Invasive meningococcal disease: For health professionals
On this page
- Key information
- Epidemiology
- Clinical manifestations
- Risk factors
- Diagnosis and laboratory testing
- Treatment
- Prevention
- Post-exposure and controlmanagement
- Surveillance and monitoring
Key information
Meningococcal disease is caused by the aerobic, gram-negative bacterium Neisseria meningitidis (meningococcus). When N. meningitidis enters normally sterile sites in the body, it becomes known as invasive meningococcal disease (IMD). Invasive meningococcal disease is a nationally notifiable disease where infection can result in:
- meningococcal meningitis (about 50% of cases)
- meningococcal sepsis or meningococcemia (about 35% to 40% of cases)
It's possible for an infection to result in both.
Meningococci also occasionally cause non-invasive infections such as conjunctivitis or urethritis.
The bacteria spreads person to person through respiratory droplets.
The National Advisory Committee on Immunization (NACI) recommends routine immunization against the disease. The Committee to Advise on Tropical Medicine and Travel (CATMAT) recommends immunization before travel to high-risk meningococcal destinations.
Learn more about:
- About CATMAT
- NACI: Meningococcal vaccines statements and publications
- Meningococcal vaccines: Canadian Immunization Guide
Epidemiology
Meningococci can be classified based on the polysaccharide capsule into 12 different serogroups. Six of these (A, B, C, X, W and Y) are associated most frequently with invasive meningococcal disease in Canada and around the globe. Incidence varies by meningococcal serogroup.
Learn more about:
- Neisseria meningitidis: Infectious substances pathogen safety data sheet
- Surveillance and monitoring of invasive meningococcal disease
Reservoir
Humans are the only reservoir.
Incubation period
Invasive meningococcal disease is characterized by a short incubation period of 2 to 10 days (usually 3 to 4 days).
Transmission
Invasive meningococcal disease is transmitted through respiratory droplets and throat secretions.
Some people are asymptomatic carriers of meningococci, both typeable and non-typeable.
Learn more about:
Clinical manifestations
Invasive meningococcal infection can result in meningitis or sepsis, or both.
Symptoms occur 2 to 10 days (usually 3 to 4 days) after exposure.
Common signs and symptoms of meningitis can include sudden onset of:
- fever
- intense headache
- nuchal rigidity
Other common symptoms of meningitis can include:
- nausea
- vomiting
- photophobia
- altered sensorium
A petechial rash with pink macules, or occasionally vesicles, may be observed as well as petechiae on the conjunctiva on the palate.
The rash can be harder to see on darker skin tones. Check paler areas, such as the:
- stomach
- soles of the feet
- roof of the mouth
- whites of the eyes
- palms of the hands
- inside of the eyelids
Meningitis may present differently or more subtly in newborns and infants. Newborns and infants may:
- vomit
- feed poorly
- be irritable
- be lethargic
- have a bulging fontanelle
- have a change in level of alertness
Symptoms of meningococcemia can include an abrupt onset of:
- fever
- chills
- myalgia
- malaise
- vomiting
- tachypnea
Symptoms also include abrupt onset of:
- prostration
- cold extremities
- limb and joint pain
- petechial non-blanchable skin rash, vesicles, and petechiae in the conjunctivae or palate
Complications of invasive meningococcal disease
Between 2012 and 2021 there were 738 cases with outcome data available. Of these, 104 deaths associated with invasive meningococcal disease were reported. This represents an overall case fatality rate of 14.1%
The case fatality rate is highest in those younger than 1 year of age, followed closely by those:
- 60 years and older
- 20 to 24 years of age
About 15% to 20% of survivors will have long-term sequalae from infection. Long-term sequalae can include:
- hearing loss
- seizures
- neurological disabilities
- behavioural and cognitive problems
- amputation of 1 or more digits or limbs
- skin scarring
Images of clinical manifestations of invasive meningococcal disease
| Image 1: Gangrene of hands and lower extremities | Image 2: Lower extremities |
|---|---|
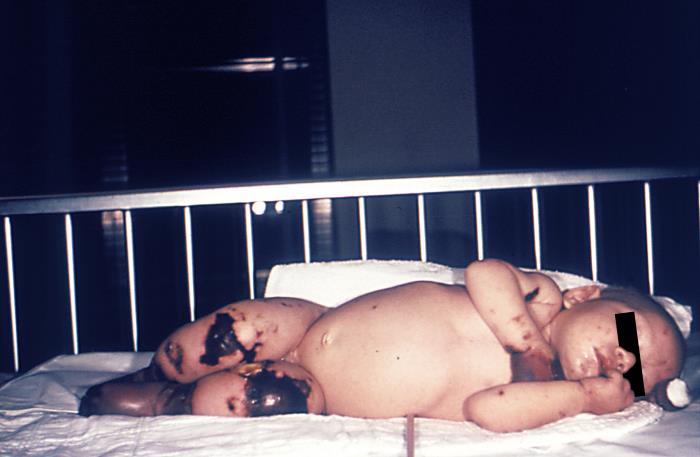 Source: the Centers for Disease Control and Prevention. |
 Courtesy of the American Academy of Pediatrics. |
Image 1 on the left shows a light-skinned 4-month old infant with gangrene of the hands and lower extremities due to meningococcemia.
Image 2 on the right shows the lower extremities of a child with the non-blanching petechial or purpuric rash caused by meningococcemia.
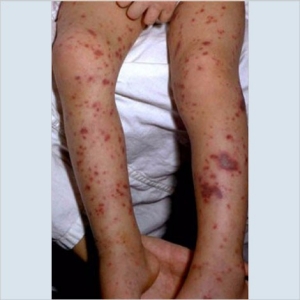
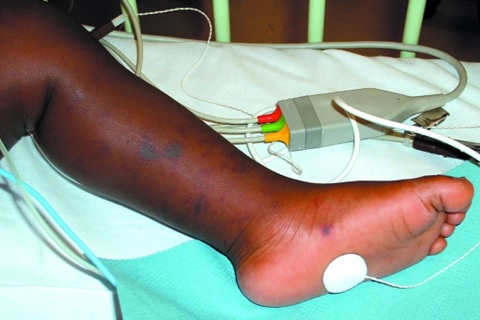
| Image 3: Purpura of the foot | Image 4: Purpura of the foot |
|---|---|
 Courtesy of the American Academy of Pediatrics. |
 Courtesy of the U.K.'s National Health Services. |
Image 3 on the left shows marked purpura in the left foot of an adolescent.
Image 4 on the right shows a rash on a darker-skinned child's leg, demonstrating that it can be harder to see on darker skin tones.
Risk factors
Individuals with increased risk of meningococcal disease because of underlying medical conditions include those with:
- functional or anatomic:
- asplenia
- sickle cell disease
- congenital immunodeficiencies such as:
- primary antibody deficiencies
- complement, properdin, factor D, combined T and B cell immunodeficiencies
- acquired complement deficiency due to receipt of the terminal complement inhibitor:
- eculizumab (Soliris™)
- ravulizumab (ULTOMIRIS®)
- HIV
Individuals at increased risk of exposure to invasive meningococcal disease include:
- travellers to areas with high rates of endemic meningococcal disease or transmission
- this includes travel to the meningitis belt of sub-Saharan Africa and pilgrims for the purposes of Hajj in Mecca or Umrah
- research, industry and clinical laboratory personnel who are potentially exposed to N. meningitidis
Age is a predictor of risk. Between 2012 and 2022 in Canada, the highest annual incidence rates were in:
- infants younger than 1 year of age
- children 1 to 4 years of age
- adolescents 15 to 19 years of age
- young adults 20 to 24 years of age
In epidemiological studies, increased incidence of the disease has also been observed in those:
- living in households with overcrowding
- who have been actively and passively smoking
- with concurrent or recent respiratory tract infection
Diagnosis and laboratory testing
A health care provider suspecting invasive meningococcal disease based on history and presentation should confirm the diagnosis with a laboratory sample.
A culture or polymerase chain reaction (PCR) from a sterile site of N. meningitidis confirms the diagnosis. The National Microbiology Laboratory in Canada confirms samples of suspected N. meningitidis to identify the serogroup and conducts further bacteriologic testing, including:
- serotyping and subtyping of all isolates
- genetic testing to identify clonal types, including clones of national and global significance
A health care provider should obtain and send a sample of:
- blood
- cerebrospinal fluid
- aspiration of pus
A sample from a purpuric or petechial lesion scraping, synovial fluid and other sterile sites may also sometimes be positive.
Other diagnostic methods can include PCR and antigen detection.
Health care providers are required to report suspected cases of invasive meningococcal disease to their local public health unit as it's a nationally notifiable disease.
Only cases meeting the national case definition are reported to the National Enhanced Invasive Meningococcal Disease Surveillance System.
Learn more about:
- National case definition: Invasive meningococcal disease
- National Enhanced Invasive Meningococcal Disease Surveillance System
Treatment
Empiric treatment for suspected invasive meningococcal disease should be started as soon as possible. There have been reported cases of penicillin-resistant invasive meningococcal disease. Consult current guidelines or consult an infectious diseases expert.
Supportive care in a hospital is usually required. Many patients require ICU and surgical care.
Learn more about:
- Treatment of Neisseria meningitidis: Infectious substances pathogen safety data sheet
- Guidelines for the management of suspected and confirmed bacterial meningitis in Canadian children older than 2 months of age (Canadian Paediatric Society)
Prevention
Immunization can reduce invasive meningococcal disease due to infection by serogroups A, B, C, W, and Y. The following meningococcal vaccine preparations are authorized for use in Canada:
- monovalent conjugate meningococcal vaccines (Men-C-C)
- quadrivalent conjugate meningococcal vaccines (Men-C-ACYW)
- serogroup B meningococcal vaccines (4CmenB and MenB-fHBP)
Vaccine effectiveness depends on the serogroup and time since vaccination. Additional doses may be recommended for certain groups.
Use of Men-C-C vaccine is recommended by NACI as a part of routine childhood immunization schedules.
NACI recommends that adolescents and young adults, depending on local epidemiology and programmatic considerations, receive a dose of monovalent conjugate meningococcal C (Men-C-C) or quadrivalent conjugate meningococcal (Men-C-ACYW) vaccine routinely at the age of 12, even if previously vaccinated as infants or toddlers.
4CMenB or MenB-fHBP vaccine may be considered on an individual basis, depending on:
- strain susceptibility
- individual preferences
- regional serogroup B epidemiology
Additionally, meningococcal vaccines are recommended for selected individuals:
- at increased risk of exposure to meningococcal disease
- with increased risk of meningococcal disease because of underlying medical conditions
For specific recommendations about meningococcal vaccines and their use, refer to:
- the Canadian Immunization Guide
- NACI statements on meningococcal vaccines
Learn more about:
- Meningococcal vaccines: Canadian Immunization Guide
- NACI: Meningococcal vaccines statements and publications
- Provincial and territorial routine and catch-up vaccination schedule for infants and children in Canada
Booster doses and re-vaccination
Additional doses of Men-C-ACYW vaccines to protect against invasive meningococcal disease may be recommended:
- when travelling to areas where meningococcal vaccine is recommended or required
- for individuals at high risk of meningococcal disease due to underlying medical conditions
- for research, industrial and clinical laboratory personnel who are potentially routinely exposed to N. meningitidis
Men-C-C vaccines or Men-C-ACYW may also be recommended:
- to contacts of a case of the disease in some circumstances
- during a community outbreak of IMD in some circumstances
Learn more about:
Adverse events and contraindications
Side effects of immunization are usually mild and resolve on their own, including local injection site reactions that may occur soon after vaccination. Some side effects may develop after vaccination, including fever and malaise.
There are very few individuals who cannot receive meningococcal vaccines. A history of anaphylaxis to the vaccine is a contraindication. Administration of meningococcal vaccine should be postponed in persons with moderate or severe acute illness.
Persons with minor acute illness, with or without fever, may be vaccinated.
Reporting of adverse events following immunization is important to inform vaccine safety surveillance and is mandatory in all provinces and territories. To enable the detection of safety concerns, reports from across the country are compiled and analyzed along with:
- expert opinions
- international data
- literature reviews
Health care providers should report serious adverse events following immunization to the local public health authority.
Learn more about:
Post-exposure and control management
Post-exposure management
Close contacts of individuals with meningococcal infections have an increased risk of developing invasive meningococcal disease. Antibiotic chemoprophylaxis is recommended for all persons having close contact with a case of the disease from 7 days before onset of symptoms in the case to 24 hours after onset, regardless of their immunization status.
Vaccination or re-vaccination of certain close contacts should be considered in addition to chemoprophylaxis when the serogroup is vaccine preventable, as it may further reduce the risk of subsequent meningococcal disease.
Learn more about:
Outbreak management
During an outbreak of invasive meningococcal disease, it is important to consult with public health officials and experts in communicable diseases.
Outbreaks may be controlled with the use of a conjugate meningococcal vaccine, depending on the serogroup causing the outbreak and the age of those being vaccinated. This can include the re-vaccination of previously vaccinated individuals.
Learn more about:
Surveillance and monitoring
Invasive meningococcal disease is endemic, but rare in Canada.
Surveillance systems in Canada
Since becoming a nationally notifiable disease in 1924, invasive meningococcal disease has affected over 23,000 people in Canada.
Cases meeting the national case definition must be reported by health care providers to their local public health unit. Information is subsequently forwarded to provincial or territorial public health officials and then to the Public Health Agency of Canada.
The National Enhanced Invasive Meningococcal Disease Surveillance System is a part of the Public Health Agency's national surveillance system. It was established to capture enhanced epidemiologic and laboratory information on invasive meningococcal disease cases for the purpose of describing annual trends, in particular serogroup trends. The system was established in 1992 and has collected anonymized data since 1985 (data from 1985 to 1991 was collected retrospectively).
Immunization programs in Canada
The implementation of invasive meningococcal disease routine immunization programs in Canada began in 2002 with the conjugate meningococcal C (Men-C-C) vaccine. By 2007, monovalent (targeting serogroup C) and quadrivalent (targeting serogroups A, C, W and Y) conjugate vaccines were being used across Canada. The multicomponent meningococcal B vaccine (4CMenB) has been used to control outbreaks.
Epidemiology in Canada
The average incidence rate of invasive meningococcal disease was:
- 0.83 cases per 100,000 population from 1997 to 2001 (the pre-vaccine era)
- 0.28 cases per 100,000 population from 2017 to 2021
This is an overall incidence rate decrease of 66% (Figure 1).
More specifically, with routine vaccination, the incidence of serogroup C has declined by 96%, (Figure 2). Canada is on track to meet its reduction target of less than 5 cases of serogroup C annually in children less than 19 years of age by 2025.
Between 2017 to 2021, serogroup B has accounted for 33% of reported cases in Canada. Serogroup B has also accounted for the majority of reported deaths. Serogroup B disproportionally affects the young (children under 1 year of age and children between 1 and 4 years of age).
Serogroup B is responsible for the majority of cases and has been declining overall since its peak in 2007. However, the incidence rates of serogroup W has been increasing. The remaining other serogroups have been relatively stable (Figure 2).
Between 2017 to 2021, serogroup W, Y and C accounted for 32%, 17% and 4% of respective cases.
Between 2017 to 2021, there was a 5.2% case-fatality rate for invasive meningococcal disease-associated deaths.

Figure 1 - Text description
| Year | Cases | Incidence rate (per 100,000 population) |
|---|---|---|
| 1997 | 265 | 0.89 |
| 1998 | 174 | 0.58 |
| 1999 | 214 | 0.7 |
| 2000 | 242 | 0.79 |
| 2001 | 366 | 1.18 |
| 2002 | 234 | 0.75 |
| 2003 | 195 | 0.62 |
| 2004 | 196 | 0.61 |
| 2005 | 182 | 0.56 |
| 2006 | 212 | 0.65 |
| 2007 | 233 | 0.71 |
| 2008 | 195 | 0.59 |
| 2009 | 212 | 0.63 |
| 2010 | 154 | 0.45 |
| 2011 | 175 | 0.51 |
| 2012 | 154 | 0.44 |
| 2013 | 121 | 0.34 |
| 2014 | 101 | 0.29 |
| 2015 | 108 | 0.3 |
| 2016 | 98 | 0.27 |
| 2017 | 119 | 0.33 |
| 2018 | 141 | 0.38 |
| 2019 | 139 | 0.37 |
| 2020 | 86 | 0.23 |
| 2021 | 49 | 0.13 |

Figure 2 - Text description
| Year | Incidence rate by serogroup (per 100,000 population) | ||||
|---|---|---|---|---|---|
| B | C | Y | W | Other | |
| 1997 | 0.36 | 0.23 | 0.11 | 0.03 | 0.14 |
| 1998 | 0.22 | 0.13 | 0.06 | 0.02 | 0.16 |
| 1999 | 0.30 | 0.21 | 0.06 | 0.04 | 0.10 |
| 2000 | 0.22 | 0.34 | 0.08 | 0.03 | 0.12 |
| 2001 | 0.28 | 0.60 | 0.10 | 0.03 | 0.17 |
| 2002 | 0.29 | 0.25 | 0.12 | 0.02 | 0.05 |
| 2003 | 0.26 | 0.15 | 0.13 | 0.05 | 0.02 |
| 2004 | 0.27 | 0.17 | 0.08 | 0.04 | 0.04 |
| 2005 | 0.30 | 0.12 | 0.07 | 0.05 | 0.02 |
| 2006 | 0.35 | 0.13 | 0.08 | 0.02 | 0.07 |
| 2007 | 0.40 | 0.09 | 0.11 | 0.04 | 0.06 |
| 2008 | 0.29 | 0.09 | 0.11 | 0.04 | 0.04 |
| 2009 | 0.38 | 0.06 | 0.10 | 0.04 | 0.05 |
| 2010 | 0.27 | 0.03 | 0.08 | 0.02 | 0.04 |
| 2011 | 0.31 | 0.01 | 0.10 | 0.03 | 0.05 |
| 2012 | 0.32 | 0.04 | 0.05 | 0.01 | 0.03 |
| 2013 | 0.23 | 0.02 | 0.07 | 0.01 | 0.02 |
| 2014 | 0.15 | 0.03 | 0.08 | 0.02 | 0.01 |
| 2015 | 0.18 | 0.01 | 0.07 | 0.03 | 0.02 |
| 2016 | 0.13 | 0.01 | 0.07 | 0.04 | 0.02 |
| 2017 | 0.13 | 0.02 | 0.06 | 0.08 | 0.03 |
| 2018 | 0.07 | 0.02 | 0.05 | 0.12 | 0.03 |
| 2019 | 0.11 | 0.01 | 0.07 | 0.12 | 0.05 |
| 2020 | 0.08 | 0.003 | 0.05 | 0.07 | 0 |
| 2021 | 0.06 | 0 | 0.005 | 0.05 | 0 |
Learn more about:
- Epidemiology of invasive meningococcal disease in Canada, 2012 to 2019, CCDR 48(5)
- Vaccine Preventable Disease (IMD): Surveillance Report to December 31, 2019
Related links
Guidelines and recommendations
- NACI: Statements and publications
- Meningococcal vaccines: Canadian Immunization Guide
- Committee to Advise on Tropical Medicine and Travel
General information
- Vaccine safety: Canadian Immunization Guide
- Immunization of travellers: Canadian Immunization Guide
- Meningococcal vaccine (Caring for kids)
- Additional Information for professionals on immunization and vaccines
- Defeating Meningitis by 2030 (World Health Organization)
- National case definition: Invasive meningococcal disease
- Pocket Guide for Immunizers: Meningococcal Vaccination (CANVax)
- Provincial and territorial routine and catch-up vaccination schedule for infants and children in Canada
- NACI update on invasive meningococcal disease epidemiology and prevention
- Highlights from the 2021 childhood National Immunization Coverage Survey (cNICS)
Page details
- Date modified: