Canadian Biosafety Guideline: Biosafety in the Elementary, Intermediate, and Secondary School Classroom
Download in PDF format
(3.83MB, 112 pages)
Organization: Public Health Agency of Canada
Published: 2020-09-11
Table of Contents
- Preface
- Abbreviations and acronyms
- Chapter 1. Introduction
- Chapter 2: Biosafety and biosecurity basics
- Chapter 3. Promoting a culture of biosafety in the classroom
- Chapter 4. Considerations before handling microbes
- Chapter 5. Strategies to safely handle microbes in the classroom
- Chapter 6. Decontamination and waste management
- Chapter 7. Considerations for science fair projects
- Chapter 8. Glossary
- Chapter 9. References and resources
- Appendix A. Risk groups and containment levels
- Appendix B. Pathogen safety data sheet template
- Appendix C. Public Health Agency of Canada biosafety posters
- Appendix D. Risk assessment template
- Appendix E. Procedures to minimize aerosol hazards
- Appendix F. General spill clean-up procedure
- Appendix G. Proper handwashing technique
- Appendix H. How to safely remove disposable gloves
- Appendix I. Suitability of chemical disinfectants
- References
Preface
Biosafety in the Elementary, Intermediate, and Secondary School Classroom is a guideline that was developed by the Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) as part of its Canadian Biosafety Guidelines series. This series of electronic publications expands upon the biosafety and biosecurity concepts discussed in the Canadian Biosafety Handbook (CBH), the companion document to the Canadian Biosafety Standard (CBS). The Biosafety in the Elementary, Intermediate, and Secondary School Classroom guideline provides recommendations for work with biological material in the classroom based on risks.
Microbes that do not cause disease in humans or animals (i.e., Risk Group 1 microorganisms) are not regulated by the PHAC or the CFIA, and persons who handle such material do not need to meet requirements described in the CBS. Nevertheless, certain activities in the classroom can result in the unintentional handling or storing of harmful microbes and may put students and personnel at risk of exposure. The Biosafety in the Elementary, Intermediate, and Secondary School Classroom guideline explains the best practices to reduce the risks of handling harmful microbes which are regulated by the PHAC and the CFIA.
In Canada, persons who handle or store human pathogens (i.e., harmful microbes) or toxins have to perform their activities in accordance with a Pathogen and Toxin Licence issued by the PHAC. The PHAC regulates these activities under the Human Pathogens and Toxins Act (HPTA) and the Human Pathogens and Toxins Regulations (HPTR). The PHAC or the CFIA also regulate the importation of animal pathogens, infected animals, and animal products or by-products (e.g., tissue, serum) or other substances that may carry an animal pathogen or toxin or a part of one. Importation is regulated under the Health of Animals Act and Health of Animals Regulations.
The following figure depicts the document hierarchy used by the PHAC and the CFIA to oversee biosafety and biosecurity operations. Each tier of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards. Acts and regulations are at the top of the pyramid as they convey the PHAC’s and the CFIA’s legal authorities. Guidance material and technical pieces are at the bottom of the pyramid as they only provide recommendations and scientific information.
Figure 1: The Government of Canada's biosafety and biosecurity document hierarchy

Figure 1: Text description
Figure in the form of a pyramid depicting the document hierarchy used by the PHAC to oversee biosafety and biosecurity operations. Each of the five tiers of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards.
At the top sits the Enabling Legislation, that is, the HPTA, HPTR, HAA, and HAR, that convey the PHAC's legal authorities. Below the acts and regulations sit Instruments in Support of Legislation, which are the Pathogen Risk Assessments. The next tier down are the Biosafety Requirements, which include the Canadian Biosafety Standard, Biosafety Directives, and Biosafety Advisories. In the second lowest tier are the Policy Documents, the Compliance and Enforcement Policy. Guidance material and technical pieces found at the bottom of the pyramid, under the Risk Communication Tools and Technical Documents heading, are intended to summarize recommendations and scientific information only. These include the Canadian Biosafety Handbook, Canadian Biosafety guidelines, and Pathogen Safety Data Sheets.
The Biosafety in the Elementary, Intermediate, and Secondary School Classroom guideline is always evolving and subject to ongoing improvement. The PHAC and the CFIA welcome questions, comments, and suggestions for incorporation into future versions. Please send the information (with references, where applicable) to:
- PHAC email: PHAC.pathogens-pathogenes.ASPC@canada.ca
Abbreviations and acronyms
- CBH
- Canadian Biosafety Handbook
- CBS
- Canadian Biosafety Standard
- CFIA
- Canadian Food Inspection Agency
- CL
- Containment level (i.e., CL1, CL2, CL3, CL4)
- ERP
- Emergency response plan
- HPTA
- Human Pathogens and Toxins Act
- HPTR
- Human Pathogens and Toxins Regulations
- PHAC
- Public Health Agency of Canada
- PPE
- Personal protective equipment
- PSDS
- Pathogen safety data sheet
- RG
- Risk Group (i.e., RG1, RG2, RG3, RG4)
- SOP
- Standard operating procedure
Chapter 1. Introduction
The words in bold type are defined in the glossary found in Chapter 8.
Hands-on science experiments are an important part of student learning. They are learning tools that help students understand the concepts taught in the classroom.Footnote 1 Biological material is often used to demonstrate a wide range of biological processes and applications that are part of the curriculum. Biological material includes microbes (i.e., microorganisms), toxins, proteins, and nucleic acids. Biological material also refers to anything in which these are present, such as soil, water, foods, environmental samples, and skin swabs. Examples of microbes are bacteria, viruses, and fungi (e.g., mushrooms, molds, yeast). While most microbes are harmless, some can infect humans and animals and make them sick when they get on or inside them. These harmful microbes are called “pathogens”. When harmful microbes manage to get on or inside a person, it is called an exposure. Exposure can occur through eating harmful microbes, breathing them in, or having them come into contact with a person’s eyes or skin.
The Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) perform risk assessments for microbes (called “pathogen risk assessments”) to find out how dangerous microbes might be to humans and animals. At the end of this assessment, the microbes are assigned to one of four risk groups. The risk group gives an indication of the measures needed to safely handle the microbe, including the containment level. Working in a containment zone means following certain rules (i.e., operational procedures) and having access to certain equipment and work spaces (i.e., meet physical requirements) to keep all the people in the work space safe from the dangers associated with the microbe.
Safe work practices, such as good microbiological laboratory practices, can help achieve a high level of safety and teach students how to properly handle biological materials.Footnote 2 Due to the low risk associated with Risk Group 1 (RG1) microbes, they are the ideal type of biological material to use in the classroom, and neither the PHAC nor the CFIA regulates them.Footnote 3
The PHAC and the CFIA do regulate biological materials classified as RG2, RG3, or RG4, as they are more dangerous. People working with these biological materials have to follow specific rules to keep themselves and the community safe. These rules are the physical containment requirements and operational practice requirements specified in the Canadian Biosafety Standard (CBS). A valid Pathogen and Toxin Licence issued by the PHAC or an animal pathogen import permit issued by the CFIA may also be required before people are allowed to work with these materials.
1.1. Scope
The Biosafety in the Elementary, Intermediate, and Secondary School Classroom guideline is meant to be used by elementary, intermediate, or secondary school teachers and support personnel such as demonstrators, teaching assistants, student teachers, and parents. This guideline is meant to help anyone planning to work with a known RG1 microbe or with environmental samples such as soil, water, and skin swabs. The guideline is designed to promote best practices, biosafety in the classroom, and awareness of the dangers associated with working with microbes. The information presented in this guideline can help keep students, teachers, personnel, and the community safe.
This guideline describes general recommendations and considerations for activities with RG1 microbes in the classroom. These recommended practices are based on risk and on evidence. The information presented here takes into account the principles of biosafety and biosecurity outlined in the Canadian Biosafety Guideline - Containment Level 1: Physical Design and Operational Practices.Footnote 4
The information provided in this guideline is intended as guidance only, to enhance biosafety in the classroom, and is not to be interpreted as requirements.
In the context of the COVID-19 pandemic, the measures described in the blue boxes throughout this document are highly recommended. These recommendations can be used in conjunction with the current public health guidance, such as the risk mitigation guidance provided by the Government of Canada for schools kindergarten to grade 12 as well as child and youth settings.Footnote 5Footnote 6
1.2. Overview of the Canadian Biosafety Standard and the Canadian Biosafety Handbook
The CBS is the national standard for the handling or storing of human and terrestrial animal pathogens and toxins in Canada. The Canadian Biosafety Handbook (CBH) provides information and guidance on how to achieve the biosafety and biosecurity requirements described in the CBS for facilities regulated by the PHAC or the CFIA. Teachers and administrators of elementary, intermediate, and secondary schools who are only working with RG1 microbes are not required to meet the requirements specified in the CBS. They can, however, consult the CBS, to understand what is required to work with regulated material, and the CBH for additional biosafety guidance.
1.3. How to use the Biosafety in the Elementary, Intermediate, and Secondary School Classroom guideline
A detailed list of all abbreviations and acronyms used in this guideline can be found at the beginning of the document. Each word or term is spelled out upon first use, with the abbreviation immediately following in brackets. After its first use, only the abbreviation is used for the rest of the document. This guideline also contains a glossary of definitions for technical terms, located in Chapter 8. The technical terms defined in the glossary appear in bold type upon first use in the guideline. A list of references and other resources is provided in Chapter 9.
Chapter 2. Biosafety and biosecurity basics
Working with microbes is necessary in public health research; it helps us learn more about harmful microbes, how to protect ourselves from infections, and how to treat and cure infections. Sometimes, this work can be dangerous because some harmful microbes can make humans sick (i.e., cause illness and disease). With harmful microbes, special care is required to avoid exposure or release. The method of exposure can depend on the microbe; for example, some microbes require an open wound, while others can be absorbed through the skin or be inhaled. A release is when a microbe gets outside of the work area where it was being handled and can spread in the community.
Biosafety is the principles and procedures that describe how to safely use biological material. These containment principles and procedures protect people, animals, and the environment from harmful microbes.Footnote 7 To maintain biosafety in the classroom, teachers can implement a basic level of operational practices that include good microbiological laboratory practices. Some physical design features, such as having a well-designed space where students can conduct their experiment, will also contribute to biosafety. Generally, RG1 microbes present a very low risk.
2.1. Types of microbes
There are different types of microbes that can be found in a classroom. They are briefly described in Table 2-1.
| TypeFootnote 1 | Characteristics | Growth conditions in a classroom laboratory |
|---|---|---|
Bacteria |
|
On solid media plates (e.g., agar) or in liquid culture (e.g., broth). |
Virus |
|
Inside another cell (i.e., cell or tissue culture) derived from various sources (e.g., animal, bacteria). |
Fungus (plural fungi) |
|
On solid media plates or in liquid culture. |
Parasite |
|
On solid media plates, in liquid culture, or inside another cell. |
Footnotes
|
||
The vast majority of microbes are harmless to humans. Some microbes can even be good for humans; for example, Lactobacillus found in yogurt can be good for the human digestive system (probiotic). As mentioned before, harmful microbes that can cause disease are called “pathogens”. When harmful microbes only cause a disease in specific circumstances, they are called opportunistic pathogens. For example, some harmful microbes can only make a person or an animal sick when they are present in a very large amount, or when the person or the animal has a weak immune system. In this guideline, “harmful microbes” refers to all microbes capable of causing disease in humans or animals.
Some harmful microbes produce poisonous substances called “toxins“. Exposure to toxins can cause a sickness known as intoxication. Examples of toxins produced by microbes include Toxic Shock Syndrome Toxin (for which symptoms can vary from fever to multiple internal organs not working) and botulinum neurotoxin (for which symptoms can vary from trouble speaking to trouble breathing). In this guideline, “toxin” only refers to toxins produced by harmful microbes.
2.2. Classification of microbes by risk groups
Risk assessments result in microbes being separated into four risk groups. This assessment looks at what makes a microbe dangerous to an individual person or animal, to public health, and the animal population. These characteristics include:
- its natural presence in the environment;
- its ability to cause a disease;
- how severe the disease is;
- how likely it is to spread in a group (e.g., from person to person);
- the availability of effective medicine to prevent infection (e.g., vaccines); and
- the availability of effective medicine to treat the disease (e.g., antibiotics).
As indicated in Table 2-2, risk groups are determined by how dangerous the microbe is to individuals (human or animal) and to the community (public health or animal populations). To determine the risk to single individuals, we consider the likelihood of a person becoming sick after being exposed and how serious the disease is. To determine the risk a microbe represents for the community, we consider how easily it can pass to another person or animal.
| Risk group | Individual risk | Community risk |
|---|---|---|
| RG1 | No or Low | Low |
| RG2 | Moderate | Low |
| RG3 | High | Low |
| RG4 | High | High |
Microbes are assigned a risk group for humans and another for animals because the two risk groups can be different. For example, Sheeppox virus is not dangerous for people (RG1 microbe for humans) but is dangerous for animals (RG3 microbe harmful to animals). More information on risk groups can be found in Appendix A. The Schedules 2 through 4 of the Human Pathogens and Toxins Act (HPTA) list examples of microbes harmful to humans according to risk group. Examples of microbes harmful for animals can be found on the CFIA’s website.Footnote 16
It is possible to find the risk group classification of thousands of microbes and toxins in the online ePATHogen – Risk Group Database.Footnote 17 It is also possible to find the risk group of a microbe in its Pathogen Safety Data Sheet (PSDS), if there is one.Footnote 18 PSDSs contain a lot of information on a microbe such as how to safely handle and dispose of it.Footnote 18 Other types of information included in the PSDS are described in the template found in Appendix B. PSDSs are available on the Government of Canada website and through the PSDS App, which can be downloaded for free from device stores (e.g., Android, Amazon, Apple, Windows). If the PSDS or the risk group for a microbe cannot be found, the PHAC can be contacted to provide relevant information before the microbe is used in a classroom.
2.3. Classification of laboratories by containment levels
The containment level of a laboratory describes both the physical design and the operational practices that protect individuals, the community, and the environment from the microbes handled there. A containment zone is an enclosed physical area that meets specific requirements for physical design and operational practices. A containment level is assigned to a containment zone that meets all the requirements specified in the CBS for physical design and operational practices.
There are four containment levels ranging from Containment Level 1 (CL1) to CL4. A basic laboratory where it is safe to work with the lowest risk biological material is considered CL1. Highly sophisticated laboratories where it is safe to work with the most dangerous microbes are CL4. In most cases, the risk group of the microbe is the same as the containment level required to work with it. For example, a harmful microbe of RG2 is usually handled in a CL2 laboratory.
The PHAC and the CFIA have established the minimum physical containment requirements, operational practice requirements, and performance and verification testing requirements for CL2, CL3, and CL4 laboratories. These requirements can also be specific to the type of activities taking place in CL2, CL3, and CL4 laboratories such as activities that involve very large volumes (i.e., large scale) or animals. These requirements are specified in the CBS. Table 2-3 presents short descriptions of all four containment levels and Appendix A provides more details. Because the risk associated with RG1 microbes is very low, the CBS does not specify requirements for CL1. Recommendations can be found however in the Canadian Biosafety Guideline - Containment Level 1: Physical Design and Operational Practices.Footnote 4
| Containment level | Minimum requirements |
|---|---|
CL1 |
|
CL2 |
|
CL3 |
|
CL4 |
|
Examples of microbes for each risk group and containment level are provided in Table 2-4. Every person who handles harmful microbes of RG2, RG3, or RG4 must have a Pathogen and Toxin Licence and/or an animal pathogen import permit, and only work with them in a laboratory of the appropriate containment level.
| Risk group for humans | Containment level | Examples Type: Species (common name or associated illness) |
|
|---|---|---|---|
RG1 |
CL1 |
Bacteria |
Bacillus subtilis |
Fungi |
Aspergillus niger |
||
Virus |
Bacteriophage of lactic acid bacteria |
||
RG2 |
CL2 |
Bacteria |
Escherichia coli O157:H7Footnote 1 |
Fungi |
Aspergillus flavus |
||
Viruses |
Influenza A virus H3N2Footnote 1 (seasonal flu) |
||
Parasite |
Giardia intestinalis (beaver fever) |
||
RG3 |
CL3 |
Bacteria |
Bacillus anthracis (anthrax) |
Fungi |
Blastomyces dermatitidis |
||
Viruses |
Influenza A virus H5N1Footnote 1 (bird flu) |
||
RG4 |
CL4 |
Virus |
Ebolavirus (Ebola) |
Footnotes
|
|||
2.4. Physical features of the classroom space for handling microbes
The majority of classrooms set up for science experiments have the recommended characteristics of a CL1 laboratory and are suitable for activities with RG1 microbes. Very few elementary, intermediate, or secondary schools meet the requirements specified in the CBS for a CL2 laboratory. The following basic physical features can contribute to a safe and functional classroom serving as a CL1 laboratory:Footnote 2Footnote 7Footnote 19
- Surfaces such as floors, benches, desks, tables, and seating are covered with or made of smooth water-resistant material like metal, vinyl (e.g., seat covers), and epoxy paint. This makes them easier to clean and decontaminate if they become contaminated with biological material. Untreated wood that may become contaminated can be coated with a sealant (e.g., varnish, paint) to make it easier to wash.
- Sinks for handwashing are available in the classroom or nearby (e.g., bathroom). If sinks are not available, students can use alcohol-based hand sanitizers until they are able to properly wash their hands.
- Storage spaces (e.g., drawers, shelves, cubbies) keep personal belongings separate from where microbes are handled. This reduces the risk of contaminating personal items, reduces clutter, and keeps floors free of tripping hazards. Solid shelves can also be installed to store reagents and reduce the risk of spills.
- Moveable furniture (e.g., benches, desks, tables) are placed in way that minimizes crowding when microbes are handled in the classroom. The furniture’s position can also be optimized to allow the teacher to supervise all activities and to allow the students to see the entire writing board, demonstration area, and instruction screen.
- Emergency eyewash stations are available to flush the eyes after an exposure to biological material. Such stations can be in the form of an eyewash water fountain or a designated space where eyewash bottles that contain a sterile solution are provided. If a sterile solution is used, it has to be in a sufficient volume to meet the applicable standards and replaced when it expires.
Additional information on a safe and functional area for work with RG1 microbes is provided in the Canadian Biosafety Guideline - Containment Level 1: Physical Design and Operational Practices.Footnote 4 While some aspects of physical design may require costly updating or renovation, it may be possible to improve safety by adapting classrooms with little or no cost.
Chapter 3. Promoting a culture of biosafety in the classroom
Biosafety is a responsibility shared between teachers and students. Training that includes biosafety practices and procedures will effectively:
- promote safe work practices and improve safety performance;
- raise awareness on the dangers related to the biological material handled;
- raise awareness on the dangers related to the work performed;
- protect the individual and the community from exposure; and
- promote a culture of biosafety.
3.1. Teacher responsibilities
Teachers are responsible for the safety of their students and for maintaining a safe classroom environment. Resources and teaching strategies that make safety a priority in the classroom often improve other components of education as well.Footnote 20 To make safety a priority, teachers can:Footnote 7Footnote 18Footnote 21Footnote 22Footnote 23
- be familiar with provincial, territorial, and federal policies, laws, and regulations related to handling biological material;
- be familiar with their board and school’s safety policies;
- communicate biosafety needs for the classroom to administrators, including any unsafe conditions or dangerous material in the classroom;
- review and supplement the school’s emergency response plan (ERP) to include response measures for the microbes handled in the classroom;
- be trained on the experimental protocols performed in the classroom;
- lead by example by following the classroom’s biosafety procedures;
- train students on the appropriate biosafety procedures (including emergency responses) for the activities taking place in the classroom;
- train students on how important it is to report incidents, even paper-cuts or spills;
- inform students of their responsibilities (as described in section 3.2); and
- never let students take biological material out of the classroom (i.e., do not bring experiments or biological material home).
3.1.1. Training students
Students need to know about the dangers present in the classroom. They also need to know the best practices (e.g., wearing PPE) and the tools made available to them to protect themselves from these dangers. Students tend to work more safely and be better at preventing accidents that lead to exposure and release of microbes when they are aware of the dangers.Footnote 19 Some of the biosafety-related instructional courses and videos produced by the PHAC may be valuable resources for teachers and students.Footnote 24 These training materials are free and available on the Government of Canada website.
A sufficient amount of time needs to be dedicated to biosafety training for it to be effective. Time needs to be allocated to teaching it and verifying that the students understand it. Biosafety training can be incorporated into the academic lesson plan and take place at the beginning of each school year. Teachers can review their students’ knowledge on biosafety on a few occasions during the year to determine if refresher training or reminders are needed. Teachers are responsible for reminding students of the importance of biosafety during every experiment to keep them safe. Before starting an experiment, teachers can let students know about:Footnote 17Footnote 18Footnote 19
- the microbe handled and the dangers that may be associated with it;
- the ePATHogen – Risk Group Database and PSDSs and how to access relevant information;
- proper techniques to handle biological material;
- the steps in the experiment with a greater risk of exposure to biological material;
- the biosafety precautions to be applied before beginning any activity;
- the PPE that will be used and how to use it;
- any special safety procedures that need to be followed;
- the consequences of violating biosafety procedures;
- how contaminated material will be treated and disposed of safely; and
- the emergency procedures (i.e., the ERP) if an incident (e.g., spills, splashes, aerosols, accidents, breakages) happens, for which instructions may include:
- a description of the emergency equipment available in or near the classroom (e.g., first aid kits, spill kits, fire extinguisher, eyewash and shower stations);
- the locations of emergency equipment and directions for their proper use;
- the persons responsible of performing first aid;
- where emergency phone numbers are posted;
- emergency exit or evacuation routes; and
- protocols for the safe removal, transport, and treatment of persons and materials that have been contaminated.
Accessible biosafety posters can be displayed around the classroom as visual reminders of these concepts and safe work practices. Examples of posters developed by the PHAC that are free to download can be found in Appendix C.
3.1.2. Students with health concerns
Students can have health concerns that may put them at a greater risk of becoming sick during activities with biological material (e.g., allergies or anaphylaxis, compromised or suppressed immune system, lack of vaccination, asthma, impaired vision, impaired hearing, immobility, epilepsy, pregnancy). Teachers need to be made aware of these issues as this allows them to evaluate the real risks associated with each experiment and prepare the safest activities for students. To allow this, teachers can ask students with health concerns to identify themselves at the beginning of the school year or when new health concerns arise. It is important for students with health issues to be given time to inform their teacher in private and for teachers to keep this information private when appropriate. Teachers can also provide students with a list of the microbes that may be handled in the classroom and ask students to consult with their doctor (or at the very least a parent or legal guardian). Based on doctor (or parent or legal guardian) recommendations, the teacher may consider excluding students who are at a higher risk of becoming sick, or preparing alternative activities for them.Footnote 23
3.2. Student responsibilities
Each student who works with biological material is responsible for their own safety and the safety of other students, teachers, the community, and the environment.Footnote 25 To keep everyone safe, students must:Footnote 2
- listen to their teacher;
- follow their teachers’ instructions, such as safety procedures;
- be careful and act in a way that prevents spills, tripping, and falls from happening, and equipment from breaking;
- only do activities with the biological material that have been approved by the teacher;
- only handle biological material under the supervision of an adult;
- immediately inform their teacher of any unsafe situations or incidents;
- keep the area where they work (e.g., desk) clean and clear of clutter. Floors also need to be kept clear of books, coats, bags, and other items. If possible, personal items can be kept in a closed cabinet or away from the biological material; and
- immediately inform their teacher if their hands, body, or clothes come into contact with biological material. The teacher can help them with the washing and decontamination procedures, or assign someone to help.
Chapter 4. Considerations before handling microbes
4.1. Risk assessment
A risk assessment is an essential step when planning an experiment. It aims to identify and characterize the dangers associated with the biological material that will be handled, the activity planned, and the consequences of an incident. For example, it can identify that an exposure to biological material may lead to illness. A risk assessment looks at all aspects of handling the biological material, including (but not limited to):Footnote 2Footnote 26
- the procedures and equipment that will be used;
- the potential of producing aerosols (airborne material) or splashes (which increases the risk);
- the amount of sample or culture (high concentrations and volumes pose greater risk);
- the possibility of biological material being contaminated with harmful microbes (which increases the risk);
- the level of student training (there is more risk with students that are less trained [e.g., in elementary school, at the beginning of a school year]); and
- the level of student competence or familiarity with a particular task (e.g., there is more risk when it is the first time performing a task versus when repeating it).
Risk assessments evaluate the measures that already exist and determine whether they are appropriate to mitigate the risks associated with the activities involving biological material. Risk assessments also help identify the additional measures that will minimize the risks.
Advice on how to perform a risk assessment for activities with microbes can be found in the Canadian Biosafety Guideline - Local Risk Assessment.Footnote 26 A template can also be found in Appendix D.
4.1.1. The spread of microbes
Microbes that are handled in the classroom can contaminate the hands and be transferred to surfaces and objects such as door handles, pens, paper, and phones. This spread of microbes presents a risk of exposure to any person that touches these contaminated surfaces or objects. Harmful microbes found on people and in the surrounding areas can also contaminate the biological material handled in the classroom and make it less safe to handle.
Some procedures, like mixing liquids vigorously, can create aerosols that contain microbes and these can be inhaled or float in the air before settling on surfaces. The smaller an aerosol droplet, the longer it can travel in the air. Larger aerosol droplets can settle on surfaces (e.g., benchtops, clothes, hands).Footnote 27 Environmental conditions such as temperature, humidity, surface material, and cleanliness can influence how long a microbe can survive on a surface.Footnote 28 Depending on the microbe, survival time can range from 1 day to 16 months on surfaces (e.g., toilets, door handles).Footnote 28 Procedures that are less likely to produce aerosols are provided in Appendix E.
Many factors can affect the transfer of microbes from one surface to another. These factors include:Footnote 29
- the type of organism;
- the type of surface;
- hand hygiene;
- the number of students in the classroom; and
- whether there are proper cleaning and decontamination procedures in place.
In one study, researchers noticed contamination was reduced by 75-90% on frequently touched surfaces when they were regularly cleaned and hand hygiene (e.g., hand sanitizers and hand washing) was implemented.Footnote 30 To help prevent the spread of microbes, teachers and students can clean the surfaces on which they work with biological material immediately after they finish their activities. Once surfaces are cleaned, hands can be cleaned with soap and water. In addition, teachers can notify the custodial staff if surfaces in the classroom may have been contaminated with microbes. This allows the custodial staff to adapt their cleaning procedures when necessary. For example, they may use disinfecting soap on surfaces where biological material has been handled and on frequently touched surfaces in the classroom (e.g., door handles).
4.1.2. Harmful microbes in environmental samples
Harmful microbes can be present in the classroom unintentionally (e.g., a student who has the flu sneezed on a surface). Harmful microbes of RG2 are also frequently found at low levels in soil, water, and food, or on the skin and surfaces such as door handles, floors, and toilets. The risk of exposure to harmful microbes is therefore higher when working with biological material taken from the environment (like swabs taken on surfaces of the classroom) since it is not known what microbes are there. Table 4-1 gives a few examples of the environmental sources for some RG2 microbes.
| Microbe | Environmental source | Disease (symptoms) |
|---|---|---|
Aspergillus fumigatus |
Soil, decaying organic matter |
Pulmonary aspergillosis |
Bacillus cereus |
Soil, foodstuff |
Gastroenteritis |
Campylobacter species |
Foodstuff, surfaces |
Gastroenteritis |
Clostridium tetani |
Soil |
Tetanus |
Escherichia coli (O157:H7 and other shigatoxigenic strains) |
Foodstuff, surfaces |
Haemolytic uremic syndrome, gastroenteritis |
Legionella species |
Water, soil |
Legionnaires’ disease |
Listeria monocytogenes |
Foodstuff, surfaces |
Listeriosis |
Rotavirus |
Surfaces |
Gastroenteritis |
Salmonella species |
Foodstuff, surfaces |
Gastroenteritis |
Staphylococcus aureus |
Skin, surfaces |
Skin and soft tissue infections, meningitis |
If samples collected from the environment are cultured, RG2 microbes may grow to a number that is sufficient to cause infection or disease.Footnote 7Footnote 32 Indeed, procedures commonly used in the classroom can unintentionally produce a culture of RG2 microbes. Cultures grown from environmental samples require notifying the PHAC if they are identified as harmful microbes of RG2 (or higher risk group). If this is done routinely, it may be necessary to apply to the PHAC for a Pathogen and Toxin Licence.
In the context of the COVID-19 pandemic, it is best to avoid experiments involving environmental samples and skin swabs to mitigate the risks of culturing harmful microbes (such as SARS-CoV-2).
Chapter 5. Strategies to safely handle microbes in the classroom
When teachers and students are aware of potential dangers and they take the appropriate precautions, hands-on experiments with biological material can be performed safely in the classroom. In such conditions, it may not be necessary to remove all risk. Rather, when finding ways to address risks, the goal is to reduce the risk to an acceptable level. An acceptable level of risk is when it is reasonable to expect that an activity can be performed safely and without incident. An acceptable level of risk also means that if an incident does occur, students, teachers, and the community are protected from exposure.
Most biological material used in the classroom are RG1 microbes. Due to the low level of risk associated with RG1 microbes, most activities can be safely performed on a desk. It is still important to treat all biological material as if they contain a harmful microbe. As mentioned in the previous chapter, harmful microbes can be encountered everywhere and they can contaminate RG1 biological material. RG1 microbes can also cause illness in very rare circumstances (e.g., in immunocompromised individuals). It is therefore important to use techniques and procedures optimized for safety (e.g., safe work practices, PPE). It is also important to work in a well-designed area that helps protect students, teachers, the community, and the environment from potential risks.Footnote 3Footnote 33
5.1. Risk mitigation strategies in the classroom
5.1.1. Elimination and substitution
The most effective way of reducing risks in the classroom is to eliminate procedures or biological material, or to substitute them for something with lower risk. Elimination means completely getting rid of the material, procedure, or equipment that leads to the unacceptable level of risk. Elimination can also mean cancelling the entire experiment when necessary. Substitution means choosing a different biological material, procedure, or equipment to lower the risk and achieve the same goal.Footnote 34 When a risk assessment identifies an unacceptable risk, substitution with less dangerous material, or elimination (if no alternative is available) can be considered as risk mitigation strategies.Footnote 35
Table 5-1 provides an example of an experiment that originally has a risk of growing harmful microbes. In this example, the risks associated with the biological material (an environmental sample placed in culture) can be eliminated by replacing the experiment with an alternative procedure.
| Objective: Investigate the effects of proper handwashing | ||
|---|---|---|
| Plan | Experiment | Comment |
| Original | Students swab their hands before and after handwashing, and streak on agar plates. Growth is compared to show the effects of handwashing. | Risk: There is the potential that harmful microbes could be cultured. |
| Alternative | Students apply to their hands a product that glows under ultraviolet light and can be washed off (or any other product allowing similar observation). The students examine their hands (e.g., under an ultraviolet light) before and after handwashing. | Why is this experiment safer? The effects of proper handwashing are demonstrated with no possibility of culturing harmful microbes. |
Another example of substitution is using known RG1 microbes in experiments instead of environmental samples that may contain harmful microbes. In fact, all experiments aiming to introduce students to microbiology procedures and concepts can be demonstrated with RG1 microbes. Examples of alternative activities for microbiology concepts are presented in Table 5-2. Using RG1 microbes can achieve the same outcome as using harmful microbes while minimizing the risks to students. Handling RG1 microbes also does not require the special equipment needed for harmful microbes.Footnote 2 Resources that include teaching plans and examples of experiment for microbiology discovery in the classroom are available online.Footnote 3
| Microbiology concept | Experiment | RG1 microbe |
|---|---|---|
Structure |
Comparison of shape (i.e., morphology) by microscopy |
Bacillus subtilis (rod shape) |
Classification |
Gram stain |
Gram negative: Escherichia coli K12 |
Bacterial isolation |
Recovery from clover root nodules |
Rhizobium species |
Sporulation |
Modifying growth conditions |
Bacillus subtilis |
Enzyme production |
Starch degradation Milk protein degradation |
Bacillus subtilis; Escherichia coli K12 Bacillus subtilis; Saccharomyces cerevisiae |
Fermentation |
Making cheese, yogurt Making sauerkraut Fermentation in a broth containing Phenol Red |
Lactobacillus species Streptococcus thermophilus Lactobacillus species, RG1 Saccharomyces species |
Molecular biology |
Bacterial conjugation, cloning, polymerase chain reaction |
Escherichia coli K12 |
When microbes are isolated from food samples (e.g., yogurt, cheese), it is important to confirm beforehand that the products were pasteurised and maintained under the recommended conditions (e.g., temperature, best before date) to reduce the risks of growing harmful microbes. RG1 microbes that are well-characterized can be purchased from a reputable supplier, and it may be preferable (i.e., safer) to obtain RG1 microbes this way. It is important to note however that some suppliers may indicate the risk group from another country instead of the Canadian risk group. Most international risk group classifications are the same as the Canadian classification. Even so, it is important to verify the Canadian risk group classification before purchasing a microbe. The risk group for a microbe can be found in its PSDS and in the ePATHogen – Risk Group Database.Footnote 17Footnote 18 The PHAC can be contacted to confirm the risk group if it cannot be found.
5.1.2. Simulations, demonstrations, and experimental setup
When introducing experiments with biological material, it may be useful to introduce different concepts progressively. Table 5-3 provides examples of teaching strategies that can be used to introduce new concepts, while reducing the risk of exposure. For example, simulations and demonstrations significantly reduce the risks for students. They are particularly useful when activities require vigilant monitoring (e.g., long-term studies) or they have a higher level of risk (e.g., aerosols). Ideally, simulations and demonstrations are followed by hands-on experiments (potentially a similar experiment with lower risk) to give students an opportunity to develop safe work practices.
| Strategies and descriptions | Benefits |
|---|---|
Simulation |
|
Teacher demonstration |
|
Dry run experiment |
|
‘Laboratory’ station |
|
Small-scale experiments |
|
In the context of the COVID-19 pandemic, simulations and demonstrations are particularly useful as they are compatible with physical distancing in the classroom and remote teaching. Where dry run and small-scale experiments take place, the risks of contamination for teachers and students are reduced when experiments allow for physical distancing. In the context of the COVID-19 pandemic, shared 'laboratory' stations may be appropriate if shared material at the stations are decontaminated between users or strict hand hygiene practices are implemented prior to handling shared material.
5.2. Safe work practices
Respecting and following general safety precautions and good microbiological laboratory practices protect teachers and students from the biological material. These precautions and practices also minimize the spread of contamination inside and outside the classroom. They include the use of safe work practices and the use of PPE.
5.2.1. Good microbiological laboratory practices
Good microbiological laboratory practices (Table 5-4) are established practices and techniques. They lay the foundation for all safe work practices involving biological material. These practices aim to protect anyone working with biological material, the environment, and even the biological material handled from becoming contaminated.Footnote 19 Teachers can apply these practices in any classroom where work with microbes takes place.
Table 5-4: Good microbiological laboratory practices dos and don’ts
Good microbiological laboratory practices dos
- Restrain or cover hair and clothing (e.g., tie back long hair, secure loose clothing such as hoodie strings and head coverings) that may become contaminated if it comes into contact with hands, specimens, containers, or equipment.
- Cover open wounds, cuts, and scratches with waterproof dressings (bandages).
- Keep work stations (e.g., desks) and work areas (e.g., floors) free of clutter so they can be easily properly cleaned and disinfected.
- Wear shoes that cover the entire foot with no or low heels and clothing that covers the entire lower body (e.g., full length pants).
- Wear required PPE, which may include a lab coat, gloves, and safety glasses.
- Store personal belongings (e.g., purses, backpacks, personal electronic devices) and street clothing (e.g., coats, scarves) separately from PPE and away from workstations.
- At every workstation, place a waste container (e.g., beaker, jar) that is break-resistant and appropriate for the type of waste produced (e.g., pipette tips).
- Inspect all containers and equipment for defects before using them (e.g., cracked glassware).
- Use techniques to prevent contamination of the biological material with harmful microbes.
- Clean and disinfect work surfaces using a suitable disinfectant before handling biological material.
- Clean and disinfect work surfaces using a suitable disinfectant after any spills and after completing work with biological material.
- Decontaminate all items (including liquid and solid waste) that have come in contact with biological material after use and prior to disposal.
- Decontaminate all clothing and PPE (including gloves) when exposure has or may have occurred.
- Wash hands after handling biological material and immediately after removing gloves. If sinks are not available, use hand sanitizer to decontaminate hands as a temporary measure until the hands can be properly washed with soap and water.
- Follow procedures for the safe use of sharps, such as using safe alternatives, discarding used sharps in designated puncture-resistant waste containers, and placing these containers close to where sharps are used.
- Clearly label all cultures, disinfectants, media, and other material with their names and dates.
- Label any dangerous items (including waste) with the appropriate warning and information on the dangers.
Good microbiological laboratory practices don'ts
- Mouth pipette (suction liquids into a pipette with the mouth).
- Put anything (e.g., hands, pens, pencils) into or near the mouth, nose, or eyes.
- Eat, drink, chew gum, smoke, vape, store food and utensils, apply cosmetics, or handle contact lenses.
- Wear any jewellery, clothing, or other items (e.g., ring, long necklace, scarf) that may come in contact with biological material or that may damage a protective glove.
- Wear contact lenses. If a student cannot avoid wearing contact lenses, the student needs to inform their teacher and wear the appropriate safety glasses or goggles.
- Wear short pants or skirts.
- Use sharps (e.g., needles, syringes, glassware) when it is avoidable.
- Bend, shear, or break needles, or recap used needles.
- Touch personal objects (e.g., cell phone, backpack, notebook) while working with biological material, or while wearing gloves.
- Create sprays or splashes.
- Run or fool around near the laboratory stations.
5.2.1.1. Aseptic techniques (to keep material free of harmful microbes)
Aseptic techniques are procedures that keep material free of harmful microbes. This prevents the contamination of biological material and protects individuals and their environment from the biological material being handled. Aseptic techniques depend on the work planned, and some practices may not be recommended in a classroom setting (e.g., use of open flames). The following are key points of aseptic technique:
- Organize the space such that biological material is moved from a cleaner area to an area where material is considered contaminated (e.g., clean materials on left, waste on right).
- Have all the required materials close to the user to avoid having to reach over the work area.
- Use only materials that are free of harmful microbes (e.g., sterilized containers, reagents, media, inoculating tools).
- Keep containers closed except when they are in use.
- Limit actions that may contaminate the cultures (e.g., speaking may spray saliva, contamination can fall from hands or sleeves into open containers).
Although flames are not recommended in most classroom laboratories, it may be safe in some settings for experienced students to use them during advanced classes. In such situations, the following aseptic techniques would also need to be in place:
- Have a flame in the work area to create an upward airflow, to sterilize inoculating loops, and to flame container (e.g., flask, vial) openings.
- Briefly flame the container mouth immediately after opening and before closing the container.
5.2.2. Preventing spills and splashes
Spills and splashes are the most common incidents with biological material. They can contaminate surfaces, equipment, samples, and individuals. Spills and splashes are frequently the result of containers (e.g., flask, beaker, vial) tipping over, being dropped, or breaking. Spills and splashes also occur during vigorous mixing (e.g., mixing with a vortex device) and when liquids are being ejected or are dropped during transfer. Procedures that can be implemented to minimize the risk of spills and splashes include the following:Footnote 38Footnote 39
- Hold containers securely and handle them over the work area to prevent dropping.
- Keep containers and racks away from the edge of a table, desk, work surface, or shelf.
- Use culture tubes that are long enough to be held by the side of the tube in the rack instead of by their caps, especially if the caps are loose to allow air exchange.
- Use a device (e.g., pipette, funnel) to transfer liquids, instead of pouring directly into a container.
- Keep containers closed except when they are in use.
- Pay close attention to avoid overfilling when liquids are transferred from one container to another.
- Use carts when transporting heavy containers or multiple containers at once.
- Use secondary containers when transporting liquids (e.g., place flasks on a tray with raised edges).
Appendix F indicates possible response actions and a general clean-up procedure in the event of a spill.
5.2.3. Hand hygiene
In all laboratories, washing hands frequently is one of the most basic and also one of the most effective safety precautions. Washing hands reduces the number of microbes that may be present on hands. This in turn prevents the contamination on hands from transferring onto surfaces in the environment. Even if gloves are worn, they wear with use and can become porous. This is why it is so important to wash hands after removing gloves; it eliminates any contamination that may have reached the hands. Appendix G provides instructions on the proper technique to wash hands.Footnote 40Footnote 41 Teachers reinforce the importance of handwashing when they:
- take the time to demonstrate proper hand hygiene to students;
- explain the value of proper hand hygiene to students; and
- consistently remind students to wash their hands.
Hand sanitizers can be used as an alternative method until hands can be properly washed at a sink. Appendix G outlines some considerations on the use of hand sanitizers.
In the context of the COVID-19 pandemic, hand hygiene is particularly important as it reduces the risk of spreading harmful microbes on shared surfaces (e.g., door handles).
5.2.4. Best practices for culturing samples in the classroom
Any time microbes are grown (i.e., cultured), there is a risk that samples may become contaminated with other microbes (including harmful microbes) present in the surrounding environment. The risk of growing contaminants can be reduced by applying best practices such as the following:Footnote 32Footnote 42
- Choose experiments and material that match the level of skill of the students and the needs of the curriculum, such as:
- printed and digital images of microbes instead of live specimens;
- prepared microscope slides of inactivated material;
- known RG1 microbes purchased from a reputable supplier for experiments requiring live specimens or open cultures; and
- microbes that occur naturally on moldy bread, cheese, or objects affected with mildew rather than those that occur on commonly touched surfaces (e.g., door handles, toilets) if environmental sampling is performed.
- Grow microbes on solid media (e.g., agar) rather than liquid (e.g., broth) to prevent spills and the formation of aerosols.
- Use a general purpose medium (e.g., nutrient agar) instead of non selective enriched media (e.g., blood agar). Alternatively, selective media to grow a specific microbe may be used (e.g., M17 agar, Rogosa agar, and MRS [de Man, Rogosa, and Sharpe] agar that help grow Lactobacillus species).
- Incubate culture plates with the agar side up (i.e., plates are “upside down”) to prevent condensation inside the lid from dripping onto the cultures.
- Grow cultures at room temperature and no higher than 32˚C. This will discourage or slow the growth of harmful microbes that prefer 37˚C and may be able to live in the human body.
- Keep cultures closed and look at growth on agar plates through the closed plate cover to prevent release.
- Tape the cover of inoculated agar plates onto the base to prevent accidental opening. It is best practice to secure culture dish covers to their bases with periodically spaced pieces of tape to allow air exchange. Avoid continuous taping around the rim as it can serve as a seal and lead to the growth of microbes which only grow in the absence of oxygen.Footnote 43Footnote 44 These microbes can be difficult to detect in a classroom setting and they are associated with infections that can be difficult to treat.Footnote 45
- Do not perform any experiments that involve intentionally culturing harmful microbes.
- Do not transfer microbes from one growth medium to another as this could result in contamination and the culturing of unknown harmful microbes.
5.3. Personal protective equipment
PPE is the equipment and clothing that protect the user from various dangers. For example, it provides a barrier between the user and the biological material handled and it is the last line of defence against exposure. The need for any PPE is based on risk assessments, which take into consideration the risks of handling unknown harmful microbes.Footnote 2 It may be determined that a certain (or all) PPE is not required for work with a particular RG1 microbe. When PPE is required, it is important that teachers provide students with the appropriate PPE for the activity and verify that it is being used correctly. To the extent possible, different sizes of PPE should be ordered to accommodate diverse body types.
Some common examples of PPE are provided below, and in Figure 5-1.Footnote 7Footnote 19
Figure 5-1: Examples of PPE
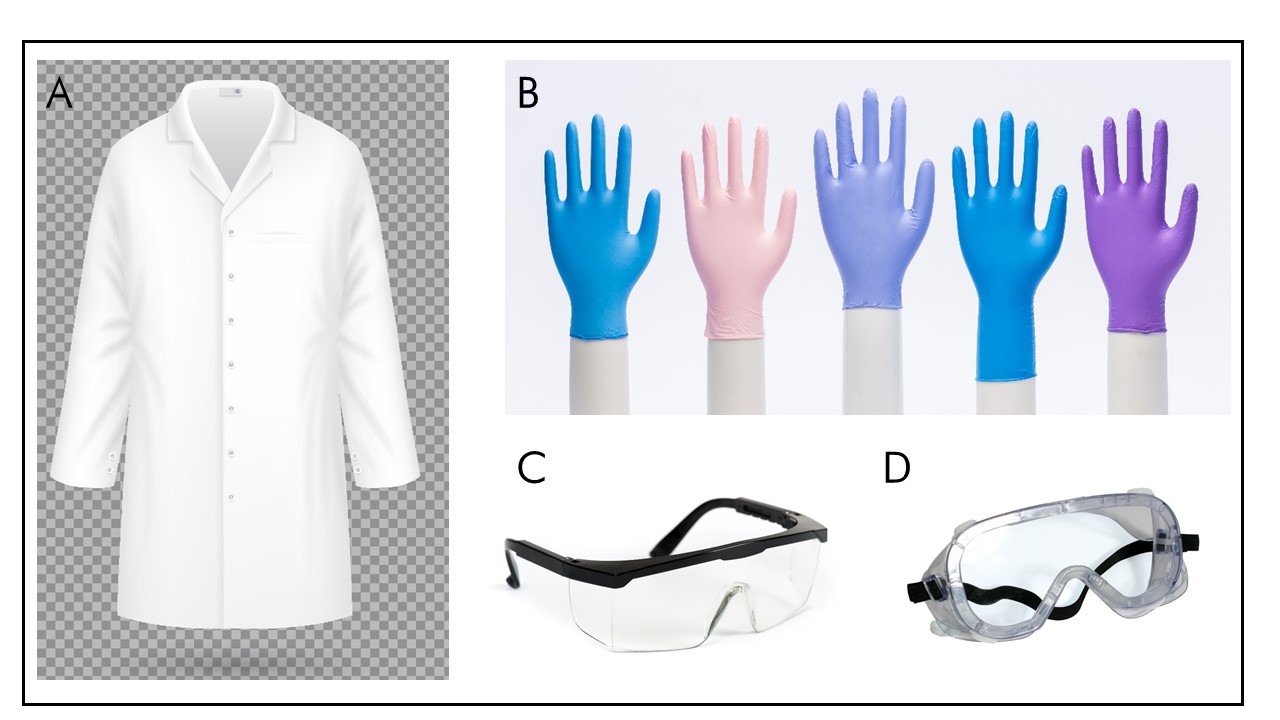
Figure 5-1: Text description
- Figure depicting a fitted lab coat that is fastened with buttons at the front and two buttons on each sleeve cuff.
- Figure depicting five different disposable gloves of different colors and sizes.
- Figure depicting safety glasses with side protection that are well adjusted behind the ears.
- Figure depicting safety goggles, which enclose the area surrounding the eyes and have a tighter fit than glasses due to the attachment that goes around the head from one side panel to the other.
(A) A properly fitted lab coat that is fastened at the front. (B) Disposable gloves. (C) Safety glasses are made of impact resistant material, with side protection. (D) Safety goggles have a tighter fit than glasses.
5.3.1. Body protection
A lab coat is the most commonly used PPE. It protects the user’s body and personal clothes against contamination. Lab coats always have to be completely fastened. Snap closures are preferred over buttons as they allow a quicker removal of the lab coat in an emergency. It is also important for lab coats to fit closely to the body and, when possible, have cuffed sleeves. This prevents the lab coat from dragging and catching on material during experiments. The material of the lab coat determines if it is intended for one use (i.e., disposable) or meant to be worn multiple times.
Personal clothing that minimizes the amount of skin that is exposed and shoes that cover the entire foot can also provide protection against exposure. For example, sandals and shorts do not provide sufficient protection when working with biological material even if a lab coat is worn over them. Depending on the experiments taking place, sandals and shorts can be prohibited.Footnote 2Footnote 46
5.3.2. Hand protection
Gloves provide a protective barrier between the skin and the biological material handled. Impermeable gloves reduce the risk of exposure associated with absorption through the skin (particularly if scratched or wounded). They also protect the hands from contamination.
The PHAC recommends that students wear gloves when handling RG1 microbes, unknown cultures, or samples that may contain harmful microbes. As for all PPE, the decision to wear gloves is nevertheless based on a risk assessment. For example, wearing gloves may be inadvisable when a Bunsen burner is being used if the risk of injury caused by gloves that melt is greater than the risk of exposure to the biological material.
Gloves are made from many different materials. The type of glove selected depends on the specific activity and dangers. For example, latex is incompatible with many commonly used chemicals. Latex gloves are also often avoided due to the increasing number of people with latex allergies. Nitrile gloves have excellent chemical resistance and they often tear when punctured. This is a good quality to have since it allows the user to easily recognize if a glove has been compromised. Regardless of the material, disposable gloves can never be reused as they become permeable with prolonged use. It is also important not to decontaminate disposable gloves while wearing them, as it can lead to microscopic holes that let microbes through.
Gloves need to be removed before touching any communal or personal things such as door handles, computer keyboards, pens, notebooks, face, and hair. Gloves are to be properly removed and thrown away before handwashing and exiting the work area.Footnote 46 It is important that teachers give instructions on how to properly use and remove gloves to reduce the spread of contamination (see Appendix H).
5.3.3. Eye protection
Eye protection protects the eyes from dangerous material that may cause them injury or lead to exposure of mucous membranes to biological material.Footnote 21 Safety goggles provide a higher level of protection than safety glasses due to their snug fit over and around the eyes. This tighter fit creates a barrier to dangerous liquids and splashes. The choice of eye protection depends on the activities planned. In general, safety glasses with side panels offer sufficient protection for activities performed in elementary, intermediate, and secondary school classrooms. The Canadian Standard Association CSA Z94.3 – Eye and face protectors outlines the basic performance requirements for eye protectors.Footnote 47
It is best not to wear contact lenses when there is a risk of splashes or aerosols, since contact lenses can interfere with first aid and eye-flushing procedures. If contact lenses must be worn, tight-fitting safety goggles are necessary to protect the eyes from exposure. A better alternative is to have students wear their prescription glasses underneath protective eye equipment.
It is good practice for teachers to remind students to never rub their eyes or touch their face during any activities with biological material. In the event of accidental contact with the eyes, eyes can be immediately flushed with clean water to reduce the risk of infection.
5.4. Safety considerations for equipment used in the classroom
A wide variety of equipment can be used when handling biological material (Figure 5-2). Equipment that is properly operated and well maintained minimizes the risk of exposure and prevents the release of biological material into the environment. In order to prevent spills, splashes, inoculations, and aerosols, students need to be trained in the correct use of equipment. This section provides guidance on the safe use of select equipment that may be found in the classroom.
Figure 5-2: Equipment used in a laboratory

Figure 5-2: Text description
- Figure depicting a pipettor with a pipette tip.
- Figure depicting two pipettes, one containing liquid and the other not.
- Figure depicting a pipette bulb.
- Figure depicting an electric pipetting controller with two buttons, one which allows to aspirate and the other which allows to expel liquid.
- Figure depicting an inoculating loop being heated by the flame of a Bunsen burner, with culture tubes in a tray in the background.
- Figure depicting a needle being disposed of by a gloved hand into a puncture-resistant container labelled with a biohazard symbol.
(A) A pipettor is a calibrated device that draws up a set amount of liquid into a disposable tip. (B) Pipettes are simple calibrated tubes in which liquids can be drawn and dispensed, and can be used with a pipetting aid including (C) a pipette bulb, and (D) an electric pipetting controller. (E) A Bunsen burner is a device that produces a single flame from a source (e.g., natural gas, propane, butane). (F) A sharp (needle) being disposed of into a labelled puncture-resistant container.
5.4.1. Pipettes and pipettors
The following recommendations may be considered for the safe use of pipettes and pipettors:Footnote 38
- Use plastic pipettes instead of glass pipettes whenever possible.
- Use caution when attaching a pipette (especially a glass pipette) to a pipetting aid.
- Use good technique and slow, fluid movements to avoid drops falling from the pipette.
- Keep pipettes upright while in use and between steps of a procedure to prevent contamination of the pipetting aid.
- Discharge liquids from the pipette as closely as possible to the wall of the container or the surface of media to avoid splashes and aerosols.
- Eject disposable pipette tips directly from the pipettor into a labelled container (e.g., bottle, beaker) for decontamination.
- Avoid the forceful aspirating (i.e., drawing the liquid up) or expelling of liquids from the pipette.
- Never bubble air from a pipette through a fluid, as this will generate aerosols.
5.4.2. Sharps
Sharps are objects that can pierce or cut the skin. Examples of sharps include scalpels, needles, syringes, blades, and broken glass. Handling contaminated sharps increases the risk of punctures or cuts, which provide a route of entry (i.e., inoculation) for microbes. Contaminated sharps pose a risk to anyone handling them prior to their decontamination and disposal.
The following points are general best practices for sharp use:Footnote 32Footnote 48
- Substitute material made of glass with material made of plastic when possible.
- Use safety-engineered devices (e.g., scalpel blades that re-sheath the blade or automatically retract once used) or disposable sharps (e.g., disposable scalpels) unless no alternative exists.
- Use scalpel blades only when they are inserted correctly into an appropriate handle or holder.
- Use cut-resistant or puncture-resistant hand protection.
- Use caution when handling sharps or glass items to avoid puncturing the skin.
- Always cut away from the fingers or hand.
- Dispose of all sharps properly into a labelled container that is resistant to punctures for decontamination and disposal (Figure 5-2).
- Use a brush and dustpan, tongs, forceps, or scoop to handle broken glass or other sharps.
- Never pick up broken glass or other sharps with hands, even while wearing gloves.
- Never try to catch a sharp object that is falling.
- Do not use a sawing motion or excessive force on a scalpel since these actions can cause the blade to snap.
- Avoid the use of sharps whenever possible.
Due to the high risk posed by needles, it is best to completely avoid using them in the classroom. Communicating best practices to students for the use of sharps, and verifying that these are followed, will reduce the risk of injury.
5.4.3. Bunsen burners
A Bunsen burner is a device that produces a single flame from a fuel source (e.g., natural gas, propane, butane). There is a risk of burns when working with an open flame, and the rapid heating of liquids in a Bunsen burner can produce aerosols containing microbes. Bunsen burners are used for heating and sterilization (e.g., inoculating loops). The use of a Bunsen burner can be avoided by replacing them with electric sterilisation systems or by using disposable inoculation loops. Teachers have to provide students with instructions on the proper use of a Bunsen burner before allowing students to use one. Raising awareness to all potential dangers will also help reduce the risks associated with its use. Teachers and students must follow these safe practices when working with Bunsen burners:Footnote 49
- Know where the fire extinguisher is and how to use it.
- Remove all flammable and combustible materials from the surrounding area.
- Inspect the burner, gas valve, and verify that appropriate tubing is attached prior to use.
- Know the proper procedure for lighting a Bunsen burner.
- Use only heat-resistant (e.g., borosilicate) glassware, and only after having verified the glassware has no cracks (discard any defective glassware).
- Verify that the gas supply is turned off when finished. If accessible, the teacher can use the main shutoff when gas is not needed.
- Allow glassware and equipment to cool after removing it from the Bunsen burner, and use tongs or heat-protective gloves to pick it up. Remember, hot glass looks exactly like cold glass.
- Refrain from placing hot glassware or equipment directly onto the working table, place it onto an insulating pad instead.
- Never leave a lit Bunsen burner unattended.
- Never reach over a flame.
- Never use a Bunsen burner when working with flammable liquids or materials (e.g., alcohol, paper).
Chapter 6. Decontamination and waste management
6.1. Considerations for Risk Group 1 microbes
Decontamination is a process that makes materials and surfaces safe to touch by removing microbes that may be present. Usually, well-characterized RG1 microbes do not need to be decontaminated before they are thrown out with normal waste since RG1 microbes do not pose a risk to the general public, animal populations, or the environment. Non-disposable or reusable containers and material (e.g., glassware) and surfaces (e.g., desks, tables, working table) that may have been exposed to RG1 microbes can simply be cleaned with soap and water. As long as there are no sharps, material may be thrown in the trash like normal waste. Just in case, it may be best to consult with authorities to know if there are any requirements about throwing out waste containing RG1 microbes. Waste is regulated at the provincial, territorial, and municipal levels.
6.2. Considerations for harmful microbes
If there is a risk that waste may be contaminated with harmful microbes, then all waste must be decontaminated before disposal or reuse. Waste includes cultures, stocks, microbe samples, and any material that may have come in contact with these. If harmful microbes are present, decontamination procedures are essential to protect students, teachers, and the community from exposure. Decontamination procedures that fail can also result in exposure to harmful microbes or their spread to other areas.Footnote 50Footnote 51Footnote 52
It is important to clean items before decontamination to remove as much gross contamination as possible. Gross contamination, such as organic matter (e.g., soil, dirt, blood), can make decontamination less effective. Factors to consider before decontaminating an item potentially contaminated with harmful microbes include:
- the type of microbes that may be present on the item;
- the quantity of microbes that may be present on the item;
- the type and state of the item;
- the temperature where the disinfection is taking place; and
- the amount of gross contamination on the item.
If waste contains harmful microbes and it cannot be decontaminated on-site, arrangements can be made with a commercial disposal company specialized in hazardous waste. Arrangements need to be made in advance to retrieve packaged waste and decontaminate it off-site.
6.2.1. Chemical disinfectants
Where harmful microbes may be present, chemical disinfectants (e.g., bleach) are most commonly used in the classroom for the decontamination of equipment, surfaces, liquids, and spills of biological material. It is important to use chemical disinfectants according to directions since they are often dangerous chemicals. Recommended directions include how to apply it, the concentration needed, contact time, PPE, first aid, and disposal. It is also important to know about the disinfectant’s chemical characteristics such as its toxicity, its storage stability, its chemical compatibility, its active ingredients, and its stock concentration. General cleaners, including those with microbicides, cannot be used as chemical disinfectants unless they have been demonstrated to be effective against the microbes handled.Footnote 53 Using an inappropriate chemical disinfectant or incorrectly using an appropriate chemical disinfectant can both lead to exposure to harmful microbes.
6.2.1.1. Decontamination with bleach
The disinfectant most commonly used for work with biological material is 0.5-1% sodium hypochlorite (diluted bleach). This can be prepared by mixing 1 part commercial bleach (that is normally between 5% and 8% sodium hypochlorite) with 9 parts of water. Diluted bleach has to be made fresh (e.g., weekly) as it breaks down quickly. Other disinfectants that can be used are solutions of 70% ethanol and 70% isopropanol, and the examples given in Appendix I. Users must be very careful regarding the contact time of certain disinfectants, especially when they can evaporate quickly (e.g., alcohols).
The following procedures may be suitable to disinfect or decontaminate classroom materials:Footnote 54
- Disposable material (e.g., pipette tips, inoculating loops) used to prepare cultures can be soaked for an appropriate amount of time in 0.5% sodium hypochlorite before disposal.
- Reusable material (e.g., glassware) can be soaked for an appropriate amount of time in 0.5% sodium hypochlorite before being washed in warm soapy water and rinsed with water.
- Closed (but not sealed) inoculated agar plates can be soaked for an appropriate amount of time in 0.5% sodium hypochlorite to allow the bleach to enter the plate and be in contact with the culture surface.
- Liquid cultures can be decontaminated by adding 1 volume of commercial bleach to 9 volumes of culture.
- Disposable PPE (e.g., gloves) can be soaked for an appropriate amount of time in 0.5% sodium hypochlorite.
- Reusable PPE (e.g., lab coat) can be soaked for an appropriate amount of time in 0.5% sodium hypochlorite, then rinsed in water and laundered.
- Desks, tables, and other potentially contaminated surfaces can be disinfected with an appropriate chemical disinfectant for a sufficient duration (e.g., 0.5% sodium hypochlorite for 10-30 minutes), then rinsed off, as appropriate.
An appropriate amount of time for 0.5% sodium hypochlorite to effectively decontaminate material can vary greatly based on the biological material present and the surface that requires decontamination. A contact time of a few minutes with 0.5% sodium hypochlorite may be sufficient for the decontamination of some harmful microbes, while for other harmful microbes a contact time of 30 minutes is necessary.Footnote 19 When a material is contaminated with unknown microbes (e.g., environmental samples), it may be preferable to have 30 minutes of contact time with 0.5% sodium hypochlorite in case there are resistant harmful microbes present. Once decontamination is complete, the chemical solution can be diluted with water to be safely poured down an all-purpose drain (e.g., in a sink for handwashing).
6.2.2. Physical decontamination
Some harmful microbes can be effectively decontaminated with high-pressure steam that is maintained at a high temperature (e.g., 121oC) for a long period of time (e.g., 60 minutes). Autoclaves are the only equipment that can safely reach these conditions. Microwave ovens, pressure cookers, and other home tools are not substitutes for an autoclave. These devices may even create infectious aerosols that can be released into the room.Footnote 37Footnote 55 In a classroom setting where no autoclave is available, chemical decontamination is the safest and most effective method of decontamination.
6.3. Waste management
Waste leaving the classroom can be:
- sent directly to the municipal waste if it only contains RG1 microbes;
- sent to the municipal waste after decontamination if it contained harmful microbes;
- moved to a designated decontamination area outside of the classroom if it may contain harmful microbes; or
- transported off-site for decontamination by a third-party biohazardous waste treatment facility if it contains harmful microbes.
Note: Waste that has been decontaminated prior to removal from the classroom may not be acceptable for the normal waste disposal procedures that lead to a local landfill or sanitary sewer system. Additional waste management considerations or requirements specified by the provincial, territorial, or local (i.e., municipal) authorities may also apply.Footnote 56
The following are best practices for waste management:
- Separate and dispose of the contaminated waste near the point where it is generated to reduce the possible spread of contamination.
- Seal waste bags and place them in leak-proof containers to prevent or contain any leaks. Reusable containers may be used if they are decontaminated and cleaned after every use.
- Develop SOPs for the separation, decontamination, disposal, and transport of dangerous solid and liquid materials. Some aspects to consider when developing an SOP for waste disposal are the quantity and type of waste that may be generated, as well as the decontamination technologies that are available.
If a harmful microbe is grown (accidently or intentionally) in the classroom, it becomes necessary to also display the biohazard symbol (Figure 6-1) on the outside of waste disposal bags or containers for the waste. Indeed, according to the HPTA and the HPTR, as a condition of a Pathogen and Toxin Licence, all applicable requirements in the CBS must be met for regulated activities with the harmful microbe. Requirements for storage, decontamination, and waste disposal of harmful microbe include keeping the harmful microbe in a closed, labeled, and leak-proof container. The biohazard symbol is an appropriate way of labeling a bag or a container that contains biohazardous waste and not general waste.
Figure 6-1: Biohazard symbol

Figure 6-1: Text description
Figure depicting the biohazard symbol.
After decontamination, the waste is rendered non-infectious. As it is now safe to handle, the biohazard symbol on waste disposal bags or storage containers must be removed or defaced.
6.3.1. Storage of waste
When harmful microbes may be present, it is recommended to decontaminate waste as soon as possible after an experiment is finished. Waste that contains microbes may be stored temporarily, provided it is stored in a designated area that is separate from other storage areas. Waste can be stored frozen or refrigerated to slow down the growth of microbes. Waste that has not been decontaminated can only be stored in freezers or refrigerators that are well identified and that are dedicated for such storage (i.e., not used for storage of food or drinks).
Chapter 7. Considerations for science fair projects
Curricula often recommend that students perform investigative science work through progressive tasks or projects. Whether inside or outside the classroom, student-conducted science activities increase the potential for biosafety issues to arise.
Ideally, science fair projects take place in the classroom under a teacher’s supervision or in a laboratory under a scientist’s supervision. It is much easier to monitor projects in these environments than when they are done at home or in another unstructured environment. When students are working on school-related or school-endorsed projects outside of the classroom, teachers can prohibit the use of dangerous equipment and harmful microbes. Often, this is already a requirement for science fairs.
7.1. Reviewing planned experiments
Teachers can implement a process to review and approve all experiments before students begin their project. This approval process can require students to submit a description of their project and the experiments planned. Some science competitions require that students submit the safety aspects of their work when it involves microbes. Teachers can also evaluate the safety planning as a component of a student’s project. The importance of biosafety is highlighted when biosafety is included in the overall assessment of the experiment. To help develop critical thinking about the safety aspects of their work, teachers can:
- require that students assess the risks associated with their project;
- help students complete this risk assessment; and
- establish with students the appropriate risk mitigation strategies.
This is a good exercise for students. Nevertheless, the teacher or science competition safety committee remains responsible for deciding whether the risks are appropriately mitigated and the work can be performed safely. If teachers are unsure, they can consult the PHAC or an expert on the topic.
7.2. Pre-experiment safety measures
After approving a student’s experiment, the following measures can be implemented by teachers to help keep students and the community safe:Footnote 57
- Inform parents, the science department, and administrators of the students’ experiment and the risks involved.
- Require that students know about all the biological materials they are handling by reviewing the appropriate resources (PSDSs, product sheet, ePATHogen – Risk Group Database), and know how to work with them safely (e.g., through review of the PHAC’s training portal).
- Require that all practical work be performed in an appropriate area (i.e., properly equipped) and supervised by a knowledgeable individual.
- Require that students review the general safety procedures of the classroom or space in which they are working.
- Prohibit students from taking home any material from the classroom, including cultures, media, and other supplies.
- Provide a place, away from other students, to store on-going experiments if they are being conducted in the classroom.
7.3. Where to conduct science projects
Allowing projects to be performed only in places approved by a teacher will help protect the health and safety of students, teachers, and the environment. Some science fairs may have their own guidelines as to where projects can be completed. Students need to review these guidelines before beginning an experiment. Ultimately, the level of risk associated with the activity and the risk group of the biological material will determine where the project can be safely performed.Footnote 57 For example, an activity that involves potentially handling a harmful microbe of RG2 may require a CL2 laboratory.
If a student will be working in a laboratory where regulated activities with harmful microbes and toxins are taking place (i.e., a facility with a Pathogen and Toxin Licence where harmful microbes of RG2 are handled), teachers need to confirm that the student will be supervised by a qualified individual (e.g., laboratory technician). Such licensed facilities are usually CL2 laboratories which, as described previously, are required to meet specific requirements in the CBS based on their activities. One of the requirements is for access to CL2 laboratories to be limited only to authorized individuals who are supervised or who have completed the required training (Matrix 4.3 of the CBS). Even under supervision, students may be asked to complete basic training (e.g., to know the required PPE) before they are allowed to enter a CL2 laboratory. Students may also receive activity-specific training based on the experiment they plan to perform (e.g., handling a harmful microbe of RG2 in a biological safety cabinet). Organizations with CL2 laboratories may also have additional policies regarding access to their facilities. Such policies need to be consulted as they may include a minimum age for laboratory access, intellectual property rights, and responsibilities, which may affect public presentation or publication of data.
Chapter 8. Glossary
It is important to note that while some of the definitions provided in the glossary are universally accepted, many of them were developed specifically for the CBS, or the CBH; therefore, some definitions may not be applicable to facilities that fall outside of the scope of the CBS.
- Accident
- An unplanned event that results in injury, harm, or damage.
- Aerobic
- Requires oxygen for survival.
- Aerosol
- A suspension of fine solid particles or liquid droplets in a gaseous medium (e.g., air) that can be created by any activity that imparts energy into a liquid or semi-liquid material.
- Anaerobic
- Does not require oxygen for survival. For some anaerobic microbes, oxygen is poisonous.
- Animal pathogen
- Any microbe that causes disease in animals, including those derived from biotechnology. In the context of the CBS and the CBH, "animal pathogen" refers only to harmful microbes that cause disease in terrestrial animals, including those that infect avian and amphibian animals, but excluding those that cause disease in aquatic animals and invertebrates.
- Biological material
- Microbes, proteins, and nucleic acids that can or cannot cause disease, as well as any biological matter that may contain microbes, proteins, nucleic acids, or parts thereof. Examples include, but are not limited to, bacteria, viruses, fungi, prions, toxins, genetically modified organisms, nucleic acids, tissue samples, diagnostic specimens, live vaccines, and isolates of a harmful microbe (e.g., pure culture, suspension, purified spores).
- Biological safety cabinet
- A primary containment device that provides protection for personnel, the environment, and the product (depending on biological safety cabinet class), when working with biological material.
- Biosafety
- Containment principles, technologies, and practices that are implemented to prevent unintentional exposure to biological material, or their accidental release. Biosafety protects people from biological material.
- Biosecurity
- Security measures designed to prevent the loss, theft, misuse, diversion, or intentional release of harmful microbes, toxins, and other related assets (e.g., personnel, equipment, non-infectious material, animals). Biosecurity protects biological material from people.
- Containment
- The combination of physical design parameters and operational practices that protect personnel, the immediate work environment, and the community from exposure to biological material. The term "biocontainment" is also used in this context.
- Containment level (CL)
- Minimum physical containment and operational practice requirements for handling biological material safely in laboratory, large-scale production, and animal work environments. There are four containment levels ranging from a basic laboratory (CL1) to the highest level of containment (CL4).
- Containment zone
- A physical area that meets the requirements for a specified containment level. A containment zone can be a single room (e.g., CL2 laboratory), a series of co-located rooms (e.g., several non-adjoining but lockable CL2 laboratory work areas), or it can be comprised of several adjoining rooms (e.g., CL3 suite with dedicated laboratory areas and separate animal rooms, or animal cubicles). Dedicated support areas, including anterooms (with showers and "clean" and "dirty" change areas, where required), are considered to be part of the containment zone.
- Contamination
- The undesired presence of biological material on a surface (e.g., working table, hands, gloves) or within other materials (e.g., laboratory samples, cell cultures).
- Decontamination
- The process by which materials and surfaces are rendered safe to handle and reasonably free of microbes, toxins, or prions; this may be accomplished through disinfection, inactivation, or sterilization.
- Disease
- A disorder of structure or function in a living human or animal, or one of its parts, resulting from infection or intoxication. It is typically manifested by distinguishing signs and symptoms.
- Disinfection
- Process that eliminates most forms of living microbes; disinfection is much less lethal to biological material than sterilization.
- Emergency Response Plan (ERP)
- A document outlining the actions to be taken and the people responsible in emergency situations such as a spill, exposure, release of biological material, animal escape, personnel injury or illness, power failure, fire, explosion, or other emergency situations (e.g., flood, earthquake, hurricane).
- Environmental sample
- Specimen collected from surroundings, which may include large volumes (e.g., soil, water) as well as swabs from surfaces of inanimate objects (e.g., door handle) or living things (e.g., skin and mucous membranes of people and animals).
- Exposure
- Contact with, or close proximity to, biological material that may result in infection or intoxication. Routes of exposure include inhalation, ingestion, inoculation, and absorption.
- Good microbiological laboratory practice
- Established practices applicable to all types of activities with biological material to protect workers and prevent contamination of the environment and the samples in use.
- Handling or storing
- "Handling or storing" harmful microbes, toxins, or biological material includes possessing, handling, using, producing, storing, permitting access to, transferring, importing, exporting, releasing, disposing of, or abandoning such material. This includes all controlled activities involving human pathogens and toxins specified in Section 7(1) of the HPTA.
- Incident
- An event or occurrence with the potential of causing injury, harm, infection, intoxication, disease, or damage. Incidents can involve biological material, infected animals, or toxins, including a spill, exposure, release of biological material, animal escape, personnel injury or illness, missing biological material, unauthorized entry into the containment zone, power failure, fire, explosion, flood, or other crisis situations (e.g., earthquake, hurricane). Incidents include accidents and near misses.
- Infectious material
- Any isolate of a harmful microbe or any biological material that contains human or animal pathogens and, therefore, poses a risk to human or animal health.
- Inoculation
- The introduction of a microbe into a living organism through its skin.
- Intoxication
- A substance-induced disorder or disease resulting in a symptomatic or asymptomatic condition or other physiological change resulting from an exposure (i.e., ingestion, inhalation, inoculation, absorption) to a toxin produced by or isolated from a microbe, or a synthetically produced microbial toxin.
- Laboratory
- An area within a facility or the facility itself where biological material is handled for scientific or medical purposes.
- Microbe (Microorganism)
- A cellular or non-cellular microbiological entity, capable of replication or transferring genetic material and that cannot be reasonably detected by the naked human eye. Microbes include bacteria, fungi, viruses, and parasites, and may be pathogenic or non-pathogenic in nature.
- Operational practice requirements
- Administrative controls and procedures followed in a containment zone to protect personnel, the environment, and ultimately the community, from biological material.
- Opportunistic pathogen
- A harmful microbe that does not usually cause disease in a healthy host but can cause disease when the host's resistance is low (e.g., compromised immune system).
- Pathogen
- A microbe, nucleic acid, or protein capable of causing disease or infection in humans or animals. Examples of human pathogens are listed in Schedules 2 to 4 of the HPTA but these are not exhaustive lists.
- Pathogen and Toxin Licence
- An authorization to conduct one or more controlled activities with human pathogens or toxins issued by the PHAC under Section 18 of the HPTA.
- Pathogen Safety Data Sheet (PSDS)
- Technical document describing the hazardous properties of harmful microbes and recommendations for their safe handling. A PSDS may include information such as pathogenicity, drug susceptibility, first aid treatment, PPE, and risk group classification. PSDSs were formerly called material safety data sheets for infectious material.
- Performance and verification testing requirements
- Performance and verification tests that are necessary to demonstrate compliance with the physical containment requirements and, in some cases, the operational practice requirements outlined in the CBS.
- Personal Protective Equipment (PPE)
- Equipment and clothing worn by personnel to provide a barrier against biological material, thereby minimizing the risk of exposure. PPE may include, but is not limited to, lab coats, gowns, full-body suits, gloves, protective footwear, safety glasses, safety goggles, masks, and respirators.
- Physical containment requirements
- Physical barriers in the form of engineering controls and facility design used to protect individuals, the environment, and the community from harmful microbes or toxins, as outlined in the CBS.
- Release
- The discharge of biological material from a containment system.
- Risk
- The probability of an undesirable event (e.g., accident, incident, breach of containment) occurring and the consequences of that event.
- Risk assessment
- Standardized approach to evaluate the potential risks that may be involved in a projected activity.
- Risk group (RG)
- The classification of biological material based on its inherent characteristics, including pathogenicity, virulence, risk of spread, and availability of effective prophylactic or therapeutic treatments, that describes the risk to the health of individuals and the public as well as the health of animals and the animal population.
- Standard operating procedure (SOP)
- A document that standardizes procedures for activities.
- Sterilization
- Process that completely eliminates all living microbes, including bacterial spores.
- Waste
- Any solid or liquid material generated by a facility for disposal.
Chapter 9. References and resources
Public Health Agency of Canada websites
Biosafety Posters (freely available; account required)
https://training-formation.phac-aspc.gc.ca/course/index.php?categoryid=30
Biosafety Training Portal (freely available; account required)
https://training-formation.phac-aspc.gc.ca/course/index.php?categoryid=2&lang=en
Canadian Biosafety Handbook, 2nd Edition
https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/handbook-second-edition.html
Canadian Biosafety Standards, 2nd Edition
https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition.html
ePATHogen – Risk Group Database
https://health.canada.ca/en/epathogen
Pathogen Safety Data Sheets (alphabetical by species name)
https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html
PSDS Risk Assessment Template
https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/pathogen-risk-assessment-template.html
Teaching plans (examples that may need to be adapted to follow Public Health Agency of Canada recommendations)
Australian Science Teachers Association. (2017). Australian school science information support for teachers and technicians: Guidelines for best practice for microbiology in Australian Schools. Retrieved 10/07, 2017 from https://assist.asta.edu.au/resource/4196/guidelines-best-practice-microbiology-australian-schools
Microbiology Society, Microbiology in Schools Advisory Committee (MiSAC). (2016). Practical Microbiology for Secondary Schools. Retrieved 05/25, 2018 from http://microbiologyonline.org/file/c89f015377ba698f508f2cbcd3db6abf.pdf
References
Alberta Ministry of Education. (2006). Safety in the Science Classroom: Kindergarten to Grade 12. Edmonton, AB, Canada: Alberta Education, Learning and Teaching Resources Branch.
American Chemical Society. (2015). Fundamentals of Hazard Assessment: Control Measures. Retrieved 08/18, 2017 from https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment/fundamentals/control-measures.html
American Chemical Society. (1995). Guide for Chemical Spill Response Planning in Laboratories. Retrieved 07/21, 2017 from https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/publications/guide-for-chemical-spill-response.html
Australian Science Teachers Association. (2017). Australian school science information support for teachers and technicians: Guidelines for best practice for microbiology in Australian Schools. Retrieved 10/07, 2017 from https://assist.asta.edu.au/resource/4196/guidelines-best-practice-microbiology-australian-schools
Bowman, D. D., & Georgi, J. R. (2008). Georgis' Parasitology for Veterinarians (9th ed.). Amsterdam, the Netherlands: Elsevier Health Sciences.
British Columbia Ministry of Education. (2016). Science Safety Resource Manual. Retrieved 07/18, 2017 from https://curriculum.gov.bc.ca/sites/curriculum.gov.bc.ca/files/pdf/science-safety-manual.pdf
British Broadcasting Corporation Bitesize. Treating, curing and preventing disease. Retrieved 02/25, 2019 from https://www.bbc.com/bitesize/guides/z2kvw6f/revision/7
California Department of Education. (2014). Science Safety Handbook for California Public Schools 2014 Edition. Sacramento, CA, USA: California Department of Education.
Canadian Food Inspection Agency. (2019). Biohazard Containment and Safety. Retrieved 01/02, 2019 from http://www.inspection.gc.ca/animals/biohazard-containment-and-safety/eng/1300121579431/1315776600051
Cangelosi, G. A., Freitag, N. E., & Buckley, M. (2005). From Outside to Inside: Environmental Microorganisms as Human Pathogens. A report of the American Academy of Microbiology, Washington, DC: American Academy of Microbiology. Retrieved 11/28, 2017 from https://www.asmscience.org/content/report/colloquia/colloquia.14
Carlberg, D. M., & Yeaman, M. R. (2006). Biosafety in the Teaching Laboratory. In Fleming, D. O. & Hunt, D. L. (Eds.), Biological Safety: Principles and Practices (4th ed., pp.531-549). Washington, DC, USA: ASM Press.
Collins, C. H., & Kennedy, D. A. (1999). Laboratory-acquired infections. In Laboratory-acquired infections: History, incidence, causes and preventions (4th ed., pp. 1-97). Oxford, UK: Betterworth-Heinemann.
Consortium of Local Education Authorities for the Provision of Science Services. (2019). Supporting practical science, D&T and art - in schools and colleges - Portable autoclave and pressure cooker buying guide. Retrieved 06/24, 2019 from http://science.cleapss.org.uk/Resource/GL126-Portable-autoclave-and-pressure-cooker-buying-guide.pdf
Council of Ontario Directors of Education. Student Safety in Secondary Science Education Grades 9-12. Retrieved 07/21, 2020 from http://www.ontariodirectors.ca/downloads/health_and_safety/English_Guides-Jun18-13/Safety%20Secondary%20Science%20Education%209-12%20June%2018.pdf
CSA Z94.3-07 (R2014), Eye and Face Protectors. (2007). Mississauga, ON, Canada: Canadian Standards Association.
deHoog, G. C., Guarro, J., Gené, J., & Figueras, M. J. (2014). Atlas of Clinical Fungi. Retrieved 11/03, 2015 from http://www.clinicalfungi.org/
Duke University. Personal Protection. Retrieved 07/19, 2017 from https://chem.duke.edu/about/personal-protection#top
Emmert, E. A. B., & ASM Task Committee on Laboratory Biosafety. (2012). Guidelines for Biosafety in Teaching Laboratories. American Society for Microbiology.
Emmert, E. A. B., & ASM Task Committee on Laboratory Biosafety. (2013). Biosafety Guidelines for Handling Microorganisms in the Teaching Laboratory: Development and Rationale. Journal of Microbiology & Biology Education, 14(1): 78-83.
Favero, M. S., & Arduino, M. J. (2006). Decontamination and Disinfection. In Fleming, D. O. & Hunt, D. L. (Eds.), Biological Safety: Principles and Practices (4th ed., pp. 373-381). Washington, DC, USA: ASM Press.
Fleming, D. O. (2006). Prudent Biosafety Practices. In Fleming, D. O. & Hunt, D. L. (Eds.), Biological Safety: Principles and Practices (4th ed., pp.361-371). Washington, DC, USA: ASM Press.
Flinn Scientific, Inc. (2012). Science Department Safety Training Notes. Retrieved 07/21, 2017 from https://www.flinnsci.com/globalassets/flinn-scientific/all-free-pdfs/sn030-bunsen-burner-and-hot-plate-safety.pdf?v=9952edfa341647f6a99d4fbcaaf00ae2
Gage, N. L. (Ed.). (1963). Handbook of Research on Teaching. Chicago, IL, USA: Rand McNally & Co.
Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines.html
Government of Canada. (2016). Canadian Biosafety Handbook (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines.html
Government of Canada. (2017). Canadian Biosafety Guideline – Containment Level 1: Physical Design and Operational Practices. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance/containment-level-1-physical-design-operational-practices-overview.html
Government of Canada. (2017). Canadian Biosafety Guideline – Local Risk Assessment. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance/canadian-biosafety-guidelines.html
Government of Canada. (2020). COVID-19 guidance for schools Kindergarten to Grade 12. Available from https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/guidance-schools-childcare-programs.html
Government of Canada. (2020). Risk mitigation tool for child and youth settings operating during the COVID-19 pandemic. Available from https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/covid-19-risk-mitigation-tool-child-youth-settings-operating-during-pandemic.html
Government of Canada. ePATHogen – Risk Group Database. Available from https://health.canada.ca/en/epathogen
Government of Canada. Laboratory Biosafety and Biosecurity. Public Health Agency of Canada Training Portal. Available from https://training-formation.phac-aspc.gc.ca/course/index.php?categoryid=2&lang=en
Government of Canada. Pathogen Safety Data Sheets. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html
Government of Canada. Pathogen Safety Data Sheet Template. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/pathogen-safety-data-sheet-template.html
Government of Ontario. (2016). C-4: The management of biomedical waste in Ontario. Retrieved 07/18, 2018 from https://www.ontario.ca/page/c-4-management-biomedical-waste-ontario
Health of Animals Act (S.C. 1990, c.21).
Health of Animals Regulations (C.R.C., c.296).
Hedberg, M., & Nord, C. E. Anaerobic Bacteria. Retrieved 10/10, 2018 from http://www.antimicrobe.org/b77.asp
Human Pathogens and Toxins Act (S.C. 2009, c.24).
Human Pathogens and Toxins Regulations (SOR/2015-44).
Judge, M. (2017). Safety Precautions for Using a Pipette. Retrieved 07/19, 2017 from http://sciencing.com/safety-precautions-using-pipette-8743742.html
Kramer, A., Schwebke, I., & Kampf, G. (2006). How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BioMedCentral Infectious Diseases, 6: 130.
Lim, D. (2003). Microbiology (3rd ed.). Dubuque, IA, USA: Kendall/Hunt Publishing Company.
Madigan, M. T., Martinko, J. M., Stahl, D. A., & Clark, D. P. (2010). Brock Biology of Microorganisms(13th ed.). San Francisco, CA, USA: Benjamin Cummings Publishing Company.
Microbiology Society. (2016). Practical Microbiology for Secondary School. London, UK: Microbiology Society. Retrieved 05/25, 2018 from http://microbiologyonline.org/file/c89f015377ba698f508f2cbcd3db6abf.pdf
Microbiology Society. (2017). Fungi. Retrieved 07/10, 2017 from http://microbiologyonline.org/about-microbiology/introducing-microbes/fungi
Microbiology Society. (2017). Good microbiological laboratory practice. Retrieved 07/18, 2017 from http://microbiologyonline.org/teachers/safety-information/good-microbiological-laboratory-practice
Miller, J. M., Astles, R., Baszler, T., Chapin, K., Carey, R., et al. (2012). Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories. Morbidity and Mortality Weekly Report, 61(1):1-101.
National Research Council (US) Committee on Hazardous Biological Substances in the Laboratory. (1989). Biosafety in the Laboratory: Prudent Practices for the Handling and Disposal of Infectious Materials. Washington, DC, USA: National Academy of Sciences.
National Science Teachers Association. (2007). Liability of Science Educators for Laboratory Safety. Retrieved 08/16, 2017 from http://www.nsta.org/about/positions/liability.aspx
Poppe, N., Markic, S., & Eilks, I. (2010). Low cost experimental techniques for science education: A guide for science teachers. Retrieved 08/22, 2017 from http://www.idn.uni-bremen.de/chemiedidaktik/salis_zusatz/material_pdf/lab_guide_low_cost_experiments_englisch.pdf
Public Health Agency of Canada. (2012). Hand Hygiene Practices in Healthcare Settings. Ottawa, ON, Canada: Public Health Agency of Canada.
Reynolds, K. A., Beamer, P. I., Plotkin, K. R., Sifuentes, L. Y., Koenig, D. W., et al. (2016). The healthy workplace project: Reduced viral exposure in an office setting. Archives of Environmental and Occupational Health, 71(3):157-162.
Russotto, V., Cortegiani, A., Raineri, S. M., & Giarratano, A. (2015). Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. Journal of Intensive Care, 3:54.
Science Buddies. (2017). Microorganisms Safety Guide. Retrieved 07/18, 2017 from https://www.sciencebuddies.org/science-fair-projects/project_ideas/Micro_Safety.shtml
Science Buddies. (2017). Projects Involving Potentially Hazardous Biological Agents. Retrieved 08/30, 2017 from https://www.sciencebuddies.org/science-fair-projects/competitions/biological-agents-regulations
Science Buddies. (2017). Stopping Superbugs! Retrieved 07/11, 2017 from https://www.sciencebuddies.org/science-fair-projects/Classroom_Activity_Student_AntibioticResistance.shtml
Science News for Students. (2015). Explainer: What is a virus? Retrieved 07/10, 2017 from https://www.sciencenewsforstudents.org/article/explainer-what-virus
Science Teachers’ Association of Ontario. (2011). Safe ON Science: A Reference Guide for Secondary School Science. Dresden, ON, Canada: Science Teachers’ Association of Ontario
See, J. R. H., Czachor, J. S., & Brown, G. R. (2006). Lactobacillus endocarditis: case report and literature review. Infectious Diseases in Clinical Practice, 14(3):134-136.
Steane, R. G. Experiments to show the growth of bacteria - basic techniques. Retrieved 02/25, 2019 from http://www.biotopics.co.uk/microbes/tech.html
Transportation of Dangerous Goods Act, 1992 (S.C. 1992, c.34).
United States Centers for Disease Control and Prevention. (2015). Show Me the Science – When & How to Use Hand Sanitizers. Retrieved 11/03, 2015 from http://www.cdc.gov/handwashing/show-me-the-science-hand-sanitizer.html
United States Centers for Disease Control and Prevention. (2016). About Parasites. Retrieved 07/11, 2017 from https://www.cdc.gov/parasites/about.html
United States Department of Health and Human Services, United States Centers for Disease Control and Prevention, & United States National Institutes of Health. (2009). Biosafety in Microbiological and Biomedical Laboratories (5th ed.). Washington, DC, USA: United States Government Printing Office.
University of Manitoba. (2014). Guidelines for the Safe Handling of Sharps. Retrieved 07/19, 2017 from http://umanitoba.ca/admin/vp_admin/risk_management/ehso/media/Sharps_Safety.pdf
U.S. Consumer Product Safety Commission, United States Centers for Disease Control and Prevention, & National Institute for Occupational Safety and Health. (2006). School Chemistry Laboratory Safety Guide. Retrieved 08/14, 2017 from https://www.cdc.gov/niosh/docs/2007-107/pdfs/2007-107.pdf
Virginia Department of Education. (2000). Safety in Science Teaching. Richmond, VA, USA: Virginia Department of Education.
Weber, A. M., Boudreau, Y., & Mortimer, V. D. (2000). A tuberculosis outbreak among medical waste workers. Journal of the American Biological Association, 5(2): 70-88.
Weese, J. S. (2009). Evaluation of Bacterial & Fungal Culture Practices in School Classrooms. The American Biology Teacher, 71(3):145-149.
World Health Organization. (2004). Laboratory Biosafety Manual (3rd ed.). Geneva, Switzerland: World Health Organization.
Appendix A. Risk groups and containment levels
The following sections describe the risk group categories for harmful microbes for both humans and animals based on the risk they pose to an individual human or animal, and the risk to the health of the community.Footnote 19 The containment levels for handling or storing microbes are also described.
A.1. Risk Group 1 (RG1; low individual and community risk)
RG1 biological material consists of microbes, nucleic acids, or proteins that are either unable to cause human or animal disease, or unlikely to do so; these are, therefore, not generally considered harmful microbes, and are not regulated by the PHAC or the CFIA. RG1 materials pose a low risk to the health of individuals and animals; as such, RG1 is the biological material of choice in elementary, intermediate, and secondary schools. However, RG1 materials may pose a risk to those with weakened or suppressed immune systems.
A.2. Risk Group 2 (RG2; moderate individual risk, low community risk)
Harmful microbes of RG2 pose a moderate risk to the health of individuals or animals, and a low risk to public health and the animal population. These harmful microbes are able to cause serious disease in a human or animal but are unlikely to do so. Effective treatment (e.g., antibiotics) and preventive measures (e.g., vaccines) are available, and the risk of spread of diseases caused by these harmful microbes is low.
Harmful microbes of RG2 are regulated by the PHAC, the CFIA, or both; the handling or storing of harmful microbes of RG2 requires a Pathogen and Toxin Licence issued by the PHAC, with the exception of a subset of activities (e.g., certain diagnostic laboratory analyses), and/or an animal pathogen import permit issued by the PHAC or the CFIA.
A.3. Risk Group 3 (RG3; high individual risk, low community risk)
Harmful microbes of RG3 pose a high risk to the health of individuals or animals, and a low risk to public health. These harmful microbes are likely to cause serious disease in a human or animal. Effective treatment and preventive measures are usually available and the risk of spread of disease caused by these harmful microbes is low for the public. The risk of spread to the animal population, however, can range from low to high depending on the harmful microbe.
Harmful microbes of RG3 are regulated by the PHAC, the CFIA, or both; the handling or storing of harmful microbes of RG3 requires a Pathogen and Toxin Licence issued by the PHAC and/or an animal pathogen import permit issued by the PHAC or the CFIA.
A.4. Risk Group 4 (RG4; high individual risk, high community risk)
Harmful microbes of RG4 pose a high risk to the health of individuals or animals and a high risk to public health. These harmful microbes are likely to cause serious disease in a human or animal, which can lead to death. Effective treatment and preventive measures are not usually available and the risk of spread of disease caused by these harmful microbes is high for the public. The risk of spread of disease to the animal population however, ranges from low to high depending on the harmful microbe.
Harmful microbes of RG4 are regulated by the PHAC, the CFIA, or both; the handling or storing of harmful microbes of RG4 requires a Pathogen and Toxin Licence issued by the PHAC and/or an animal pathogen import permit issued by the CFIA.
A.5. Containment Level 1
Work with RG1 biological material can be safely performed in a basic laboratory work area, often described as CL1, and can include a classroom. There are no physical or operational requirements for CL1 facilities due to the low risk associated with RG1 biological material. Biosafety is primarily achieved through the use of good microbiological laboratory practices in addition to basic physical design elements (e.g., sinks available for handwashing) that serve to protect individuals and the environment from the biological material being handled. These practices encompass the basics of biosafety and are the foundation of the mandatory practices required in higher containment levels.
A.6. Containment Level 2
Biosafety and biosecurity at CL2 are achieved by meeting the minimum applicable physical containment requirements, operational practice requirements, and performance and verification testing requirements specified in the CBS. Physical design requirements include the location of the laboratory, surface finishes, access control, and availability of biosafety equipment, based on work activities. Operational practices for CL2 include administrative controls (e.g., training of all individuals, having SOPs in place that detail the steps of routine procedures for safe work practices), use of PPE, and decontamination.
A.7. Containment Level 3
Biosafety and biosecurity at CL3 are achieved through comprehensive physical containment, operational practice, and performance and verification testing requirements specified in the CBS. CL3 requires stringent facility design and engineering controls, as well as specialized biosafety equipment to prevent the release of dangerous biological material into surrounding rooms and the environment. Operational practices at CL3 build upon those required for CL2, while taking into account the increased risks associated with the biological material and laboratory activities being carried out with harmful microbes of RG3.
A.8. Containment Level 4
CL4 is the highest level of containment available. A CL4 laboratory requires a highly complex facility design such as a self-contained area within a building or, when necessary, a separate building, that meets the applicable requirements specified in the CBS. It includes enhanced engineering controls, specialized biosafety equipment, and redundant biosafety features. CL4 requires the maximum level of operational practices that build upon those required at CL3.
Appendix B. Pathogen safety data sheet template
The following template can be used to develop a PSDS for biological material handled in the classroom. A downloadable, editable PSDS template is also available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/pathogen-safety-data-sheet-template.html
A template for a pathogen risk assessment is available at https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/pathogen-risk-assessment-template.html
The Canadian Biosafety Guideline Pathogen Risk Assessment is available at https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance/pathogen-risk-assessment.html
Section II. Hazard identification
Pathogenicity / toxicity
Duration of the illness/disease associated with the infectious agent in humans and animals. Symptoms of the disease, including severity and prevalence. Mortality rate of the disease.
Variations of the disease and clinical presentations. Other ailments associated with the disease. Potential acute and chronic effects.
Predisposing factors: Conditions or cofactors that may predispose to infection, disease, or more severe disease (e.g., pregnancy, immune status).
Epidemiology
Whether the disease is maintained in human or animal populations and, if so, where.
Where the disease is localised geographically.
Any specific outbreaks of the disease, and their magnitude.
Host range
Natural Host(s): Primary (definitive), secondary (intermediate), and dead-end hosts.
Other Host(s): Other hosts, including experimentally infected hosts, if applicable.
Infectious dose
The number of organisms or concentration of organisms required to cause disease (typically ID50) in the natural host(s).
Incubation period
The duration between contact with the infectious agent and presentation of the earliest clinical signs of the disease in the natural host(s) (usually measured in days).
Communicability
The ways in which the infectious agent can be transmitted from one host to another (e.g., ingestion, injection, mucous membrane/skin contact, inhalation.)
Likelihood of transmission by direct (intimate, casual) or indirect (fomites, vectors) contact.
Preferred mode of transmission.
Section III. Dissemination
Reservoir
Organisms (often a species of small mammal or bird) in which the infectious agent is maintained without causing any obvious clinical symptoms.
Zoonosis / reverse zoonosis
Whether the disease is spread between animals and humans; the direction of spread, and between which species.
Vectors
Species that can carry and transmit the pathogen to humans or animals. Typically, this refers to an arthropod that transmits by biting or laying eggs but could refer to a “mechanical vector”.
Section IV. Stability and viability
Drug susceptibility
Drugs/pharmaceutical agents that are effective and available for treating infection or disease.
Drug resistance
Known drug resistance or multi-drug resistance.
Susceptibility to disinfectants
Disinfectants capable of destroying the pathogen, including its toxins and/or spores (if applicable) and, if known, what conditions are necessary to achieve disinfection (e.g., concentration, contact time, and temperature). If unknown, disinfectants that are effective against a class of pathogens (e.g., against Gram positive bacteria).
Disinfectants or classes of disinfectant to which the pathogen is resistant.
Physical inactivation
Other means to inactivate the infectious material (e.g., UV irradiation, gamma irradiation, dry or moist heat, pH) and, if known, the effective parameters (method, duration, environmental conditions). If unknown, physical inactivation methods that are effective against a class of pathogens.
Survival outside host
Survival times for the infectious agent outside of its host environment (e.g., dried blood, on surfaces, in aerosol form).
Section V. First aid and medical
Surveillance
How the pathogen can be detected/diagnosed in an infected individual.
Symptoms to look for. The recommendations for surveillance.
First aid / treatment
How the infection/disease can be treated in an infected individual. Whether treatment is typically undertaken for infected animals.
Specific first aid/treatment that is recommended.
Immunization
Preventative and/or post-exposure immunizations that are recommended for those working with the pathogen. Whether these recommendations are universal, or based on the activities being performed or other factors.
Specific cofactors (e.g., pregnancy) that would change the recommendations. Whether animals are typically vaccinated against the pathogen.
Prophylaxis
Recommended pre- or post-exposure prophylaxis. Whether these recommendations are universal, or based on the activities being performed or other factors. Specific cofactors (e.g., pregnancy) that would change the recommendations.
Section VI. Laboratory hazards
Laboratory-acquired infections
Documented evidence of laboratory (research, diagnostic, healthcare) acquired infections with the infectious agent, including how many and under what circumstances.
Sources / specimens
The primary biological samples and specimens likely to contain the infectious agent (e.g., blood, urine, semen, mucous, faeces, necropsy tissues).
Primary hazards
The primary exposure hazard with infectious material (e.g., ingestion, exposure of mucous membranes/skin, autoinoculation, inhalation of airborne or aerosolized infectious material, in animal waste or animal carcasses, or bites/scratches of an infected animal).
Special hazards
Other hazards that an individual should be aware of when dealing with this pathogen.
Section VII. Exposure controls and personal protection
Risk Group classification
Risk Group classification in humans and animals for the pathogen.
Containment
The containment requirements for working with the pathogen (i.e., the containment level).
Specific requirements for certain activities (e.g., using a biological safety cabinet).
Protective clothing
Whether, when and what specific PPE is to be used when working with this pathogen (e.g., respirators, gloves, lab coat).
Other precautions
Precautions, other than PPE, that should be considered when working with the pathogen.
Section VIII. Handling and storage recommendations
Spills
Spill procedure to be followed in the event of a spill with the pathogen, including the type and quantity of disinfectant that should be used (from Section IV – Stability and Viability).
Disposal
How the infectious or potentially infectious material should be disposed of.
Specific decontamination procedure(s) that should be followed prior to disposal.
Storage
How to store the infectious material.
Section IX. Regulatory and other information
Regulatory information
The regulatory authorities for the use, storage, import, export, transport, transfer, disposal, or other activities involving the pathogen (e.g., PHAC, CFIA, Health Canada, Environment Canada, Transport Canada, the Department of Foreign Affairs, Trade and Development.) Users are responsible for ensuring they are compliant with all relevant acts, regulations, guidelines, and standards, including Federal, Provincial/Territorial, and Municipal.
Updated
Date of last update
Prepared by
Name of user and their institution
References
List of references used in the order that they were cited in the text.
Appendix C. Public Health Agency of Canada biosafety posters
Full-size versions of the following PHAC biosafety posters are freely available.
Users are required to register for a free account to access these resources.
Poster 1: Biosafety in action

Poster 1: Text description
Exposure to biohazards can happen in the following ways:
- absorption (through eyes or skin)
- ingestion (through mouth)
- inhalation (through nose/mouth)
- injection or puncture wound
How to protect yourself and others when using biohazards:
Do:
- tie back long hair
- wear safety glasses (no contact lenses)
- wear gloves
- wear a lab coat
- wear closed-toe shoes
Don’t:
- touch personal objects with contaminated gloves
- create fine spray with biohazards
- bring food into the room
- bend, shear or recap used needles
Remember to always wash hands and decontaminate workspaces after all activities!
Learn more! To learn more, visit publichealth.gc.ca/pathogens for free instructional courses, videos and other tools.
Poster 2: Biosafety in the laboratory

Poster 2: Text description
Personal protection
- Lab coat: Lab coats must be worn and fastened until all experiments have been completed. Laboratory clothing must not be worn outside the laboratory.
- Gloves: Gloves must be worn when handling infectious material. Gloves must be removed and properly disposed of before leaving the laboratory.
- Eye and face protections: Eye protection must be worn when there is a potential risk of splashes or flying objects.
- Footwear: Suitable footwear with closed toes and heels must be worn in the laboratory.
Hygienic practices
- Wearing jewellery is not recommended in the laboratory.
- Cover any open wounds, cuts or scratches with waterproof dressings.
- Tie back long hair.
Laboratory working area
There are a number of actions that are never permitted in the laboratory.
- Never apply cosmetics.
- Never smoke.
- Never remove or insert contact lenses within the lab area.
- Never eat.
- Never store food or drinks, personal belongings, or utensils.
- Never drink.
- Never mouth pipette any substance.
Hand washing
- Hand washing is the most important practice.
- Hands must be washed after the removal of gloves.
- Hand washing should last as long as it takes to sing the happy birthday song to yourself twice!
Cleanup and disposal procedures
- Work surfaces must be cleaned thoroughly with a suitable disinfectant at the end of every experiment as well as after any spill.
- Most glassware instruments and laboratory clothing can be reused or recycled after appropriate decontamination.
- Broken glass must be disposed in a puncture-resistant sharps container.
Emergency procedures
- Report any lab incident to the laboratory supervisor.
Appendix D. Risk assessment template
The template below, with an example provided, can be used to assist in completing a risk assessment. The sections and the level of information can be adapted to fit the classroom needs.
Step 1: Hazard identification
Hazard
Describe an identified hazard (e.g., potential propagation of an RG2 organism by culture of a soil sample).
Step 2: Risk identification and assessment
Risk scenarios
Describe the potential consequences as a result of the hazard (e.g., exposure and infection of students by an RG2 organism).
Risk rating
Indicate the relative level of risk associated with the activity (e.g., low, moderate, high).
Step 3: Develop and implement risk mitigation
Type(s) of strategies
Elimination
- Use of an inactivated biological material.
- Use of a non-pathogenic/non-toxic surrogate.
(e.g., sterilize the soil sample prior to adding a known RG1 organism)
Reduction or substitution
- Use a less dangerous biological material.
(e.g., culture a sample from yogurt instead of soil)
Procedural
- Use of building controls.
(e.g., keeping plates closed)
Administrative controls
- Use of safe work practices, good microbiological laboratory practices, SOPs and training.
(e.g., preventing aerosols, avoiding touching mouth, nose, and eyes)
PPE
- Use activity-specific PPE.
(e.g., gloves, lab coat)
Step 4: Review risk assessment
Review strategies
- Determine if the protocol or equipment has changed.
- Determine if any incidents have occurred since implementation of the procedure.
- As appropriate, update the risk assessment or initiate a new risk assessment. If no changes are needed, document the fact.
Appendix E. Procedures to minimize aerosol hazards
Users are required to register for a free account to access these resources.
Poster 3: Procedures to minimize aerosol hazards

Poster 3: Text description
Opening tubes:
- Manipulate infectious materials within a biological safety cabinet.
- Upon opening, unscrew the cap slightly and wait a few seconds before removing it.
Inoculating loop:
- Use a microincinerator or a disposable loop instead of a Bunsen burner.
- Allow the inoculating loop to cool before any procedures.
Syringes/needles:
- Withdraw needles from bottle using disinfectant-soaked absorbent pads wrapped around the bottle cap.
- Use locking syringes.
Pipetting:
- Use “to deliver” pipettes calibrated to retain the last drop.
- Use pipettes with plugs.
- Discharge pipettes close to the fluid level and let the contents run down the wall of the container.
- Never forcefully expel infectious materials from the pipette.
Centrifugation:
- Centrifuge infectious material in closed containers, placed in sealed safety cups or rotors.
- Open cups in a biological safety cabinet.
- Maintain the centrifuge to ensure that it is clean and the gaskets and O-rings are not compromised.
- Wait 5 minutes before opening the centrifuge after each run to allow any aerosols to settle.
Breakage:
- Avoid the use of glassware where possible.
- Use plastic tubes, flasks and bottles.
- Use screw-capped tubes and bottles rather than plugs or snap caps.
Mixing and homogenizing:
- Ensure the lab blender has a gasket lid and leak proof bearings.
- Wait a few seconds before opening a lid after mixing.
- Use a vortex, instead of inverting the cultures.
Pouring infectious materials:
- Perform your work over plastic-backed absorbent material.
- Wipe the rim of the tube with disinfectant-soaked absorbent paper to remove potential contamination on the outside of the tube.
Appendix F. General spill clean-up procedure
Spill response
The procedures to follow when a spill occurs can be included in the ERP and taught to students as a part of the safety training at the beginning of each school year, and again as a refresher prior to beginning any experiment.Footnote 42
Having a pre-assembled “biological spill kit” that contains all items needed to contain, clean-up, and decontaminate a spill will facilitate a timely and effective spill response. The decontamination protocol following a spill depends on where it occurred, its size, and the nature of the material contaminated. However, a typical biological spill kit might include:
- appropriate PPE (e.g., gloves, disposable gowns, shoe covers);
- effective disinfecting agent (e.g., household bleach) that is within its expiry date;
- absorbent material (e.g., paper towels);
- disposal material (e.g., dustpan, broom, tongs, waste bags); and
- a waterproof copy of the spill clean-up SOP.
All spill decontamination procedures are to be based on the outcome of a risk assessment. An important consideration is whether aerosols containing potentially infectious biological material have been generated. In these cases, the risk of exposure can be minimized by evacuating the classroom, closing the door, and decontaminating all PPE worn by those leaving the classroom. Waiting a sufficient time before re-entering the classroom (generally 30 minutes) will allow droplets to settle and aerosols to clear.Footnote 58 Decontamination of surfaces is important to prevent the transfer of settled microbes.
Large spills and those involving potentially dangerous biological material may require more expertise and specialized material. In such a case, it would be best to evacuate the classroom and report the spill to the appropriate internal authorities who can coordinate the clean-up.
General spill clean-up procedures
The steps below can be followed in order to contain a spill of biological material, clean it up, and decontaminate the affected area:Footnote 58
- Use PPE appropriate to the risk, which may include gloves, protective clothing, respiratory protection, and eye protection.
- Assemble the required clean-up materials (e.g., biological spill kit) and bring them to the spill.
- Cover the spill with cloth or paper towels to contain it.
- Starting at the outer margin of the spill area and working concentrically toward the center, pour an appropriate disinfectant (i.e., sufficient concentration and effective against the biological material spilled) over the cloth or paper towels and the immediately surrounding area. Using alcohol (as a disinfectant) is not recommended due to the risk of fire or explosion with large volumes.
- After the appropriate contact time, clear away the absorbent material and debris. If there is broken glass or other sharps, use a dustpan or pieces of stiff cardboard to collect and deposit the material into a puncture-resistant container for disposal. Only handle glass fragments with forceps. Dustpans can be autoclaved or decontaminated using an effective disinfectant.
- Clean and disinfect the area of the spill and any area where a splash could have reached.
- Dispose of all contaminated materials in a leak-proof, puncture-resistant waste disposal container, and decontaminate the container prior to disposal.
- Remove and decontaminate PPE.
Appendix G. Proper handwashing technique
Handwashing is the most common method for decontamination of hands and the most effective means for preventing the transmission of infection. When done properly, handwashing using soap and clean running water is an effective way to remove visible soil and organic material, and harmful microbes from the surface of the hands.
Procedure for handwashing (soap and water)Footnote 40
- Hands are wet under running water.
- Enough soap is used to lather all surfaces of the hands, including fingers, fingertips, between fingers, palms, backs of hands and thumbs, base of thumb, and if a ring is worn, on and under the ring.
- The palms and backs of each hand are rubbed vigorously, interlocking and interfacing fingers so that fingers and thumbs are rubbed for 15 to 30 seconds, or about the time it takes to sing the “Happy Birthday” song, twice.
- Hands are rinsed thoroughly, with fingers pointing downward, under running water.
- Hands are dried thoroughly by patting with a single-use towel.
- Manual faucets are turned off with paper towel, so that hands are not recontaminated in the process.
- Skin products can be applied regularly to maintain healthy skin.
- The complete handwashing procedure (wetting hands, applying soap, lathering, rinsing, and drying) takes 40 to 80 seconds.
Figure G: Procedure for handwashing

Figure G: Text description
Figure depicting the proper handwashing procedure with soap and water in six images.
- Figure depicting two hands being wet under running water in a handwashing sink.
- Figure depicting a right hand dispensing soap from a liquid soap dispenser.
- Figure depicting two hands being rubbed together to lather all surfaces of the hands. The palm of the left hand is rubbing the back of the right hand with soap. Water is running from the faucet of the handwashing sink in the background.
- Figure depicting two hands with fingers interlaced to lather soap between the fingers, with a liquid soap dispenser and running water from the faucet of the handwashing sink in the background.
- Figure depicting two hands being dried with a brown paper towel once they have been rinsed, with a liquid soap dispenser and running water from the faucet of the handwashing sink in the background.
- Figure depicting a left hand turning off the hot water faucet with a brown paper towel.
To reduce the potential of recontamination, consider the following hands-free options:
- mounted automated liquid soap dispensers rather than squeeze bottles or bar soap; and
- one person with clean hands may be tasked with dispensing liquid soap directly into other students’ hands.
Considerations on the use of alcohol-based hand sanitizers
- While the use of alcohol-based hand sanitizers may be appropriate in certain situations and can reduce the number of organisms, they are not as effective as handwashing with soap and water and cannot eliminate all types of harmful microbes.Footnote 41
- Alcohol-based hand sanitizers may not be as effective as handwashing when hands are visibly dirty or greasy.
- Hand sanitizers are less effective if they are mixed with water, so consider keeping dispensers away from handwashing sinks to prevent them from being confused with soap dispensers.
- A hand sanitizer that has been demonstrated to be effective against the biological material in use in the laboratory may be a suitable alternative where handwashing sinks are not easily accessible to avoid the spread of contamination. In this instance, handwashing should follow as soon as a suitable handwashing sink is available.
- Applying alcohol-based hand sanitizers to wet hands will dilute the alcohol and render the sanitizer less effective or ineffective.
- For a sanitizer to be effective, generally, all hand surfaces have to be rubbed until the product has dried to allow for the appropriate contact time, and paper towels are not used to dry the hands or wipe off the sanitizer.
- Alcohol-based hand sanitizers are flammable and must be allowed to dry prior to contact with an oxygen-rich environment to prevent ignition.
- Hand wipes (impregnated with soap, antimicrobials, or alcohol) are not an alternative to antimicrobial soaps or alcohol-based hand sanitizers for hand antisepsis.
Appendix H. How to safely remove disposable gloves
Gloves are removed using a glove-to-glove and skin-to-skin technique.
- Grasp the outside of the glove near the wrist (but away from the edge) and peel away.
- Peel the glove inside-out down to fingertips.
- Leave the fingertips of the glove in place.
- Using the gloved fingertips (and thumb), grasp the outside of the second glove near the wrist; ensure that the skin is only exposed to the inside of the gloves.
- Peel down to the fingertips.
- Discard both gloves into the waste receptacle, and wash hands.
Figure H: How to safely remove disposable gloves

Figure H: Text description
Figure depicting how to safely remove disposable gloves in six steps.
- Figure depicting two gloved hands. The right hand is grasping the outside of the left glove near the wrist (but away from the edge).
- Figure depicting two gloved hands. The right hand is peeling the left glove inside-out to uncover the palm of the left hand.
- Figure depicting a right gloved hand removing a glove off a left hand down to the fingertips, leaving the fingertips of the glove in place.
- Figure depicting a left hand with a glove covering the fingertips grasping the outside of a glove on a right hand near the wrist.
- Figure depicting a left hand with a glove covering the fingertips peeling a glove on a right hand down to the fingertips.
- Figure depicting a left hand holding two inside-out gloves.
Appendix I. Suitability of chemical disinfectants
| Chemical disinfectant | Suitability | Disadvantages |
|---|---|---|
Chlorine |
Effective against most microbes; can be used for disinfecting contaminated liquids and equipment that is not visibly soiled. |
|
Alcohol |
Effective against bacteria, and some viruses and fungi; limited or no effectiveness against fungal and bacterial spores. |
|
Quaternary ammonium compounds (QACs) |
Not effective on sporulating microbes. Useful for cleaning of smooth surfaces and floors. |
|
Phenolics |
Variable effectiveness against bacteria, viruses, and fungi. |
|
Glutaraldehyde (2% acidic solution) |
Effective against most microbes. |
|
Formaldehyde |
Effective against most microbes. |
|
Hydrogen peroxide (H2O2) (30% solution) |
Effective against most microbes. |
|
References
Footnotes
- Footnote 1
-
Gage, N. L. (Ed.). (1963). Handbook of Research on Teaching. Chicago, IL, USA: Rand McNally & Co.
- Footnote 2
-
Emmert, E. A. B., & ASM Task Committee on Laboratory Biosafety. (2013). Biosafety Guidelines for Handling Microorganisms in the Teaching Laboratory: Development and Rationale. Journal of Microbiology & Biology Education, 14(1):78-83.
- Footnote 3
-
Microbiology Society. (2016). Practical Microbiology for Secondary School. London, UK: Microbiology Society. Retrieved 05/25, 2018 from http://microbiologyonline.org/file/c89f015377ba698f508f2cbcd3db6abf.pdf
- Footnote 4
-
Government of Canada. (2017). Canadian Biosafety Guideline – Containment Level 1: Physical Design and Operational Practices. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance/containment-level-1-physical-design-operational-practices-overview.html
- Footnote 5
-
Government of Canada. (2020). COVID-19 guidance for schools Kindergarten to Grade 12. Available from https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/guidance-schools-childcare-programs.html
- Footnote 6
-
Government of Canada. (2020). Risk mitigation tool for child and youth settings operating during the COVID-19 pandemic. Available from https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/covid-19-risk-mitigation-tool-child-youth-settings-operating-during-pandemic.html
- Footnote 7
-
Carlberg, D. M., & Yeaman, M. R. (2006). Biosafety in the Teaching Laboratory. In Fleming, D. O., & Hunt, D. L. (Eds.), Biological Safety: Principles and Practices (4th ed., pp.531-549). Washington, DC, USA: ASM Press.
- Footnote 8
-
Madigan, M. T., Martinko, J. M., Stahl, D. A., & Clark, D. P. (2010). Brock Biology of Microorganisms (13th ed.). San Francisco, CA, USA: Benjamin Cummings Publishing Company.
- Footnote 9
-
Lim, D. (2003). Microbiology (3rd ed.). Dubuque, IA, USA: Kendall/Hunt Publishing Company.
- Footnote 10
-
See, J. R. H., Czachor, J. S., & Brown, G. R. (2006). Lactobacillus endocarditis: case report and literature review. Infectious Diseases in Clinical Practice, 14(3):134-136.
- Footnote 11
-
Science News for Students. (2015). Explainer: What is a virus? Retrieved 07/10, 2017 from https://www.sciencenewsforstudents.org/article/explainer-what-virus
- Footnote 12
-
Microbiology Society. (2017). Fungi. Retrieved 07/10, 2017 from http://microbiologyonline.org/about-microbiology/introducing-microbes/fungi
- Footnote 13
-
deHoog, G. C., Guarro, J., Gené, J., & Figueras, M. J. (2014). Atlas of Clinical Fungi. Retrieved 11/03, 2015 from http://www.clinicalfungi.org/
- Footnote 14
-
Bowman, D. D., & Georgi, J. R. (2008). Georgis' Parasitology for Veterinarians (9th ed.). Amsterdam, the Netherlands: Elsevier Health Sciences.
- Footnote 15
-
United States Centers for Disease Control and Prevention. (2016). About Parasites. Retrieved 07/11, 2017 from https://www.cdc.gov/parasites/about.html
- Footnote 16
-
Canadian Food Inspection Agency. Biohazard Containment and Safety. Retrieved 01/02, 2019 from http://www.inspection.gc.ca/animals/biohazard-containment-and-safety/eng/1300121579431/1315776600051
- Footnote 17
-
Government of Canada. ePATHogen – Risk Group Database. Available from https://health.canada.ca/en/epathogen
- Footnote 18
-
Government of Canada. Pathogen Safety Data Sheets. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html
- Footnote 19
-
Government of Canada. (2016). Canadian Biosafety Handbook (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 20
-
British Columbia Ministry of Education. (2016). Science Safety Resource Manual. Retrieved 08/14, 2017 from https://curriculum.gov.bc.ca/sites/curriculum.gov.bc.ca/files/pdf/science-safety-manual.pdf
- Footnote 21
-
California Department of Education. (2014). Science Safety Handbook for California Public Schools 2014 Edition. Sacramento, CA, USA: California Department of Education.
- Footnote 22
-
Virginia Department of Education. (2000). Safety in Science Teaching. Richmond, VA, USA: Virginia Department of Education.
- Footnote 23
-
National Science Teachers Association. (2007). Liability of Science Educators for Laboratory Safety. Retrieved 08/16, 2017 from http://www.nsta.org/about/positions/liability.aspx
- Footnote 24
-
Government of Canada. Laboratory Biosafety and Biosecurity. Public Health Agency of Canada Training Portal. Available from https://training-formation.phac-aspc.gc.ca/course/index.php?categoryid=2&lang=en
- Footnote 25
-
Microbiology Society. (2017). Good microbiological laboratory practice. Retrieved 07/18, 2017 from http://microbiologyonline.org/teachers/safety-information/good-microbiological-laboratory-practice
- Footnote 26
-
Government of Canada. (2017). Canadian Biosafety Guideline – Local Risk Assessment. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance/canadian-biosafety-guidelines.html
- Footnote 27
-
Miller, J. M., Astles, R., Baszler, T., Chapin, K., Carey, R., et al. (2012). Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories. Morbidity and Mortality Weekly Report, 61(1):1-102.
- Footnote 28
-
Kramer, A., Schwebke, I., & Kampf, G. (2006). How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BioMedCentral Infectious Diseases, 6:130.
- Footnote 29
-
Russotto, V., Cortegiani, A., Raineri, S. M., & Giarratano, A. (2015). Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. Journal of Intensive Care, 3:54.
- Footnote 30
-
Reynolds, K. A., Beamer, P. I., Plotkin, K. R., Sifuentes, L. Y., Koenig, D. W., et al. (2016). The healthy workplace project: Reduced viral exposure in an office setting. Archives of Environmental and Occupational Health, 71(3):157-162.
- Footnote 31
-
Cangelosi, G. A., Freitag, N. E., & Buckley, M. (2005). From Outside to Inside: Environmental Microorganisms as Human Pathogens. A report of the American Academy of Microbiology, Washington, DC: American Academy of Microbiology. Retrieved 11/28, 2017 from https://www.asmscience.org/content/report/colloquia/colloquia.14
- Footnote 32
-
Alberta Ministry of Education. (2006). Safety in the Science Classroom: Kindergarten to Grade 12. Edmonton, AB, Canada: Alberta Education, Learning and Teaching Resources Branch.
- Footnote 33
-
Council of Ontario Directors of Education. Student Safety in Secondary Science Education Grades 9-12. Retrieved 07/21, 2020 from http://www.ontariodirectors.ca/downloads/health_and_safety/English_Guides-Jun18-13/Safety%20Secondary%20Science%20Education%209-12%20June%2018.pdf
- Footnote 34
-
American Chemical Society. (2015). Fundamentals of Hazard Assessment: Control Measures. Retrieved 08/18, 2017 from https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment/fundamentals/control-measures.html
- Footnote 35
-
National Research Council (US) Committee on Hazardous Biological Substances in the Laboratory. (1989). Biosafety in the Laboratory: Prudent Practices for the Handling and Disposal of Infectious Materials. Washington, DC, USA: National Academy of Sciences.
- Footnote 36
-
Poppe, N., Markic, S., & Eilks, I. (2010). Low cost experimental techniques for science education: A guide for science teachers. Retrieved 08/22, 2017 from http://www.idn.uni-bremen.de/chemiedidaktik/salis_zusatz/material_pdf/lab_guide_low_cost_experiments_englisch.pdf
- Footnote 37
-
Science Buddies. (2017). Microorganisms Safety Guide. Retrieved 07/18, 2017 from https://www.sciencebuddies.org/science-fair-projects/project_ideas/Micro_Safety.shtml
- Footnote 38
-
Fleming, D. O. (2006). Prudent Biosafety Practices. In Fleming, D. O. & Hunt, D. L. (Eds.), Biological Safety: Principles and Practices (4th ed., pp. 361-371). Washington, DC, USA: ASM Press.
- Footnote 39
-
American Chemical Society. (1995). Guide for Chemical Spill Response Planning in Laboratories. Retrieved 07/21, 2017 from https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/publications/guide-for-chemical-spill-response.html
- Footnote 40
-
Public Health Agency of Canada. (2012). Hand Hygiene Practices in Healthcare Settings. Ottawa, ON, Canada: Public Health Agency of Canada.
- Footnote 41
-
United States Centers for Disease Control and Prevention. (2015). Show Me the Science – When & How to Use Hand Sanitizers. Retrieved 11/03, 2015 from http://www.cdc.gov/handwashing/show-me-the-science-hand-sanitizer.html
- Footnote 42
-
Science Teachers' Association of Ontario. (2011). Safe ON Science: A Reference Guide for Secondary School Science. Dresden, ON, Canada: Science Teachers' Association of Ontario.
- Footnote 43
-
British Broadcasting Corporation Bitesize. Treating, curing and preventing disease. Retrieved 02/25, 2019 from https://www.bbc.com/bitesize/guides/z2kvw6f/revision/7
- Footnote 44
-
Steane, R. G. Experiments to show the growth of bacteria - basic techniques. Retrieved 02/25, 2019 from http://www.biotopics.co.uk/microbes/tech.html
- Footnote 45
-
Hedberg, M. & Nord, C. E. Anaerobic Bacteria. Retrieved 10/10, 2018 from http://www.antimicrobe.org/b77.asp
- Footnote 46
-
Duke University. Personal Protection. Retrieved 07/19, 2017 from https://chem.duke.edu/about/personal-protection#top
- Footnote 47
-
CSA Z94.3-07 (R2014), Eye and Face Protectors. (2007). Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 48
-
University of Manitoba. (2014). Guidelines for the Safe Handling of Sharps. Retrieved 07/19, 2017 from http://umanitoba.ca/admin/vp_admin/risk_management/ehso/media/Sharps_Safety.pdf
- Footnote 49
-
Flinn Scientific, Inc. (2012). Science Department Safety Training Notes. Retrieved 07/21, 2017 from https://www.flinnsci.com/globalassets/flinn-scientific/all-free-pdfs/sn030-bunsen-burner-and-hot-plate-safety.pdf?v=9952edfa341647f6a99d4fbcaaf00ae2
- Footnote 50
-
Weber, A. M., Boudreau, Y., & Mortimer, V. D. (2000). A tuberculosis outbreak among medical waste workers. Journal of the American Biological Safety Association, 5(2):70-88.
- Footnote 51
-
Collins, C. H., & Kennedy, D. A. (1999). Laboratory-acquired infections. In Laboratory-acquired infections: History, incidence, causes and preventions (4th ed., pp. 1-97). Oxford, UK: Betterworth-Heinemann.
- Footnote 52
-
United States Department of Health and Human Services, United States Centers for Disease Control and Prevention, & United States National Institutes of Health. (2009). Biosafety in Microbiological and Biomedical Laboratories (5th ed.). Washington, DC, USA: United States Government Printing Office.
- Footnote 53
-
Weese, J. S. (2009). Evaluation of Bacterial & Fungal Culture Practices in School Classrooms. The American Biology Teacher, 71(3):145-149.
- Footnote 54
-
Australian Science Teachers Association. (2017). Australian school science information support for teachers and technicians: Guidelines for best practice for microbiology in Australian Schools. Retrieved 07/10, 2017 from https://assist.asta.edu.au/resource/4196/guidelines-best-practice-microbiology-australian-schools
- Footnote 55
-
Consortium of Local Education Authorities for the Provision of Science Services. (2019). Supporting practical science, D&T and art - in schools and colleges - Portable autoclave and pressure cooker buying guide. Retrieved 06/24, 2019 from http://science.cleapss.org.uk/Resource/GL126-Portable-autoclave-and-pressure-cooker-buying-guide.pdf
- Footnote 56
-
Government of Ontario. (2016). C-4: The management of biomedical waste in Ontario. Retrieved 07/18, 2018 from https://www.ontario.ca/page/c-4-management-biomedical-waste-ontario
- Footnote 57
-
Science Buddies. (2017). Projects Involving Potentially Hazardous Biological Agents. Retrieved 08/30, 2017 from https://www.sciencebuddies.org/science-fair-projects/competitions/biological-agents-regulations
- Footnote 58
-
World Health Organization. (2004). Laboratory Biosafety Manual (3rd ed.). Geneva, Switzerland: World Health Organization.
Page details
- Date modified:
