (proprietary 33016-PF Madin-Darby Canine Kidney [MDCK] cell lines) adapted to grow freely in suspension in culture medium.
There is currently only 1 RIV authorized for use in Canada: Supemtek (RIV4), a quadrivalent unadjuvanted, baculovirus-expressed seasonal influenza vaccine that contains 45 µg of HA per strain (compared to 15 µg HA per strain in a standard dose) and authorized for adults 18 years of age and older. RIV contains recombinant HAs produced in an insect cell line using genetic sequences from cell-derived influenza viruses. The production of RIV does not depend on egg supply.
The NACI annual statement on seasonal influenza vaccine contains a full description of vaccines available for use in Canada.
Concurrent administration with other vaccines
The NACI annual statement on seasonal influenza vaccine contains a full description of concurrent administration of influenza vaccines with other vaccines. Briefly, all seasonal influenza vaccines may be given at the same time as, or any time before or after administration of other vaccines, including COVID-19 and pneumococcal vaccines.
Data are limited regarding concurrent administration of newer adjuvanted influenza vaccines with other adjuvanted or non-adjuvanted vaccines.
Recombinant zoster vaccine (RZV) (Shingrix®, GlaxoSmithKline) is authorized for use in Canada in adults 50 years of age and older, and in adults 18 years of age or older who are or will be at increased risk of herpes zoster (HZ) due to immunodeficiency or immunosuppression caused by known disease or therapy; therefore, the target age group for herpes zoster vaccine and influenza vaccine overlap. RZV has been shown to be safe and effective when given concurrently with unadjuvanted, standard dose influenza vaccinesFootnote 31. However, no studies have been conducted that have assessed the concurrent administration of RZV with adjuvanted or high dose influenza vaccineFootnote 32. It should be noted that RZV and IIV-Adj contain the adjuvants AS01B and MF59 respectively. How these adjuvants may interact when RZV and IIV-Adj are administered concurrently is not known.
NACI will continue to review the evidence and update guidance accordingly.
Efficacy and effectiveness
To answer the policy question addressed in this statement, ACIP and DSEN MAGIC results evaluating the relative benefits and harms of IIV-HD, IIV-Adj, IIV-cc and RIV, as compared with one another and with IIV-SD were presented in the narrative summary. Of note, DSEN MAGIC conducted a network meta-analysis (NMA) to evaluate the efficacy of influenza vaccines in adults 65 years of age and older. However, due to the difficulties in interpreting the NMA results arising from the presence of sparse and disconnected networks, and the challenges of comparing influenza efficacy in different seasons, only the pairwise meta-analysis and single study results were presented in the narrative summary. For further information, please refer to the original publication by Veroniki et al (2023)Footnote 15.
ACIP and DSEN MAGIC appraised articles using the Cochrane risk of bias tools. Study limitations of articles included in the evidence synthesis are reported in Table 6.
Overall, in the ACIP review the level of certainty of the evidence for outcomes reporting on vaccine efficacy and effectiveness was rated as low to very low and was primarily downgraded due to limited availability of randomized studies. For additional details regarding the summary of findings and assessments of the quality of the evidence please refer to GRADE: Higher Dose and Adjuvanted Influenza Vaccines for Persons Aged ≥65 Years and to the Evidence to Recommendations (EtR) Framework: Higher Dose and Adjuvanted Influenza Vaccines for Persons Aged ≥65 Years.
In the DSEN MAGIC review, certainty of evidence of NMA estimates was assessed using the Confidence in Network Meta-Analysis (CINeMA) approach and confidence in pairwise estimates for which a NMA could not be performed was assessed using the GRADE approach (i.e., IIV-HD vs. IIV-SD). The GRADE certainty of evidence for outcomes reporting on vaccine efficacy of IIV-HD compared to IIV-SD were rated low to high which were primarily downgraded due to imprecision and risk of bias. For additional details regarding the GRADE assessments and other supplementary materials for quality of evidence appraisals that were conducted, please refer to the original publication by Veroniki et al (2023)Footnote 15 and the framework webpage.
Please note that analyses on vaccine efficacy and effectiveness were conducted on the overall population of adults 65 years of age and older, and not by risk groups (e.g., population with comorbid conditions, sex, previous vaccination, and adults 80 years of age and older) due to data limitation, including the number of studies reporting for each outcome.
Efficacy and effectiveness of high dose, recombinant and adjuvanted influenza vaccines compared to standard-dose inactivated influenza vaccines
Summary of study characteristics
Overall, the ACIP review identified 31 studies (9 RCTsFootnote 33Footnote 34Footnote 35Footnote 36Footnote 37Footnote 38Footnote 39Footnote 40Footnote 41, including 2 cluster RCTsFootnote 40Footnote 41, and 22 observational studiesFootnote 42Footnote 43Footnote 44Footnote 45Footnote 46Footnote 47Footnote 48Footnote 49Footnote 50Footnote 51Footnote 52Footnote 53Footnote 54Footnote 55Footnote 56Footnote 57Footnote 58Footnote 59Footnote 60Footnote 61Footnote 62Footnote 63) reporting data on influenza vaccine efficacy/effectiveness outcomes in adults 65 years of age and older. Their systemic review provided data on influenza illness (n=4) defined as LCI or influenza-like illness (ILI) syndrome without laboratory confirmation of viral etiology, influenza-associated outpatient and/or emergency department (ED) visits (n=8), influenza-associated hospitalization (n=21), and influenza-associated deaths (n=2).
The DSEN MAGIC systematic review considered only RCTs and identified 10 studies reporting data on influenza vaccine efficacy outcomes comparing IIV-HD, IIV-Adj, and RIV to IIV-SD in adults 65 years of age and olderFootnote 33Footnote 34Footnote 35Footnote 64Footnote 65Footnote 66Footnote 67Footnote 68Footnote 69Footnote 70. Their systemic review provided data on influenza LCI (n=5), ILI syndrome without laboratory confirmation of viral etiology (n=5), influenza-associated outpatient visits (n=1), influenza-associated hospitalization (n=4), influenza-associated deaths (n=1), and influenza-associated vascular events (n=7).
Summary of vaccine efficacy/effectiveness against influenza
Overall, ACIP included 4 RCTs reporting data on influenza illness in adults 65 years of age and older. Of those, 1 RCT compared IIV3-HD to IIV3-SD against LCIFootnote 33, 1 compared IIV3-Adj to IIV3-SD against ILIFootnote 35, and 2 RCTs compared RIV to IIV-SD against LCIFootnote 34Footnote 36. The DSEN MAGIC included 4 RCTs reporting data comparing IIV-HD to IIV-SD against LCI (n=3) Footnote 33Footnote 64Footnote 65 and ILI (n=3) Footnote 33Footnote 65Footnote 66, as well as 2 RCTs comparing RIV4 to IIV-SD against LCI and ILIFootnote 34Footnote 71 in adults 65 years of age and older.
Both the ACIP and DSEN MAGIC reviews found that IIV-HD was associated with relative vaccine efficacy of approximately 25% compared to IIV-SD against LCI. The ACIP review used data from 1 RCT by DiazGranados 2014Footnote 33 while the DSEN MAGIC pooled estimates from 3 RCTs, also including DiazGranados 2014 RCTFootnote 33Footnote 64Footnote 65, with both groups demonstrating beneficial effects of IIV-HD compared to IIV-SD. ACIP reported a relative vaccine efficacy of 18% (95% CI: -17 to 43%) against LCI combining 2 RCTsFootnote 34Footnote 36 comparing RIV to IIV-SD. The DSEN MAGIC observed a potential beneficial protective effect of RIV4 over IIV-SD against LCI combining 2 RCTs though the estimate lacked precision (pooled relative vaccine efficacy of 30%, 95% CI: -18 to 58%)Footnote 34Footnote 71.
ACIP reported no difference in vaccine efficacy between IIV3-Adj and IIV-SD against ILI from 1 RCT (relative vaccine efficacy of -3%, 95% CI: -19 to 11%)Footnote 35. Finally, the DSEN MAGIC did not identify a difference between IIV3-HD (pooled relative vaccine efficacy of 2%, 95% CI: -2 to 7%) Footnote 33Footnote 64Footnote 66, RIV (relative vaccine efficacy of 1%, 95% CI: -9 to 11%, and 4%, 95% CI: -65 to 45%)Footnote 34Footnote 71, and IIV-Adj (relative vaccine efficacy of -3%, 95% CI: -21 to 13%)Footnote 35 when compared to IIV-SD for the prevention of ILI syndrome without laboratory confirmation of viral etiology.
Summary of vaccine efficacy/effectiveness against influenza-associated outpatient and/or emergency department visits
Overall, ACIP included 8 observational studies reporting data on influenza-associated outpatient and/or ED visits defined by clinical diagnosis. Of those, 5 compared IIV3-HD to IIV-SD Footnote 43Footnote 44Footnote 45Footnote 46Footnote 47, and 4 compared IIV3-Adj to IIV-SDFootnote 42Footnote 45Footnote 48Footnote 49. The DSEN MAGIC included 1 RCT comparing IIV-HD to IIV-SD against influenza-associated outpatient visits defined by clinical diagnosisFootnote 64.
From the ACIP review, pooled results from 4 retrospective cohort studies demonstrated a beneficial effect of IIV-HD compared to IIV-SD with a relative vaccine effectiveness (rVE) of 13% (95% CI: 1 to 24%)Footnote 44Footnote 45Footnote 46Footnote 47. They also identified 1 test-negative case-control study comparing IIV-HD to IIV-SD that found a rVE of 9% (95% CI: -12 to 27%)Footnote 43. The DSEN MAGIC did not find a difference between IIV3-HD and IIV3-SD for the prevention of outpatient visits for ILI in 1 RCT (relative vaccine efficacy of 3%, 95% CI: -14 to 18%)Footnote 64.
Evidence comparing IIV-Adj to IIV-SD against outpatient and/or ED visits for ILI from the ACIP review was inconsistent. Evidence derived from 2 observational studies indicated a beneficial protective effect of IIV-Adj compared to IIV-SD (pooled rVE of 36%, 95% CI: 21 to 48%)Footnote 42Footnote 48. However, evidence derived from 2 retrospective cohort studies did not identify a difference between IIV-Adj and IIV-SD for the prevention of outpatient and/or ED visits for ILI (pooled rVE of 0%, 95% CI: -3 to 3%)Footnote 45Footnote 49.
Summary of vaccine efficacy/effectiveness against influenza-associated hospitalizations
Overall, ACIP included 4 RCTsFootnote 38Footnote 39Footnote 40Footnote 72 and 15 observational studiesFootnote 45Footnote 47Footnote 50Footnote 51Footnote 52Footnote 53Footnote 54Footnote 55Footnote 56Footnote 57Footnote 58Footnote 59Footnote 60Footnote 61Footnote 62 reporting data on influenza-associated hospitalization including laboratory-confirmed, code-based, and clinical case definitions. Of those, 13 compared IIV3-HD to IIV-SDFootnote 38Footnote 39Footnote 40Footnote 45Footnote 47Footnote 50Footnote 51Footnote 52Footnote 53Footnote 54Footnote 55Footnote 56Footnote 57, 7 compared IIV3-Adj to IIV-SDFootnote 45Footnote 51Footnote 58Footnote 59Footnote 60Footnote 61Footnote 72, and 1 compared RIV to IIV-SDFootnote 51. The DSEN MAGIC included 3 RCTsFootnote 33Footnote 64Footnote 66 comparing IIV-HD to IIV-SD and 1 comparing RIV to IIV-SDFootnote 34 against ILI (n=2) and LCI (n=3) hospitalization.
In the ACIP review, most data were available for influenza hospitalizations among all outcomes examined. Their evidence demonstrated protective beneficial effects for IIV-HD, IIV-Adj, and RIV when compared to IIV-SD, though the depth of data varied as most of the data were for IIV-HD (n=13), less for IIV-Adj (n=7), and least for RIV (n=1). The DSEN MAGIC review also demonstrated a beneficial protective effect of IIV-HD compared to IIV-SD against ILI hospitalization (pooled relative vaccine efficacy of 28%, 95%: 8 to 43%)Footnote 33Footnote 64. Evidence for IIV-HD (relative vaccine efficacy of 40%, 95% CI: -65 to 78%, and 0%, 95% CI: -1570 to 94%)Footnote 33Footnote 66 and RIV (relative vaccine efficacy of 67%, 95% CI: -221 to 96%)Footnote 34 compared to IIV-SD against hospitalization for LCI was only available from single studies in the DSEN MAGIC review and did not demonstrate protective effects as estimates were imprecise with large confidence interval.
Summary of vaccine efficacy/effectiveness against influenza-associated deaths
Overall, ACIP included 2 retrospective cohort studiesFootnote 46Footnote 59 comparing IIV3-HD to IIV-SD, and the DSEN MAGIC included 1 RCT comparing IIV-Adj to IIV-SD against influenza-associated deaths defined by clinical diagnosis codesFootnote 35.
ACIP demonstrated a beneficial protective effect of IIV3-HD compared to IIV-SD against influenza-associated deaths (pooled rVE of 31%, 95% CI: 16 to 43%)Footnote 46Footnote 59. The DSEN MAGIC identified a study reporting a point estimate of lower influenza related deaths for IIV3-Adj compared with IIV3-SD, though the effect was very imprecise with wide confidence intervals (vaccine efficacy of 25%, 95% CI: -236 to 83%) Footnote 35.
Summary of vaccine efficacy/effectiveness against vascular events
Data on influenza-associated vascular events, which include various cardiovascular outcomes associated with influenza infection, as defined by the individual study (e.g., myocardial infarction, heart failure, stroke, etc.) were only available from the DSEN MAGIC review. Overall, they included 7 RCTs reporting data on vascular eventsFootnote 33Footnote 34Footnote 35Footnote 64Footnote 67Footnote 68Footnote 73. Of those, 4 compared IIV3-HD to IIV-SDFootnote 33Footnote 64Footnote 68Footnote 73, 2 compared IIV3-Adj to IIV-SDFootnote 35Footnote 67, and 1 compared RIV to IIV-SDFootnote 34. The 3 vaccines IIV-HD (pooled rate ratio of 0.75, 95% CI: 0.43 to 1.29), IIV-Adj (pooled rate ratio of 0.83, 95% CI: 0.54 to 1.27) and RIV (odds ratio of 0.89, 95% CI: 0.30 to 2.60) were associated with a lower number of vascular events compared to IIV-SD, though the associations were not statistically significant with wide confidence intervals.
Efficacy and effectiveness of high dose, recombinant and adjuvanted influenza vaccines compared to one another
Summary of study characteristics
The ACIP systematic review identified 7 studies (1 RCT and 6 observational studies) that assessed the efficacy/effectiveness of IIV-HD, IIV-Adj and RIV against one another. Of those, 2 reported data comparing IIV3-Adj to RIV4Footnote 37Footnote 51, 7 reported data comparing IIV3-HD to IIV3-AdjFootnote 37Footnote 45Footnote 49Footnote 50Footnote 51Footnote 62Footnote 63 and 2 reported data comparing IIV3-HD to RIV4Footnote 37Footnote 51. Their systemic review provided data on LCI (n=1), influenza-associated outpatient and/or ED visits (n=3), and influenza-associated hospitalization (n=4).
The DSEN MAGIC identified 2 RCTs reporting on the efficacy of IIV-HD, IIV-Adj and RIV with one another against LCI. Of those studies, 1 reported data comparing IIV3-HD to IIV3-Adj and RIV4Footnote 37, and another reported data comparing IIV3-HD to IIV3-AdjFootnote 74.
No studies were identified that compared the efficacy/effectiveness of these vaccines with one another against influenza-associated deaths and vascular events. Few studies reported data comparing the efficacy/effectiveness of IIV-HD, IIV-Adj and RIV against one another thus limiting the generalizability of findings to all or most influenza seasons.
Summary of vaccine efficacy/effectiveness against laboratory-confirmed influenza
The ACIP review identified a single RCT that compared the efficacy of IIV-HD vs IIV-Adj (relative vaccine efficacy of 66%, 95% CI: -213 to 96%), IIV-HD vs RIV (relative vaccine efficacy of 74%, 95% CI: -118 to 97%) and IIV-Adj vs RIV (relative vaccine efficacy of 25%, 95% CI: -207 to 82%) against LCIFootnote 37. Nevertheless, the study did not demonstrate a beneficial protective effect associated with IIV-HD, IIV-Adj, or RIV compared with one another due to the important imprecision associated with these vaccine efficacy estimates.
The DSEN MAGIC review identified 2 RCTs comparing IIV-HD to IIV-Adj (relative vaccine efficacy of -210%, 95% CI: -3,080 to 70%), RIV to IIV-Adj (relative vaccine efficacy of 28%, 95% CI: -254 to 85%), and RIV to IIV-HD (relative vaccine efficacy of 77%, 95% CI: -121 to 98%) against LCIFootnote 37Footnote 74. Similar to the ACIP review, the studies did not demonstrate a beneficial protective effect against LCI associated with IIV-HD, IIV-Adj, or RIV compared with one another due to the wide confidence intervals associated with the vaccine efficacy estimates.
Summary of vaccine efficacy/effectiveness against influenza-associated outpatient and/or emergency department visits
The ACIP review included 3 retrospective cohort studies comparing IIV3-HD to IIV3-Adj against influenza-associated outpatient and/or ED visits defined using diagnostic and procedural codes associated with a prescription of antiviral (i.e., oseltamivir)Footnote 45Footnote 49Footnote 63. A meta-analysis involving those studies did not demonstrate a beneficial protective effect against influenza-associated outpatient and/or ED visits with IIV-HD over IIV-Adj (pooled rVE of –6%, 95% CI: -23 to 8%).
The DSEN MAGIC review did not identify any RCTs reporting data on influenza-associated outpatient visits comparing IIV-HD, IIV-Adj, and RIV with one another.
Summary of vaccine efficacy/effectiveness against influenza-associated hospitalization
The ACIP review identified 4 retrospective cohort studies reporting data on influenza-associated hospitalizations defined by clinical diagnosis codes comparing IIV3-HD to IIV3-Adj (n=4)Footnote 45Footnote 50Footnote 51Footnote 62, IIV3-HD to RIV4 (n=1)Footnote 51 and IIV3-Adj to RIV4 (n=1)Footnote 51. All of these studies assessed influenza associated hospitalizations through diagnostic codes. The DSEN MAGIC review did not identify any RCTs reporting data on influenza-associated hospitalization comparing IIV-HD, IIV-Adj, and RIV to one another.
One (1) retrospective cohort study demonstrated a relative benefit of RIV compared to IIV-HD and IIV-Adj against influenza-associated hospitalizations during the 2019-20 influenza seasonFootnote 51. A meta-analysis of 4 observational studies conducted over 4 influenza seasons did not find a difference between IIV3-HD and IIV3-Adj against influenza-associated hospitalization (rVE of 4%, 95% CI: -1 to 10%)Footnote 45Footnote 50Footnote 51Footnote 62.
Vaccine safety
Safety outcomes evaluated in the systematic review conducted by the US ACIP were rated as low to very low and most were downgraded for imprecision due to low number of events, small sample size, and wide confidence intervals around the effect estimateFootnote 14. For additional details regarding the summary of findings and assessments of the quality of the evidence please refer to GRADE: Higher Dose and Adjuvanted Influenza Vaccines for Persons Aged ≥65 Years and to the Evidence to Recommendations (EtR) Framework: Higher Dose and Adjuvanted Influenza Vaccines for Persons Aged ≥65 Years.
Please note that subgroup analyses on vaccine safety were conducted on the overall population of adults 65 years of age and older, and not by risk groups (e.g., population with comorbid conditions, sex, previous vaccination, and adults 80 years of age and older) due to data limitations, including the number of studies reporting for each outcome.
Vaccine safety of high dose, recombinant and adjuvanted influenza vaccines compared to standard-dose inactivated influenza vaccines
Summary of study characteristics
The ACIP review included 23 RCTsFootnote 33Footnote 34Footnote 35Footnote 36Footnote 39Footnote 67Footnote 68Footnote 71Footnote 73Footnote 75Footnote 76Footnote 77Footnote 78Footnote 79Footnote 80Footnote 81Footnote 82Footnote 83Footnote 84Footnote 85Footnote 86Footnote 87Footnote 88 and 1 retrospective cohort studyFootnote 89 that reported safety data comparing IIV-HD, IIV-Adj, and RIV to IIV-SD in adults 65 years of age and older. Of those, 8 compared IIV-HD to IIV-SDFootnote 33Footnote 36Footnote 39Footnote 68Footnote 73Footnote 78Footnote 86Footnote 87, 12 compared IIV-Adj to IIV-SDFootnote 35Footnote 67Footnote 71Footnote 75Footnote 79Footnote 80Footnote 81Footnote 82Footnote 85Footnote 87Footnote 88Footnote 89, and 7 compared RIV to IIV-SDFootnote 34Footnote 71Footnote 76Footnote 77Footnote 83Footnote 84Footnote 87. Their systematic review provided data on any solicited systemic events grade 3 or higher (n=7), Guillain-Barré Syndrome (n=4), any serious adverse events (n=18), and any solicited injection site events grade 3 or higher (n=6).
Summary of vaccine safety
Any solicited systemic events grade 3 or higher following immunization
The ACIP review included 7 RCTs reporting data on solicited systemic adverse events grade 3 or higher comparing HD-IIV3 (n=3)Footnote 36Footnote 86Footnote 87, IIV3-Adj (n=5) Footnote 67Footnote 71Footnote 75Footnote 87Footnote 88 or RIV3 (n=1)Footnote 87 to IIV-SD in adults 65 years of age and older. Together, these studies showed that HD-IIV3, IIV3-Adj and RIV3 may lead to a decrease in solicited systemic adverse events grade 3 or higher when compared to IIV-SD though all the estimates lack precision (pooled risk ratio [RR] of 0.95, 95% CI: 0.20 to 4.53, 0.77, 95% CI: 0.34 to 1.76, and RR of 0.28, 95% CI: 0.05 to 1.67, respectively).
Guillain-Barré syndrome
The ACIP review included 2 RCTsFootnote 35Footnote 39 and 2 observational studiesFootnote 77Footnote 89 reporting data on Guillain-Barré Syndrome (GBS) comparing IIV3-HD, IIV3-Adj or RIV3 to IIV-SD. One (1) RCT comparing IIV3-HD (n=2,606) to IIV-SD (n=2,604) did not identify any cases of GBS among 5,210 vaccine recipientsFootnote 39. One (1) RCT found a non-significant decreased risk of GBS with IIV3-Adj compared to IIV-SD (RR of 0.33, 95% CI: 0.01 to 8.16)Footnote 35. One (1) observational study comparing IIV3-Adj (n=88,449) to IIV-SD (n=82,539) did not identify any cases of GBS among 170,988 vaccine recipientsFootnote 89. Another observational study comparing RIV3 to IIV3-SD identified 4 GBS cases among 283,683 IIV3-SD recipients and none among 21,976 RIV3 recipientsFootnote 77. Of note, as GBS occurs very rarely in the general population, it is not expected that studies of these sizes would be sufficiently powered to detect a difference in the risk of GBS between groups.
Any serious adverse events (SAE) following immunization
The ACIP review included 18 RCTs reporting data on any SAE comparing IIV3-HD (n=7)Footnote 33Footnote 36Footnote 39Footnote 68Footnote 73Footnote 78Footnote 87, IIV3-Adj (n=8)35}Footnote 67Footnote 79Footnote 80Footnote 81Footnote 82Footnote 85Footnote 87 or RIV3 (n=5)Footnote 34Footnote 71Footnote 83Footnote 84Footnote 87 to IIV-SD in adults 65 years of age and older. A meta-analysis of 7 RCTs showed that IIV-HD was associated with a lower risk of SAE compared to IIV-SD (pooled RR of 0.91, 95% CI: 0.85 to 0.97). No differences were observed in SAE with IIV-Adj and RIV compared to IIV-SD though the estimates lacked precision (pooled RR of 1.07, 95% CI: 0.92 to 1.26 and 1.03, 95% CI: 0.84 to 1.26, respectively).
Any solicited injection site events grade 3 or higher following immunization
The ACIP review included 6 RCTs reporting solicited injection site events grade 3 or higher comparing IIV3-HD, IIV3-Adj or RIV to IIV-SD in adults 65 years of age and olderFootnote 36Footnote 67Footnote 71Footnote 85Footnote 87Footnote 88. A meta-analysis of 4 RCTs showed that IIV3-Adj led to an increase in reactogenicity events compared to IIV-SD (pooled RR of 3.39, 95% CI: 1.32 to 8.72)Footnote 67Footnote 85Footnote 87Footnote 88. Similarly, a meta-analysis of 2 RCTs showed that IIV3-HD may lead to an increase in reactogenicity events compared to IIV-SD though the estimate lacked precision (pooled RR of 5.03, 95% CI: 0.88 to 28.74)Footnote 36Footnote 87. Conversely, results suggested that RIV may lead to a decrease in solicited injection sites events grade 3 or higher when compared to IIV-SD, however the estimates also lacked precision (pooled RR of 0.67, 95% CI: 0.27 to 1.69)Footnote 71Footnote 87.
Vaccine safety of high dose, recombinant and adjuvanted influenza vaccines compared to one another
Summary of study characteristics
The ACIP review included 3 RCTs reporting safety data comparing IIV3-HD, IIV3-Adj, and RIV4 to one anotherFootnote 76Footnote 87Footnote 90. Of those, 2 compared IIV3-HD to IIV3-AdjFootnote 76Footnote 87, 2 compared IIV3-HD to RIV4Footnote 87Footnote 90, and 1 compared RIV4 to IIV3-AdjFootnote 87. Their systematic review provided data on any solicited adverse events grade 3 or higher (n=3), any serious adverse events (n=3), and any solicited injection site events grade 3 or higher (n=3). No study comparing IIV3-HD, IIV3-Adj, and RIV4 to one another with data on GBS was identified in this review.
Summary of vaccine safety
Any solicited systemic adverse events grade 3 or higher following immunization
The ACIP review included 3 RCTs reporting data on solicited systemic adverse events grade 3 or higher comparing IIV3-HD, IIV3-Adj, and RIV4 to one anotherFootnote 76Footnote 87Footnote 90. Two (2) meta-analyses showed that IIV3-HD was less likely to cause solicited systemic adverse events compared to IIV3-Adj (n=2)Footnote 76Footnote 87 and RIV4 (n=2)Footnote 87Footnote 90, however both estimates were imprecise (pooled RR of 0.73, 95% CI: 0.29 to 1.80, and pooled RR of 0.86, 95% CI: 0.22 to 3.32, respectively). Additionally, 1 RCT reported that IIV3-Adj may lead to an increased risk of solicited systemic adverse events when compared to RIV3 though the estimate lacked precision (RR of 4.62, 95% CI: 0.24 to 89.17)Footnote 87.
Any serious adverse events (SAEs) following immunization
The ACIP review included 3 RCTs reporting on SAEs comparing IIV3-HD to IIV3-Adj (n=2)Footnote 76Footnote 87, IIV3-HD to RIV4 (n=2)Footnote 87Footnote 90 and IIV3-Adj to RIV4 (n=1)Footnote 87. One (1) meta-analysis and 1 single study showed that IIV3-HD and IIV3-Adj were associated with higher risks of SAEs when compared to RIV4, however the estimates lacked precision (pooled RR of 1.77, 95% CI: 0.73 to 4.27 and RR of 1.81, 95% CI: 0.58 to 5.65, respectively). Additionally, 1 meta-analysis reported that IIV3-HD may be associated with a lower risk of SAEs compared to IIV3-Adj though the pooled estimate also lacked precision (pooled RR of 0.65, 95% CI: 0.32 to 1.30).
Any solicited injection site events grade 3 or higher following immunization
The ACIP review included 3 RCTs that reported data on solicited injection site events grade 3 or higher comparing IIV3-HD to IIV3-Adj (n=2)Footnote 76Footnote 87, IIV3-HD to RIV4 (n=2)Footnote 87Footnote 90, and IIV3-Adj to RIV4 (n=1)Footnote 87. One (1) meta-analysis and 1 RCT showed that IIV3-HD and IIV3-Adj may be associated with more reactogenicity compared to RIV4, however the estimates lacked precision (pooled RR of 5.92, 95% CI: 0.32 to 109.56, and RR of 4.62, 95% CI: 0.24 to 89.17, respectively). Additionally, 1 meta-analysis reported that IIV3-HD may be associated with less reactogenicity compared to IIV3-Adj though the estimate also lacked precision (pooled RR of 0.88, 95% CI: 0.45 to 1.75).
Economics
Two (2) economic analyses are summarized below. The first is a published systematic review of the cost-effectiveness of influenza vaccination among adults 65 years of age and olderFootnote 91. Studies on influenza vaccines approved for use in the United States or in Canada published as full-text peer reviewed articles up to October 29, 2020, were included. All included studies compared the cost-effectiveness of quadrivalent or high-dose/adjuvanted vaccine strategies to an IIV3-SD strategy. The second is an economic evaluation published by the Comité sur l'immunisation du Québec (CIQ)Footnote 92.
Systematic review of economic evaluations
A high-level overview of the published systematic reviewFootnote 91 is presented here with additional NACI commentary. All costs are reported in 2019 Canadian dollars. For a primer on the interpretation of economic evaluation findings and cost-effectiveness thresholds, please refer to the NACI interpretation guide to health economics for decision-makersFootnote 93. A brief overview of key terminology is presented in Appendix A.
Summary of included studies
Overall, 19 studies, consisting of 16 cost-utility analysesFootnote 94Footnote 95Footnote 96Footnote 97Footnote 98Footnote 99Footnote 100Footnote 101Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109, 2 cost-benefit analysesFootnote 110Footnote 111 and 1 cost-effectiveness analysisFootnote 112, were included. Among the included studies, 8 were conducted in North AmericaFootnote 91Footnote 92Footnote 96Footnote 97Footnote 101Footnote 103Footnote 106Footnote 107, 5 were conducted in EuropeFootnote 97Footnote 98Footnote 99Footnote 108Footnote 112, 5 were conducted in AsiaFootnote 94Footnote 102Footnote 103Footnote 104Footnote 106, and 1 was conducted in South AmericaFootnote 109. All studies but 1Footnote 112 were published between 2014 and 2020. All included studies were appraised to be of high (n=13)Footnote 94Footnote 95Footnote 96Footnote 97Footnote 100Footnote 101Footnote 102Footnote 103Footnote 104Footnote 105Footnote 107Footnote 108Footnote 109 or moderate (n=6)Footnote 98Footnote 99Footnote 106Footnote 110Footnote 111Footnote 112 quality.
Figure 1 shows the comparisons of vaccine products made (note multiple comparisons were possible within 1 study).
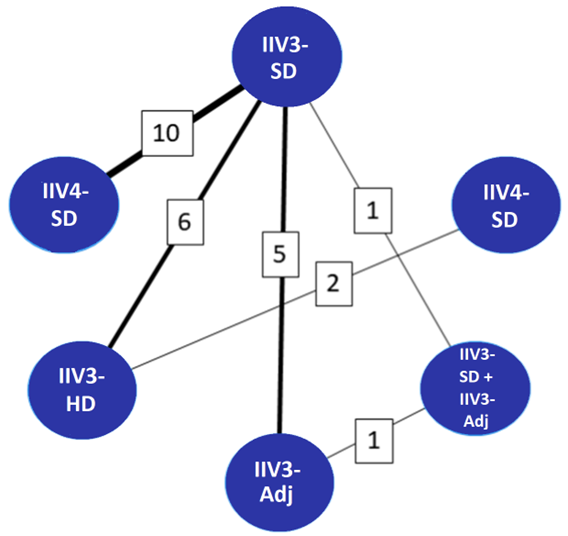
Figure 1 : Descriptive text
| Pairwise vaccine comparisons (n=6)Footnote a | Number of studies reporting on each comparisonFootnote b |
|---|---|
| IIV4-SD and IIV3-SD | 10 |
| IIV3-SD and IIV3-HD | 6 |
| IIV3-SD and IIV3-Adj | 5 |
| IIV3-HD and IIV4-SD | 2 |
| IIV3-SD and IIV3-SD + IIV3-Adj | 1 |
| IIV3-Adj and IIV3-SD + IIV3-Adj | 1 |
| Total pair-wise vaccine comparisons | 6 |
| Total number of studies included | 19 |
|
|
Note: "IIV3-SD + IIV3-Adj" refers to IIV3-SD for individuals aged 65 to 74 years and IIV3-Adj for adults aged 75 years of age and olderFootnote 98; IIV4-SD vs IIV3-SDFootnote 94Footnote 97Footnote 99Footnote 101Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 108; IIV3-HD vs IIV3-SDFootnote 95Footnote 96Footnote 100Footnote 107Footnote 110Footnote 111; IIV3-Adj vs IIV3-SDFootnote 99Footnote 104Footnote 108Footnote 109Footnote 112; IIV3-HD vs IIV4-SDFootnote 96Footnote 107. Line thickness represents the number of studies reporting data for a given comparison, also indicated in boxes.
The following perspectives were adopted for analyses:
- Societal perspective: n=13studiesFootnote 94Footnote 95Footnote 96Footnote 97Footnote 99Footnote 100Footnote 101Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107
- Healthcare payer perspective: n=12 studies
- Healthcare provider perspective: n=1 studyFootnote 102
The time horizon used for analysis in the included studies varied from 1 influenza seasonFootnote 110 to lifetimeFootnote 97Footnote 99Footnote 104, with 6 studiesFootnote 94Footnote 95Footnote 96Footnote 100Footnote 107Footnote 112 applying different time horizons for costs and effects (e.g., a time horizon of 1 influenza season for cost and a lifetime time horizon for effect). Four (4) studies did not report the time horizon used for analysisFootnote 98Footnote 101Footnote 102Footnote 111.
Most studies were funded by industry (n=13)Footnote 95Footnote 96Footnote 97Footnote 99Footnote 100Footnote 101Footnote 103Footnote 105Footnote 108Footnote 109Footnote 110Footnote 111Footnote 112. Three (3)Footnote 98Footnote 104Footnote 107 were supported by public funding sources and 1 reported a mix of both industry and public fundingFootnote 94. Two (2) studies did not specify the funding sourcesFootnote 102Footnote 106.
Model-specific appraisal
Key model parameters included influenza vaccine coverage, influenza attack rate, influenza-related complications (e.g., pneumonia, bronchitis, cardiovascular disease, central nervous system complications), need for prescription drugs to treat influenza-related complications, medical consultations, ED visits, hospitalizations, and influenza-associated mortality. Influenza vaccine coverage ranged from 27%Footnote 94 to 82%Footnote 103Footnote 104 across the included studies.
A minority of studies accounted for cross protection (n=5)Footnote 97Footnote 99Footnote 101Footnote 104Footnote 105 and community immunity (i.e., herd effect) (n=1)Footnote 105. No studies accounted for frailty, vaccine wastage, the availability of multi-dose and single-dose formats, and the availability of various influenza vaccines on the market.
Summary of results
A summary of the findings from included cost-utility studies is provided in Table 1 (n=16)Footnote 94Footnote 95Footnote 96Footnote 97Footnote 98Footnote 99Footnote 100Footnote 101Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109. In general, IIV4-SD, IIV3-Adj, and IIV3-HD were found to be cost-effective options compared to IIV3-SD from the healthcare and societal perspectives. The included cost-effectiveness analysis (n=1)Footnote 112 and cost-benefit analyses (n=2)Footnote 110Footnote 111 had similar conclusions which were that IIV3-HD and IIV3-Adj were cost-effective compared to IIV3-SD.
| Outcome | IIV4-SD vs IIV3-SD | IIV3-Adj vs IIV3-SD | IIV3-HD vs IIV3-SD | IIV3-HD vs IIV4-SD | IIV-SD+IIV-Adj vs IIV-SD | IIV-Adj vsIIV-SD+IIV-Adj |
|---|---|---|---|---|---|---|
| Healthcare payer perspective | ||||||
| Number of ICERs | 4Footnote 97Footnote 99Footnote 101Footnote 108 | 2Footnote 108Footnote 109 | 3Footnote 95Footnote 96Footnote 100 | 1Footnote 96 | 1Footnote 98 | 1Footnote 98 |
| ICER (Minimum) | $28,524/ QALY gainedFootnote 97 | $3,406/ QALY gainedFootnote 109 | IIV3-HD dominated IIV3-SDFootnote 95Footnote 100 | $5,709/ QALY gainedFootnote 96 | $9,771/ QALY gainedFootnote 98 | $13,804/ QALY gainedFootnote 98 |
| ICER (Maximum) | $224,000/ QALY gainedFootnote 108 | $7,692/ QALY gainedFootnote 108 | $13,537/ QALY gainedFootnote 96 | - | - | - |
| Proportion of estimates CE at $10,000/QALY | 0% | 100%Footnote 108Footnote 109 | 67%Footnote 95Footnote 100 | 100% Footnote 96 | 100%Footnote 98 | 0% |
| Proportion of estimates CE at $40,000/QALY | 75%Footnote 96Footnote 97Footnote 99 | 100%Footnote 108Footnote 109 | 100%Footnote 95Footnote 96Footnote 100 | 100%Footnote 96 | 100%Footnote 98 | 100%Footnote 98 |
| Proportion of estimates CE at $50,000/QALY | 75%Footnote 96Footnote 97Footnote 99 | 100%Footnote 108Footnote 109 | 100%Footnote 95Footnote 96Footnote 100 | 100%Footnote 96 | 100%Footnote 98 | 100%Footnote 98 |
| Societal perspective | ||||||
| Number of ICERs | 9Footnote 94Footnote 97Footnote 99Footnote 101Footnote 102 Footnote 103Footnote 104Footnote 105Footnote 106 | 2Footnote 99Footnote 104 | 4Footnote 95Footnote 96Footnote 100Footnote 107 | 2Footnote 96Footnote 107 | 0 | 0 |
| ICER (Minimum) | IIV4-SD dominated IIV3-SDFootnote 103Footnote 105 | IIV3-Adj dominated IIV3-SDFootnote 99Footnote 104 | IIV3-HD dominated IIV3-SDFootnote 95Footnote 100 | IIV3-HD dominated IIV4-SDFootnote 96 | - | - |
| ICER (Maximum) | $55,865/QALY gainedFootnote 106 | - | $36,967/QALY gainedFootnote 107 | $40,824/QALY gainedFootnote 107 | - | - |
| Proportion of estimates CE at $10,000/QALY | 33%Footnote 94Footnote 103Footnote 105 | 100%Footnote 99Footnote 104 | 75%Footnote 95Footnote 100Footnote 101 | 50%Footnote 96 | - | - |
| Proportion of estimates CE at $40,000/QALY | 89%Footnote 94Footnote 97Footnote 99Footnote 101Footnote 102 Footnote 103Footnote 104Footnote 105 | 100%Footnote 99Footnote 104 | 100%Footnote 95Footnote 100Footnote 101Footnote 107 | 50%Footnote 96 | - | - |
| Proportion of estimates CE at $50,000/QALY | 89%Footnote 94Footnote 97Footnote 99Footnote 101 Footnote 102Footnote 103Footnote 104Footnote 105 | 100%Footnote 99Footnote 104 | 100%Footnote 95Footnote 100Footnote 101Footnote 107 | 100%Footnote 107 | - | - |
| Healthcare provider perspective | ||||||
| Number of ICERs | 1Footnote 102 | 0 | 0 | 0 | 0 | 0 |
| ICER (Minimum) | $29,562/QALY gainedFootnote 102 | - | - | - | - | - |
| ICER (Maximum) | - | - | - | - | - | - |
| Proportion of estimates CE at $10,000/QALY | 0% | - | - | - | - | - |
| Proportion of estimates CE at $40,000/QALY | 100%Footnote 102 | - | - | - | - | - |
| Proportion of estimates CE at $50,000/QALY | 100%Footnote 102 | - | - | - | - | - |
Abbreviations: CE, cost-effective; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year Notes: "IIV3-SD + IIV3-Adj" refers to IIV3-SD for individuals 65 to 74 years of age and IIV3-Adj for adults 75 years of age and olderFootnote 98. "Intervention A dominated Intervention B" means that Intervention A is less costly and more effective than Intervention B. |
||||||
Results from studies considered highly generalizable to a Canadian setting
Four (4) studies were considered highly generalizable to a Canadian setting: 2 were conducted in CanadaFootnote 95Footnote 101 and 2 were conducted in the United Kingdom (UK)Footnote 97Footnote 98, of which, only 1 was not industry-fundedFootnote 98. Details on how generalizability was assessed can be found in Appendix B. The findings from the 4 studies are presented below.
Healthcare payer perspective: All 4 studies conducted analyses from the healthcare payer perspective (Table 2)Footnote 95Footnote 97Footnote 98Footnote 101. Comparing a IIV4-SD to a IIV3-SD strategy, the incremental cost-effectiveness ratio (ICER) estimates ranged from $28,524Footnote 97 to $39,599Footnote 101 per quality-adjusted life year (QALY) gained. These estimates can be considered cost-effective under commonly used thresholds (Appendix A). Comparing the IIV3-HD strategy to the IIV3-SD strategy in Canada, the IIV3-HD strategy was less costly and more effective among adults (1) 65 years of age and older, (2) living with a cardiorespiratory condition, and (3) living with 1 or more comorbid conditionsFootnote 95. Further, the IIV3-HD strategy was cost-effective among adults 75 years and older compared to the IIV3-SD strategyFootnote 95. Comparing a mixed intervention approach (IIV3-SD for individuals 65 to 74 years of age and IIV3-Adj for adults 75 years of age and older) to IIV3-Adj and to IIV3-SD, the mixed approach was cost-effective under commonly used thresholds ($9,771 per QAFootnote 99 to $13,084 per QALY)Footnote 98.
Societal perspective: Three (3) studies conducted analyses from the societal perspective (Table 2)Footnote 95Footnote 97Footnote 101. Comparing an IIV4-SD strategy to an IIV3-SD strategy, the ICER estimates ranged from $26,288Footnote 101 to $36,115Footnote 97 per QALY gained. Comparing an IIV3-HD strategy to an IIV3-SD strategy in Canada, IIV3-HD strategy was less costly and more effective in adults (1) 65 years of age and older, (2) 75 years of age and older, (3) living with a cardiorespiratory condition, and (4) living with 1 or more comorbid conditionsFootnote 95.
Estimated ICER values were consistently lower from a societal perspective than a healthcare payer perspective. The former often included productivity loss for patients and/or caregivers.
| Author, year, country | Funding | Population | Time horizon | Findings: Healthcare payer perspective | Findings: Societal perspective |
|---|---|---|---|---|---|
| IIV4-SD (intervention) vs IIV3-SD (comparator) | |||||
| Chit et al., 2015aFootnote 101, Canada | Sanofi Pasteur (Industry) | Adults 65 years of age and older | Not reported | $39,599/QALY gained | $36,115/QALY gained |
| Meier et al., 2015Footnote 97, UK | GlaxoSmithKline Biologicals SA (Industry) | Adults 65 years of age and older | Lifetime | $28,524/QALY gained | $26,288/QALY gained |
| IIV3-HD (intervention) vs IIV3-SD (comparator) | |||||
| Becker et al., 2016Footnote 95, Canada | Sanofi Pasteur (Industry) | Adults 65 years of age and older | One (1) influenza season for cost and lifetime for effect | All participants 65 years of age and older: IIV3-HD dominated IIV3-SD Subgroup analyses Participants 75 years of age and older: $87/QALY gained Participants living with a cardiorespiratory condition: IIV3-HD dominated IIV3-SD Participants living with 1 or more comorbidities: IIV3-HD dominated IIV3-SD |
All participants 65 years of age and older: IIV3-HD dominated IIV3-SD Subgroup analyses Participants 75 years of age and older: IIV3-HD dominated IIV3-SD Participants living with a cardiorespiratory condition: IIV3-HD dominated IIV3-SD Participants living with 1 or more comorbidities: IIV3-HD dominated IIV3-SD |
| IIV3-SD + IIV3-Adj (intervention)Footnote a vs IIV3-SD (comparator) | |||||
| Thorrington et al., 2019Footnote 98, UK | Multiple sources (Public) | Adults 65 years of age and older | Not reported | $9,771/QALY gained | Analysis not conducted |
| IIV3-Adj (intervention) vs IIV3-SD + IIV3-Adj (comparator)Footnote a | |||||
| Thorrington et al., 2019Footnote 98, UK | Multiple sources (Public) | Adults 65 years of age and older | Not reported | $13,084/QALY gained | Analysis not conducted |
Footnotes:
|
|||||
Results from studies considered less generalizable to a Canadian setting
A summary of the 15 studiesFootnote 94Footnote 96Footnote 99Footnote 100Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109Footnote 110Footnote 111Footnote 112 deemed to have limited generalizability to a Canadian setting can be found in Appendix C. The findings from these 15 studies were broadly similar to the 4 studies above. These studies also found IIV4-SD, IIV3-Adj, and IIV3-HD strategies to be cost-effective compared to an IIV3-SD strategy.
Results from studies on higher risk older adults
Table 3 shows the 5 studies that assessed older adults with a higher risk of influenza infection and/or complications such as those with comorbidities or in congregate livingFootnote 95Footnote 100Footnote 104Footnote 110Footnote 112. IIV3-HD and IIV3-Adj strategies were either cost-effective or less costly and more effective compared to an IIV3-SD strategy depending on the population. An IIV4-SD strategy was cost-effective compared to an IIV3-SD strategy under commonly used thresholds.
| Author, year, country | Funding | Study type | Time horizon | Findings: Healthcare payer perspective | Findings: Societal perspective |
|---|---|---|---|---|---|
| IIV4-SD (intervention) vs IIV3-SD (comparator) | |||||
| Yun et al., 2019Footnote 104, South Korea | Korea Centers for Disease Control and Prevention (Public) | Cost-utility | Lifetime for cost and effect | Analysis not conducted | Participants at high risk of seasonal influenza infection and/or influenza-related complications or hospitalizations: $1 327/QALY gained |
| IIV3-Adj (intervention) vs IIV3-SD (comparator) | |||||
| Yun et al., 2019Footnote 104, South Korea | Korea Centers for Disease Control and Prevention (Public) | Cost-utility | Lifetime for cost and effect | Analysis not conducted | Participants at high risk of seasonal influenza infection and/or influenza-related complications or hospitalizations: IIV3-Adj dominated IIV3-SD |
| Piercy et al., 2004Footnote 112, France | Chiron Vaccines (Industry) | Cost-effectiveness | One (1) year for cost and lifetime for effect | Participants suffering from heart or lung disease: $44,492 per death avoided $8,943 per life year gained |
Analysis not conducted |
| IIV3-HD (Intervention) vs IIV3-SD (ComparatoFootnote 96Becker et al., 2016Footnote 95, Canada | Sanofi Pasteur (Industry) | Cost-utility | One (1) influenza season for cost and lifetime for effect | Participants living with a cardiorespiratory condition: IIV3-HD dominated IIV3-SD Participants living with 1 or more comorbidities: IIV3-HD dominated IIV3-SD |
Participants living with a cardiorespiratory condition: IIV3-HD dominated IIV3-SD Participants living with 1 or more comorbidities: IIV3-HD dominated IIV3-SD |
| Chit et al., 2015bFootnote 100, US | Sanofi Pasteur (Industry) | Cost-utility | One (1) year for cost and lifetime for effect | Participants living with 1 or more comorbidities: IIV3-HD dominated IIV3-SD Participants living with a cardiorespiratory condition: IIV3-HD dominated IIV3-SD |
Participants living with 1 or more comorbidities: IIV3-HD dominated IIV3-SD Participants living with a cardiorespiratory condition: IIV3-HD dominated IIV3-SD |
| Shireman et al., 2019Footnote 110, US | Sanofi Pasteur (Industry) | Cost-benefit analysis | One (1) influenza season for cost and effect | Nursing home residents: Positive net monetary benefit | Analysis not conducted |
Influential factors that affect cost-effectiveness
Most of the included studies, apart from 2Footnote 105Footnote 106, conducted sensitivity analyses to test model assumptions and the robustness of study results. In most cases, the results from the sensitivity analyses supported the base case conclusions (i.e., interventions that were cost-effective under base case analysis remained cost-effective during sensitivity analyses). However, cost-effectiveness was found to be sensitive to a number of variables including mismatch between seasonal influenza vaccines and circulating strainsFootnote 97Footnote 99Footnote 102Footnote 103Footnote 104, relative vaccine effectiveness (e.g., against symptomatic disease, hospitalizations, mortality)Footnote 94Footnote 103Footnote 104, vaccine costsFootnote 102Footnote 104, level of vaccine cross protection in the event of type B lineage mismatch, and influenza mortality rateFootnote 99.
Discussion
The current review of economic evaluation studies summarizes the cost-effectiveness of seasonal influenza vaccines among adults 65 years of age and older. Studies on budget impact (i.e., analyses on the likely change in expenditure to a specific budget holder when a vaccination program is implemented) were not included. In total, 19 studies of moderate to high quality were included in this review. The directionality of cost-effectiveness results remained consistent across all studies. IIV4-SD, IIV3-HD, and IIV3-Adj strategies were cost-effective compared to an IIV3-SD strategy under commonly used thresholds. The findings of the current review are supported by 2 recent literature reviews in older adults conducted by Sugishita and Sugiwara (2021)Footnote 113 and Postma et al. (2023)Footnote 114.
A trend emerged in 2 studies showing that IIV4-SD became increasingly more cost-effective (i.e., lower ICER) compared to IIV3-SD with increasingly older age groupsFootnote 102Footnote 104. Generally, immune function declines with older age, resulting in the increased risk of influenza infection and influenza-related hospitalization and/or complications among older adultsFootnote 115Footnote 116Footnote 117Footnote 118. Additionally, the average number of comorbidities and level of frailty per individual tend to increase with older ageFootnote 119Footnote 120Footnote 121Footnote 122. Individuals living with 1 or more comorbidity and/or higher levels of frailty are at increased risk for more severe influenza-related outcomes, including hospitalizations, functional decline, and death following infectionFootnote 20Footnote 123Footnote 124Footnote 125. More effective vaccines administered to older adults can result in fewer influenza-related hospitalizations and complications, reducing associated healthcare utilization costs and resulting in more favourable cost-effectiveness results with increasing age.
Thirteen of the 19 included studies were conducted from a societal perspective, often incorporating productivity loss for the older adult and their caregiver(s). Other studies assumed that income loss was minimal among older adults who may be retired by 65 years of ageFootnote 126. The societal perspective resulted in additional cost-savings and lower ICER estimates.
Several studies conducted their analyses from a short time horizon based on the length of the influenza seasonFootnote 103Footnote 108Footnote 109Footnote 110. A short time horizon can be appropriate in many cases given that consequences and costs of influenza often occur within a single yearFootnote 127. However, long term consequences and costs associated with influenza infection would not be well-captured in these studies, such as long-term disability, loss of independence, reduced health-related quality of life, need for nursing home placement or home care, and death post-infectionFootnote 128Footnote 129.
No studies accounted for frailty in their analyses. Frailty is a complex, dynamic, multifactorial syndrome characterized by an increased risk of adverse outcomes compared to other individuals in the same age groupFootnote 129Footnote 130Footnote 131Footnote 132. While vaccine effectiveness and odds of recovery from influenza infection decline with increasing frailty, frailer adults tend to have increased vaccine coverageFootnote 133. A previous Canadian study conducted among older adults found that influenza vaccination provided good protection against influenza hospitalization among non-frail older adults, and those on the milder end of the frailty spectrumFootnote 133. Not adjusting for frailty tends to underestimate vaccine effectiveness in the older adult populationFootnote 133. The underestimation of vaccine effectiveness may subsequently impact cost-effectiveness results, particularly when indication bias is considered.
For instance, to improve immune response, frail older adults may be more likely to receive influenza vaccines designed specifically to enhance immunogenicity compared to non-frail older adults, and this indication bias is difficult to fully account for, especially in the absence of adjusting the models for frailty. The difference in type of influenza vaccine received between frail and non-frail adults (i.e., if frail older adults who are most at risk of adverse outcomes are also more likely to have received the enhanced vaccines) may underestimate the benefit of these vaccines compared to standard vaccine products, potentially underestimating their cost-effectiveness compared to standard vaccine strategies.
Four (4) of the 19 included studies (from Ontario, CanadaFootnote 95Footnote 101 and UKFootnote 97Footnote 98) were considered to be highly generalizable to a Canadian setting based on participant demographics, vaccine availability, and the healthcare resources consumed and associated costs, among other generalizability assessment criteria (Appendix B)Footnote 95Footnote 97Footnote 98Footnote 101. Several studies were conducted in non-OECD countries (n=4)Footnote 94Footnote 102Footnote 106Footnote 109, 1 of which was conducted in the southern hemisphereFootnote 109 and may have limited generalizability due to differing formulations of the vaccine products, healthcare costs, and differing seasonality of influenza. Although the US has similar demographics and influenza epidemiology compared to Canada, there are differences in healthcare systems (e.g., payer) and discrepancies in the cost of vaccines and healthcare services. For economic evaluations conducted from a societal perspective, out-of-pocket costs and productivity loss can also vary across countries and regions.
The reported ICERs comparing IIV4-SD to IIV3-SD strategies may be of limited generalizability to the present time and setting considering the absence of confirmed detections of B/Yamagata since March 2020. The review included studies up until 2020 when both the B/Victoria and B/Yamagata lineages were still circulating. The relevance of IIV4-SD cost-effectiveness for the future is unclear. Further, Health Canada authorizations and industry determine which products are available on the Canadian market, and so at present, provinces and territories do not choose between IIV4 or IIV3 for the standard dose vaccines.
The small variation in ICER estimates may be due to assumptions and data sources used for influential variables such as vaccine mismatch, vaccine effectiveness, vaccine costs, level of vaccine cross protection, vaccine coverage, and influenza mortality rate. In particular, vaccine effectiveness, seasonality, and the match between the vaccine and circulating influenza strains within a single country or region vary across influenza seasons and by type of vaccine product, so outcome estimates may vary depending on the year(s) of data used and the vaccine products compared.
Notably, only 1 of the 19 included studies accounted for potential benefits of community immunity (also known as herd immunity) arising from immunization of older adults in their analysisFootnote 105. Community immunity refers to the concept that once a certain proportion of the population is vaccinated against a specific disease, the remaining individuals in the population who are not immunized experience indirect protection against the disease because the infectious organism is less able to circulate. Its inclusion may be particularly important in economic evaluations of close congregate settings (e.g., nursing homes, long term care facilities) where residents are in close contact with shared caregivers and staffFootnote 134Footnote 135. A previously published conference abstract by Yang and Tan (2014) found that IIV4-SD cost US$35,851 more than IIV3-SD for each QALY gained when community immunity was not considered during data analysisFootnote 136. This ICER estimate decreased to US$32,660 per QALY gained when community immunity was incorporated into the analysisFootnote 136. Without accounting for community immunity, studies may be undervaluing vaccine effectiveness and the associated incremental benefit of vaccination, and subsequently undervaluing the cost-effectiveness of the vaccine.
Other factors including vaccine wastage (which may decrease vaccine availability and supply), and the availability of various influenza vaccines each season were not accounted for in any of the included studies. Vaccine wastage refers to the under-usage of purchased vaccines or the over-purchase of a vaccine product, which typically expires after each influenza season. As an example, in Canada, seasonal influenza vaccines are available in multi-dose and single-dose formatsFootnote 137. Compared to single-dose formats, multi-dose formats have been associated with increased safety concerns, increased risk of contamination, and potentially higher costs associated with waste disposal, storage, and vaccine wasteFootnote 138. The incorporation of programmatic factors such as vaccine wastage, the availability and use of multi-dose and single-dose formats, and the availability of different seasonal influenza vaccine products may impact costs, leading to changes in cost-effectiveness estimates.
The current review had several limitations. First, most of the identified economic evaluations on the use of seasonal influenza vaccines in older adults used IIV3-SD as the comparator. There is a need for future economic analyses to compare newer vaccine products with comparators that are currently available in Canada for older adults. For example, Canadian provinces such as Alberta, Ontario, Prince Edward Island, Saskatchewan, Nova Scotia, Yukon, and New Brunswick, among others, provide IIV4-HD to all adults 65 years of age and older as part of their publicly funded seasonal influenza vaccination programsFootnote 139Footnote 140Footnote 141Footnote 142Footnote 143Footnote 144. Other vaccine interventions of interest for comparison include IIV4-cc, RIV4, IIV3-Adj, IIV4-SD, and IIV4-Adj are needed. Second, the majority of the included studies were funded by industry. In this review, findings from industry-funded studies (n=13, 68%) were similar to non-industry-funded studies. There is also a lack of cost-effectiveness estimates by age subgroups, presence of comorbidities, and frailty within the older adult population. Finally, vaccine prices vary by product and jurisdiction. As such, vaccine prices used in studies conducted outside of Canada may not be applicable to a Canadian setting.
Economic evaluation by the Comité sur l'immunisation du Québec
A high-level overview of the Comité sur l'immunisation du Québec (CIQ) economic evaluation (cost-utility analysis) is presented below. The economic exercise was 1 component of CIQ's decision-making. A literature review of vaccine efficacy was also conducted, which found no head-to-head RCT comparing IIV3-Adj and IIV-SD against LCI and found the observational literature for IIV3-Adj was not of high quality. CIQ concluded that there is insufficient evidence to support that IIV3-Adj is superior to IIV-SD or that rVE of IIV3-Adj is similar to IIV-HD.
Decision problem and methods
The analysis assessed the cost-effectiveness of vaccinating the older adult population (65 years of age and older) in Quebec using enhanced vaccines compared to standard dose vaccines for the prevention of influenza morbidity and mortality. In the CIQ analyses, the terminology of "enhanced vaccines" was used, not specific to either high-dose or adjuvanted vaccines. Rather, they referred to vaccines that were designed to enhanced immunogenicity. As such, the same terminology is used here in the reporting of CIQ results. The enhanced vaccines had the following hypothetical characteristics: relative vaccine effectiveness compared to standard dose between 0% and 100% (base case 25%); price differential compared to standard dose between $0 and $50 (base case $30); duration of protection of 1 year. The standard dose vaccines had a VE of 40% (base case). The vaccination program had 100% coverage. The study population was stratified by age (65 to 74 years of age vs. 75 years of age and older) and by the presence of at least 1 chronic illness that increases the risk of influenza complications (specific chronic illnesses not listed; proportion of population with at least 1 condition 52% among 65 to 74 years of age, and 58% for 75 years of age and older).
The analysis was conducted from a publicly funded health system perspective, which included costs of hospitalization, ICU, medications, and the vaccination program. Costs and consequences of caregivers and productivity loss of the study population were not included. The analysis used burden data and other parameters based on a CIQ influenza report from 2018Footnote 145.
Summary of results
Results are presented as ICERs using QALYs as well as numbers of consultations, hospitalizations and deaths averted. All costs are reported in 2022 Canadian dollars. Results presented are discounted at 3% (which deviates from NACI and other Canadian recommendations of 1.5% for base case analysis), but also presented at 0% (in line with NACI and other Canadian recommendations for sensitivity analysis)Footnote 93Footnote 146. Discounting allows the analysis to account for time preferences (i.e., costs and consequences in the future are usually valued less than the present).
The base case results showed that among individuals 65 to 74 years of age, enhanced vaccines prevented 571 consultations, 155 hospitalization, and 4 deaths compared to standard dose vaccine. Among people 75 years of age and older, enhanced vaccines prevented 541 consultations, 533 hospitalizations, and 28 deaths. Table 4 shows the ICERs of enhanced vaccines compared to standard dose among older adults in Quebec, stratified by age and by presence of chronic illness. ICERs of non-base case analyses were not reported.
| Demographic | ICER ($ per QALY), discounted at 3% | ICER ($ per QALY), undiscounted |
|---|---|---|
| All ages (65 years of age and older) | NR | NR |
| 65 to 74 years of age, all | 609,927 | 480,604 |
| 65 to 74 years of age with chronic illness(es) | 345,297 | 270,784 |
| 65 to 74 years of age without chronic illness(es) | 2,648,381 | 2,166,967 |
| 75 years of age and older, all | 100,618 | 84,805 |
| 75 years of age and older with chronic illness(es) | 56,173 | 47,308 |
| 75 years of age and older without chronic illness(es) | 496,177 | 421,085 |
Abbreviations: ICER, incremental cost-effectiveness ratio; NR, not reported; QALY, quality-adjusted life year. Notes: Base case used a relative VE of 25% for enhanced vaccines, VE of 40% for standard dose, price differential of $30, duration of protection of 1 year, vaccine coverage of 100%. |
||
Individuals 75 years of age and older with chronic illness had the lowest ICER (i.e., most value for money) (discounted: $56,173 per QALY) among the stratified study populations. The other stratified groups had ICERs over 6 times that ICER (discounted range: $345,297 per QALY to $2,648,381 per QALY).
Discussion
Based on commonly used cost-effectiveness thresholds, the use of enhanced vaccines (relative VE of 25%) in Quebec does not appear to be cost-effective compared to standard dose. Of the 4 stratified groups, use of enhanced vaccines among individuals 75 years of age and older with chronic illness was the most cost-effective strategy compared to standard dose ($56,173 per QALY).
The presence of chronic illness appeared to drive the ICERs lower (i.e., better value for money), more so than older age. However, implementing a vaccination strategy to identify individuals by chronic illness may not be feasible. Conversely, implementing a vaccination strategy that is age-based does not appear cost-effective (ICERs for individuals 65 to 74 years of age versus 75 years of age and older were $609,927 per QALY and $100,618 per QALY, respectively) in this analysis.
While the analysis used a previously recommended discount rate of 3% instead of current recommendations of 1.5% (as of 2017 and 2023)Footnote 93Footnote 146, the discount rate does not appear to be an influential parameter as it did not change the results of enhanced vaccines from being not cost-effective to markedly cost-effective.
Results from the Quebec economic evaluation may be generalizable to other provinces and territories unless the decision-maker believes the epidemiology or health system costs are marked differently across Canadian jurisdictions. The authors of the analysis noted that the majority of model inputs were based on parameters from the CIQ influenza report from 2018Footnote 145; hence, it is possible that there are changes in disease burden or changes in the management of the health system not reflected in the analysis.
As noted, cost-effectiveness is 1 consideration in CIQ's decision-making. Based on the clinical literature review, CIQ found that there was insufficient evidence to support that vaccine efficacy of adjuvanted vaccine is superior to that of standard dose. Based on the totality of evidence, CIQ preferentially recommends high dose vaccine over adjuvanted vaccine and standard dose vaccine for people 75 years of age and older with chronic illnesses. Given implementation considerations, CIQ recommends that high dose vaccine may be offered to all people 75 years of age and older despite the much higher costs and lack of cost-effectiveness.
Discussion
The present statement comprehensively examines the available evidence on the efficacy, effectiveness, and safety of influenza vaccines designed to enhance protection in older adults (i.e., IIV-HD and IIV-Adj), as well as those leveraging technology and heightened antigen concentrations to increase immune responses (i.e., RIV).
The analysis focused on comparisons and evidence from studies conducted during regular influenza seasons. Only influenza vaccines approved for older adults were included, with the primary research question comparing IIV-HD, IIV-Adj, RIV and IIV-cc to IIV-SD, as well as against each other, to determine their relative performance in adults 65 years of age and older. Of note, no study included in this review compared IIV-cc to other influenza vaccines. RCTs identified in the DSEN MAGIC review that addressed IIV-cc did not report on critical outcomes or meaningful vaccine comparisons involving the desired vaccines of interest within their corresponding study armsFootnote 147Footnote 148Footnote 149. Furthermore, the review conducted by ACIP did not evaluate evidence on IIV-cc. Consequently, it was not possible to make a recommendation on the preferential use of IIV-cc in adults 65 years of age and older.
Overall, the results presented suggest a general trend favouring influenza vaccines aimed at enhancing protection for older adults, pointing to potential benefits associated with IIV-HD, IIV-Adj, and RIV when compared to IIV-SD, with most available evidence for IIV-HD. However, evidence comparing IIV-HD, IIV-Adj and RIV to one another is limited. Both the ACIP and DSEN MAGIC reviews found that IIV-HD was approximately 25% more effective than IIV-SD against LCI (relative vaccine efficacy of 24%, 95% CI: 10 to 36%; and pooled relative vaccine efficacy of 25%, 95% CI: 12 to 37%, respectively). For RIV, DSEN MAGIC and ACIP reported a pooled relative vaccine efficacy for RIV versus IIV-SD of 30% (95% CI: -18 to 58%) and 18% (95% CI: -17 to 43%) against LCI, respectively.
However, there were no studies available reporting on the efficacy or effectiveness of IIV-Adj compared to IIV-SD against LCI. ACIP reported 2 meta-analyses of observational studies comparing IIV-Adj to IIV-SD against influenza-associated outpatient/ED visits defined by clinical diagnosis. One (1) meta-analysis of 2 studies demonstrated a beneficial protective effect of IIV-Adj compared to IIV-SD against influenza-associated outpatient/ED visits (pooled rVE of 36%, 95% CI: 21 to 48%). However, another meta-analysis of 2 retrospective cohort studies did not identify a difference between IIV-Adj and IIV-SD against this outcome (pooled rVE of 0%, 95% CI: -9 to 3%).
Nevertheless, IIV-Adj demonstrated effectiveness in reducing influenza-associated hospitalizations compared to IIV-SD in 2 meta-analyses of observational studies (pooled rVE of 12%, 95% CI: 3 to 20% and 25%, 95% CI: 3 to 42%). There are few RCTs comparing IIV-HD, IIV-Adj, and RIV to one another against LCI. Notably, both reviews did not demonstrate a beneficial protective effect against LCI associated with IIV-HD, IIV-Adj and RIV compared to one another due to the wide confidence intervals associated with the vaccine efficacy estimates. While the results from both reviews suggest potential advantages associated with IIV-HD, IIV-Adj and RIV compared to IIV-SD, the current available evidence directly comparing these vaccines to one another is insufficient to establish with certainty that 1 vaccine consistently outperforms the others.
This review also examined the comparative safety of seasonal influenza vaccines. Data for IIV-HD, IIV-Adj, and RIV against IIV-SD demonstrated a comparable safety profile with regards to SAEs. However, recipients of IIV-HD and IIV-Adj exhibited a higher frequency of injection site and systemic events when compared with those receiving IIV-SD. Conversely, evidence regarding RIV did not indicate an increase in injection site or systemic events in comparison to IIV-SD.
The NACI Secretariat applied the Committee's EEFA Framework to assess the implications of ethics, equity, feasibility, and acceptability of its recommendation on influenza vaccination in adults 65 years of age and older in Canada. There were no potential inequities or ethical considerations identified that could arise with the recommendation of age-appropriate preferential use of high-dose, adjuvanted, recombinant influenza vaccines. In fact, acceptability may be increased for certain sub-groups (i.e., frailty) who benefit by the preferential vaccine's real or perceived increased efficacy. Potential feasibility issues for providers and policymakers are limited to the increased costs of the preferred vaccines but as these vaccines are already in use across some jurisdictions this is unlikely a wide-spread issue.
In addition to EEFA factors, economic considerations were evaluated to supplement the evidence base for programmatic factors. Published economic assessment determined that both IIV3-HD and IIV3-Adj are cost-effective in comparison to IIV3-SD. No economic evidence was available for RIV, resulting in the absence of data reporting on RIV's cost-effectiveness when compared to IIV-SD. Moreover, no economic evidence identified in this review directly compared IIV-HD, IIV-Adj and RIV to one another.
One (1) notable limitation in both reviews is the absence of data on frail older adults, an important factor for understanding the impact of influenza vaccination in adults 65 years of age and older at higher risk of severe influenza-related outcomes and complications. The efficacy and effectiveness of influenza vaccines in older adults can vary due to multiple factors, which may be more pronounced among frail older adults. As adults age, immune function declines, increasing the vulnerability to influenza infection and related complicationsFootnote 115Footnote 116Footnote 117Footnote 118.
Furthermore, among older age groups, an increase is observed in both the average number of comorbidities present, and the level of frailty exhibited. Individuals living with elevated levels of frailty, or 1 or more comorbidities are at increased risk for severe influenza-related outcomes, including hospitalizations, functional decline, and death following infection. Nonetheless, due to the complexities of the frailty spectrum, influenza-related morbidity in frail older adults might be underestimatedFootnote 20Footnote 123Footnote 124Footnote 125. Limited studies exist that provide insights into the impact of influenza vaccines among frail older adults. Notably, 2 RCTs conducted in the US and Canada reported decreasing vaccine efficacy with increasing frailty, indicating an association between frailty and lower protection against influenza infection and its complicationsFootnote 133Footnote 150.
A similar trend was observed in a case-control study comparing vaccine effectiveness in patients with low vs. high frailty scores, showing lower VE among those with higher scoresFootnote 151. Additionally, despite the previously reported reduced vaccine efficacy in frail older adults, DiazGranados et al. reported favourable relative efficacy for IIV-HD over IIV-SD in this populationFootnote 150. A recent test-negative study conducted in Canada found, in an exploratory analysis, a higher relative vaccine effectiveness comparing IIV-Adj to IIV-SD against LCI hospitalization among adults 65 years of age and older after accounting for frailty (rVE of 25%,95% CI: 8 to 39%)Footnote 152. Similarly, Nace et al. reported higher geometric mean titers and seroconversion rates associated with IIV-HD as opposed to IIV-SD in frail residents of long-term care facilitiesFootnote 78.
These findings provide reassurance that the greater efficacy of IIV-HD and IIV-Adj observed across the literature in the general older population remains consistent in frail older adults. The mentioned studies offer valuable insight into the relationship between frailty and influenza vaccine efficacy/effectiveness. As frailty increases, influenza vaccine effectiveness and the probability of recovering from influenza infection tend to decline. However, the cited studies show a trend of improved vaccine protection associated with IIV-HD and IIV-Adj compared to IIV-SD among frail older adults. Taken together, these findings highlight the potential benefits of administering influenza vaccines designed to enhance protection in older adults to reduce the burden of influenza illness within this population. As further research is required, some of the following methodological considerations may be pertinent to the older adult population. For instance, the presence of a "frailty bias" in studies of vaccine efficacy/effectiveness – that is, the differential susceptibility to adverse health outcomes due to frailties, underlines the importance of accounting for frailty as a confounding factor in future research on influenza vaccines in older adults. Frail older adults tend to be under-represented in trials, especially RCTs; hence, literature reviews and meta-analyses that focus only on RCTs will tend to not fully represent frail populations - even if they do attempt to include a frailty measure. As such, well-designed and rigorously conducted observational studies continue to play an important role.
Other limitations include the limited number of studies reporting on the vaccine of interest; limited number of influenza seasons represented within each comparison; variability of vaccine formulations between influenza seasons; differences in outcome definitions within the 2 reviews; downgrading of certainty due to study design; and imprecise effect estimates. Most available studies focused on IIV-HD, less on IIV-Adj, while data on RIV was limited. The limited number of studies available per comparison resulted in the inclusion of a low number of influenza seasons for each, especially for pooled-effect estimates involving RCT data, which can affect the generalizability of the results. Another contributing factor influencing the generalizability of findings is the variability in vaccine effectiveness in each influenza season. The differences in outcome definitions between the reviews are mostly attributed to the inclusion of influenza outcomes defined by LCI, clinical diagnostic or ILI in both reviews, leading to challenges in summarizing and interpreting the results due to differences in sensitivity, specificity, and the susceptibility to misclassification bias of the different influenza case definitions. Another difference between ACIP's review and DSEN MAGIC's is that DSEN MAGIC included only RCTs, which generally tend to exclude older and frailer adults. As such, downgrading certainty due to observational study design and imprecise effect estimates presents a limitation as it may make interpretation of the results less robust.
While randomized trials generally provide the highest certainty of evidence as they are less susceptible to bias, they are usually conducted over few influenza seasons. Therefore, it cannot be assumed that results from 1 or few seasons will be generalizable to all or most seasons due to the constant evolution of influenza viruses. Observational studies are more numerous and bring other advantages including representation of larger and more diverse populations and results across a larger range of influenza seasons than RCTs. Hence, it is important to recognize the value of observational studies in providing a more comprehensive understanding of the relative benefits of IIV-HD, IIV-Adj and RIV to one another and to IIV-SD within the real-world setting despite providing lower certainty of evidence.
Recommendations
Following the thorough review of available evidence summarized above, as well as the assessment of ethics, equity, feasibility, and acceptability considerations with the EEFA Framework, the following section outlines the evidence-informed recommendation made by NACI regarding the use of influenza vaccines in adults 65 years of age and older. NACI will continue to carefully monitor the scientific developments related to influenza vaccines, as well as ongoing vaccine pharmacovigilance, and will update recommendations as evidence evolves.
Please note:
- A strong recommendation applies to most populations/individuals and should be followed unless a clear and compelling rationale for an alternative approach is present
- A discretionary recommendation may be considered for some populations/individuals in some circumstances. Alternative approaches may be reasonable
Please see Table 8 for a more detailed explanation of strength of NACI recommendations and grade of the body of evidence.
The following recommendation for population-level and individual-level vaccination decisions regarding annual influenza vaccination for adults 65 years of age and older supplements NACI's overarching recommendation for influenza vaccination, available in the NACI Seasonal Influenza Vaccine Statement, which is that an age-appropriate influenza vaccine should be offered annually to anyone 6 months of age and older (Strong NACI Recommendation), noting product-specific contraindications.
NACI recommends that IIV-HD, IIV-Adj, or RIV should be offered over other influenza vaccines for adults 65 years of age and older. If a preferred product is not available, any of the available age-appropriate influenza vaccine should be used. (Strong NACI recommendation)
- Where supply of IIV-HD, IIV-Adj, or RIV is limited, consideration can be given to prioritizing groups at highest risk of severe outcomes from influenza among adults 65 years of age and older, such as advanced-age older adults (e.g., 75 years of age and older), those with 1 or more comorbidities, older frail adults, and residents of nursing homes and other chronic care facilities.
Summary of evidence and rationale:
- IIV-HD, IIV-Adj, and RIV appear to have increased vaccine efficacy/effectiveness as compared to IIV-SD.
- No definitive conclusion can be reached regarding the superiority of any of these vaccines over one another as there is a limited number of studies directly comparing IIV-HD, IIV-Adj, and RIV against each other. Notably, IIV-HD has the most substantial body of supporting evidence, followed by IIV-Adj, and then RIV.
- IIV-HD, IIV-Adj, and RIV are effective alternatives to IIV-SD, with no identified difference in safety, based on direct evidence among adults 65 years of age and older.
- IIV-HD and IIV-Adj are cost-effective when compared to IIV-SD.
Research priorities
Research to address the following outstanding knowledge gaps is encouraged:
- Additional safety, efficacy, and effectiveness data for newer vaccine technologies, including cell culture and recombinant influenza vaccines among adults 65 years of age and older.
- Vaccine comparisons (pairwise or comparisons between multiple vaccines) of safety, efficacy and effectiveness between newer influenza vaccines approved for use among adults aged 65 years and older that are currently available in the Canadian market (e.g., IIV-cc, RIV, IIV-Adj, IIV-SD, and IIV-HD).
- Data on the efficacy and effectiveness of influenza vaccines in subpopulations of adults 65 years of age and older at higher risk of severe influenza-related outcomes and complications, such as older adults, individuals living with 1 or more chronic medical conditions, and that consider the role of frailty in older adults.
- Consideration of frailty on VE estimates and the impact of longer-term functional outcomes among adults 65 years of age and older.
- National-level influenza surveillance data among adults 65 years of age and older in Canada, which will help to better define the burden of disease for older adults, especially those at higher risk of severe influenza-related outcomes, such as individuals living with 1 or more chronic medical conditions, and frail older adults.
- Timing of influenza immunization in adults 65 years of age and older with respect to duration or waning of protection against infection and severe disease.
- Incorporation and investigation of the impact of community immunity on cost-effectiveness of seasonal influenza vaccines among adults 65 years of age and older.
- Consideration of frailty into cost-effectiveness and the impact of longer-term functional outcomes.
- Vaccine confidence and acceptability among adults 65 years of age and older in Canada, especially among racialized groups.
- Range and complex interplay of factors that influence acceptability of influenza immunization in general and for high-risk groups among adults 65 years of age and older.
Surveillance issues
Ongoing and systematic data collection, analysis, interpretation, and timely dissemination is fundamental to planning, implementation, evaluation, and evidence-based decision-making. To support such efforts, NACI encourages ongoing surveillance and continued expansion of surveillance details in the epidemiology of influenza in Canada.
In Canada, FluWatch, the national surveillance system, monitors the spread of influenza and influenza-like illness (ILI) by province/territory and age group, including adults 65 years of age and older. Robust enhanced surveillance data including health status and granularity for older age groups, who might be at higher risk of severe influenza-related outcomes due to the decline in immune function is limited. Therefore, initiatives are needed to collect data on influenza infection (e.g., ILI and LCI incidence, viral strain, hospitalization, detailed health status including frailty, and assessment of outcomes over both short- and long- time horizons) from adults 65 years of age and older who are at higher risk of severe influenza-related outcomes, to determine vaccine effectiveness in these groups and to inform appropriate public health efforts such as targeted vaccination campaigns and education. Importantly, increased use of enhanced influenza vaccine products will underscore the need for ongoing surveillance and research on product-specific effectiveness as well as continued rigorous product-specific safety monitoring. It will thus be all the more important to ensure robust and feasible means of verifying which product a person has received, such as through immunization registries that can be readily linked to both clinical and administrative data.
Tables
| Study | Outcome | Vaccine | Study design | Participants (n/N) | Summary of key findings |
|---|---|---|---|---|---|
| Efficacy | |||||
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Lab-confirmed influenza (LCI) | IIV3-HD vs. IIV-SD | RCT (n=1) | IIV3-HD: n=228/15990 (1.4%) IIV-SD: n= 301/15993 (1.9%) |
The relative effect estimate (95% CI) was a RR: 0.76 (0.64 to 0.90) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza-like illness (ILI) | IIV3-Adj vs. IIV-SD | RCT (n=1) | IIV3-Adj: n= 322/3479 (9.3%) IIV-SD: n=314/3482 (9.0%) |
The relative effect estimate (95% CI) was a RR: 1.03 (0.89 to 1.19) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Lab-confirmed influenza (LCI) | RIV3 vs. IIV-SD | RCT (n=2) | RIV3: n= 53/2168 (2.4%) IIV-SD: n=64/2143 (3.0%) |
The pooled relative effect estimate (95% CI) was a RR: 0.82 (0.57 to 1.17) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Lab-confirmed influenza (LCI) | IIV3-HD vs. IIV3-Adj | RCT (n=1) | IIV3-HD: n= 1/29 (3.4%) IIV3-Adj: n=3/30 (10.0%) |
The relative effect estimate (95% CI) was a RR: 0.34 (0.04 to 3.13) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Lab-confirmed influenza (LCI) | IIV3-HD vs. RIV4 | RCT (n= 1) | IIV3-HD: n= 1/29 (3.4%) RIV4: n=4/30 (13.3%) |
The relative effect estimate (95% CI) was a RR: 0.26 (0.03 to 2.18) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Lab-confirmed influenza (LCI) | IIV-Adj vs. RIV4 | RCT (n= 1) | IIV-Adj: n= 3/30 (10.0%) RIV4: n= 4/30 (13.3%) |
The relative effect estimate (95% CI) was a RR: 0.75 (0.18 to 3.07) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-HD vs. IIV-SD | RCT (n= 2) | IIV3-HD: n=14/18596 (0.1%) IIV-SD: n=14/18597 (0.1%) |
The relative pooled effect estimate (95% CI) was a RR: 1.00 (0.48 to 2.09) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-HD vs. IIV-SD | Cluster RCT (n= 1) | IIV3-HD: n=247/19127 IIV-SD: n=309/19129 |
The relative effect estimate (95% CI) was a rate ratio of 0.79 (0.66 to 0.95) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-Adj vs. IIV-SD | RCT (n= 1) | IIV3-Adj: n=242/24926 IIV-SD: n=309/25806 |
The relative effect estimate (95% CI) was a rate ratio of 0.79 (0.65 to 0.96) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Lab-confirmed influenza (LCI) | IIV3-HD vs. IIV3-SD | RCT (n=3) | IIV3-HD: n=252/22394 IIV3-SD: n=329/19359 |
The relative pooled-effect estimate (95% CI) was an OR: 0.75 (0.63 to 0.88) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Lab-confirmed influenza (LCI) | RIV vs. IIV-SD | RCT (n=2) | RIV: n= -/- IIV-SD: n= -/- |
The relative pooled effect estimate (95% CI) was an OR: 0.70 (0.42 to 1.18) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Influenza-like illness (ILI) | IIV-HD vs. IIV-SD | RCT (n=3) | IIV-HD: n=-/41209 IIV-SD: n=-/41209 |
The relative pooled effect estimate (95% CI) was an OR: 0.98 (0.93 to 1.02) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Influenza-like illness (ILI) | RIV vs. IIV-SD | RCT (n=1) | RIV4: n= -/9003 IIV4-SD: n= -/9003 |
The relative effect estimate (95% CI) was an OR: 0.99 (0.89 to 1.09) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Influenza-like illness (ILI) | RIV vs. IIV-SD | RCT (n=1) | RIV3: n= -/870 IIV3-SD: n= -/870 |
The relative effect estimate (95% CI) was an OR: 0.96 (0.55 to 1.65) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Influenza-like illness (ILI) | IIV3-Adj vs. IIV3-SD | RCT (n=1) | IIV3-Adj: n= -/6961 IIV3-SD n= -/6961 |
The relative effect estimate (95% CI) was an OR: 1.03 (0.87 to 1.21) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Hospitalization for LCI | IIV3-HD vs. IIV4-SD | RCT (n=1) | IIV3-HD: n= -/68 IIV4-SD: n= -/68 |
The relative effect estimate (95% CI) was an OR: 1 (0.06 to 16.7) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Hospitalization for LCI | IIV3-HD vs. IIV3-SD | RCT (n=1) | IIV3-HD: n= -/31983 IIV3-SD: n= -/31983 |
The relative effect estimate (95% CI) was an OR: 0.6 (0.22 to 1.65) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Hospitalization for LCI | RIV4 vs. IIV4-SD | RCT (n=1) | RIV4: n= -/ 9003 IIV4-SD: n= -/9003 |
The relative effect estimate (95% CI) was an OR: 0.33 (0.04 to 3.21) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Hospitalization for ILI | IIV3-HD vs. IIV3-SD | RCT (n=2) | IIV3-HD: n=127/22098 IIV3-SD: n=155/19043 |
The relative pooled effect estimate (95% CI) was an OR: 0.72 (0.57 to 0.92) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Outpatient Visit | IIV3-HD vs. IIV3-SD | RCT (n=1) | IIV3-HD: n=439/6108 IIV3-SD: n=226/3050 |
The relative effect estimate (95% CI) was an OR: 0.97 (0.82 to 1.14) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Number of vascular events | IIV3-HD vs. IIV3-SD | RCT (n=4) | IIV3-HD: n=166/23551 IIV3-SD: n= 169/19847Footnote a |
The relative pooled effect estimate (95% CI) was a rate ratio of 0.75 (0.43 to 1.29) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Number of vascular events | IIV3-Adj vs. IIV3-SD | RCT (n=2) | IIV3-Adj: n=39/3422Footnote a IIV3-SD: n= 47/3427Footnote a |
The relative pooled effect estimate (95% CI) was a rate ratio of 0.83 (0.54 to 1.27) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Number of vascular events | RIV4 vs. IIV4-SD | RCT (n=1) | RIV4: n= -/ 9003 IIV4-SD: n= -/9003 Total N= 9003 |
The relative effect estimate (95% CI) was an OR: 0.89 (0.30 to 2.60) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Influenza-related death | IIV3-Adj vs. IIV3-SD | RCT (n=1) | IIV3-Adj: n= -/6961 IIV3-SD: n= -/6961 Total N= 6961 |
The relative effect estimate (95% CI) was an OR: 0.75 (0.17 to 3.36) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Lab-confirmed influenza (LCI) | IIV-HD vs. IIV-Adj | RCT (n=1) | IIV-HD: n= -/152 IIV-Adj: n= -/152 Total N= 152 |
The relative effect estimate (95% CI) was an OR: 3.1 (0.30 to 31.8) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Lab-confirmed influenza (LCI) | RIV vs. IIV-Adj | RCT (n=1) | RIV: n= -/89 IIV-Adj: n= -/89 |
The relative effect estimate (95% CI) was an OR: 0.72 (0.15 to 3.54) |
Veroniki/Tricco et al. (2023)Footnote 15 Efficacy of the trivalent and quadrivalent influenza vaccines relative to one another among adults 60 years of age and older: A systematic review and network meta-analysis. Submitted. |
Lab-confirmed influenza (LCI) | RIV vs. IIV-HD | RCT (n=1) | RIV: n= -/89 IIV-HD: n=-/89 Total N= 89 |
The relative effect estimate (95% CI) was an OR: 0.23 (0.02 to 2.20) |
| Effectiveness | |||||
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-HD vs. IIV-SD | Observational (n= 8) | IIV3-HD: n=-/43519865Footnote a IIV-SD: n=-/20193377Footnote a |
The relative pooled effect estimate (95% CI) was a rate ratio of 0.92 (0.90 to 0.94) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-HD vs. IIV-SD | Observational (n= 2) | IIV3-HD: n=177/24334 (0.7%) IIV-SD: n=201/24197 (0.8%) |
The relative pooled effect estimate (95% CI) was RR: 0.71 (0.57 to 0.88) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-Adj vs. IIV-SD | Observational (n= 4) | IIV3-Adj: n=/6133023Footnote a IIV-SD: n=-/4861653Footnote a |
The relative pooled effect estimate (95% CI) was a rate ratio of 0.88 (0.80 to 0.97) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-Adj vs. IIV-SD | Observational (n= 2) | IIV3-Adj: n=230/85483 IIV-SD: n=35/79610 |
The relative pooled effect estimate (95% CI) was a rate ratio of 0.75 (0.58 to 0.97) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | RIV3 vs. IIV-SD | Observational (n=1) | RIV3: n=640/608433 IIV-SD: n=2309/1584451 |
The relative effect estimate (95% CI) was a rate ratio of 0.83 (0.76 to 0.91) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-HD vs. IIV-Adj | Observational (n= 4) | IIV3-HD: n=-/25467741Footnote a IIV-Adj: n=-/6356816Footnote a |
The relative pooled effect estimate (95% CI) was a rate ratio of 0.96 (0.90 to 1.01) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV3-HD vs. RIV | Observational (n= 1) | IIV3-HD: n=81492/7173433 RIV: n=640/608433 |
The relative effect estimate (95% CI) was a rate ratio of 1.12 (1.03 to 1.21) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza hospitalization | IIV-Adj vs. RIV | Observational (n= 1) | IIV-Adj: n=2783/2565513 RIV: n=640/608433 |
The relative effect estimate (95% CI) was a rate ratio of 1.12 (1.03 to 1.22) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza outpatient and/or ED visits | IIV3-Adj vs. IIV-SD | Observational (n= 2) | IIV3-Adj: n=-/1701231Footnote a IIV-SD: n=-/2035149Footnote a |
The relative pooled effect estimate (95% CI) indicated a rate ratio of 1.00 (0.97 to 1.03) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza outpatient and/or ED visits | IIV3-Adj vs. IIV-SD | Observational (n= 2) | IIV3-Adj: n=344/1333 IIV-SD: n=197/988 |
The relative pooled effect estimate (95% CI) was RR: 0.64 (0.52 to 0.79) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza outpatient and/or ED visits | IIV3-HD vs. IIV-SD | Observational (n= 1) | IIV3-HD: n= 593/3141 (18.9%) IIV-SD: n= 580/2840 (20.4%) |
The relative pooled effect estimate (95% CI) was RR: 0.91 (0.73 to 1.12) |
Grohskopf et al. (2022)Footnote 16 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza outpatient and/or ED visits | IIV3-HD vs. IIV-SD | Observational (n= 4) | IIV3-HD: n=-/11001581Footnote a IIV-SD: n=-/5658869Footnote a |
The relative pooled effect estimate (95% CI) was a rate ratio of 0.87 (0.76 to 0.99) |
Grohskopf et al. (2022)Footnote 16 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza outpatient and/or ED visits | IIV3-HD vs. IIV3-Adj | Observational (n=3) | IIV3-HD: n=-/11430788Footnote a IIV-Adj: n=-/2262474Footnote a |
The relative pooled effect estimate (95% CI) was a rate ratio of 1.06 (0.92 to 1.23) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Influenza-related death | IIV3-HD vs. IIV-SD | Observational (n= 2) | IIV3-HD: n= -/2755395Footnote a IIV-SD: n= -/3922569Footnote a |
The relative pooled effect estimate (95% CI) was a rate ratio of 0.69 (0.57 to 0.84) |
| Safety | |||||
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Any Serious Adverse Event | IIV3-HD vs. IIV-SD | RCT (n=7) | IIV3-HD: n=1518/22109 (6.7%) IIV-SD: n=1582/20811 (7.5%) |
The relative effect estimate (95% CI) was RR: 0.91 (0.85 to 0.97) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Any Serious Adverse Event | IIV3-Adj vs. IIV-SD | RCT (n=8) | IIV3-Adj: n=300/5266 (5.7%) IIV-SD: n=277/5055 (5.5%) |
The relative effect estimate (95% CI) was RR: 1.07 (0.92 to 1.26) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Any Serious Adverse Event | RIV3 vs. IIV-SD | RCT (n=5) | RIV3: n=191/6513 (2.9%) IIV-SD: n=190/6697 (2.8%) |
The relative effect estimate (95% CI) was RR: 1.03 (0.84 to 1.26) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Any Serious Adverse Event | IIV3-HD vs. IIV3-Adj | RCT (n=2) | IIV3-HD: n=13/887 (1.5%) IIV3-Adj: n=20/886 (2.3%) |
The relative effect estimate (95% CI) was RR: 0.65 (0.32 to 1.30) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Any Serious Adverse Event | IIV3-HD vs. RIV | RCT (n=2) | IIV3-HD: n=16/663 (2.4%) RIV: n=7/486 (1.4%) |
The relative effect estimate (95% CI) was RR: 1.77 (0.73 to 4.27) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Any Serious Adverse Event | IIV-Adj vs. RIV | RCT (n=1) | IIV-Adj: n=11/508 (2.2%) RIV: n=4/335 (1.2%) |
The relative effect estimate (95% CI) was RR: 1.81 (0.58 to 5.65) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Guillain-Barré syndrome | IIV3-Adj vs. IIV-SD | RCT (n= 1) | IIV3-Adj: n=0/3545 (0.0%) IIV-SD: n=1/3537 (0.0%) |
The relative effect estimate (95% CI) was RR: 0.33 (0.01 to 8.16) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Guillain-Barré syndrome | IIV3-Adj vs. IIV-SD | Observational (n= 1) | IIV3-Adj: n=0/88449 (0.0%) IIV-SD: n=0/82539 (0.0%) |
The relative effect estimate was not estimable |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited injection site events grade 3 or higher | IIV3-HD vs. IIV-SD | RCT (n=2) | IIV3-HD: n=7/560 (1.3%) IIV-SD: n=1/559 (0.2%) |
The relative effect estimate (95% CI) was RR: 5.03 (0.88 to 28.74) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited injection site events grade 3 or higher | IIV3-Adj vs. IIV-SD | RCT (n=4) | IIV3-Adj: n=18/952 (1.9%) IIV-SD: n=5/957 (0.5%) |
The relative effect estimate (95% CI) was RR: 3.39 (1.32 to 8.72) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited injection site events grade 3 or higher | RIV3 vs. IIV-SD | RCT (n=2) | RIV3: n=7/771 (0.9%) IIV-SD: n=11/941 (1.2%) |
The relative effect estimate (95% CI) was RR: 0.67 (0.27 to 1.69) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited injection site events grade 3 or higher | IIV3-HD vs. IIV3-Adj | RCT (n=2) | IIV3-HD: n=15/887 (1.7%) IIV3-Adj: n=17/886 (1.9%) |
The relative effect estimate (95% CI) was RR: 0.88 (0.45 to 1.75) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited injection site events grade 3 or higher | IIV3-HD vs. RIV | RCT (n=2) | IIV3-HD: n=4/663 (0.6%) RIV: n=0/486 (0.0%) |
The relative effect estimate (95% CI) was RR: 5.92 (0.32 to 109.56) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited injection site events grade 3 or higher | IIV-Adj vs. RIV | RCT (n=1) | IIV-Adj: n=3/508 (0.6%) RIV: n=0/335 (0.0%) |
The relative effect estimate (95% CI) was RR: 4.62 (0.24 to 89.17) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited systemic events grade 3 or higher | IIV3-HD vs. IIV-SD | RCT (n=2) | IIV3-HD: n=3/766 (0.4%) IIV-SD: n=3/767 (0.4%) |
The relative effect estimate (95% CI) was RR: 0.95 (0.20 to 4.53) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited systemic events grade 3 or higher | IIV3-Adj vs. IIV-SD | RCT (n=4) | IIV3-Adj: n=10/1016(1.0%) IIV-SD: n=13/1026 (1.3%) |
The relative effect estimate (95% CI) was RR: 0.77 (0.34 to 1.76) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited systemic events grade 3 or higher | RIV3 vs. IIV-SD | RCT (n=2) | RIV3: n=1/771 (0.1%) IIV-SD: n=6/941 (0.6%) |
The relative effect estimate (95% CI) was RR: 0.28 (0.05 to 1.67) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited systemic events grade 3 or higher | IIV3-HD vs. IIV3-Adj | RCT (n=2) | IIV3-HD: n=8/887 (0.9%) IIV3-Adj: n=11/886 (1.2%) |
The relative effect estimate (95% CI) was RR: 0.73 0.29 to 1.80) |
Grohskopf et al. (2022)Footnote 14 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited systemic events grade 3 or higher | IIV3-HD vs. RIV4 | RCT (n=2) | IIV3-HD: n=4/663 (0.6%) RIV4: n=4/486 (0.8%) |
The relative effect estimate (95% CI) was RR: 0.86 (0.22 to 3.32) |
Grohskopf et al. (2022)Footnote 16 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep 2022 |
Solicited systemic events grade 3 or higher | IIV-Adj vs. RIV | RCT (n=1) | IIV-Adj: n=3/508 (0.6%) RIV: n=0/335 (0.0%) |
The relative effect estimate (95% CI) was RR: 4.62 (0.24 to 89.17) |
Footnotes:
|
|||||
Abbreviations: CI, confidence interval; ED, emergency department; IIV3-Adj, adjuvanted trivalent inactivated influenza vaccine (egg-based); IIV3-HD, high-dose trivalent inactivated influenza vaccine (egg-based); IIV3-SD, standard-dose trivalent inactivated influenza vaccine (egg-based); IIV-Adj, adjuvanted inactivated influenza vaccine (egg-based); IIV-HD, high-dose inactivated influenza vaccine (egg-based); IIV-SD, standard-dose inactivated influenza vaccine (egg-based); ILI, influenza-like illness; LCI, laboratory-confirmed influenza infection; OR, odds ratio; RCT, randomized controlled trial; RIV, recombinant influenza vaccine; RIV3, trivalent recombinant influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine; RR, risk ratio; vs, versus. |
|||||
| Author, year | Funding | Study design | Country | Intervention | Comparison | Outcomes | Study limitations (risk of bias)Footnote aFootnote b |
|---|---|---|---|---|---|---|---|
| Balasubramani 2020Footnote 43 | US government | Observational (TNCC) | US | IIV3-HD | IIV3-SD IIV4-SD | Outpatient/ER | ModerateFootnote a |
| Belongia 2020Footnote 37 | US government | RCT | US | IIV3-HD IIV3-Adj RIV4 |
IIV3-Adj RIV4 |
LCI | HighFootnote a |
| Cocchio 2020Footnote 58 | No external funding. | Observational (Retro. cohort) | Italy | IIV3-Adj | IIV-SD | Hospitalization | SeriousFootnote a |
| Couch 2007Footnote 86 | US government | RCT | US | IIV3-HD | IIV3-SD | Any Solicited Systemic AE Grade ≥3 | UnclearFootnote a |
| Cowling 2020Footnote 87 | US government | RCT | Hong Kong | IIV3-HD IIV3-Adj RIV4 |
IIV4-SD IIV3-Adj RIV4 |
Any Solicited Systemic AE Grade ≥3 | LowFootnote a |
| Any Solicited injection site AE Grade ≥3 | LowFootnote a | ||||||
| Any SAE | LowFootnote a | ||||||
| DiazGranados 2014Footnote 33 | Sanofi Pasteur | RCT | US/ Canada | IIV3-HD | IIV3-SD | LCI | LowFootnote a |
| Any SAE ER visits for ILI LCI ILI |
LowFootnote aFootnote b | ||||||
| Hospitalizations for ILI Outpatient visit | Some concernsFootnote b | ||||||
| DiazGranados 2015Footnote 38 | Sanofi Pasteur | RCT | US/ Canada | IIV3-HD | IIV3-SD | Hospitalization | UnclearFootnote a |
| De Bruijn 2007Footnote 79 | Unclear; Solvay authors. | RCT | Germany, Sweden, Lithuania, Bulgaria | IIV3-Adj | IIV3-SD | Any SAE | HighFootnote a |
| De Donato 1999Footnote 80 | Unclear; Chiron authors. | RCT | Italy | IIV3-Adj | IIV3-SD | Any SAE | HighFootnote a |
| Della Cioppa 2012Footnote 85 | Novartis | RCT | Poland, Belgium, Germany | IIV3-Adj | IIV3-SD | Any SAE | HighFootnote a |
| Doyle 2021Footnote 52 | US government | Observational (TNCC) | US | IIV3-HD | IIV-SD | Hospitalization | SeriousFootnote a |
| Dunkle 2017Footnote 34 | BARDA; Protein Sciences authors | RCT | US | RIV4 | IIV4-SD | LCI cases | LowFootnote a - HighFootnote b |
| ILI cases | HighFootnote b | ||||||
| Any SAE | LowFootnote a | ||||||
| Falsey 2009Footnote 68 | Sanofi Pasteur | RCT | US | IIV3-HD | IIV3-SD | Any SAE | UnclearFootnote a |
| Number of vascular events | Some concernsFootnote b | ||||||
| Frey 2014Footnote 35 | Novartis | RCT | US Colombia, Panama Philippines |
IIV3-Adj | IIV3-SD | ILI cases | LowFootnote a – Some concernsFootnote b |
| Any SAE | LowFootnote a | ||||||
| GBS | LowFootnote a | ||||||
| Gravenstein 2017Footnote 40 | Sanofi Pasteur | Cluster RCT | US | IIV3-HD | IIV3-SD | Hospitalization | UnclearFootnote a |
| Hansen 2020Footnote 77 | Protein Sciences | Observational (Retro. cohort) | US | RIV3 | IIV3-SD | GBS | ModerateFootnote a |
| Iob 2005Footnote 48 | Not stated | Observational (Cohort) | Italy | IIV3-Adj | IIV3-SD | Outpatient/ER Visit | SeriousFootnote a |
| Izikson 2015Footnote 83 | BARDA; Protein Sciences authors | RCT | US | RIV3 | IIV3-SD | Any SAEs | UnclearFootnote a |
| Izurieta 2015Footnote 44 | US government | Observational (Retro. cohort) | US | IIV3-HD | IIV3-SD | Outpatient/ER visit | SeriousFootnote a |
| Izurieta 2019Footnote 45 | US government | Observational (Retro. cohort) | US | IIV3-HD | IIV4-SD | Outpatient/ER visit, Hospitalization | SeriousFootnote a |
| IIV3-Adj | IIV4-SD | Hospitalization | SeriousFootnote a | ||||
| IIV3-HD | IIV3-Adj | Hospitalization | SeriousFootnote a | ||||
| Izurieta 2020Footnote 50 | US government | Observational (Retro. cohort) | US | IIV3-HD IIV3-Adj RIV4 |
IIV4-SD | Hospitalization | SeriousFootnote a |
| IIV3-HD | IIV3-Adj | Hospitalization | SeriousFootnote a | ||||
| IIV3-Adj | IIV-SD | Hospitalization | SeriousFootnote a | ||||
| Izurieta 2021Footnote 51 | US government | Observational (Retro. Cohort) | US | RIV4 | IIV4-SD | Hospitalization | SeriousFootnote a |
| IIV3-HD | IIV3-Adj | Hospitalization | SeriousFootnote a | ||||
| IIV3-HD | RIV4 | Hospitalization | SeriousFootnote a | ||||
| IIV3-Adj | RIV4 | Hospitalization | SeriousFootnote a | ||||
| Keitel 2006Footnote 36 | US government | RCT | US | IIV3-HD | IIV3-SD | Any SAEs | UnclearFootnote a |
| Any Solicited Systemic AE Grade ≥3 | UnclearFootnote a | ||||||
| Any Solicited Injection Site AE Grade ≥3 | UnclearFootnote a | ||||||
| Keitel 2010Footnote 71 | Unclear; Protein Sciences author | RCT | US | RIV3 | IIV3-SD | Any SAEs | UnclearFootnote a |
| Any Solicited Systemic AE Grade ≥3 | UnclearFootnote a | ||||||
| Any Solicited Injection Site AE Grade ≥3 | UnclearFootnote a | ||||||
| LCI cases | UnclearFootnote a Some concernsFootnote b | ||||||
| ILI cases | Some concernsFootnote b | ||||||
| Li 2008Footnote 81 | Novartis | RCT | China | IIV3-Adj | IIV3-SD | Any SAEs | HighFootnote a |
| Loeb 2020Footnote 65 | National Institute on Aging, National Institutes of Health | RCT | US/Canada | IIV3-HD | IIV3-SD | LCI cases | Some concernsFootnote aFootnote b |
| Number of vascular events | LowFootnote b | ||||||
| Lu 2019Footnote 53 | US government | Observational (Retro. cohort) | US | IIV3-HD | IIV-SD | Hospitalization | SeriousFootnote a |
| Mannino 2012Footnote 60 | Novartis | Observational (Prospective cohort) | Italy | IIV3-Adj | IIV-SD | Hospitalization | SeriousFootnote a |
| McConeghy 2020Footnote 72 | Seqirus | Cluster RCT | US | IIV3-Adj | IIV3-SD | Hospitalization | UnclearFootnote a |
| McLean 2021Footnote 74 | Centers for Disease Control and Prevention and the Marshfield Clinic Research Institute | RCT | US | IIV3-HD | IIV3-Adj | LCI cases | UnclearFootnote b |
| Menegon 1999Footnote 88 | Ministry grant | RCT | Italy | IIV3-Adj | IIV3-HD | Any Solicited injection Site AE Grade ≥3 | UnclearFootnote a |
| Nace 2015Footnote 78 | Sanofi Pasteur | RCT | US | IIV3-HD | IIV3-SD | Any SAEs | HighFootnote a |
| Paudel 2020Footnote 54 | Sanofi Pasteur | Observational (Retro. cohort) | US | IIV3-HD | IIV-SD | Hospitalization | SeriousFootnote a |
| Pebody 2020Footnote 61 | UK Government | Observational (TNCC) | UK | IIV3-Adj | IIV-SD | Hospitalization | ModerateFootnote a |
| Pelton 2020Footnote 49 | Seqirus | Observational (Retro. cohort) | US | IIV3-HD | IIV3-Adj | Outpatient/ER visit | SeriousFootnote a |
| IIV3-Adj | IIV4-SD | Outpatient/ER visit | SeriousFootnote a | ||||
| Pelton 2021Footnote 63 | Funding not stated. Seqirus authors. | Observational (Retro. cohort) | US | IIV3-HD IIV3-Adj |
IIV3-Adj | Outpatient/ER visit | SeriousFootnote a |
| Richardson 2015Footnote 55 | US government | Observational (Retro. cohort) | US | IIV3-HD | IIV-SD | Hospitalization | SeriousFootnote a |
| Robison 2018Footnote 56 | Funding not stated. No industry authors. | Observational (Matched cohort) | US | IIV3-HD | IIV-SD | Hospitalization | SeriousFootnote a |
| Shay 2017Footnote 46 | US government | Observational (Retro. cohort) | US | IIV3-HD | IIV3-SD | Outpatient/ER visit Death |
SeriousFootnote a |
| Scheifele 2013Footnote 67 | Canadian government | RCT | Canada | IIV3-Adj | IIV3-SD | Any SAEs | LowFootnote a |
| Any Solicited Systemic AE Grade ≥3 | LowFootnote a | ||||||
| Any Solicited Injection Site AE Grade ≥3 | LowFootnote a | ||||||
| Schmader 2021Footnote 76 | US government | RCT | US | IIV3-HD | IIV3-Adj | Any SAEs | LowFootnote a |
| IIV3-HD | IIV3-Adj | - | - | ||||
| IIV3-HD | IIV3-Adj | GBS | LowFootnote a | ||||
| IIV3-HD | IIV3-Adj | Any Solicited Injection Site AE Grade ≥3 | LowFootnote a | ||||
| Seo 2014Footnote 75 | S. Korean government | RCT | S. Korea | IIV3-Adj | IIV3-SD | Any Solicited Systemic AE Grade ≥3 | UnclearFootnote a |
| Sindoni 2009Footnote 82 | Not stated. No industry authors. | RCT | Italy | IIV3-Adj | IIV3-SD | Any SAEs | UnclearFootnote a |
| Shinde 2022Footnote 90 | Novavax | RCT | US | IIV3-HD | RIV4 | Any SAEs GBS |
LowFootnote a |
| Any Solicited Systemic AE Grade ≥3 | LowFootnote a | ||||||
| Teh 2021Footnote 66 | Sanofi Pasteur | RCT | Australia | IIV3-HD | IIV4-SD | Hospitalization for LCI ILI cases LCI cases |
LowFootnote b |
| Treanor 2006Footnote 84 | US government; Protein Sciences author | RCT | US | RIV3 | IIV3-SD | Any SAEs | UnclearFootnote a |
| Tsang 2014Footnote 73 | Sanofi Pasteur | RCT | US | IIV3-HD | IIV3-SD | Any SAEs | UnclearFootnote a |
| Number of vascular events | Some concernsFootnote b | ||||||
| Van Aalst 2020Footnote 62 | Sanofi Pasteur | Observational (Retro. cohort) | US | IIV3-HD IIV3-Adj |
IIV3-Adj | Hospitalization | SeriousFootnote a |
| Van Buynder 2013Footnote 42 | Unrestricted grant, Novartis; Fraser Health Authority | Observational (TNCC) | Canada | IIV3-Adj | IIV3-SD | Outpatient/ER | SeriousFootnote a |
| Vardeny 2021Footnote 39 | US government, Sanofi Pasteur | RCT | US, Canada | IIV3-HD | IIV4-SD | Hospitalization | UnclearFootnote a |
| Villa 2013Footnote 89 | Unclear; Novartis authors. | Observational (Retro. cohort) | Italy | IIV3-Adj | IIV3-SD | GBS | SeriousFootnote a |
| Young-Xu 2018Footnote 47 | Sanofi Pasteur; Sanofi authors | Observational (Retro. cohort) | US | IIV3-HD | IIV3-SD | Outpatient/ER Hospitalizations |
SeriousFootnote a |
| Young-Xu 2019Footnote 57 | Unrestricted grant, Sanofi Pasteur; Sanofi authors | Observational (Retro. cohort) | US | IIV3-HD | IIV3-SD | Hospitalization | SeriousFootnote a |
| Young-Xu 2020Footnote 59 | Unrestricted grant, Sanofi Pasteur; Sanofi authors | Observational (Retro. cohort) | US | IIV3-HD | IIV3-SD | Death | ModerateFootnote a |
Footnotes:
Abbreviations: IIV3-Adj, adjuvanted trivalent inactivated influenza vaccine (egg-based); IIV3-HD, high-dose trivalent inactivated influenza vaccine (egg-based); IIV3-SD, standard-dose trivalent inactivated influenza vaccine (egg-based); IIV-Adj, adjuvanted inactivated influenza vaccine (egg-based); IIV-HD, high-dose inactivated influenza vaccine (egg-based); IIV-SD, standard-dose inactivated influenza vaccine (egg-based); ILI, influenza-like illness; LCI, laboratory-confirmed influenza infection; RCT, randomized controlled trial; RIV, recombinant influenza vaccine; RIV3, trivalent recombinant influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine; Retro. Cohort, retrospective cohort; TNCC, test-negative case-control. |
|||||||
| GRADE rating | Description |
|---|---|
| High | Very confident that the true effect lies close to that of the effect estimate. |
| Moderate | Moderately confident: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. |
| Low | Limited confidence: the true effect may be substantially different from the effect estimate. |
| Very low | Verry little confidence: the true effect is likely to be substantially different from the effect estimate. |
| Abbreviation: GRADE, Grading of Recommendations Assessment, Development and Evaluation. | |
| Strength of NACI recommendation based on factors not isolated to strength of evidence (e.g., public health need) | Strong | Discretionary |
|---|---|---|
| Wording | "should/should not be offered" | "may/may not be offered" |
| Rationale | Known/anticipated advantages outweigh known/anticipated disadvantages ("should"), OR Known/Anticipated disadvantages outweigh known/anticipated advantages ("should not") | Known/anticipated advantages are closely balanced with known/anticipated disadvantages, OR uncertainty in the evidence of advantages and disadvantages exists |
| Implication | A strong recommendation applies to most populations/individuals and should be followed unless a clear and compelling rationale for an alternative approach is present. | A discretionary recommendation may be considered for some populations/individuals in some circumstances. Alternative approaches may be reasonable. |
List of abbreviations
- ACIP
- Advisory Committee on Immunization Practices
- cc
- Cell cultured
- CE
- Cost-effectiveness
- CI
- Confidence interval
- CIG
- Canadian Immunization Guide
- CINeMA
- Confidence in network meta-analysis
- CIQ
- Comité sur l'immunisation du Québec
- DSEN
- Drug Safety and Effectiveness Network
- ED
- Emergency department
- EEFA
- Ethics, equity, feasibility, and acceptability
- GBS
- Guillain-Barré syndrome
- GRADE
- Grading of Recommendations, Assessment, Development, and Evaluation
- HA
- Hemagglutinin
- HZ
- Herpes zoster
- ICER
- Incremental cost-effectiveness ratio
- ICU
- Intensive care unit
- IIV
- Inactivated influenza vaccine
- IIV3
- Trivalent inactivated influenza vaccine
- IIV4
- Quadrivalent inactivated influenza vaccine
- IIV-Adj
- Adjuvanted inactivated influenza vaccine (egg-based)
- IIV-cc
- Mammalian cell culture-based inactivated influenza vaccine
- IIV-HD
- High-dose inactivated influenza vaccine (egg-based)
- IIV-SD
- Standard dose inactivated influenza vaccine (egg-based)
- IIV3-Adj
- Adjuvanted trivalent inactivated influenza vaccine (egg-based)
- IIV3-HD
- High-dose trivalent inactivated influenza vaccine (egg-based)
- IIV3-SD
- Standard dose trivalent inactivated influenza vaccine (egg-based)
- IIV4-Adj
- Adjuvanted quadrivalent inactivated influenza vaccine (egg-based)
- IIV4-cc
- Mammalian cell culture-based quadrivalent inactivated influenza vaccine
- IIV4-HD
- High-dose quadrivalent inactivated influenza vaccine (egg-based)
- IIV4-SD
- Standard dose quadrivalent inactivated influenza vaccine (egg-based)
- ILI
- Influenza-like illness
- IM
- Intramuscular
- LCI
- Laboratory-confirmed influenza
- MDCK
- Madin-Darby Canine Kidney
- MAGIC
- Methods and Applications Group for Indirect Comparisons
- NACI
- National Advisory Committee on Immunization
- NMA
- Network Meta-analysis
- NOC
- Notice of Compliance
- OECD
- Organisation for Economic Co-operation and Development
- PHAC
- Public Health Agency of Canada
- QALY
- Quality-adjusted life year
- RCT
- Randomized controlled trial
- RIV
- Recombinant influenza vaccine
- RIV3
- Recombinant trivalent influenza vaccine
- RIV4
- Recombinant quadrivalent influenza vaccine
- RR
- Risk ratio
- RT-PCR
- Reverse transcription polymerase chain reaction
- rVE
- Relative vaccine efficacy
- RZV
- Recombinant zoster vaccine
- SAE
- Serious adverse event
- UK
- United Kingdom
- US
- United States
- VE
- Vaccine effectiveness
Acknowledgements
This statement was prepared by: P Doyon-Plourde, A Gil, A Sinilaite, W Siu and J Papenburg, on behalf of the NACI Influenza Working Group and approved by NACI
NACI gratefully acknowledges the contribution of: M Hersi, A Howarth, K Gusic, N Moqueet, B Pe Benito, A Stevens, C Tremblay, N Sicard, M Tunis, A Tuite, M W Yeung, K Young, R Yorke and M Xi.
NACI Influenza Working Group
Members: J Papenburg (Chair), M Andrew, P De Wals, I Gemmill, R Harrison, J Langley, and A McGeer.
Former Members: D Fell
Liaison representatives: L Grohskopf (Centers for Disease Control and Prevention [CDC], United States), D Moore (Canadian Paediatric Society).
Ex-officio representatives: L Lee (Centre for Immunization and Respiratory Infectious Diseases [CIRID], PHAC), K Daly (First Nations and Inuit Health Branch [FNIHB], Indigenous Services Canada [ISC]), B Warshawsky (Vice President's Office, Infectious Disease Prevention and Control Branch [IDPCB]), and M Russell (Biologics and Genetic Therapies Directorate [BGTD], Health Canada [HC]).
NACI
NACI Members: S Deeks (Chair), R Harrison (Vice-Chair), M Andrew, J Bettinger, N Brousseau, H Decaluwe, P De Wals, E Dubé, V Dubey, K Hildebrand, K Klein, M O'Driscoll, J Papenburg, A Pham-Huy, B Sander, and S Wilson
Liaison Representatives: L Bill (Canadian Indigenous Nurses Association), LM Bucci (Canadian Public Health Association), E Castillo (Society of Obstetricians and Gynaecologists of Canada), J Comeau (Association of Medical Microbiology and Infectious Disease Control), L Dupuis (Canadian Nurses Association), E Adams (Indigenous Physicians Association of Canada), J Hui (College of Family Physicians of Canada), M Lavoie (Council of Chief Medical Officers of Health), D Moore (Canadian Paediatric Society), M Naus (Canadian Immunization Committee), A Ung (Canadian Pharmacists Association)
Ex-Officio Representatives: V Beswick-Escanlar (National Defence and the Canadian Armed Forces), E Henry (Centre for Immunization and Respiratory Infectious Diseases (CIRID), PHAC), M Lacroix (Public Health Ethics Consultative Group, PHAC), C Lourenco (Biologic and Radiopharmaceutical Drugs Directorate, Health Canada), S Ogunnaike-Cooke (CIRID, PHAC), K Robinson (Marketed Health Products Directorate, HC), M Routledge (National Microbiology Laboratory, PHAC), and T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada).
Appendix A: Key terminology for understanding economic evaluation evidence
| Term | Description |
|---|---|
| Cost-benefit analysis | "In healthcare evaluation, cost-benefit analysis (CBA) is a comparison of interventions and their consequences in which both costs and resulting benefits (health outcomes and others) are expressed in monetary terms. This enables 2 or more treatment alternatives to be compared using the summary metric of net monetary benefit, which is the difference between the benefit of each treatment (expressed in monetary units) less the cost of each. Monetary valuations of benefits are commonly obtained through willingness to pay (WTP) surveys or discrete choice experiments (DCEs). Although popular in other fields, CBA is not commonly used in health technology assessment due to difficulty of associating monetary values with health outcomes such as (increased) survival. Most commonly CBAs have been used to assess large capital development projects (new hospital facilities) or interventions that improve waiting times or location/access to services Footnote 153." The outcome of a CBA is expressed as the incremental net monetary benefit (NMB). "NMB is a summary statistic that represents the value of an intervention in monetary terms when a willingness to pay threshold for a unit of benefit (for example a measure of health outcome or QALY) is known. NMB is calculated as (incremental benefit x threshold) – incremental cost. Incremental NMB measures the difference in NMB between alternative interventions, a positive incremental NMB indicating that the intervention is cost-effective compared with the alternative at the given willingness-to-pay threshold. In this case the cost to derive the benefit is less than the maximum amount that the decision-maker would be willing to pay for this benefit Footnote 153." |
| Cost-effectiveness analysis | "Cost-effectiveness analysis (CEA) evaluates the effectiveness of 2 or more treatments relative to their cost Footnote 153." CEA is an economic evaluation in which health outcomes are expressed in natural units (e.g., infections avoided). The outcome of a CEA is expressed as the incremental cost-effectiveness ratio (ICER). The ICER is a ratio calculated by dividing the difference in mean expected costs by the difference in mean expected health outcomes or effects between 2 alternatives being compared in an economic evaluation. |
| Cost-utility analysis | Cost-utility analysis is an economic evaluation in which health outcomes are expressed in quality-adjusted life years (QALYs), or other generic measure of health-related utility. It is sometimes referred to as a cost-effectiveness analysis (CEA), or CEA with QALYs. This is the form of economic evaluation favoured by public health care decision-makers in Canada. The outcome of a CUA is expressed as an ICER, (also sometimes referred to as an incremental cost-utility ratio [ICUR] in a CUA specifically). The ICER is a ratio calculated by dividing the difference in mean expected costs by the difference in mean expected QALYs between 2 alternatives being compared in an economic evaluation. ICERs can be compared to cost-effectiveness thresholds (see below) to assess if the new intervention is an efficient use of resources. |
| Dominant | An intervention is dominant if it is less costly and more effective (i.e., results in relatively more benefits) than the comparator. Conversely, an intervention that is both more costly and less effective is said to be "dominated." |
| Perspective | The viewpoint from which an economic evaluation will be conducted. The perspective determines the outcomes and costs that will be included in the analysis. NACI's draft Guidelines for the Economic Evaluation of Vaccination Programs in Canada (2022) recommends the adoption of 2 reference case analyses: 1 conducted from the publicly funded health system perspective and the other conducted from the societal perspective. In these guidelines, health system refers to both healthcare treatment services and Public Health. The purpose of these reference cases is to encourage the use of a standard set of methods when conducting economic evaluations of vaccination programs and to ensure that decision-makers are able to compare results between different vaccination programs. The health system perspective includes all resources within the publicly funded health system that are consumed through the delivery of the vaccination program, and resources that are consumed or saved as a result of its implementation (e.g., healthcare costs, public health costs). The provider perspective is a narrower perspective that includes resources and outcomes associated with the healthcare provider (e.g., hospital). The societal perspective is broader and accounts for the full range of benefits associated with vaccination programs, including those that accrue to non-health sectors. It includes elements such as out-of-pocket costs, productivity loss (e.g., individual, caregiver, macroeconomic), and non-medical consumption. |
| Time horizon | "The time horizon used for an economic evaluation is the duration over which health outcomes and costs are calculated. The choice of time horizon is an important decision for economic modelling and depends on the nature of the disease and intervention under consideration and the purpose of the analysis. Longer time horizons are applicable to chronic conditions associated with on-going medical management, rather than a cure. A shorter time horizon may be appropriate for some acute conditions, for which long-term consequences are less important Footnote 153." |
| Cost-effectiveness threshold | Cost-effectiveness thresholds can be used to assess if an intervention represents sufficient value for money to merit adoption into the health system. Some agencies advocate for their use because they are practical, whereas others dismiss their use arguing they are arbitrary. There are several approaches to estimating a cost-effectiveness threshold, basing it on: (1) willingness-to-pay for a unit of outcome; (2) value of interventions already funded in the system; and (3) opportunity costs in terms of forgone health benefits (e.g., cost per QALY gained forgone). In Canada, no explicit cost-effectiveness threshold has been formally adopted by federal or provincial/ territorial agencies. In the literature, common cost-effectiveness thresholds range from $20,000 to $100,000 CAD per QALY gained 40 with $50,000 per QALY being commonly citedFootnote 154. |
Appendix B: Methods for quality appraisal and generalizability assessment
The following sections contain additional information regarding the methods and findings of the quality appraisal and generalizability assessment of the included studies.
The systematic review methodology was developed in collaboration with the Methods and Applications Group for Indirect Comparisons (MAGIC) team through the Drug Safety and Effectiveness Network (DSEN). The systematic review followed existing NACI guidelines for systematic reviews on economic evaluations of vaccination programs. The methods were specified a priori in a written protocol that included the research question, search strategy, inclusion and exclusion criteria, and quality assessment (registered in PROSPERO, CRD42020177337).
Quality appraisal
The quality of included studies was assessed using the 11-item Joanna Briggs Institute (JBI) Appraisal Checklist for Economic EvaluationsFootnote 155. All included studies were of high or moderate quality. Thirteen studies were considered to be high quality, reporting 10 or more of the 11 items (>90%) in the JBI Appraisal Checklist for Economic EvaluationsFootnote 94Footnote 95Footnote 96Footnote 97Footnote 100Footnote 101Footnote 102Footnote 103Footnote 104Footnote 105Footnote 107Footnote 108Footnote 109. The remaining 6 studies were of moderate quality and reported 7 to 9 of the 11 items (64% to 82%) in the JBI Appraisal Checklist for Economic EvaluationsFootnote 98Footnote 99Footnote 106Footnote 110Footnote 111Footnote 112. Main issues related to quality assessment included unclear or insufficient information to confirm whether all important and relevant costs and outcomes for each intervention and comparator were identified (n=4)Footnote 98Footnote 106Footnote 111Footnote 112 and whether the time horizon and discount rate were identified and justified (n=4)Footnote 98Footnote 99Footnote 106Footnote 111.
Generalizability of studies to a Canadian setting
Using the Heyland Generalizability AssessmentFootnote 156, 4 studies, including 2 conducted in CanadaFootnote 95Footnote 101 and 2 conducted in UK settingsFootnote 97Footnote 98 had high applicability to a Canadian setting. The remaining studies were found to have low generalizability to a Canadian setting. Most of the remaining studies satisfied less than half of the Heyland Generalizability Assessment criteria. The following elements of the Heyland Generalizability Assessment were scored poorly when comparing the article being assessed and the setting of interest (i.e., Canada):
- Similar patient preferences: n=19 studiesFootnote 94Footnote 95Footnote 96Footnote 97Footnote 98Footnote 99Footnote 100Footnote 101Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109Footnote 110Footnote 111Footnote 112
- Exchange rates could be converted appropriately: n=17 studiesFootnote 94Footnote 96Footnote 97Footnote 98Footnote 99Footnote 100Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109Footnote 110Footnote 111Footnote 112
- Similar average cost per patient: n=16 studiesFootnote 94Footnote 96Footnote 98Footnote 99Footnote 100Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109Footnote 110Footnote 111Footnote 112
- Similar resource consumption: n=15 studiesFootnote 94Footnote 96Footnote 99Footnote 100Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109Footnote 110Footnote 111Footnote 112
- Similar unit price of relevant resources: n=15 studiesFootnote 94Footnote 96Footnote 99Footnote 100Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109Footnote 110Footnote 111Footnote 112
- Similar perspective of analysis: n=14 studiesFootnote 94Footnote 96Footnote 99Footnote 100Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107Footnote 108Footnote 109Footnote 110Footnote 111
Appendix C: Findings from remaining included economic evaluation studies deemed to have less generalizability to a Canadian setting
Findings among cost-utility, cost-benefit, and cost-effectiveness studies that were deemed to have less generalizability to a Canadian setting are described below.
Results among cost-utility analysis studies considered less generalizable to a Canadian setting
Among the remaining 12 cost-utility studies conducted in settings with limited generalizability to a Canadian setting, the following perspectives were taken. Note that a single study can include multiple analyses from different perspectives.
- Healthcare payer: n=5 studiesFootnote 96Footnote 99Footnote 100Footnote 108Footnote 109
- Societal: n=10 studiesFootnote 94Footnote 96Footnote 99Footnote 100Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106Footnote 107
- Healthcare provider: n=1 studyFootnote 102
The results are described below. Results were similar to the above studies deemed generalizable to the Canadian context.
Results among cost-utility analysis studies with limited generalizability to a Canadian setting: Healthcare payer perspective
The 2 Italian studies comparing an IIV4-SD strategy to an IIV3-SD strategy had differing results, with ICER estimates ranging from $32,617 per QALY gained over a lifetime time horizonFootnote 99 to $224,000 per QALY gained over a 1-year time horizonFootnote 108 (Table A2).
ICER values for an IIV3-Adj strategy versus an IIV3-SD strategy ranged from $3,406Footnote 109 to $7,692Footnote 108 per QALY gained (Table A2). These estimates can be considered cost-effective under commonly used thresholds.
Among the 2 studies comparing an IIV3-HD strategy to an IIV3-SD strategy, 1Footnote 100 found an IIV3-HD strategy to be less costly and more effective than an IIV3-SD strategy and the other studyFootnote 96 estimated an ICER value of $13,537 per QALY gained (Table A2).
One (1) study comparing an IIV3-HD strategy to an IIV4-SD strategy estimated an ICER value of $5,709 per QALY gained (Table A2)Footnote 96.
| Author, year, country | Funding | Population | Time horizon | Findings |
|---|---|---|---|---|
| IIV4-SD (intervention) vs IIV3-SD (comparator) | ||||
| Mennini et al., 2018Footnote 99, Italy | Sanofi Pasteur (Industry) | Adults 65 years of age and older | Lifetime | $32,617/QALY gained |
| Capri et al., 2018Footnote 108, Italy | Seqirus SRL (Industry) | Adults 65 years of age and older | One (1) year | $224,000/QALY gained |
| IIV3-Adj (intervention) vs IIV3-SD (comparator) | ||||
| Capri et al., 2018Footnote 108, Italy | Seqirus SRL (Industry) | Adults 65 years of age and older | One (1) year | $7,692/QALY gained |
| Nguyen et al., 2020Footnote 109, Argentina | Seqirus USA Inc. (Industry) | Adults 65 years of age and older | One (1) year | $3,406/QALY gained |
| IIV3-HD (intervention) vs IIV3-SD (comparator) | ||||
| Chit et al., 2015bFootnote 100, US | Sanofi Pasteur (Industry) | Adults 65 years of age and older | One (1) year for cost and lifetime for effect | IIV3-HD dominated IIV3-SD Participants living with 1 or more comorbidities: IIV3-HD dominated IIV3-SD Participants living with a cardiorespiratory condition: IIV3-HD dominated IIV3-SD |
| Chit et al., 2015cFootnote 96, US | Sanofi Pasteur (Industry) | Adults 65 years of age and older | One (1) influenza season for cost and lifetime for effect | $13,537/QALY gained |
| IIV3-HD (intervention) vs IIV4-SD (comparator) | ||||
| Chit et al., 2015cFootnote 96, US | Sanofi Pasteur (Industry) | Adults 65 years of age and older | One (1) influenza season for cost and lifetime for effect | $5,709/QALY gained |
| Note: "Intervention A dominated Intervention B" means that Intervention A is less costly and more effective than Intervention B. | ||||
Results among cost-utility analysis studies with limited generalizability to a Canadian setting: Societal perspective
The following comparisons were analyzed from studies that carried out analyses from a societal perspective:
- IIV4-SD vs IIV3-SD: n=7 studiesFootnote 94Footnote 99Footnote 102Footnote 103Footnote 104Footnote 105Footnote 106
- IIV3-Adj vs IIV3-SD: n=2 studiesFootnote 99Footnote 104
- IIV3-HD vs IIV3-SD: n=3 studiesFootnote 96Footnote 100Footnote 107
- IIV3-HD vs IIV4-SD: n=2 studiesFootnote 96Footnote 107
Among the 7 studies comparing an IIV4-SD strategy to an IIV3-SD strategy, 2 found an IIV4-SD strategy to be less costly and more effective than an IIV3-SD strategy (Table A3)Footnote 103Footnote 105. ICER estimates for the remaining 6 studies ranged from $8,087Footnote 94 to $55,865Footnote 106 per QALY gained over time horizons ranging from 1 year to lifetime. Notably, the 2 studies that conducted subgroup analyses by age group found an IIV4-SD strategy to be increasingly more cost-effective than an IIV3-SD strategy for older age groupsFootnote 102Footnote 104.
The 2 studies comparing an IIV3-Adj strategy to an IIV3-SD strategy found an IIV3-Adj strategy to be less costly and more effective than an IIV3-SD strategy over a lifetime time horizon for adults 65 years of age and older (Table A3) Footnote 99Footnote 104. These findings were robust in the 1 study that conducted subgroup analyses by age group (65–74 years, 75–84 years, and 85 years of age and older) and for individuals at high risk of seasonal influenza infection and/or influenza-related complications or hospitalizationFootnote 104.
Of the 3 studies comparing an IIV3-HD strategy to an IIV3-SD strategy, 1 found an IIV3-HD strategy to be less costly and more effective than an IIV3-SD strategy (Table A3)Footnote 100. The remaining 2 studies found an IIV3-HD strategy to cost $6,930Footnote 96 to $36,967Footnote 107 for each QALY gained compared to an IIV3-SD strategy.
Two (2) studies compared an IIV3-HD strategy to an IIV4-SD strategy (Table A3). One (1) study found an IIV3-HD strategy to be less costly and more effective than an IIV4-SD strategyFootnote 96 while the second study found an IIV3-HD strategy to cost $40,824Footnote 107 more than an IIV4-SD strategy for each QALY gained.
| Author, year, country | Funding | Population | Time horizon | Findings |
|---|---|---|---|---|
| IIV4-SD (intervention) vs IIV3-SD (comparator) | ||||
| Jiang et al., 2020Footnote 94, China | China Postdoctoral Science Foundation China (Public and Industry) | Adults 69 years of age and older | One (1) year for cost and lifetime for effect | $8,087/QALY gained |
| You et al., 2015Footnote 102, Hong Kong | Not reported | Adults 65 years of age and older | Not reported | All participants 65 years of age and older: $16,424/QALY gained Subgroup analyses Participants 65-79 years of age: $40,221/QALY gained Participants 80 years of age and older: IIV4-SD dominated IIV3-SD |
| Kim et al., 2018Footnote 103, South Korea | GlaxoSmithKline Biologicals SA (Industry) | Adults 65 years of age and older | One (1) year for cost and effect | Broad set of ICD-10 codes used to identify influenza infection: IIV4-SD dominated IIV3-SD Narrow set of ICD-10 codes used to identify influenza infection: IIV4-SD dominated IIV3-SD |
| Yun et al., 2019Footnote 104, South Korea | Korea Centers for Disease Control and Prevention (Public) | Adults 65 years of age and older | Lifetime for cost and effect | All participants 65 years of age and older: $22,656/QALY gained Subgroup analyses Participants 65-74 years of age: $31,869/QALY gained Participants 75-84 years of age: $13,304/QALY gained Participants 85 years of age and older: $4,392/QALY gained |
| Brogan et al., 2017Footnote 105, US | GlaxoSmithKline Biologicals SA (Industry) | Adults 65 years of age and older | 10 years for cost and effect | IIV4-SD dominated IIV3-SD |
| You et al., 2014Footnote 106, Hong Kong | None | Adults 65 years of age and older | 9 years for cost and effect | Assuming IIV4-SD cost an additional $1 USD compared to IIV3-SD in 2010, the ICER was $55,865/QALY gained for adults aged 65-79 and IIV4-SD dominated IIV3-SD for adults 85 years of age and older. |
| Mennini et al., 2018Footnote 99, Italy | Sanofi Pasteur (Industry) | Adults 65 years of age and older | Lifetime for cost and effect | $32,617/QALY gained |
| IIV3-Adj (intervention) vs IIV3-SD (comparator) | ||||
| Yun et al., 2019Footnote 104, South Korea | Korea Centers for Disease Control and Prevention (Public) | Adults 65 years of age and older | Lifetime for cost and effect | All participants 65 years of age and older: IIV3-Adj dominated IIV3-SD Subgroup analyses Participants 65-74 years of age: IIV3-Adj dominated IIV3-SD Participants 75-84 years of age: IIV3-Adj dominated IIV3-SD Participants 85 years of age and older: IIV3-Adj dominated IIV3-SD IIV3-Adj dominated IIV3-SD |
| Mennini et al., 2018Footnote 99, Italy | Sanofi Pasteur (Industry) | Adults 65 years of age and older | Lifetime for cost and effect | IIV3-Adj dominated IIV3-SD |
| IIV3-HD (intervention) vs IIV3-SD (comparator) | ||||
| Chit et al., 2015bFootnote 100, US | Sanofi Pasteur (Industry) | Adults 65 years of age and older | One (1) year for cost and lifetime for effect | IIV3-HD dominated IIV3-SD Participants living with 1 or more comorbidities: IIV3-HD dominated IIV3-SD Participants living with a cardiorespiratory condition: IIV3-HD dominated IIV3-SD |
| Chit et al., 2015cFootnote 96, US | Sanofi Pasteur (Industry) | Adults 65 years of age and older | One (1) year for cost and lifetime for effect | $6,930/QALY gained |
| Raviotta et al., 2016Footnote 107, US | National Institute of General Medical Sciences of the National Institutes of Health Award (Industry) | Adults 65 years of age and older | One (1) year for cost and lifetime for effect | $36,967/QALY gained |
| IIV3-HD (intervention) vs IIV4-SD (comparator) | ||||
| Chit et al., 2015cFootnote 96, US | Sanofi Pasteur (Industry) | Adults 65 years of age and older | One (1) year for cost and lifetime for effect | IIV3-HD dominated IIV4-SD |
| Raviotta et al., 2016Footnote 107, US | National Institute of General Medical Sciences of the National Institutes of Health Award (Industry) | Adults 65 years of age and older | One (1) year for cost and lifetime for effect | $40,824/QALY gained |
| Note: "Intervention A dominated Intervention B" means that Intervention A is less costly and more effective than Intervention B. | ||||
Results among cost-utility analysis studies with limited generalizability to a Canadian setting: Healthcare provider perspective
One (1) study was conducted from a healthcare provider perspective and did not report the time horizon used for analysisFootnote 102. The study found an IIV4-SD strategy to cost $29,562 more than an IIV3-SD strategy for each QALY gainedFootnote 102. The study found an IIV4-SD strategy to be increasingly more cost-effective (i.e., lower ICER) compared to an IIV3-SD strategy with increasing ageFootnote 102.
Results from cost-benefit analysis studies
The 2 cost-benefit analyses concluded that an IIV3-HD strategy was cost-effective (i.e., positive net monetary benefit) compared to an IIV3-SD strategy in the US (Table A4)Footnote 110Footnote 111.
| Author, year, country | Funding | Population | Perspective | Time horizon | Findings |
|---|---|---|---|---|---|
| IIV3-HD (intervention) vs IIV3-SD (comparator) | |||||
| Shireman et al., 2019Footnote 110, US | Sanofi Pasteur (Industry) | Adults 65 years of age and older, nursing home residents | Healthcare payer | One (1) influenza season for cost and effect | Positive net monetary benefit |
| van Aalst et al., 2019Footnote 111, US | Multiple sources (Industry) | Adults 65 years of age and older, veterans | Healthcare payer | Not specified | Positive net monetary benefit |
Results from cost-effectiveness analysis studies
One (1) cost-effectiveness study comparing an IIV3-Adj strategy to an IIV3-SD strategy was identified Footnote 112. The study was conducted from a French healthcare payer perspective over a 1 year time horizon for cost and lifetime time horizon for effectFootnote 112. The study estimated ICERs of $44,492 per death avoided and $8,943 per life year gained for an IIV3-Adj strategy compared to an IIV3-SD strategyFootnote 112.
References
- Footnote 1
-
Schanzer DL, McGeer A, Morris K. Statistical estimates of respiratory admissions attributable to seasonal and pandemic influenza for Canada. Influenza Other Respir Viruses. 2013 Sep;7(5):799-808. https://doi.org/10.1111/irv.12011.
- Footnote 2
-
Schanzer DL, Sevenhuysen C, Winchester B, Mersereau T. Estimating influenza deaths in Canada, 1992-2009. PLoS One. 2013 Nov 27;8(11):e80481. https://doi.org/10.1371/journal.pone.0080481.
- Footnote 3
-
Mitchell R, Taylor G, McGeer A, Frenette C, Suh KN, Wong A, et al. Understanding the burden of influenza infection among adults in Canadian hospitals: A comparison of the 2009-2010 pandemic season with the prepandemic and postpandemic seasons. Am J Infect Control. 2013 Nov;41(11):1032-7. https://doi.org/10.1016/j.ajic.2013.06.008.
- Footnote 4
-
National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2022–2023 [Internet]. Ottawa (ON): Public Health Agency of Canada; 2023 Jun 08 [cited 2023 Aug 14]. Available from: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2022-2023.html.
- Footnote 5
-
National Advisory Committee on Immunization (NACI). Recommendations on the use of MF59-Adjuvanted Trivalent Influenza Vaccine (Fluad®). Can Commun Dis Rep. 2011 Oct 21;37(ACS-6):1-68. https://doi.org/10.14745/ccdr.v37i00a06.
- Footnote 6
-
National Advisory Committee on Immunization (NACI). A Review of the Literature of High Dose Seasonal Influenza Vaccine for Adults 65 Years and Older [Internet]. Ottawa (ON): Government of Canada; 2016 [cited 2023 Aug 15]. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/a-review-literature-high-dose-seasonal-influenza-vaccine-adults-65-years-older.html.
- Footnote 7
-
National Advisory Committee on Immunization (NACI). Literature Review Update on the Efficacy and Effectiveness of High-Dose (Fluzone®High-Dose) and MF59-Adjuvanted (Fluad®) Trivalent Inactivated Influenza Vaccines in Adults 65 Years of Age and Older [Internet]. Ottawa (ON): Government of Canada; 2018 May [cited 2023 Oct 18]. Available from: https://publications.gc.ca/site/eng/9.852907/publication.html.
- Footnote 8
-
Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006 Feb 20;24(8):1159-69. https://doi.org/10.1016/j.vaccine.2005.08.105.
- Footnote 9
-
Beyer WEP, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus ADME. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine. 2013 Dec 05;31(50):6030-3. https://doi.org/10.1016/j.vaccine.2013.09.063.
- Footnote 10
-
Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy, and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012 Jan;12(1):36-44. https://doi.org/10.1016/S1473-3099(11)70295-X.
- Footnote 11
-
Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017 Jan;81:101-10. https://doi.org/10.1016/j.jclinepi.2016.09.009.
- Footnote 12
-
Tricco A, Straus S, Hutton B, Corry M, Veroniki AA, Muller M, et al. Comparative effectiveness of influenza vaccines in adults 65 years of age and older: a systematic review and network meta-analysis. PROSPERO. 2020.
- Footnote 13
-
Isaranuwatchai W, Loong D, Masucci L, Deena D, Tricco A, Radhakrishnan A, et al. Systematic Review on the Cost-Effectiveness of Seasonal Influenza Vaccines in Older Adults. PROSPERO. 2020. https://doi.org/https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020177337.
- Footnote 14
-
Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2022-23 Influenza Season. MMWR Recomm Rep. 2022 Aug 26;71(1):1-28. https://doi.org/10.15585/mmwr.rr7101a1.
- Footnote 15
-
Veroniki AA, Thirugnanasampanthar SS, Konstantinidis M, Dourka J, Ghassemi M, Neupane D, et al. Comparing trivalent and quadrivalent seasonal influenza vaccine efficacy in persons 60 years of age and older: A systematic review and network meta-analysis. medRxiv. 2023 Nov 29. https://doi.org/10.1101/2023.11.29.23299123.
- Footnote 16
-
Groves HE, Piché-Renaud P, Peci A, Farrar DS, Buckrell S, Bancej C, et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg Health Am. 2021 Sep;1:100015. https://doi.org/10.1016/j.lana.2021.100015.
- Footnote 17
-
Schanzer DL, Saboui M, Lee L, Nwosu A, Bancej C. Burden of influenza, respiratory syncytial virus, and other respiratory viruses and the completeness of respiratory viral identification among respiratory inpatients, Canada, 2003-2014. Influenza Other Respir Viruses. 2018 Jan;12(1):113-21. https://doi.org/10.1111/irv.12497.
- Footnote 18
-
Schanzer DL, Tam TWS, Langley JM, Winchester BT. Influenza-attributable deaths, Canada 1990-1999. Epidemiol Infect. 2007 Oct;135(7):1109-16. https://doi.org/10.1017/S0950268807007923.
- Footnote 19
-
Hamilton MA, Liu Y, Calzavara A, Sundaram ME, Djebli M, Darvin D, et al. Predictors of all-cause mortality among patients hospitalized with influenza, respiratory syncytial virus, or SARS-CoV-2. Influenza Other Respir Viruses. 2022 Nov;16(6):1072-81. https://doi.org/10.1111/irv.13004.
- Footnote 20
-
Lees C, Godin J, McElhaney JE, McNeil SA, Loeb M, Hatchette TF, et al. Frailty Hinders Recovery from Influenza and Acute Respiratory Illness in Older Adults. J Infect Dis. 2020 Jul 06;222(3):428-37. https://doi.org/10.1093/infdis/jiaa092.
- Footnote 21
-
Public Health Agency of Canada (PHAC). FluWatch report: April 30 to May 20, 2023 (weeks 18-20) [Internet]. Ottawa (ON): Government of Canada; 2023 May 26 [cited 2023 Aug 17]. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/fluwatch/2022-2023/weeks-18-20-april-30-may-20-2023.html.
- Footnote 22
-
Buckrell S, Moussa MB, Bui T, Rahal A, Schmidt K, Lee L, et al. National Influenza Annual Report, Canada, 2021–2022: A brief, late influenza epidemic. CCDR. 2022 Oct 26;48(10):473-83. https://doi.org/10.14745/ccdr.v48i10a07.
- Footnote 23
-
Lee L. Personal Communication. Feedback on epi burden for NACI Influenza Working Group ppt for adults age 65+. 2023 Jun 14.
- Footnote 24
-
Public Health Agency of Canada (PHAC). Highlights from the 2021–2022 Seasonal Influenza (Flu) Vaccination Coverage Survey [Internet]. Ottawa (ON): Government of Canada; 2022 Nov 21 [cited 2023-08-17]. Available from: https://www.canada.ca/en/public-health/services/immunization-vaccines/vaccination-coverage/seasonal-influenza-survey-results-2021-2022.html.
- Footnote 25
-
National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2016-2017 [Internet]. Ottawa (ON): Public Health Agency of Canada; 2016 Oct 18 [cited 2023 Aug 15]. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/canadian-immunization-guide-chapter-on-influenza-statement-on-seasonal-influenza-vaccine-2016-2017-advisory-committee-statement.html.
- Footnote 26
-
National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on influenza and statement on seasonal influenza vaccine for 2021–2022 [Internet]. Ottawa (ON): Public Health Agency of Canada; 2021 May 31 [cited 2023 Sep 07]. Available from: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2021-2022.html.
- Footnote 27
-
Evidence-based recommendations for immunization-methods of the National Advisory Committee on Immunization. An Advisory Committee Statement (ACS). Can Commun Dis Rep. 2009 Jan;35(ACS-1):1-10.
- Footnote 28
-
Product monograph: FLUAD Pediatric™ and FLUAD®(Influenza Vaccine, Surface Antigen, Inactivated, Adjuvanted with MF59C.1) [Internet]. Maidenhead (UK): Seqirus; 2017 Apr 28 [cited 2023 Aug 15]. Available from: https://pdf.hres.ca/dpd_pm/00070120.PDF.
- Footnote 29
-
National Advisory Committee on Immunization (NACI). Statement on Seasonal Influenza Vaccine for 2011-2012: An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI)†. Can Commun Dis Rep. 2011 Oct 14;37(ACS-5):1-55. https://doi.org/10.14745/ccdr.v37i00a05.
- Footnote 30
-
National Advisory Committee on Immunization (NACI). Statement on Seasonal Influenza Vaccine for 2014-2015 [Internet]. Ottawa (ON): Government of Canada; 2021 [cited 2023 Aug 15]. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/statement-on-seasonal-influenza-vaccine-2014-2015.html.
- Footnote 31
-
Schwarz TF, Aggarwal N, Moeckesch B, Schenkenberger I, Claeys C, Douha M, et al. Immunogenicity and Safety of an Adjuvanted Herpes Zoster Subunit Vaccine Coadministered With Seasonal Influenza Vaccine in Adults Aged 50 Years or Older. J Infect Dis. 2017 Dec 12;216(11):1352-61. https://doi.org/10.1093/infdis/jix481.
- Footnote 32
-
National Advisory Committee on Immunization (NACI). Updated Recommendations on the Use of Herpes Zoster Vaccines [Internet]. Ottawa (ON): Government of Canada; 2018 Aug 27 [cited 2023 Aug 17]. Available from: https://www.canada.ca/en/services/health/publications/healthy-living/updated-recommendations-use-herpes-zoster-vaccines.html.
- Footnote 33
-
DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014 Aug 14;371(7):635-45. https://doi.org/10.1056/NEJMoa1315727.
- Footnote 34
-
Dunkle LM, Izikson R, Patriarca P, Goldenthal KL, Muse D, Callahan J, et al. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N Engl J Med. 2017 Jun 22;376(25):2427-36. https://doi.org/10.1056/NEJMoa1608862.
- Footnote 35
-
Frey SE, Reyes MRAL, Reynales H, Bermal NN, Nicolay U, Narasimhan V, et al. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine. 2014 Sep 03;32(39):5027-34. https://doi.org/10.1016/j.vaccine.2014.07.013.
- Footnote 36
-
Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006 May 22;166(10):1121-7. https://doi.org/10.1001/archinte.166.10.1121.
- Footnote 37
-
Belongia EA, Levine MZ, Olaiya O, Gross FL, King JP, Flannery B, et al. Clinical trial to assess immunogenicity of high-dose, adjuvanted, and recombinant influenza vaccines against cell-grown A(H3N2) viruses in adults 65 to 74 years, 2017-2018. Vaccine. 2020 Mar 30;38(15):3121-8. https://doi.org/10.1016/j.vaccine.2020.02.055.
- Footnote 38
-
DiazGranados CA, Robertson CA, Talbot HK, Landolfi V, Dunning AJ, Greenberg DP. Prevention of serious events in adults 65 years of age or older: A comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015 Sep 11;33(38):4988-93. https://doi.org/10.1016/j.vaccine.2015.07.006.
- Footnote 39
-
Vardeny O, Kim K, Udell JA, Joseph J, Desai AS, Farkouh ME, et al. Effect of High-Dose Trivalent vs Standard-Dose Quadrivalent Influenza Vaccine on Mortality or Cardiopulmonary Hospitalization in Patients With High-risk Cardiovascular Disease: A Randomized Clinical Trial. JAMA. 2021 Jan 05;325(1):39-49. https://doi.org/10.1001/jama.2020.23649.
- Footnote 40
-
Gravenstein S, Davidson HE, Taljaard M, Ogarek J, Gozalo P, Han L, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med. 2017 Sep;5(9):738-46. https://doi.org/10.1016/S2213-2600(17)30235-7.
- Footnote 41
-
McConeghy KW, Davidson HE, Canaday DH, Han L, Saade E, Mor V, et al. Cluster-randomized Trial of Adjuvanted Versus Nonadjuvanted Trivalent Influenza Vaccine in 823 US Nursing Homes. Clin Infect Dis. 2021 Dec 06;73(11):e4237-43. https://doi.org/10.1093/cid/ciaa1233.
- Footnote 42
-
Van Buynder PG, Konrad S, Van Buynder JL, Brodkin E, Krajden M, Ramler G, et al. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine. 2013 Dec 09;31(51):6122-8. https://doi.org/10.1016/j.vaccine.2013.07.059.
- Footnote 43
-
Balasubramani GK, Choi WS, Nowalk MP, Zimmerman RK, Monto AS, Martin ET, et al. Relative effectiveness of high dose versus standard dose influenza vaccines in older adult outpatients over 4 seasons, 2015-16 to 2018-19. Vaccine. 2020 Sep 29;38(42):6562-9. https://doi.org/10.1016/j.vaccine.2020.08.011.
- Footnote 44
-
Izurieta HS, Thadani N, Shay DK, Lu Y, Maurer A, Foppa IM, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis. 2015 Mar;15(3):293-300. https://doi.org/10.1016/S1473-3099(14)71087-4.
- Footnote 45
-
Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, et al. Relative Effectiveness of Cell-Cultured and Egg-Based Influenza Vaccines Among Elderly Persons in the United States, 2017-2018. J Infect Dis. 2019 Sep 13;220(8):1255-64. https://doi.org/10.1093/infdis/jiy716.
- Footnote 46
-
Shay DK, Chillarige Y, Kelman J, Forshee RA, Foppa IM, Wernecke M, et al. Comparative Effectiveness of High-Dose Versus Standard-Dose Influenza Vaccines Among US Medicare Beneficiaries in Preventing Postinfluenza Deaths During 2012-2013 and 2013-2014. J Infect Dis. 2017 Feb 15;215(4):510-7. https://doi.org/10.1093/infdis/jiw641.
- Footnote 47
-
Young-Xu Y, Van Aalst R, Mahmud SM, Rothman KJ, Snider JT, Westreich D, et al. Relative Vaccine Effectiveness of High-Dose Versus Standard-Dose Influenza Vaccines Among Veterans Health Administration Patients. J Infect Dis. 2018 May 05;217(11):1718-27. https://doi.org/10.1093/infdis/jiy088.
- Footnote 48
-
Iob A, Brianti G, Zamparo E, Gallo T. Evidence of increased clinical protection of an MF59-adjuvant influenza vaccine compared to a non-adjuvant vaccine among elderly residents of long-term care facilities in Italy. Epidemiol Infect. 2005 Aug;133(4):687-93. https://doi.org/10.1017/s0950268805003936.
- Footnote 49
-
Pelton SI, Divino V, Shah D, Mould-Quevedo J, DeKoven M, Krishnarajah G, et al. Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017-2018 Influenza Season. Vaccines (Basel). 2020 Aug 07;8(3):446. https://doi.org/10.3390/vaccines8030446.
- Footnote 50
-
Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, et al. Relative Effectiveness of Influenza Vaccines Among the United States Elderly, 2018-2019. J Infect Dis. 2020 Jun 29;222(2):278-87. https://doi.org/10.1093/infdis/jiaa080.
- Footnote 51
-
Izurieta HS, Lu M, Kelman J, Lu Y, Lindaas A, Loc J, et al. Comparative Effectiveness of Influenza Vaccines Among US Medicare Beneficiaries Ages 65 Years and Older During the 2019-2020 Season. Clin Infect Dis. 2021 Dec 06;73(11):e4251-9. https://doi.org/10.1093/cid/ciaa1727.
- Footnote 52
-
Doyle JD, Beacham L, Martin ET, Talbot HK, Monto A, Gaglani M, et al. Relative and Absolute Effectiveness of High-Dose and Standard-Dose Influenza Vaccine Against Influenza-Related Hospitalization Among Older Adults-United States, 2015-2017. Clin Infect Dis. 2021 Mar 15;72(6):995-1003. https://doi.org/10.1093/cid/ciaa160.
- Footnote 53
-
Lu Y, Chillarige Y, Izurieta HS, Wei Y, Xu W, Lu M, et al. Effect of Age on Relative Effectiveness of High-Dose Versus Standard-Dose Influenza Vaccines Among US Medicare Beneficiaries Aged ≥65 Years. J Infect Dis. 2019 Sep 26;220(9):1511-20. https://doi.org/10.1093/infdis/jiz360.
- Footnote 54
-
Paudel M, Mahmud S, Buikema A, Korrer S, Van Voorhis D, Brekke L, et al. Relative vaccine efficacy of high-dose versus standard-dose influenza vaccines in preventing probable influenza in a Medicare Fee-for-Service population. Vaccine. 2020 Jun 15;38(29):4548-56. https://doi.org/10.1016/j.vaccine.2020.05.020.
- Footnote 55
-
Richardson DM, Medvedeva EL, Roberts CB, Linkin DR. Comparative effectiveness of high-dose versus standard-dose influenza vaccination in community-dwelling veterans. Clin Infect Dis. 2015 Jul 15;61(2):171-6. https://doi.org/10.1093/cid/civ261.
- Footnote 56
-
Robison SG, Thomas AR. Assessing the effectiveness of high-dose influenza vaccine in preventing hospitalization among seniors, and observations on the limitations of effectiveness study design. Vaccine. 2018 Oct 29;36(45):6683-7. https://doi.org/10.1016/j.vaccine.2018.09.050.
- Footnote 57
-
Young-Xu Y, Snider JT, van Aalst R, Mahmud SM, Thommes EW, Lee JKH, et al. Analysis of relative effectiveness of high-dose versus standard-dose influenza vaccines using an instrumental variable method. Vaccine. 2019 Mar 07;37(11):1484-90. https://doi.org/10.1016/j.vaccine.2019.01.063.
- Footnote 58
-
Cocchio S, Gallo T, Del Zotto S, Clagnan E, Iob A, Furlan P, et al. Preventing the Risk of Hospitalization for Respiratory Complications of Influenza among the Elderly: Is There a Better Influenza Vaccination Strategy? A Retrospective Population Study. Vaccines (Basel). 2020 Jun 28;8(3):344. https://doi.org/10.3390/vaccines8030344.
- Footnote 59
-
Young-Xu Y, Thornton Snider J, Mahmud SM, Russo EM, Van Aalst R, Thommes EW, et al. High-dose influenza vaccination and mortality among predominantly male, white, senior veterans, United States, 2012/13 to 2014/15. Euro Surveill. 2020 May;25(19):1900401. https://doi.org/10.2807/1560-7917.ES.2020.25.19.1900401.
- Footnote 60
-
Mannino S, Villa M, Apolone G, Weiss NS, Groth N, Aquino I, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012 Sep 15;176(6):527-33. https://doi.org/10.1093/aje/kws313.
- Footnote 61
-
Pebody R, Whitaker H, Zhao H, Andrews N, Ellis J, Donati M, et al. Protection provided by influenza vaccine against influenza-related hospitalisation in ≥65 year olds: Early experience of introduction of a newly licensed adjuvanted vaccine in England in 2018/19. Vaccine. 2020 Jan 10;38(2):173-9. https://doi.org/10.1016/j.vaccine.2019.10.032.
- Footnote 62
-
van Aalst R, Gravenstein S, Mor V, Mahmud SM, Wilschut J, Postma M, et al. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: A retrospective cohort study. Vaccine. 2020 Jan 10;38(2):372-9. https://doi.org/10.1016/j.vaccine.2019.09.105.
- Footnote 63
-
Pelton SI, Divino V, Postma MJ, Shah D, Mould-Quevedo J, DeKoven M, et al. A retrospective cohort study assessing relative effectiveness of adjuvanted versus high-dose trivalent influenza vaccines among older adults in the United States during the 2018-19 influenza season. Vaccine. 2021 Apr 22;39(17):2396-407. https://doi.org/10.1016/j.vaccine.2021.03.054.
- Footnote 64
-
DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity, and relative efficacy during the 2009-2010 season. Vaccine. 2013 Jan 30;31(6):861-6. https://doi.org/10.1016/j.vaccine.2012.12.013.
- Footnote 65
-
Loeb N, Andrew MK, Loeb M, Kuchel GA, Haynes L, McElhaney JE, et al. Frailty Is Associated With Increased Hemagglutination-Inhibition Titers in a 4-Year Randomized Trial Comparing Standard- and High-Dose Influenza Vaccination. Open Forum Infect Dis. 2020 May;7(5):ofaa148. https://doi.org/10.1093/ofid/ofaa148.
- Footnote 66
-
Teh BW, Leung VKY, Mordant FL, Sullivan SG, Joyce T, Harrison SJ, et al. A Randomized Trial of Two (2) 2-Dose Influenza Vaccination Strategies for Patients Following Autologous Hematopoietic Stem Cell Transplantation. Clin Infect Dis. 2021 Dec 06;73(11):e4269-77. https://doi.org/10.1093/cid/ciaa1711.
- Footnote 67
-
Scheifele DW, McNeil SA, Ward BJ, Dionne M, Cooper C, Coleman B, et al. Safety, immunogenicity, and tolerability of 3 influenza vaccines in older adults: results of a randomized, controlled comparison. Hum Vaccin Immunother. 2013 Nov;9(11):2460-73. https://doi.org/10.4161/hv.25580.
- Footnote 68
-
Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009 Jul 15;200(2):172-80. https://doi.org/10.1086/599790.
- Footnote 69
-
Keitel WA, Treanor JJ, El Sahly HM, Gilbert A, Meyer AL, Patriarca PA, Cox,M.M. Comparative immunogenicity of recombinant influenza hemagglutinin (rHA) and trivalent inactivated vaccine (TIV) among persons > or =65 years old. Vaccine. 2009;28(2):379.
- Footnote 70
-
Tsang P, Gorse GJ, Strout CB, Sperling M, Greenberg DP, Ozol-Godfrey A, et al. Immunogenicity and safety of Fluzone® intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine. 2014 May 01;32(21):2507-17. https://doi.org/10.1016/j.vaccine.2013.09.074.
- Footnote 71
-
Keitel WA, Treanor JJ, El Sahly HM, Gilbert A, Meyer AL, Patriarca PA, et al. Comparative immunogenicity of recombinant influenza hemagglutinin (rHA) and trivalent inactivated vaccine (TIV) among persons > or =65 years old. Vaccine. 2009 Dec 11;28(2):379-85. https://doi.org/10.1016/j.vaccine.2009.10.037.
- Footnote 72
-
McConeghy KW, Huang SS, Miller LG, McKinnell JA, Shireman TI, Mor V, et al. Hospital Influenza Admissions as a Harbinger for Nursing Home Influenza Cases. J Am Med Dir Assoc. 2020 Jan;21(1):121-6. https://doi.org/10.1016/j.jamda.2019.06.025.
- Footnote 73
-
Tsang P, Gorse GJ, Strout CB, Sperling M, Greenberg DP, Ozol-Godfrey A, et al. Immunogenicity and safety of Fluzone® intradermal and high-dose influenza vaccines in older adults ≥65 years of age: a randomized, controlled, phase II trial. Vaccine. 2014 May 01;32(21):2507-17. https://doi.org/10.1016/j.vaccine.2013.09.074.
- Footnote 74
-
McLean HQ, Levine MZ, King JP, Flannery B, Belongia EA. Serologic response to sequential vaccination with enhanced influenza vaccines: Open label randomized trial among adults aged 65-74 years. Vaccine. 2021 Dec 03;39(49):7146-52. https://doi.org/10.1016/j.vaccine.2021.10.072.
- Footnote 75
-
Seo YB, Choi WS, Lee J, Song JY, Cheong HJ, Kim WJ. Comparison of the immunogenicity and safety of the conventional subunit, MF59-adjuvanted, and intradermal influenza vaccines in the elderly. Clin Vaccine Immunol. 2014 Jul;21(7):989-96. https://doi.org/10.1128/CVI.00615-13.
- Footnote 76
-
Schmader KE, Liu CK, Harrington T, Rountree W, Auerbach H, Walter EB, et al. Safety, Reactogenicity, and Health-Related Quality of Life After Trivalent Adjuvanted vs Trivalent High-Dose Inactivated Influenza Vaccines in Older Adults: A Randomized Clinical Trial. JAMA Netw Open. 2021 Jan 04;4(1):e2031266. https://doi.org/10.1001/jamanetworkopen.2020.31266.
- Footnote 77
-
Hansen J, Goddard K, Timbol J, Zhang L, Lewis N, Dunkle L, et al. Safety of Recombinant Influenza Vaccine Compared to Inactivated Influenza Vaccine in Adults: An Observational Study. Open Forum Infect Dis. 2020 May 20;7(6):ofaa179. https://doi.org/10.1093/ofid/ofaa179.
- Footnote 78
-
Nace DA, Lin CJ, Ross TM, Saracco S, Churilla RM, Zimmerman RK. Randomized, controlled trial of high-dose influenza vaccine among frail residents of long-term care facilities. J Infect Dis. 2015 Jun 15;211(12):1915-24. https://doi.org/10.1093/infdis/jiu622.
- Footnote 79
-
de Bruijn I, Meyer I, Gerez L, Nauta J, Giezeman K, Palache B. Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. Vaccine. 2007 Dec 21;26(1):119-27. https://doi.org/10.1016/j.vaccine.2007.10.051.
- Footnote 80
-
De Donato S, Granoff D, Minutello M, Lecchi G, Faccini M, Agnello M, et al. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999 Aug 06;17(23-24):3094-101. https://doi.org/10.1016/s0264-410x(99)00138-3.
- Footnote 81
-
Li R, Fang H, Li Y, Liu Y, Pellegrini M, Podda A. Safety, and immunogenicity of an MF59-adjuvanted subunit influenza vaccine in elderly Chinese subjects. Immun Ageing. 2008 Feb 20;5:2. https://doi.org/10.1186/1742-4933-5-2.
- Footnote 82
-
Sindoni D, La Fauci V, Squeri R, Cannavò G, Bacilieri S, Panatto D, et al. Comparison between a conventional subunit vaccine and the MF59-adjuvanted subunit influenza vaccine in the elderly: an evaluation of the safety, tolerability, and immunogenicity. J Prev Med Hyg. 2009 Jun;50(2):121-6.
- Footnote 83
-
Izikson R, Leffell DJ, Bock SA, Patriarca PA, Post P, Dunkle LM, et al. Randomized comparison of the safety of Flublok® versus licensed inactivated influenza vaccine in healthy, medically stable adults ≥ 50 years of age. Vaccine. 2015 Nov 27;33(48):6622-8. https://doi.org/10.1016/j.vaccine.2015.10.097.
- Footnote 84
-
Treanor JJ, Schiff GM, Couch RB, Cate TR, Brady RC, Hay CM, et al. Dose-related safety, and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis. 2006 May 01;193(9):1223-8. https://doi.org/10.1086/503050.
- Footnote 85
-
Della Cioppa G, Nicolay U, Lindert K, Leroux-Roels G, Clement F, Castellino F, et al. Superior immunogenicity of seasonal influenza vaccines containing full dose of MF59® adjuvant: results from a dose-finding clinical trial in older adults. Hum Vaccin Immunother. 2012 Feb;8(2):216-27. https://doi.org/10.4161/hv.18445.
- Footnote 86
-
Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007 Nov 01;25(44):7656-63. https://doi.org/10.1016/j.vaccine.2007.08.042.
- Footnote 87
-
Cowling BJ, Thompson MG, Ng TWY, Fang VJ, Perera RAPM, Leung NHL, et al. Comparative reactogenicity of enhanced influenza vaccines in older adults; 32407535. J Infect Dis. 2020;222(8):1383-91. https://doi.org/10.1093/infdis/jiaa255.
- Footnote 88
-
Menegon T, Baldo V, Bonello C, Dalla Costa D, Di Tommaso A, Trivello R. Influenza vaccines: antibody responses to split virus and MF59-adjuvanted subunit virus in an adult population. Eur J Epidemiol. 1999 Jul;15(6):573-6. https://doi.org/10.1023/a:1007594911541.
- Footnote 89
-
Villa M, Black S, Groth N, Rothman KJ, Apolone G, Weiss NS, et al. Safety of MF59-adjuvanted influenza vaccination in the elderly: results of a comparative study of MF59-adjuvanted vaccine versus nonadjuvanted influenza vaccine in northern Italy. Am J Epidemiol. 2013 Oct 01;178(7):1139-45. https://doi.org/10.1093/aje/kwt078.
- Footnote 90
-
Shinde V, Cho I, Plested JS, Agrawal S, Fiske J, Cai R, et al. Comparison of the safety and immunogenicity of a novel Matrix-M-adjuvanted nanoparticle influenza vaccine with a quadrivalent seasonal influenza vaccine in older adults: a phase 3 randomised controlled trial. Lancet Infect Dis. 2022 Jan;22(1):73-84. https://doi.org/10.1016/S1473-3099(21)00192-4.
- Footnote 91
-
Loong D, Pham B, Amiri M, Saunders H, Mishra S, Radhakrishnan A, et al. Systematic Review on the Cost-Effectiveness of Seasonal Influenza Vaccines in Older Adults. Value Health. 2022 Aug;25(8):1439-58. https://doi.org/10.1016/j.jval.2022.03.011.
- Footnote 92
-
Comité sur l'immunisation du Québec. Utilisation des vaccins à haute dose ou adjuvantés dans le Programme d'immunisation contre l'influenza [Internet]. Quebec (CA): Institut national de santé publique du Québec (INSPQ); 2023 Aug 17 [cited 2023 Sep 03]. Available from: https://www.inspq.qc.ca/publications/3370.
- Footnote 93
-
National Advisory Committee on Immunization. National Advisory Committee on Immunization (NACI): Methods and process [Internet]. Ottawa (ON): Government of Canada; 2023 Apr 12 [cited 2023 Jun 26]. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/methods-process.html.
- Footnote 94
-
Jiang M, Li P, Wang W, Zhao M, Atif N, Zhu S, et al. Cost-effectiveness of quadrivalent versus trivalent influenza vaccine for elderly population in China. Vaccine. 2020 Jan 29;38(5):1057-64. https://doi.org/10.1016/j.vaccine.2019.11.045.
- Footnote 95
-
Becker DL, Chit A, DiazGranados CA, Maschio M, Yau E, Drummond M. High-dose inactivated influenza vaccine is associated with cost savings and better outcomes compared to standard-dose inactivated influenza vaccine in Canadian seniors. Hum Vaccin Immunother. 2016 Dec;12(12):3036-42. https://doi.org/10.1080/21645515.2016.1215395.
- Footnote 96
-
Chit A, Roiz J, Briquet B, Greenberg DP. Expected cost effectiveness of high-dose trivalent influenza vaccine in US seniors. Vaccine. 2015 Jan 29;33(5):734-41. https://doi.org/10.1016/j.vaccine.2014.10.079.
- Footnote 97
-
Meier G, Gregg M, Poulsen Nautrup B. Cost-effectiveness analysis of quadrivalent influenza vaccination in at-risk adults and the elderly: an updated analysis in the U.K. J Med Econ. 2015;18(9):746-61. https://doi.org/10.3111/13696998.2015.1044456.
- Footnote 98
-
Thorrington D, van Leeuwen E, Ramsay M, Pebody R, Baguelin M. Assessing optimal use of the standard dose adjuvanted trivalent seasonal influenza vaccine in the elderly. Vaccine. 2019 Apr 03;37(15):2051-6. https://doi.org/10.1016/j.vaccine.2019.03.002.
- Footnote 99
-
Mennini FS, Bini C, Marcellusi A, Rinaldi A, Franco E. Cost-effectiveness of switching from trivalent to quadrivalent inactivated influenza vaccines for the at-risk population in Italy. Hum Vaccin Immunother. 2018;14(8):1867-73. https://doi.org/10.1080/21645515.2018.1469368.
- Footnote 100
-
Chit A, Becker DL, DiazGranados CA, Maschio M, Yau E, Drummond M. Cost-effectiveness of high-dose versus standard-dose inactivated influenza vaccine in adults aged 65 years and older: an economic evaluation of data from a randomised controlled trial. Lancet Infect Dis. 2015 Dec;15(12):1459-66. https://doi.org/10.1016/S1473-3099(15)00249-2.
- Footnote 101
-
Chit A, Roiz J, Aballea S. An Assessment of the Expected Cost-Effectiveness of Quadrivalent Influenza Vaccines in Ontario, Canada Using a Static Model. PLoS One. 2015 Jul 29;10(7):e0133606. https://doi.org/10.1371/journal.pone.0133606.
- Footnote 102
-
You JHS, Ming W, Chan PKS. Cost-effectiveness of quadrivalent influenza vaccine in Hong Kong - A decision analysis. Hum Vaccin Immunother. 2015;11(3):564-71. https://doi.org/10.1080/21645515.2015.1011016.
- Footnote 103
-
Kim Y, Song JY, Jang H, Kim TH, Koo H, Varghese L, et al. Cost Effectiveness of Quadrivalent Influenza Vaccines Compared with Trivalent Influenza Vaccines in Young Children and Older Adults in Korea. Pharmacoeconomics. 2018 Dec;36(12):1475-90. https://doi.org/10.1007/s40273-018-0715-5.
- Footnote 104
-
Yun J, Choi MJ, Shin G, Lim J, Noh JY, Kim Y, et al. Cost-effectiveness of influenza vaccine strategies for the elderly in South Korea. PLoS One. 2019 Jan 25;14(1):e0209643. https://doi.org/10.1371/journal.pone.0209643.
- Footnote 105
-
Brogan AJ, Talbird SE, Davis AE, Thommes EW, Meier G. Cost-effectiveness of seasonal quadrivalent versus trivalent influenza vaccination in the United States: A dynamic transmission modeling approach. Hum Vaccin Immunother. 2017 Mar 04;13(3):533-42. https://doi.org/10.1080/21645515.2016.1242541.
- Footnote 106
-
You JHS, Ming W, Chan PKS. Cost-effectiveness analysis of quadrivalent influenza vaccine versus trivalent influenza vaccine for elderly in Hong Kong. BMC Infect Dis. 2014 Nov 25;14:618. https://doi.org/10.1186/s12879-014-0618-9.
- Footnote 107
-
Raviotta JM, Smith KJ, DePasse J, Brown ST, Shim E, Nowalk MP, et al. Cost-Effectiveness and Public Health Effect of Influenza Vaccine Strategies for U.S. Elderly Adults. J Am Geriatr Soc. 2016 Oct;64(10):2126-31. https://doi.org/10.1111/jgs.14323.
- Footnote 108
-
Capri S, Barbieri M, de Waure C, Boccalini S, Panatto D. Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly Italian population. Hum Vaccin Immunother. 2018 Jun 03;14(6):1331-41. https://doi.org/10.1080/21645515.2018.1438792.
- Footnote 109
-
Nguyen VH, Vizzotti C, Uruena A, Giglio N, Magneres C, Richmond H. Cost-effectiveness of introducing an MF59-adjuvanted trivalent influenza vaccine for older adults in Argentina. Vaccine. 2020 Apr 29;38(20):3682-9. https://doi.org/10.1016/j.vaccine.2020.02.081.
- Footnote 110
-
Shireman TI, Ogarek J, Gozalo P, Zhang T, Mor V, Davidson HE, et al. Cost Benefit of High-Dose vs Standard-Dose Influenza Vaccine in a Long-Term Care Population During an A/H1N1-Predominant Influenza Season. J Am Med Dir Assoc. 2019 Jul;20(7):874-8. https://doi.org/10.1016/j.jamda.2018.12.003.
- Footnote 111
-
van Aalst R, Russo EM, Neupane N, Mahmud SM, Mor V, Wilschut J, et al. Economic assessment of a high-dose versus a standard-dose influenza vaccine in the US Veteran population: Estimating the impact on hospitalization cost for cardio-respiratory disease. Vaccine. 2019 Jul 26;37(32):4499-503. https://doi.org/10.1016/j.vaccine.2019.06.066.
- Footnote 112
-
Piercy J, Ryan J, Megas F. Economic evaluation of MF59 adjuvanted vaccine against influenza in the high-risk elderly population in France. Journal of Medical Economics. 2004 Jan;7(1-4):1-18. https://doi.org/10.3111/200407001018.
- Footnote 113
-
Sugishita Y, Sugawara T. Effectiveness, and cost-effectiveness of influenza vaccination for elderly people. Vaccine. 2021 Dec 20;39(52):7531-40. https://doi.org/10.1016/j.vaccine.2021.09.054.
- Footnote 114
-
Postma M, Fisman D, Giglio N, Márquez-Peláez S, Nguyen VH, Pugliese A, et al. Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion. Vaccines (Basel). 2023 Jun 11;11(6):1089. https://doi.org/10.3390/vaccines11061089.
- Footnote 115
-
Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018 Mar 31;391(10127):1285-300. https://doi.org/10.1016/S0140-6736(17)33293-2.
- Footnote 116
-
Nichols MK, Andrew MK, Hatchette TF, Ambrose A, Boivin G, Bowie W, et al. Influenza vaccine effectiveness to prevent influenza-related hospitalizations and serious outcomes in Canadian adults over the 2011/12 through 2013/14 influenza seasons: A pooled analysis from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS Network). Vaccine. 2018 Apr 12;36(16):2166-75. https://doi.org/10.1016/j.vaccine.2018.02.093.
- Footnote 117
-
McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012 Mar 09;30(12):2060-7. https://doi.org/10.1016/j.vaccine.2012.01.015.
- Footnote 118
-
Czaja CA, Miller L, Alden N, Wald HL, Cummings CN, Rolfes MA, et al. Age-Related Differences in Hospitalization Rates, Clinical Presentation, and Outcomes Among Older Adults Hospitalized With Influenza-U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET). Open Forum Infect Dis. 2019 Jul 01;6(7):ofz225. https://doi.org/10.1093/ofid/ofz225.
- Footnote 119
-
Divo MJ, Martinez CH, Mannino DM. Ageing, and the epidemiology of multimorbidity. Eur Respir J. 2014 Oct;44(4):1055-68. https://doi.org/10.1183/09031936.00059814.
- Footnote 120
-
Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011 May 17;183(8):487. https://doi.org/10.1503/cmaj.101271.
- Footnote 121
-
Theou O, Brothers TD, Rockwood MR, Haardt D, Mitnitski A, Rockwood K. Exploring the relationship between national economic indicators and relative fitness and frailty in middle-aged and older Europeans. Age Ageing. 2013 Sep;42(5):614-9. https://doi.org/10.1093/ageing/aft010.
- Footnote 122
-
Rohrmann S. Epidemiology of Frailty in Older People. In: Veronese N, editor. Frailty and Cardiovascular Diseases : Research into an Elderly Population. Cham: Springer International Publishing; 2020. p. 21-7.
- Footnote 123
-
Walker TA, Waite B, Thompson MG, McArthur C, Wong C, Baker MG, et al. Risk of Severe Influenza Among Adults With Chronic Medical Conditions. J Infect Dis. 2020 Jan 02;221(2):183-90. https://doi.org/10.1093/infdis/jiz570.
- Footnote 124
-
Andrew MK, MacDonald S, Godin J, McElhaney JE, LeBlanc J, Hatchette TF, et al. Persistent Functional Decline Following Hospitalization with Influenza or Acute Respiratory Illness. J Am Geriatr Soc. 2021 Mar;69(3):696-703. https://doi.org/10.1111/jgs.16950.
- Footnote 125
-
Iwai-Saito K, Sato K, Aida J, Kondo K. Association of frailty with influenza and hospitalization due to influenza among independent older adults: a longitudinal study of Japan Gerontological Evaluation Study (JAGES). BMC Geriatr. 2023 Apr 26;23(1):249. https://doi.org/10.1186/s12877-023-03979-y.
- Footnote 126
-
Earle A, Heymann J. The cost of caregiving: wage loss among caregivers of elderly and disabled adults and children with special needs. Community, Work and Family. 2012 Aug 01;15(3):357-75. https://doi.org/10.1080/13668803.2012.674408.
- Footnote 127
-
Newall AT, Chaiyakunapruk N, Lambach P, Hutubessy RCW. WHO guide on the economic evaluation of influenza vaccination. Influenza Other Respir Viruses. 2018 Mar;12(2):211-9. https://doi.org/10.1111/irv.12510.
- Footnote 128
-
Andrew MK, MacDonald S, Ye L, Ambrose A, Boivin G, Diaz-Mitoma F, et al. Impact of Frailty on Influenza Vaccine Effectiveness and Clinical Outcomes: Experience From the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) Network 2011/12 Season. Open Forum Infect Dis. 2016 Oct 24;3:710. https://doi.org/10.1093/ofid/ofw172.573.
- Footnote 129
-
McElhaney JE, Andrew MK, McNeil SA. Estimating influenza vaccine effectiveness: Evolution of methods to better understand effects of confounding in older adults. Vaccine. 2017 Nov 01;35(46):6269-74. https://doi.org/10.1016/j.vaccine.2017.09.084.
- Footnote 130
-
Hogan DB. Chapter 3 - Models, Definitions, and Criteria for Frailty. In: Ram JL, Conn PM, editors. Conn's Handbook of Models for Human Aging (Second Edition). Academic Press; 2018. p. 35-44.
- Footnote 131
-
Strandberg TE, Pitkälä KH. Frailty in elderly people. Lancet. 2007 Apr 21;369(9570):1328-9. https://doi.org/10.1016/S0140-6736(07)60613-8.
- Footnote 132
-
Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):731-7. https://doi.org/10.1093/gerona/62.7.731.
- Footnote 133
-
Andrew MK, Shinde V, Ye L, Hatchette T, Haguinet F, Dos Santos G, et al. The Importance of Frailty in the Assessment of Influenza Vaccine Effectiveness Against Influenza-Related Hospitalization in Elderly People. J Infect Dis. 2017 Aug 15;216(4):405-14. https://doi.org/10.1093/infdis/jix282.
- Footnote 134
-
Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003 Apr 01;36(7):870-6. https://doi.org/10.1086/368197.
- Footnote 135
-
Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017 Sep;11(5):356-66. https://doi.org/10.1111/irv.12464.
- Footnote 136
-
Yang MC, Tan CH. Cost-Effectiveness Of Quadrivalent Influenza Vaccination Program For The Elderly Aged 65 Years Or Older In Taiwan. Value in Health. 2014 May;17(3):A276. https://doi.org/10.1016/j.jval.2014.03.1606.
- Footnote 137
-
National Advisory Committee on Immunization (NACI). Statement on Seasonal Influenza Vaccine for 2023–2024 [Internet]. Ottawa (ON): Public Health Agency of Canada; 2023 May 31 [cited 2023 Aug 16]. Available from: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-statement-seasonal-influenza-vaccine-2023-2024.html.
- Footnote 138
-
Lee BY, Norman BA, Assi T, Chen S, Bailey RR, Rajgopal J, et al. Single versus multi-dose vaccine vials: an economic computational model. Vaccine. 2010 Jul 19;28(32):5292-300. https://doi.org/10.1016/j.vaccine.2010.05.048.
- Footnote 139
-
BC Centre for Disease Control. Fluzone®High-Dose Quadrivalent Influenza Vaccine Question and Answer Document Updated – August 2021 [Internet]. Vancouver (BC): BCCDC; 2021 Aug [cited 2022 Mar 16]. Available from: http://www.bccdc.ca/resource-gallery/Documents/Guidelines%20and%20Forms/Guidelines%20and%20Manuals/Immunization/Vaccine%20Info/Archived_Fluzone_HD_Q_A_August_2021.pdf.
- Footnote 140
-
Publicly funded seasonal inactivated influenza vaccine information for health care providers 2023-24 [Internet]. Halifax (NS): Province of Nova Scotia; 2023 Sep 26 [cited 2023 Oct 23]. Available from: https://novascotia.ca/dhw/cdpc/documents/Publicly-Funded-Seasonal-Inactivated-Influenza-Vaccine-Information.pdf.
- Footnote 141
-
Ontario Agency for Health Protection and Promotion (Public Health Ontario). Focus On: Influenza vaccines for the 2023-24 Influenza season [Internet]. Toronto (ON): King's Printer for Ontario; 2023 Sep [cited 2023 Oct 23]. Available from: https://www.publichealthontario.ca/-/media/Documents/V/2023/vaccines-influenza-season.pdf?rev=fc6a30901525496c9cb2656e7362069c&sc_lang=en.
- Footnote 142
-
Saskatchewan influenza immunization policy 2023-2024 [Internet]. Regina (SK): Government of Saskatchewan; 2023 Sep [cited 2023 Oct 23]. Available from: https://www.ehealthsask.ca/services/Manuals/Documents/2023-24%20Saskatchewan%20Influenza%20Immunization%20Policy.pdf.
- Footnote 143
-
Yukon Immunization Program Manual: Section 8 - Biological Products - Influenza [Internet]. Whitehorse (YT): Government of Yukon; 2023 Jul [cited 2023 Oct 23]. Available from: https://yukon.ca/sites/yukon.ca/files/hss/hss-section-8-influenza-vaccine_0.pdf.
- Footnote 144
-
Public Health Plan – Publicly Funded Seasonal Influenza Vaccine (2023-2024) [Internet]. Fredericton (NB): Government of New Brunswick; 2023 Aug 30 [cited 2023 Oct 23]. Available from: https://www2.gnb.ca/content/gnb/en/departments/health/MedicarePrescriptionDrugPlan/TheNewBrunswickPrescriptionDrugProgram/Public-Health-Plan-Publicly-Funded-Seasonal-Influenza-Vaccine-2022-2023.html.
- Footnote 145
-
Comité sur l'immunisation du Québec. Révision du Programme d'immunisation contre l'influenza au Québec [Internet]. Quebec (CA): Institut national de santé publique du Québec (INSPQ); 2018 Jun 20 [cited 2023 Sep 05]. Available from: https://www.inspq.qc.ca/publications/2415.
- Footnote 146
-
Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada — 4th Edition [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2023 Aug 15 [cited 2023 Sep 05]. Available from: https://www.cadth.ca/guidelines-economic-evaluation-health-technologies-canada-4th-edition.
- Footnote 147
-
Bart S, Cannon K, Herrington D, Mills R, Forleo-Neto E, Lindert K, et al. Immunogenicity and safety of a cell culture-based quadrivalent influenza vaccine in adults: A Phase III, double-blind, multicenter, randomized, non-inferiority study. Hum Vaccin Immunother. 2016 Sep;12(9):2278-88. https://doi.org/10.1080/21645515.2016.1182270.
- Footnote 148
-
Szymczakiewicz-Multanowska A, Groth N, Bugarini R, Lattanzi M, Casula D, Hilbert A, et al. Safety and immunogenicity of a novel influenza subunit vaccine produced in mammalian cell culture. J Infect Dis. 2009 Sep 15;200(6):841-8. https://doi.org/10.1086/605505.
- Footnote 149
-
Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Casula D, Sparacio M, Kovacs C, et al. Safety assessment and immunogenicity of a cell-culture-derived influenza vaccine in adults and elderly subjects over 3 successive influenza seasons. Hum Vaccin Immunother. 2012 May;8(5):645-52. https://doi.org/10.4161/hv.19493.
- Footnote 150
-
DiazGranados CA, Dunning AJ, Robertson CA, Talbot HK, Landolfi V, Greenberg DP. Efficacy and immunogenicity of high-dose influenza vaccine in older adults by age, comorbidities, and frailty. Vaccine. 2015 Aug 26;33(36):4565-71. https://doi.org/10.1016/j.vaccine.2015.07.003.
- Footnote 151
-
Petrie JG, Ohmit SE, Cheng CK, Martin ET, Malosh RE, Lauring AS, et al. Influenza Vaccine Effectiveness Against Antigenically Drifted Influenza Higher Than Expected in Hospitalized Adults: 2014-2015. Clin Infect Dis. 2016 Oct 15;63(8):1017-25. https://doi.org/10.1093/cid/ciw432.
- Footnote 152
-
Pott H, Andrew MK, Shaffelburg Z, Nichols MK, Ye L, ElSherif M, et al. Vaccine Effectiveness of non-adjuvanted and adjuvanted trivalent inactivated influenza vaccines in the prevention of influenza-related hospitalization in older adults: A pooled analysis from the Serious Outcomes Surveillance (SOS) Network of the Canadian Immunization Research Network (CIRN). Vaccine. 2023 Sep 09:S0264-5. https://doi.org/10.1016/j.vaccine.2023.08.070.
- Footnote 153
-
York Health Economics Consortium. Glossary: Health Economic Terms [Internet]. York (UK): YHEC; 2023 [cited 2023 Aug 16]. Available from: https://yhec.co.uk/resources/glossary/.
- Footnote 154
-
Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992 Feb 15;146(4):473-81.
- Footnote 155
-
Gomersall JS, Jadotte YT, Xue Y, Lockwood S, Riddle D, Preda A. Conducting systematic reviews of economic evaluations. Int J Evid Based Healthc. 2015 Sep;13(3):170-8. https://doi.org/10.1097/XEB.0000000000000063.
- Footnote 156
-
Heyland DK, Kernerman P, Gafni A, Cook DJ. Economic evaluations in the critical care literature: do they help us improve the efficiency of our unit? Crit Care Med. 1996 Sep;24(9):1591-8. https://doi.org/10.1097/00003246-199609000-00025.
Page details
- Date modified:
