Screening Assessment for The Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Decamethylcyclopentasiloxane
(D5)
Chemical Abstracts Service Registry Number
541-02-6
Environment Canada
Health Canada
November 2008
Table of Contents
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on decamethylcyclopentasiloxane (D5), Chemical Abstracts Service Registry Number 541-02-6. During the categorization process, this substance was identified as a high priority for screening assessment and included in the Ministerial Challenge because it had been initially found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity (PBiT) to non-human organisms and it is known to be in commerce in Canada.
Although the categorization exercise did not determine D5 to be a priority for assessment of potential risks to human health, a human health assessment of D5 was also conducted due to its structure and use pattern similarity to D4, octamethylcyclotetrasiloxane (also known as D4), a high priority for assessment for both human health and ecological risks under CEPA 1999.
D5 is an industrial chemical which was not manufactured in Canada in 2006 in a quantity above the reporting threshold of 100 kg, but which is imported into the country as an essentially pure substance, in mixtures with other cyclic siloxanes, as a residual in silicone polymers, and in finished consumer products. From responses to a notice published under section 71 of CEPA 1999, it was determined that between 1 000 000 and 10 000 000 kg of D5 were imported into Canada in 2006.
D5 may be released to the environment from industrial processes such as blending, formulation and packaging operations, and from its use as an industrial defoamer and degreaser. It is also released from the use and disposal of personal care products. Air, wastewater, and agriculture soil are the principal receiving environmental media for D5 based on its physical-chemical properties and its use patterns.
In air, D5 is persistent with calculated atmospheric half-lives of more than 3 days. D5 has the potential to be transported over long-distances in the atmosphere. However, it has a low potential to be deposited in water or soil in remote regions. The hydrolysis half-lives for D5 under Canadian water conditions ranged from 1 to 733 days, indicating the substance is persistent under certain Canadian water conditions, especially in cooler and neutral water (5-10 ºC). D5 is also judged to be persistent in sediment, with half-lives of 49 to 588 days estimated under realistic Canadian sediment conditions (temperature of 5-25 °C) based on information to structurally similar analogue, D4, indicating the substance may be persistent in sediment. D5 is not considered persistent in soil, based on evidence of clay-catalysed degradation, with dimethylsilanediol being the stable hydrolysis product. Therefore, D5 has been determined to meet the persistence criterion as set out in the Persistence and Bioaccumulation Regulations.
The empirical bioconcentration factor and modelled bioaccumulation factor are both above 5000, indicating D5 may have a high potential to accumulate in aquatic organisms. However, data from a biomagnification study in fish and a biota-sediment accumulation study in invertebrates suggest that the bioaccumulation potential of D5 may be lower, possibly due to reduced bioavailability. Therefore, while D5 has the potential to accumulate in biota, it is not possible to conclude at this time that D5 meets the criterion for bioaccumulation as set out in the Persistence and Bioaccumulation Regulations based on consideration of the conflicting evidence from laboratory studies and predictive models.
Adverse effects from exposure to D5 in sediment-dwelling organisms were observed at a concentration of 160 mg/kg. The experimental toxicity data showed no adverse effects to pelagic aquatic organisms at concentrations up to 0.015 mg/L, its approximate water solubility limit. However, it is possible that toxicity may manifest at the solubility limit if sufficient exposure and sensitive species were present. Risk quotients derived from exposure scenarios involving discharges of D5 from both consumer use and industrial operations show that a total of 65 sites (~6.8%) evaluated across Canada have predicted environmental concentrations in water higher than the 0.015 mg/L predicted no-effect concentration, without the use of an application factor, for aquatic organisms. Considering the persistence of D5 under colder Canadian water conditions and its potential to bioaccumulate in biota, long-term environmental exposure to D5 may potentially cause adverse effects to aquatic organisms in certain Canadian environments.
Based principally on the weight of evidence-based assessment of the Danish EPA, a potential effect for repeated-dose toxicity is carcinogenicity, as observed in a 2-yr rat study. It is noted that the uterine tumours in this study were observed at higher levels than the effects identified for the lung and liver in several other toxicity studies. The lung was identified as a target organ for inhalation exposures of D5 whereas the liver was identified as a target organ for oral and inhalation exposures. The critical effect level for repeated dose toxicity via the inhalation route was based on a significant increased incidence of pulmonary vascular mineralization as observed in a rat reproduction study. Comparison of the critical effect level for repeated dose effects via inhalation and the conservative upper-bounding exposure estimate via inhalation for decamethylcyclotetrasiloxane, results in adequate margins of exposure. The critical effect level for repeated-dose toxicity was based on increased liver weight in a 90-day rat study and using as support, the determinations of oral critical effect levels for the similar compounds, octamethylcyclotetrasiloxane and dodecamethylcyclohexasiloxane. Based on an independent review of a refined exposure assessment for personal care products, an adequate margin of exposure was derived by comparison of the critical effect level for repeated dose effects via the oral route and a conservative upper-bounding estimate of daily intake of D5 via use of personal care products
Based on the available information on its potential to cause ecological harm, it is concluded that D5 is entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity.
Based on the available information on its potential to cause harm to human health, it is concluded that D5 is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
This substance will be included in the upcoming Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment and, where appropriate, the performance of potential control measures identified during the risk management phase.
Based on the information available, it is concluded that D5 meets one or more of the criteria set out in section 64 of the Canadian Environmental Protection Act, 1999.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance decamethylcyclopentasiloxane, also known as D5, was identified as a high priority for assessment of ecological risk as it was found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was issued in the Canada Gazette on May 12, 2007 (Canada 2007). A substance profile was released at the same time. The substance profile presented the technical ecological information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, more than 100 submissions of information were received for this substance pertaining to its physical and chemical properties, bioaccumulation potential, persistence, ecotoxicology, quantity in commerce and so on.
Although the categorization exercise did not determine D5 to be a priority for assessment with respect to risks to human health, it was recommended that a human health assessment also be conducted due to its structure and use pattern similarity to D4, also known as octamethylcyclotetrasiloxane, a high priority for assessment for both human health and ecological risks under CEPA 1999. Therefore, this assessment focuses on information relevant to the evaluation of ecological risks and risks to human health.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to August 2008 for both human health and ecological sections of the document. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessments from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight-of-evidence assessments of other agencies that were used for prioritization of the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. This assessment has undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from Toxicology Excellence for Risk Assessment (TERA). While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this document, decamethylcyclopentasiloxane will be referred to as D5, an abbreviated name derived from the siloxane notation developed by General Electric (Hurd 1946).
D5 belongs to a group of cyclic volatile methyl siloxanes (cVMS) with relatively low molecular weight < 600 g/mol) and high vapour pressure. These cVMS are volatile, low-viscosity silicone fluids consisting of [-Si(CH3)2O-]× structural units in a cyclic configuration. D5 consists of five of these [-Si(CH3)2O-] structural units (x = 5) as shown in the chemical structure below (Table 1).
| Chemical Abstracts Service Registry Number (CAS RN) | 541-02-6 |
|---|---|
| [1]National Chemical Inventories (NCI). 2006: AICS (Australian Inventory of Chemical Substances); ASIA-PAC (Asia-Pacific Substances Lists); ECL (Korean Existing Chemicals List); EINECS (European Inventory of Existing Commercial Chemical Substances); PICCS (Philippine Inventory of Chemicals and Chemical Substances); NZIoC New Zealand Inventory of Chemicals; and TSCA (Toxic Substances Control Act Chemical Substance Inventory). | |
| Name on Domestic Substances List (DSL) | Cyclopentasiloxane, decamethyl- |
| National Chemical Inventories(NCI) names[1] | Cyclopentasiloxane, 2,2,4,4,6,6,8,8,10,10-decamethyl- (TSCA); Cyclopentasiloxane, decamethyl- (AICS, PICCS, ASIA-PAC, NZIoC); Decamethylcyclopentasiloxane (EINECS); Decamethyl cyclopentasiloxane (ECL) |
| Other names | Cyclic dimethylsiloxane pentamer; Cyclo-decamethylpentasiloxane; D5; Dimethylsiloxane pentamer; Pentacyclomethicone |
| Major chemical class or use | Organosilicon compounds |
| Major chemical sub-class | Cyclic volatile methyl-siloxanes (cVMS) |
| Chemical formula | C10H30O5Si5 |
| Chemical structure |  |
| Simplified Molecular Input Line Entry System (SMILES) | C[Si]1(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O[Si](C)(C)O1 |
| Molecular mass | 370.78 g/mol |
It should be noted that D5 is also contained under another Chemical Abstracts Service Registry Number. This registry number, CAS RN 69430-24-6, refers to a mixture of dimethyl-substituted cyclosiloxanes of the general structure [-Si(CH3)2O-]x in a cyclic configuration, where × is generally less than 8, and more commonly × is 3-7 (SEHSC 2007b). This CAS number is associated with the following names: cyclopolydimethylsiloxane, cyclopolydimethylsiloxane (DX), cyclosiloxanes di-Me, dimethylcyclopolysiloxane, polydimethyl siloxy cyclics, polydimethylcyclosiloxane, cyclomethicone and mixed cyclosiloxane. In this report it will be referred to as cyclomethicone, a term commonly used for the mixture in the cosmetics industry.
For this assessment, data from analogue D4 has also been used based on structural similarity as shown in the table below:
| D4 | D5 |
|---|---|
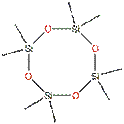 |
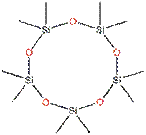 |
Table 2 contains experimental and modelled physical and chemical properties of D5 that are relevant to its environmental fate.
| Property | Type | Value[1] | Temperature (°C) | Reference |
|---|---|---|---|---|
| [1] If different, values and units in parentheses represent the original values as reported by the authors or as estimated by the models. [*] Values used in modelling for this screening assessment. |
||||
| Melting point (°C) |
Experimental | -38[*] | PhysProp 2006 | |
| Modelled | -5.19 | MPBPWIN 2000 | ||
| Boiling point (°C) |
Experimental | 210[*] | PhysProp 2006 | |
| Modelled | 196.78 | MPBPWIN 2000 | ||
| Density (kg/m3) |
Experimental | 954 | SEHSC 2005a | |
| Vapour pressure (Pa) |
Experimental | 26.66 (0.20 mm Hg) |
25 | Flaningam 1986 |
| 33.2[*] (0.249 mm Hg) |
25 | SEHSC 2005a | ||
| Modelled | 29.06 (0.22 mm Hg) |
25 | MPBPWIN 2000 | |
| Henry's Law constant (Pa·m3/mol) |
Experimental | 3 350 000[*] (33.1 atm·m3/mol) |
Calculated from Kaw value of Xu and Kropscott 2007 | |
| 13 444 (0.133 atm·m3/mol) |
23 | David et al. 2000 | ||
| 32 317 (0.319 atm·m3/mol) |
26 | Kochetkov et al.2001 | ||
| 29 831 (0.294 atm·m3/mol) |
26 | Kochetkov et al.2001 | ||
| Modelled | 12 159 (0.12 atm·m3/mol) |
25 | HENRYWIN 2000 | |
| Log Kaw (Air-water partition coefficient) (dimensionless) | Experimental | 3.13[*] | 24.6 | Xu and Kropscott 2007 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Experimental | 5.2 | Bruggeman et al. 1984 | |
| 8.03[*] | 25.3 | Kozerski 2007 | ||
| 4.76 | 22.4 | Sible 2006 | ||
| Modelled | 5.71 | KOWWIN 2000 | ||
| Log Koc (Organic carbon-water partition coefficient) (dimensionless) |
Experimental | 5.17 | SEHSC 2005a | |
| Modelled | 5.16 | PCKOCWIN 2000 | ||
| Water solubility (mg/L) |
Experimental | 0.017[*] | 23 | Varaprath et al. 1996 |
| Modelled | 0.05 | 25 | WSKOWWIN 2000 | |
| Log Koa (Octanol-air partition coefficient) (dimensionless) |
Experimental | 5.06[*] | 24 | Xu 2006 |
Recently, empirical log Kow values for D5 were received and were critically evaluated. A recent experimental log Kow value of 8.03 for D5 (99.19% purity) at 25.3°C was determined by using the slow stir method following OECD Draft Guideline 123 (Kozerski 2007). The measurement of Kowwas carried out in triplicate with one blank control. Two-litre borosilicate glass vessels were used in performing the equilibrium and were charged with 1.6 L high-purity water followed by adding 0.10 L of 1-octanol carefully to minimize droplet formation. The test was initiated by adding ~5 mL of D5 spiking solution (119.68 mg D5/g) in 1-octanol to the test vessel. The temperature was maintained at between 25.2 and 25.6°C during the study. It was concluded that equilibrium was achieved 24 hours after test initiation. The weighted average Kow was calculated to be 8.03. A headspace of ~0.3 L (15% total volume) was present in the test vessels, indicating that the D5 may have volatized into the headspace from the water phase (high vapour pressure, low water solubility). However, a flask mass balance check suggested that the total vaporized D5 was less than 2%. Therefore, the study is considered acceptable and the log Kow of 8.03 will be used for this screening assessment report.
The experimental log Kow of 4.76 was determined by using the slow stir method following OECD Draft Guideline 123 (Sible 2006). Radio-labelled 14C-D5 (99.8% radiochemical purity) diluted with unlabelled D5 (99.77%) was used in the test. The measurement of Kow was carried out in triplicate with one blank control. The temperature was maintained constant at 22.4 ± 0.5°C throughout the study. Equilibrium was achieved in 7 days and a log Kow of 4.76 was obtained based on the average measured log Kow from day 8 to day 11. The analysis of radio-labelled 14C-D5 in water and octanol was conducted by using liquid scintillation counting (LSC). LSC analysis does not distinguish between a parent compound and its potential hydrolysis products. Since D5 may undergo slow hydrolysis under the test condition (Durham 2006), the D5 concentration in water may have been overestimated, which may have resulted in underestimation of the log Kow value.
A log Kow value of 5.2 was determined by Bruggeman et al. (1984) using a high-performance liquid chromatography (HPLC)-retention time method. The measurement was performed on an octadecylsilyl-bonded silica column with 90:10 methanol:water as the mobile phase. Homologous series of n-alkylbenzenes with known log Kow values were used as reference compounds to calibrate the method. The experimental details of this study are currently unavailable.
Recent experiments on the air-water partition coefficient for D4 and D5 were conducted by Xu (Xu and Kropscott 2007). The partitioning equilibrium among air, water and an organic phase (octanol) was simultaneously achieved during the experiment; the log Koa was calculated to be 4.94 and the log Kow was calculated to be 8.07 for D5. Both values are reasonably consistent with the measured experimental values reported by Xu (2006, Table 2) and Kozerski (Kozerski 2007, Table 2). The study is therefore considered acceptable and the log Kaw of 3.13 for D5 from the study will be used for this screening assessment report. A simultaneous determination of the partitioning equilibrium among air, water, and organic phase (octanol) has been conducted by Xu (Xu and Kropscott 2007). A custom-made double syringe system was designed for measuring the partitioning equilibrium among the three phases. The system consists of two air-tight syringes with the left syringe containing ~5 mL octanol-saturated water, 14C-labelled D5 in octanol on top of the water phase, and a gas phase of ~70-80 cm3. The right syringe contained a ~60-80 mL octanol-saturated water phase and a ~20-40 cm3 air phase. The air phases between the two syringes were connected during the test. The equilibrium between the air and water phase was accelerated by the slow stirring of water and was reached after 20 hours. The average log Kaw of 3.13 for D5 at 24.6°C was thus determined by the total D5 radioactivity in air and water. This value is in good agreement with the equilibrium of log Kow = log Koa + log Kaw. The experimental Kaw gives a Henry's Law constant of 3 350 000 Pa·m3/mol at ~25°C.
For D5, other modelled physical and chemical properties are in good agreement with its measured experimental data. Except for the data discussed above, the most conservative experimental data, when applicable, are used in various model predictions in this screening assessment report.
There are no known natural sources of D5.
D5 is an industrial chemical which was not manufactured by any company in Canada in 2006 in a quantity above the reporting threshold of 100 kg, but which is imported into the country as an essentially pure substance, in mixtures with other cyclic siloxanes, as a residual in silicone polymers, and in finished consumer products. From responses to a notice published under section 71 of CEPA 1999, it was determined that between 1 000 000 and 10 000 000 kg of D5 were imported into Canada in 2006, as raw materials or in finished products (Environment Canada 2007). The quantities of D5 imported into Canada have increased significantly since the DSL nomination (Environment Canada 1988).
D5 is a constituent of CAS RN 69430-24-6, termed cyclomethicone in the cosmetics industry. Although cyclomethicone was not directly surveyed under CEPA section 71 by Environment Canada and Health Canada in 2007, it is evident that in some cases, responses to the notice published under section 71 of CEPA 1999 for the 2006 calendar year contained data on the quantity of D5 used or imported as CAS RN 69430-24-6 (Environment Canada 2007).
The quantity of CAS RN 69430-24-6 reported in commerce in Canada during the 1986 calendar year was 2 220 000 kg (Environment Canada 1988). In 2005, Canada was a net importer of 11 500 000 kg of all types of silicone polymers and siloxanes (Will et al. 2007).
D5 is an intermediate in the production of polydimethylsiloxanes (PDMS) silicone polymers, and all PDMS polymers contain residual amounts of volatile cyclosiloxanes, including D5. The lower molecular weight (and consequently lower viscosity) polymers may contain from < 0.1% to 0.5% volatile cyclosiloxanes, and higher molecular weight (and consequently higher viscosity) polymers may contain 1-3% volatile cyclosiloxanes. The proportion of the volatile cyclosiloxanes that consists of D5 is highly product-specific. Release of D5 from some applications of PDMS is expected to occur once the PDMS product is in use (SEHSC 2007b).
Detection of D5 at sewage treatment plants, landfills and near industrial plants, as well as in indoor and ambient air away from industrial activity, is evidence that both point sources and disperse sources contribute to the concentration of D5 in the environment (Norden 2005; Kaj et al. 2005; personal communication, Environment Canada, Canadian Centre for Inland Waters, 2007 [unreferenced]). The application of D5-containing pesticides on crops and the disposal of sewage sludge on agricultural lands, by incineration and by deposit in landfills will result in the release of D5 to environmental media. There is some evidence that D5 is a transient degradation product of PDMS in contact with soil, while the principal degradation products are silanols prior to complete mineralization (Herner et al. 2002). Thus, in addition to release of residual D5 from PDMS manufacture there may be de novosynthesis of D5 occurring in landfills and agricultural lands where sewage sludge containing PDMS is spread, although the overall contribution of PDMS degradation is not considered significant under environmental conditions.
D5 has been identified as a high production volume (HPV) chemical by the Organisation for Economic Co-operation and Development (OECD 2007), the US Environmental Protection Agency (US EPA 2007), and the European Chemicals Bureau (ECB 2007).
In the United States, there is a trend toward the increased use of volatile methyl siloxanes, including D5, because of their exemption from volatile organic compound (VOC) legislation in 1994 (US EPA 1994). Volatile methyl siloxanes were used as an alternative to chlorofluorocarbons (CFCs) as a means of reducing the regulated VOC content in products (specifically, precision and electronic cleaning applications). According to information from the US EPA, the import/production of D5 in the United States was in the range of 4500-22 500 tonnes in 1986, 1990 and 1994. The import/production increased to 22 500-45 000 tonnes in 1998, and to 45 000-225 000 tonnes in 2002.
In Europe, four companies have been identified as producers/importers of D5: Bayer AG of Germany, Dow Corning Limited of the United Kingdom, Rhodia Chimie of France and Amway Europe of Belgium (ECB 2007). The quantity of D5 used in the European Union as a site-limited intermediate during 2003-2004 is confidential information.
The predominant use, worldwide and in Canada, of D5 is in blending and formulating consumer products (Environment Canada 2007). D5 is also used as an intermediate in the production of PDMS polymers and a small number of commercial dry cleaners in Canada use D5 as a dry-cleaning fluid (Green Earth 2008). Also, all silicone polymers contain trace residual amounts of volatile cyclosiloxanes, including D5. As indicated above, D5 is also a constituent of CAS RN 69430-24-6, termed cyclomethicone in the cosmetics industry.
Cyclomethicone is a mixture of low molecular weight volatile cyclic siloxanes, the principal ingredients of which are octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5) and dodecamethylcyclohexasiloxane (D6), in varying proportions. In Canada, the most important uses of the mixtures of low molecular weight volatile cyclic siloxanes, which may contain a high percentage of D4 or of D5, are in the preparation of personal care products, including hair and skin care products and antiperspirants (Environment Canada 2007).
Silicone polymers that contain trace amounts of D5 can be grouped as fluids, gums and resins. Uses of such polymers are described below.
Important uses of silicone fluids include as a formulation component of personal care products for hair and skin care, antiperspirants and deodorants; pharmaceuticals; processing aids such as defoamers; surfactants and mould release agents; lubricants; polishes and coatings on a range of substrates including textiles, carpeting and paper; sealants; architectural coatings; mechanical, heat transfer and dielectric fluids; and reprography (Will et al. 2007).
While it is anticipated that higher molecular weight polymers are used in most of these applications, D5 was reported for use as a defoamer (Environment Canada 2007). Defoamers are employed often at parts per million levels in a range of processing industries including pulp and paper, food, petrochemical, petroleum, and chemical manufacture as well as water treatment. Silicones are also used as defoamers in household products such as cleaners and detergents (Will et al. 2007).
The use of silicone formulants containing D5 in certain pesticide products is regulated in Canada under the Pest Control Products Act (PMRA 2007).
Silicone fluid/gel mixtures are used use for several types of cosmetics and medical devices. Silicone fluids have been approved as active and non-active ingredients in pharmaceuticals in Canada (DPD 2007), the most common use being in anti-flatulence drugs. Other biomedical uses of silicone fluids in Canada are in blood-handling equipment, as a blood defoaming agent, as protective barriers and lubricants, and for surface treatment of wound dressings.
Silicone gums are used in the production of elastomers that are used as sealants and adhesives, and in moulded silicone rubber, coatings and encapsulation. Silicone elastomers are used in the manufacture of consumer products such as pacifiers. Silicone elastomers are also used in a large number of biomedical applications including short- and long-term implants and prostheses, catheters, contact lenses and dentures (Will et al. 2007).
Silicone resins are primarily used in specialty coatings applications, and in the production of silicone-modified polymers (Will et al. 2007). Consumers may be exposed to D5 through the use of these products and by occupying enclosed spaces where coatings, caulking, sealants and silicone rubber are used as building materials or are present in consumer products.
D5 is not reported as part of Environment Canada's National Pollutant Release Inventory. This substance belongs to a chemical group used in various industry and consumer applications that are associated with the potential for widespread releases.
D5 may be emitted to the environment from industrial processes in which it is reacted to form silicone polymers and co-polymers and from blending, formulation and packaging operations. All of these operations take place in Canada (Environment Canada 2007). Industrial releases of D5 may also occur when silicone polymers are used in process industries as foam control agents, as mould release agents, as lubricants, and in other applications (Environment Canada 2007). The use of D5 as a commercial dry-cleaning fluid (even though its current scale of use indicates that its contribution is not significant) and as an industrial degreaser may lead to atmospheric releases. The releases from industrial processes are expected to be to the atmosphere and wastewater. D5 will be released during the use of personal care products such as hair and skin care products, antiperspirants and others, and these releases will be to air and wastewater. It is estimated that more than 90% of D5 used in personal care products enters the atmosphere (Allen et al. 1997). The releases of D5 to sewage treatment plants as wastewater can lead to its association with sludges that may then be sent to landfills, incinerated or be applied to agricultural soils as soil enrichment. Disposal of consumer and industrial products containing D5 can also lead to the transfer of D5 to landfills.
Detection of D5 at sewage treatment plants, landfills and near industrial plants as well as in indoor and ambient air away from industrial activity is evidence that both point sources and disperse sources contribute to the concentration of D5 in the environment (Norden 2005, Kaj et al. 2005). The application of D5-containing pesticides of crops and the disposal of sewage sludge on agricultural lands, by incineration and by deposit in landfills will result in the release of D5 to environmental media. There is some evidence that D5 is a transient degradation product of PDMS in contact with soil, while the principal degradation products are silanols (Xu et al.1998). Thus, in addition to release of residual D5 from PDMS manufacture, there may be de novo synthesis of D5 occurring in landfills and agricultural lands where sewage sludge containing PDMS is spread, although the overall contribution of PDMS degradation is not considered significant under environmental conditions.
Mass Flow Tool
To estimate the potential release of D5 to the environment at different stages of its life cycle, a Mass Flow Tool was used. Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of release to the different environmental media are estimated, as are the proportions of the substance chemically transformed or sent for waste disposal. Assumptions and input parameters used in making these estimates are based on information obtained from a variety of sources including responses to regulatory surveys, Statistics Canada, manufacturers' websites and technical databases. Of particular relevance are emission factors, which are generally expressed as the fraction of a substance released to the environment, particularly during its manufacture, transformation, and use associated with industrial processes. Sources of such information include emission scenario documents, often developed under the auspices of the Organisation for Economic Co-operation and Development (OECD), and default assumptions used by different international chemical regulatory agencies. It is noted that the level of uncertainty in the mass of substance and quantity released to the environment generally increases further down the life cycle.
| Fate | Proportion of the mass (%)[1] | Major life cycle stage involved[2] |
|---|---|---|
| [*]Wastewater before any form of treatment [1] For D5, information from the following OECD emission scenario documents was used to estimate releases to the environment and distribution of the substance as summarized in this table: OECD 2004; OECD 2006. Values presented for releases to environmental media do not account for possible mitigation measures that may be in place in some locations (e.g., partial removal by sewage treatment plants). Specific assumptions used in the derivation of these estimates are summarized in Environment Canada 2008a. [2] Applicable stage(s): production, formulation, industrial use, consumer use, service life of article/product, waste disposal. |
||
| Releases to environment: | ||
| To soil | 0 | - |
| To air | 77.5 | Industrial use and consumer use |
| To sewer[*] | 12.2 | Formulation, industrial use and consumer use |
| Chemically transformed | 0.8 | Industrial use |
| Transferred to waste disposal sites (e.g., landfill, incineration) | 9.5 | Waste disposal |
Based on the information available, the substance is mainly released to air and wastewater (sewer). Air receives the highest proportion of releases, approximately 77.5%. This is a result of the use of consumer products such as skin creams, sun creams or polishes (SPIN 2007). Releases to wastewater are estimated to be approximately 12.2% from industry use during product formulation and from the use of personal care products. Another 9.5% of the substance is transferred for waste disposal.
Based on its physical and chemical properties (Table 2) and the results of Level III fugacity modelling (Table 4; model input parameters are listed in Appendix 5 of this screening assessment), D5 may partition in significant quantities to any environmental medium, depending on the compartment of release.
| Substance released to | Fraction of substance partitioning into each compartment | ||||
|---|---|---|---|---|---|
| Air | Water | Soil | Sediment | ||
| Air (100%) | 100 | 0.0 | 0.0 | 0.0 | |
| Water (100%) | 4.4 | 30.9 | 0.0 | 64.7 | |
| Soil (100%) | 71.2 | 0.0 | 28.7 | 0.0 | |
Based on the information available (Table 3), the environmental release of D5 is estimated to be mainly to air. A vapour pressure of 26.66-33.2 Pa and a Henry's Law constant of 3 350 000 Pa·m3/mol, as well as a long half-life in air, indicate that when D5 is released to air it will remain there (100%) (Table 4).
When D5 is released to water (Table 4), it is expected to adsorb to suspended solids, such as sewage sludge and sediments, based on its high empirical log Koc values (> 5). Results of the Level III fugacity simulation for release to water show that approximately 64.7% will reside in the solid phase (suspended sediment and bed sediments) and 30.9% will reside in the aqueous phase (water column). The log Koc for the compound is in the high sorption range, and the moderate hydrolysis rate of D5 in water at ambient temperature reduces the fraction that is expected to be adsorbed to sediments. Volatilization from water surfaces is also expected based upon the air-water partition coefficient (Kaw); the mass fraction expected to partition to air from volatilization at 25oC is 4.4% (Table 4).
When D5 is released to moist soils through, for example, the application of sewage sludge on agricultural lands, ~71% of the mass fraction will partition to air. This estimate is consistent with the observation by Xu (1999) that volatilization is the major loss process of cyclic siloxanes from moist soils. Approximately 28.7% will remain in soil associated with solids, for the same reasons as described for sediment. In dry soil, D5 is expected to be quickly hydrolyzed by clay minerals to form dimethylsilanediol as the final breakdown substance (Xu 1999, Xu and Chandra 1999).
Environmental Persistence
Atmospheric Degradation
The Level III fugacity model results indicate that D5, when released to air, will remain in air, where it is expected to be slowly oxidized by the gas-phase reaction with photochemically produced hydroxyl (OH) radicals. The empirically derived half-life for D5 in the gas-phase hydroxyl radical reaction is 6.9 days (Atkinson 1989; see Table 5a). This is based on an experimental reaction rate of 1.55 × 10-12 cm3/mol.sec (Atkinson 1989), which may be converted to an estimated half-life of 6.9 days, assuming first-order kinetics, a 12-hour day, and 1.5 × 106 OH/cm3. D5 is not expected to react, or react appreciably, with other photo-oxidative species in the atmosphere, such as O3, nor is it likely to degrade via direct photolysis (Atkinson 1991). Therefore, it is expected that reactions with hydroxyl radicals will be the most important fate process in the atmosphere for this substance.
Recent field measurements of the hydroxyl radical concentrations in an urban environment (Ren et al. 2003, Kramp and Volz-Thomas 1997, Rivett et al. 2003) suggested that there is a higher OH concentration in the urban atmosphere than that observed in the rural and marine atmosphere due to higher OH radical precursors in polluted urban areas (SEHSC 2008b). Ren et al. (2003) have measured the concentration of hydroxyl radical in the summer atmosphere in New York City, NY, USA. The measurement was conducted over a 34-day period. The average maximum hydroxyl radical concentration was reported to be 7 × 106 OH/cm3 and was comparable to those measured (1-10 × 106 OH/cm3) in similar urban environments of the United States (Los Angeles, CA and Nashville, TN; SEHSC 2008b) and in European countries (Kramp and Volz-Thomas 1997, Rivett et al. 2003). However, most of these measurements were carried out during the summer, when the sunlight was strong and the atmospheric photochemistry was active. The OH radical concentration measured by Ren et al. (2006) in the winter in New York City was ~5 times lower than in the summer at the same site. The measurement was conducted over a 28-day period and the average maximum concentration was 1.4 × 106 OH/cm3. Therefore, half-lives of 3.0 to 14.8 days can be calculated assuming first-order kinetics, a 12-hour day, and a daily average hydroxyl concentration of 3.5 × 106 OH/cm3 and 0.7 × 106 OH/cm3 (daily average concentration = maximum concentration/2), in summer and winter respectively. It is therefore concluded that D5 could be degraded more rapidly in urban centers in summer seasons when the atmospheric hydroxyl radicals are most abundant. However, when a yearly average removal half-life is considered, it is consistent with the half-life of 6.9 days estimated with a hydroxyl radical concentration of 1.5 × 106 OH/cm3. The degradation half-life of 6.9 days is considered critical and will be used for D5 in environmental fate modelling.
Navea et al. (2007) have investigated the effects of ozone, aerosols and solar radiation on the fate of D4 and D5 in a simulated environment chamber. They concluded that mineral aerosols such as kaolinite and hematite can significantly accelerate the removal of D4 and D5 from the gas phase of the atmosphere, especially under daytime conditions. The finding also indicated that ozone can further accelerate these removal processes for D4 and D5. Although obtained data suggested that mineral aerosols, combined with ozone, may have significant effects on the environmental fate of cVMS in the air, it is difficult to quantitatively extrapolate the results of the simulations to realistic environmental conditions. First, it should be noted that the study was conducted under unrealistically high concentrations of cVMS, mineral aerosols and ozone. Second, mineral and carbon black samples used in the study were high-purity (>99%) analytical samples that provided maximum surface area and thus maximally available sorption sites, i.e., ideal conditions for D4/D5 absorption. The degree to which these pure minerals are representative of the particulate matter in air is questionable. Third, it is reasonable to believe that minerals such as kaolinite and hematite can be found in atmospheric particular matter (PM); however, they are unlikely to be the most common and abundant components in atmospheric dust. In addition, it should be mentioned that the study was conducted in a simulated environment chamber and involved reacting the mineral aerosols with only one cVMS (D4 or D5) at a time. Under actual environmental conditions, thousands of chemicals compete for aerosols' adsorption sites. Therefore, in such conditions, the "effectiveness" of D4/D5 removal from the ambient air could be significantly lower than that observed in the chamber's mono-component atmosphere.
Thus, it may be concluded that the degree to which aerosols and ozone accelerate the degradation of cVMS in air under realistic environmental conditions is uncertain.
The AOPWIN (2000) model (Table 5b) also provides evidence indicating the potential for persistence of this substance, with a predicted atmospheric oxidation half-life of 3-15 days. Thus, the empirical and model data demonstrate that this substance is persistent in air (half-life > 2 days) in accordance with the Persistence and Bioaccumulation Regulations (Canada 2000).
| Medium | Fate process | Degradation value | Degradation endpoint /Units | Reference |
|---|---|---|---|---|
| Air | OH reaction | 6.9 | Half-life (days) | Atkinson 1989 |
| Air | OH reaction | 3.0-14.8 | Half-life (days) | Ren et al. 2003, 2006 |
| Water | Biodegradation | 0.14% | 28 d degradation | Springborn Smithers Laboratories 2004 |
| Water | Hydrolysis | 0.9-733 | Half-life (days) pH 6-9 5-25°C |
Durham 2006 Kozerski 2008 Bidleman 2008 |
| Water/sediments | Abiotic degradation | 49-588 | Read-across half-life (days) from D4 neutral pH; 5-25°C |
Xu and Miller 2008 |
| Soil (Wahiawa soils from Hawaii) | Clay-catalyzed hydrolysis | ~2 hours (32% relative humidity) |
Half-life (hours) | Xu 1999 Xu and Chandra 1999 |
| Soil (Londo soils from Michigan) | Clay-catalyzed hydrolysis | 3.54 days (32% relative humidity); 5.25 days (93% relative humidity) |
Read-across half-life (days) from D4 | Xu and Chandra 1999 |
| Medium | Fate process | Degradation value | Degradation endpoint / Units | Model |
|---|---|---|---|---|
| [1] Results from CATABOL biodegradation simulation show that D5 is in the global parameter domain and metabolic domain, but out of the structural domain. The most important of these domains is the metabolic domain, and CATABOL suggests that the substance will not be degraded, as the probability of stable methyl group and aromatic ring oxidation products is low. [2] Atmospheric oxidation half-lives re-calculated with measured OH radical concentrations from New York City in summer and winter, respectively. |
||||
| Air | Atmospheric oxidation | 7.15 | Half-life (days) | AOPWIN 2000 |
| Air | Atmospheric oxidation | 3.1-15.3 [2] | Half-life (days) | AOPWIN 2000 |
| Water | Biodegradation | 37.5 | Half-life (days) | BIOWIN 2000 Ultimate survey |
| Water | Biodegradation | 0 (does not biodegrade fast) | Probability | BIOWIN 2000, MITI Linear probability |
| Water | Biodegradation | 0.0003 (does not biodegrade fast) | Probability | BIOWIN 2000, MITI Non-linear probability |
| Water | Biodegradation | 2.3 | Percent biochemical oxygen demand (BOD) (MITI 301C)[1] | CATABOL c2004-2008 |
| Soil | Biodegradation (anaerobic) | 0.04 (does not biodegrade fast) | Probability | BIOWIN 2000 |
Degradation in Water and Sediment
The empirical hydrolysis data for D5 (Durham 2006) were critically reviewed by internal experts (Bidleman 2008); the results of these reviews are summarized below. The hydrolysis kinetics of D5 were determined by measuring the disappearance of radio-labelled 14C-decamethylcyclopentasiloxane (D5, substance purity 98.9%) and the formation of hydrolysis products as a function of time based on OECD Guideline 111. Reactions were investigated in flame-sealed borosilicate glass tubes at pH values of 4, 5.5, 7, 8 and 9 and temperatures of 10°C, 25°C and 35°C. The initial test concentration was targeted at 6 µg/L upon spiking, corresponding to 1/2-1/3 of the water solubility of D5. Tetrahydrofuran (THF) was used as a solubilizer at a concentration of less than 1% v/v. A similar hydrolysis kinetics study was also conducted for radio labelled 14C-octamethylcyclotetrasiloxane (D4) at the same laboratory (Durham and Kozerski 2005). The hydrolysis rates of D4 and D5 were reported to be pH-dependent and followed pseudo first-order kinetics. Both D4 and D5 were found to undergo rapid hydrolysis under acidic (pH 4) and basic (pH 9) conditions, with average half-lives (t½) ranging from minutes to less than 6.5 hours for D4 and from hours to less than 6 days for D5 at 10-35°C. Two additional hydrolysis tests were performed for D5 at near-neutral (pH 5.5 and 8) conditions at 25°C and their t½ were approximately 15 and 9 days, respectively. The half-lives at neutral pH 7 conditions increased significantly for D4 and D5. The t½ for D4 at 10-25°C was calculated to be 3.3-23 days. The t½ for D5 at 10-25°C was calculated to be approximately 74-416 days. The hydrolysis products were intermediates dimethylsiloxane-alpha, and omega-diol oligomers HO(Me2SiO)nH (n=2-4 or 5), while dimethylsilanediol (DMSD) was the final hydrolysis product. Although loss of parent compounds and poor reproducibility were reported at neutral pH, loss rates at neutral pH may be estimated using the second-order rate constants for the acid- and base-catalyzed reactions. The hydrolysis studies for D4 and D5 are thus considered reliable for this screening assessment. An error in the calculation of the hydroxide catalytic rate constant, kOH, at temperatures other than 25°C has, however, been identified by Bidleman (2008). Table 6 lists revised second-order rate constants for hydronium and hydroxide-catalyzed hydrolysis of D5 (Kozerski 2008).
| Rate constant (M-1h-1) | Temperature (°C) | |||
|---|---|---|---|---|
| 5 | 10 | 25 | 35 | |
| kH | 141 | 210 | 742 | 1600 |
| kOH | 1360 | 1730 | 3170 | 5020 |
The pseudo first-order rate constants, kobs, for the hydrolysis of D5 can be calculated using the following kinetic equation (assuming negligible contribution of uncatalyzed hydrolysis, as confirmed by the experiments at a pH of 7):
kobs = kH+[H+] + kOH-[OH-]
The calculated half-lives for D5 (Table 7) under realistic Canadian environmental conditions (pH 6-9, temperature 5-25°C) (GEMStat 2008, NOAA 2008) are in the range of 0.9-733 days.
| Temperature (°C) | Water dissociation constant pKw | pH | Rate constant k (h-1) |
Half-life (days) |
|---|---|---|---|---|
| 25 | 14 | 6 | 7.74E-04 | 37 |
| 7 | 3.91E-04 | 74 | ||
| 8 | 3.18E-03 | 9.1 | ||
| 9 | 3.17E-02 | 0.9 | ||
| 10 | 14.52 | 6 | 2.15E-04 | 134 |
| 7 | 7.32E-05 | 395 | ||
| 8 | 5.24E-04 | 55 | ||
| 9 | 5.22E-03 | 5.5 | ||
| 5 | 14.73 | 6 | 1.44E-04 | 201 |
| 7 | 3.94E-05 | 733 | ||
| 8 | 2.54E-04 | 114 | ||
| 9 | 2.53E-03 | 11 |
New information received on microbial degradation indicates that D5 is not likely to be biodegraded in water. The 28-day ready-biodegradability test was performed in sealed vessels in accordance with OECD Draft Guideline 310 and data showed limited biodegradation (0.14%) of D5 over 28 days in a ready-biodegradation test (Springborn Smithers Laboratories 2004). These data are further supported by two of the models in Table 5b. These models indicate that the probability of biodegradation of D5 in water is effectively zero. Also, BIOWIN reported an overall weighted conclusion of "not readily biodegradable" based on the combined results of the BIOWIN3 and BIOWIN5 models.
Experimental and modelled biodegradation data indicate that D5 has little potential to biodegrade in aqueous environments. Therefore, hydrolysis is the major degradation process for D5 in water. The evidence suggests that D5 may be degraded relatively rapidly by hydrolysis when the water temperature is elevated. However, D5 is expected to persist for relatively long periods at low temperatures and neutral or slightly acidic water conditions in Canada (pH 6-7, temperature 5-10°C). It is therefore concluded that D5 meets the criterion of persistence in water (t½ > 182 days) under the Persistence and Bioaccumulation Regulations (Canada 2000).
No sediment degradation information was available for D5. However, a preliminary degradation study for D4 in a water/sediment system has been received recently (Xu and Miller 2008). A modified OECD 308 guideline was followed. The study was conducted at ambient temperature (22-25°C) with natural sediment (sandy silt, high OC content, ~70% water content and 11% organic matter, pH ~7) and water collected from deep under an uncontaminated lake. Radio-labelled D4 in diethylene glycol methyl ether was added via syringe at 10-15 spots on the surface sediment in each flask after the overlying water was carefully removed. Overlying water was again added onto the spiked sediment with minimum disturbance of sediment. Spiking of sediment instead of water ensured the substance's distribution in sediment. This properly addressed the substance's specific physical and chemical properties (high volatility and potential hydrolysis) and improved the reproducibility of the study. The concentrations of D4 measured from day 6 to day 22 (test termination) indicated that a steady state had been reached between water and sediment, with more than 95% D4 and radioactivity being detected in sediment. As demonstrated in the hydrolysis study of D4, the degradation products in sediment/water were intermediate diol oligomers HO(Me2SiO)nH (n=2-4), while dimethylsilanediol (DMSD) was the final degradation product. The calculated half-life for D4 degradation in sediment was 49 days at 22-25°C. The same degradation products observed in the study and in the hydrolysis study of D4 suggested that hydrolysis was the major degradation process in the sediment/water system. The major uncertainty in the study is the lack of test replicates. Since no data were available for degradation half-lives at lower temperatures, a read-across approach using the D4 hydrolysis data was applied. The hydrolysis half-lives of D4 in water were ~6-12 times longer when water temperatures were decreased to 5-10°C from 25°C. Assuming a similar trend of decreases in sediment, the estimated half-lives for D4 in sediment are 294 and 588 days, at 10°C and 5°C, respectively.
The sediment degradation half-lives of D4 are considered as read-across for D5 based on the similarity of the two substances. It is therefore concluded that the lack of fast degradation of D5 under some environmental conditions, especially in colder Canadian environments, will result in a half-life in sediment of t½ > 365 days. D5 is thus considered persistent in water and sediment in accordance with criteria defined under the Persistence and Bioaccumulation Regulations (Canada 2000). The extrapolation from the D4 sediment degradation at lower temperatures based on hydrolysis, however, is not without uncertainty. The assumption that the loss of D4 in sediments is solely a function of hydrolysis in the pore water does not take into account the fraction that may be sorbed to the solid phase–the degradation processes and rate of which are unknown.
Degradation in Soil
Although no empirical data on biodegradation in soils are available, biodegradation of D5 in water is negligible as noted above, based on the ready-biodegradation test (Springborn Smithers Laboratories 2004) and predictions of five of the six biodegradation models (Table 5b).
Xu (Xu 1999, Xu and Chandra 1999) has extensively investigated potential degradation pathways of cyclosiloxanes, including D4, D5 and D6, in Wahiawa soil from Hawaii at room temperature and 32% relative humidity. He concluded that the ring-opening polymerization reaction to form polydimethylsiloxane (PDMS) and the demethylation for cyclosiloxanes were insignificant in soils at concentrations of < 200 mg/kg dry weight. Clay-catalyzed hydrolysis of D5 was observed in highly weathered Wahiawa soils under dry soil conditions. It was suggested that the dryness of soil severely limits biological activity but promotes abiotic reactions such as surface-acid-catalyzed hydrolysis of PDMS, a polymer with the same dimethylsiloxane backbone as cyclosiloxanes (Xu 1999). The degradation rates of cyclosiloxanes were determined by soil moisture, clay type and clay content, as well as the size of the siloxane molecules that determine the rate of diffusion to the surface catalytic sites. The hydrolysis degradation half-life of D5 on Wahiawa soil (55% clay content, 2.1% water content) was approximately 2 hours at 22°C under dry soil conditions. The degradation rate of D5 was comparable to that of D4 under the same soil conditions (~1 hour half-life) and supported the statement that degradation rates among the three cyclosiloxanes were expected to decrease as the molecular weight increases: D4 > D5 >> D6. The hydrolysis rate of D5 in temperate Londo soils from Michigan (22% clay content) was not reported. The degradation half-lives of D4 in Londo soil were 3.54-5.25 days at relative humidity of less than 93%. Comparable degradation half-lives would be expected for D5 in the temperate Londo soil. The weight of evidence suggests that D5 will undergo clay-catalyzed hydrolysis and is not likely to be persistent under dry soil conditions. The lack of hydrolysis degradation of D4 in water-saturated Londo soil also suggests that degradation of D5 is negligible under the same conditions. Volatilization becomes the major loss mechanism for D5 under such soil conditions in an open system.
While investigating the influence of clay types on the degradation potential of polydimethylsiloxanes (PDMS), Xu et al. (1998) demonstrated that PDMS were degraded by clay minerals even though their catalytic activities varied. The widespread presence of these clay minerals suggests that D5 will undergo clay-catalyzed degradation in soil as long as critical soil conditions such as low moisture content are present, despite the tremendous diversity of Canadian soils.
Based on the available empirical studies that show the potential for rapid clay-catalyzed hydrolysis in surface soils, D5 is not considered persistent in soil since its degradation half-life is less than the criterion of t½ > 182 days stated in the Persistence and Bioaccumulation Regulations (Canada 2000).
The available empirical and modelled data indicate that D5 meets the persistence criteria for air (half-life ≥ 2 days), water (half life ≥ 182 days) and sediments (half-life ≥ 365 days) as set out in the Persistence and Bioaccumulation Regulations(Canada 2000), but that it does not meet the half-life criterion for soil (i.e., its half-life is < 182 days).
Long-range Transport Potential
The Transport and Persistence Level III model (TaPL3 2000), a regional model, was used to estimate the characteristic travel distance (CTD) of D5. CTD is defined as the maximum distance travelled in air by 63% of the substance. Beyer et al.(2000) have proposed that CTDs of > 2000 km represent high long-range atmospheric transport potential (LRATP), those of 700-2000 km represent moderate LRATP, and those of <700 km represent low LRATP. Based on the CTD estimate of 3447 km, the long-range atmospheric transport potential of D5 is judged to be high. This means that D5 is subject to atmospheric transport to remote regions such as the Arctic.
| Characteristic travel distance | Model (reference) |
|---|---|
| 3447 km | TaPL3 v. 2.10 (TaPL3 2000) |
| 3438 km | OECD LRTP POPs Tool v.2.0 (Scheringer et al.2006) |
The OECD POPs Screening Model can be used to help identify chemicals with high persistence and long-range transport potential (Scheringer et al. 2006). The OECD model is a global model which compartmentalizes the earth into air, water and soil. This model is "transport-oriented" rather than "target-oriented," as it simply identifies the CTD without indicating specifically where a substance may be transported (Fenner et al. 2005). Klasmeier et al. (2006) have suggested that a threshold of 5098 km, based on the model's CTD estimate for 2,2',3',4,4',5,5'-Heptachlorobiphenyl (PCB-180), can be used to identify substances with high long-range transport potential. PCB-180 has been found in remote regions. The CTD calculated for D5 using the OECD model is 3438 km, indicating that D5 still has a significant potential for transport in air, but is below the boundary suggested for global pollutants by Klasmeier et al. (2006).
The OECD POPs Screening Model also calculates the transfer efficiency (TE), which is the percentage of emission flux to air that is deposited to the surface (water and soil) in a remote region (TE = D/E × 100, where E is the emission flux to air and D is the deposition flux to surface media in a target region). The TE for D5 was calculated to be 1.9E-06%, which is well below the boundary of 4.65E-04% (2,4,4'-trichlorobiphenyl, or PCB-28) established for the model's reference substances that are empirically known to be deposited from air to soil or water. The low TE means that D5 has the potential for long-range travel in the atmosphere without being deposited to Earth's surface in any particular remote region. In addition, the log Koa and log Kaw of D5 suggest that it will also have a low Arctic contamination potential (ACP) when examined using chemical partitioning space plots as described by Wania (2003, 2006).
A preliminary monitoring study of a remote ecosystem was conducted in Lake Opeongo, the largest lake in Algonquin Provincial Park, Ontario, Canada. The lake is relatively remote from potential sources of cVMS from sewage and runoff. Therefore, it was assumed that the only significant source of cVMS to the lake would be from atmosphere deposition (Powell 2008). Preliminary analysis of sediments and zooplankton samples for cVMS found no D5, suggesting that atmospheric deposition is not a significant source of D5 to Lake Opeongo.
Limits of detection were 15.9 ng (background corrected mass) for both sediments and zooplankton.
It is therefore concluded that D5 has the potential to be transported over long distances in the atmosphere. However, the modelled TE for D5 is low, which suggests that it lacks the potential to be deposited in water or soil in remote regions. The monitoring results of Lake Opeongo also supported the low atmospheric deposition potential for D5. It is expected that airborne D5 will be eventually degraded by hydroxyl radicals in air.
Potential for Bioaccumulation
The empirical and modelled log Kow values for D5 (Table 2) suggest that this substance has the potential to bioaccumulate in biota.
In the Aquatic Compartment
New empirical data indicate that D5 has the ability to bioconcentrate in aquatic organisms. A bioconcentration study for D5 was conducted on fathead minnows (Pimephales promelas) in a flow-through system (Drottar 2005). The uptake of radio-labelled [14C] D5 in fish tissue was investigated at concentrations of 1.1 µg/L and 15 µg/L (measured) over a 35-day period and depuration was monitored over a 70-day period. The mean steady-state bioconcentration factor (BCFss) was calculated to be 7060 L/kg based on concentrations measured from day 14 to day 35. The kinetic bioconcentration factor (BCFk) was calculated to be 13 300 L/kg based on the uptake and depuration rates (k1/k2) for the 1.1 µg/L treatment groups (Table 7a). Fish tissue analysis also indicated that the depuration half-life for radio-labelled D5 was 24 days. There is uncertainty as to whether steady-state was reached on day 14 of the uptake phase as claimed. While no statistically significant differences were noted in the concentration of D5 in fish for days 14, 21, 28 and 35, the data was variable and the average concentrations continued to increase with time. This, plus the inconsistency noted between the kinetic and steady-state BCF (~2 times) and the long depuration half-life suggests that steady-state may not have been reached on day 14. Therefore, the kinetic BCFk of 13 300 L/kg is considered more reliable in this screening assessment report.
A separate study investigating the metabolic rate of radio-labelled [14C] D5 in rainbow trout (Oncorhynchus mykiss) was conducted through oral administration (Springer 2007). A nominal dose of 15 mg D5/kg (9.4-13 mg D5/kg measured) body weight of [14C] D5 (radio purity 97.6%) in corn oil was fed to three mature trout over a 96-hour period. The majority of metabolites in fish were found in bile, with < 1% of radioactivity contributed by the parent compound, while 56%, 60% and 76% of radioactivity in egg sacs, liver and digestive tract was attributed to parent D5, respectively. Insignificant metabolism was found in fish fat and blood. The administered D5 was eliminated through urine and feces. The calculated metabolism in trout over the 96-hour period was estimated to be 14% based on the total metabolism in µg (tissues, bile and urine) divided by the total µg equivalents recovered in carcass, tissues (including bile) and urine. The calculated metabolic rate constant, kM, determined from this study was 0.166 day-1 (personal communication, Environment Canada, Dow Cornind Corporation, Health and Environmental Sciences, 2008, unreferenced). However, it is not clear how the metabolic rate was calculated since the calculated metabolic rate is equivalent to a 4.2-day total metabolism, while the total metabolism observed in the study was 14% in 4 days.
Experimental data have also been received on a dietary bioaccumulation study in aquatic organisms (Drottar 2006; see Table 9a of this assessment). A dietary bioaccumulation study of 14C-decamethylcyclopentasiloxane (radiochemical purity 98.7%, 6.09 mCi/g) in rainbow trout (Oncorhynchus mykiss) was carried out in a flow-through system (Drottar 2007) for 35 days, followed by 42 days of depuration. Fish were fed trout chow dosed with an average measured parent concentration of 458 µg/g. The higher feeding rate was modified to provide better instrument detection and is considered justified. Although the temperature dropped below 8.6°C (value recommended in protocol is 12 ± 2°C) on day 15 of the test and several measurements of dissolved oxygen were below 60%, no adverse effects on the fish were observed throughout the study. The entire extracted radioactivity from fish tissue was identified as parent D5, while unknown metabolites were detected in fish liver and digestive tract. This result indicates that D5 had been metabolized as observed in the D5 fish BCF (Drottar 2005) and metabolic studies (Springer 2007), even though the metabolic rates of D5 are not considered significant (<17% D5 was metabolized). The calculated elimination rate constant indicated that a period of 57 days would be required to achieve 90% of steady state instead of the 35 day uptake in the test. Therefore, a fish residue at day 57 uptake was extrapolated and the corrected fish biomagnifications factor, BMF (lipid-normalized), was 0.82. The kinetic BMF was calculated by a model accounting for fish growth rates during the uptake and the depuration phases of the study, the amount of D5 in the fish over time, the mass of the fish over time, as well as the food consumption rate. The metabolic rate constant, kM, was assumed to be zero. Fish growth rates were calculated by using linear expression (Domoradzki 2008a, 2008b; SEHSC 2008c). The resultant kinetic BMF (lipid-normalized) was 0.91. The BMF values agreed reasonably well. It is therefore considered that D5 did not show biomagnification potential in the laboratory fish dietary study.
| Test organism | Endpoint[1] | Value | Reference |
|---|---|---|---|
| [1] BCFss: steady-state bioconcentration factor; BCFk: kinetic bioconcentration factor; BMF: biomagnification factor; BSAF: biota-sediment accumulation factor | |||
| Pimephales promelas (fathead minnow) | BCFss | 7060 L/kg wet wt | Drottar 2005 |
| Pimephales promelas (fathead minnow) |
BCFk | 13 300 L/kg wet wt | Drottar 2005 |
| Oncorhynchus mykiss (rainbow trout) | BMF | 0.82-0.91 (lipid normalized) | Drottar 2006; Domoradzki 2008a, 2008b; SEHSC 2008c |
| Chironomus riparius(midge) | BSAF | 0.46-1.2 | Springborn Smithers Laboratories 2003b |
The Arnot-Gobas model (Arnot and Gobas 2003) can be used to predict the bioaccumulation factor (BAF) of this substance, while taking into account any potential metabolism using a metabolic rate constant (kM). The available BCF and BMF in vivo tests data were used to derive an in vivo-based metabolic rate constant according to the method of Arnot et al. (2008a). In this method, kM is derived according to the following equation:
kM = (k1f/BCF) - (k2 + kE + kG) (1)
where
kM = the metabolic rate constant (1/days)
k1 = the uptake rate constant (Arnot and Gobas 2003)
f = fraction of freely dissolved chemical in water (Arnot and Gobas 2003)
BCF = the available empirical bioconcentration factor
k2 = the elimination rate constant (Arnot and Gobas 2003)
kE = fecal egestion rate constant (Arnot and Gobas 2003)
kG = growth rate constant (Arnot and Gobas 2003)
The method of Arnot et al. (2008a) provides for the estimation of confidence factors (CF) for the kM to account for error associated with the in vivo data (i.e., measurement variability, parameter estimation uncertainty and model error). A CF of ± 2.6 was calculated for the available BCF and BMF data.
Because metabolic potential can be related to body weight and temperature (e.g., Hu and Layton 2001, Nichols et al.2007), the kM was further normalized to 15oC and then corrected for the body weight of the middle trophic level fish in the Arnot-Gobas model (184 g) (Arnot et al. 2008b). The middle trophic level fish was used to represent overall model output as suggested by the model developer (Arnot, personal communication to Bonnell M. of Environment Canada, 2008, unreferenced) and is most representative of fish weight likely to be consumed by an avian or terrestrial piscivore. After normalization routines, the kM ranges from ~0.001 to 0.01 with a median value of ~0.004.
| kM (middle trophic level normalized) day-1 | log Kow used | Arnot-Gobas BCF | Arnot-Gobas BAF |
|---|---|---|---|
| 1.30E-03 (2.5%) | 8.0 | 4265 | 2 630 268 |
| 3.90E-03 (average) | 8.0 | 1905 | 1 023 293 |
| 1.09E-02 (97.5%) | 8.0 | 759 | 269 153 |
The calculated kM values based on in vivoexperiments suggest that the rate of metabolism of D5 is quite low (≤ 0.01 day-1 at best). The experimental BCF study on fathead minnows (Drottar 2005), the dietary bioaccumulation study on rainbow trout (Drottar 2006), and the metabolic study (Springer 2007) demonstrated metabolism, though limited, of D5. The calculated BCF of 4265 using the lower percentile rate constant (~0.001) is approximately 50% lower than the BCF values reported by Drottar (2005), but is within the acceptable range of the experimental values. A corresponding BAF of 2 630 268 was calculated for D5 for fish in Canadian waters using this metabolic rate constant.
In the Sediment Compartment
No bioaccumulation information for D5 was available for the soil compartment.
In the Terrestrial Compartment
The Gobas mass-balance bioaccumulation model for terrestrial organisms (Gobas et al. 2003) uses a chemical's octanol-air and octanol-water partition coefficient (Koaand Kow) to estimate the chemical's biomagnification (BMF) potential in terrestrial food chains. It was estimated that chemicals with a log Koa > 5 can biomagnify in terrestrial food chains if log Kow > 2 and the rate of chemical transformation or metabolism is low. A log Koa of 5.06 indicates that D5 may have the potential to biomagnify in terrestrial food chains. However, the metabolism of cVMS demonstrated in laboratory mammals could reduce the biomagnification potential in the terrestrial food web.
Summary of the Bioaccumulation Potential of D5
Overall, the empirical steady-state and kinetic fish BCF study, although optimized for water-borne exposure, has shown that the bioconcentration potential for D5 from water is high (i.e., ≥ 5000). Although the log Kow for D5 would suggest that dietary uptake will be significant and likely predominate, it is still at the limit of log Kow values where bioconcentration in laboratory studies has been observed to be significant for many chemicals (e.g., Arnot and Gobas 2006). Empirical values exceed 5000, which suggests that there may be potential for high bioconcentration in other organisms at different trophic levels as well as fish, especially those with lower growth or metabolic rates (e.g., autotrophs).
Predicted BCF values, corrected for metabolism, are close to but less than 5000 and are lower than empirical BCF values. Predicted BAF values are also high and exceed 5000. As with other models, some uncertainty exists with predicted BCF and BAF values (e.g., uncertainty increases at higher log Kow values, as few chemicals have been studied for bioaccumulation in this range). Higher confidence is attributed to the predicted BAF value with a corresponding predicted BCF that most closely compares with the empirical BCF data (i.e., BAF = ~2.6E06). The mass-balance kinetic model used is based on "first principles," meaning that the most important domain of the model is that a chemical obeys the principal mechanism of the model, in this case passive diffusion. D5 meets this domain and is within the model's log Kowand molecular weight boundaries as well. Therefore, the predictions for bioaccumulation are considered to be applicable to D5. However, there is less agreement between metabolism-corrected BCF values and empirical BCF values (compared with D4), suggesting that BCF and BAF model results for D5 are more uncertain due to the relative absence of empirical BCF and BAF values at D5's range of log Kow used in the model (i.e. ≥ 8). Also, the possibility that super-hydrophobic chemicals do not reach steady state in the environment (which the model assumes), adds to the uncertainty (Arnot and Gobas 2006).
BSAF values for D5 would suggest a relatively low level of accumulation in sediment macroinvertebrates. As this is the only sediment bioaccumulation test available for cVMS and there are no predictive models for sediment organisms, testing or field evidence at more realistic environmental loadings would help verify these values. The BMF values generated for D5 are less than 1, which suggests that there may be low biomagnification potential in fish for D5, but there is currently no evidence to suggest that this may be the case for other trophic levels. Field mesocosm studies are currently under way to examine trophic transfer of cVMS in aquatic food webs, but these data are not yet available for full evaluation and were not considered for this assessment.
Finally, there is conflicting evidence on the bioaccumulation potential of D5 tested under laboratory conditions as well as that determined from predictive models. BMF studies in fish and BSAF studies in invertebrates suggest that the bioaccumulation potential of D5 is low, possibly due to reduced bioavailability. Available optimized BCF test data suggest there may be potential for significant bioconcentration of D5 in fish and potentially at lower levels of an aquatic food web. Predicted BCF values are much lower than measured values for fish. Therefore, it is reasonable to conclude that D5 has bioaccumulation potential in biota. However, considering the conflicting evidence, it is not possible to conclude that D5 meets the criterion for bioaccumulation (BCF or BAF ≥ 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
A - In the Aquatic Compartment
New empirical ecotoxicity data have been received for D5 and are discussed in detail below (Table 10a). These studies indicate that this substance does not exhibit adverse effects on fish and Daphnia exposed at concentrations up to its water solubility limit (0.017 mg/L).
The aquatic toxicity of D5 to rainbow trout (Oncorhynchus mykiss) was conducted under a flow-through system (Springborn Laboratories 2000). Juveniles of rainbow trout (mean wet weight 2.3 g and mean length 61mm) were exposed to concentrations of D5 (99.35% purity) of 2.1, 3.1, 5.0, 8.6 and 16 µg/L (measured) for 14 days. The test system was sealed and filled to capacity to minimize the loss of D5 from the test solution. Acetone was used as a solubilizer at a concentration of 0.1 mL/L. The pH was maintained between 6.3 and 6.9 and the temperature oscillated between 13 and 15°C. Fish were fed ~2% of the total biomass of the test fish once daily. The temperature during the test deviated slightly from the OECD protocol (12 ± 2°C) and the dissolved oxygen concentration fell below 60% of saturation for more than 24 hours, but these factors were not considered to interfere with the test. There was 5-10% mortality in the negative and solvent controls, as well 10% mortality in the 0.005 mg/L and 15% mortality in the 0.0086 mg/L treatment groups. However, the mortality observed was not considered test-substance-related since no mortality was found in the highest concentration treated (0.016 mg/L).
| Test organism | Type of test | Duration | Endpoint[1] | Value (mg/L) | Reference |
|---|---|---|---|---|---|
| [1] LC50: the lowest concentration causing 50% mortality; EC50: the lowest concentration causing sublethal effects in 50% of the population; NOEC: no-observed-effect concentration | |||||
| Rainbow trout Oncorhynchus mykiss |
Acute | 14 d | LC50 | > 0.016 | Springborn Laboratories 2000 |
| Rainbow trout Oncorhynchus mykiss |
Acute | 14 d | NOEC | 0.016 | Springborn Laboratories 2000 |
| Water flea Daphnia magna |
Acute | 48 hr | LC50 | > 0.003 | Springborn Smithers Laboratories 2002 |
| Water flea Daphnia magna |
Chronic | 21 d | NOEC | 0.015 | Springborn Smithers Laboratories 2003a |
| Freshwater algae Pseudokirchneriella subcapitata | Acute | 96 hr | EC50 | invalid | Springborn Laboratories 2001 |
Both acute and chronic toxicity studies of D5 were carried out for Daphnia magna. Juveniles of Daphnia magnawere exposed to D5 (99.35% purity) concentrations of 1.6, 1.8, 2.1, 2.5 and 2.9 µg/L (measured) for 48 hours under flow-through conditions (Springborn Smithers Laboratories 2002). The exposure system was designed to have minimum volatilization of the test substance. Acetone was used as a solubilizer at a concentration of 0.1 mg/L. The pH was maintained between 7.7 and 8.0, the temperature oscillated between 20 and 21°C and dissolved oxygen remained at > 60%. The test water had a hardness of 160-180 mg CaCO3/L. A mortality of ≤ 10% was observed at the control and test concentrations, resulting in an EC50≥ 2.9 µg/L.
A full life-cycle toxicity test of D5 with Daphnia magna was conducted under static-renewal conditions (Springborn Smithers Laboratories 2003a). Juveniles of Daphnia magna were exposed to radio-labelled 14C-D5 (100% radiochemical purity, 5.758 mCi/g specific activity) at concentrations of 1.0, 1.7, 3.5, 7.2 and 15 µg/L (measured) for 21 days under static-renewal conditions, and the test solutions were renewed every 24 hours. The test system was designed to minimize the loss of D5 from the test solution. Acetone was used as a solubilizer at a concentration of 0.1 mL/L. The pH was maintained between 6.6 and 8.7, the temperature oscillated between 20 and 23°C and dissolved oxygen remained at > 60%. The test water had a hardness of 160-170 mg CaCO3/L. At the end of the 21-day exposure period, the mean offspring per female were 150, 148, 145, 138 and 139, respectively for treatment concentrations of 1.0, 1.7, 3.5, 7.2 and 15 µg/L. The offspring number decreased slightly at treatment concentrations of 7.2 and 15 µg/L compared to negative and solvent controls (mean offspring number 146), though it was not considered statistically significant. No mortality, growth or other biologically significant effects were observed at the highest test concentration of 15 µg/L (no-observed-effect concentration ≥ 15 µg/L). The temperature during the test deviated slightly from the OECD protocol (20 ± 1°C), but was not considered to interfere with the test. However, the feeding during the test was 0.41 mg C/daphnid/day–higher than the OECD guideline of 0.1-0.2 mg C/daphnid/day–which is considered favourable to the health of the test organisms in resistance to toxic effects. A higher carbon load to the test system may also act to reduce bioavailability of D5 by providing a sorption substrate. Nonetheless, the experimental data were considered acceptable for the purposes of the screening assessment, noting possible test limitations as mentioned.
New experimental data were received on an acute toxicity study on freshwater algae, Pseudokirchneriella subcapitata(formerly known as Selenastrum capricornutum) under a 96-hour static condition (Springborn Laboratories 2001). The toxicity test was performed following Toxic Substances Control Act (TSCA) and Organisation for Economic Co-operation and Development (OECD) test standards for algae. Algae were exposed to a saturated solution of D5 (99.35% active ingredient). The test system was sealed and filled to capacity to minimize the loss of D5 from the test solution. Acetone was used as a solubilizer at a concentration of 0.1 mL/L. However, the concentrations of the test solution decreased steadily, from 12 µg/L at test initiation to ~2 µg/L at the end of the 96-hour test. It was hypothesized that the loss of D5 may be attributed to volatilization, adsorption of D5 to algae biomass or to its consumption as a carbon source for algae. Hydrolysis under alkaline conditions may also contribute to the rapid loss of D5, as the pH increased from 7.5 at test initiation to 9.4 during the 96-hour test, which reflected photosynthesis and respiration of the algae. The temperature during the study was maintained at 24°C. It is concluded that no decrease of cell density or growth rate was observed during the test. An additional 500 mg/L sodium bicarbonate was added to provide sufficient dissolved bicarbonate to compensate for cell growth in a closed system. Considering the significant reduction of D5 concentrations during the test and all the uncertainties associated with the study, the results of the test is considered invalid.
The acute and chronic toxicity of D5 was predicted using ECOSAR (2004) model. Predicted results are given in Table 10b.
| Organism | Type of test | Endpoint[1] | Duration | Concentration (mg/L) | Reference |
|---|---|---|---|---|---|
| [1] EC50: the lowest concentration causing sublethal effects in 50% of the population; ChV is the geometric mean of the NOEC (no-observed-effect concentration) and LOEC (lowest-observed-effect concentration). | |||||
| Fish | Chronic | ChV | 30 d | 0.0002 | ECOSAR 2004 |
| Daphnia | Chronic | EC50 | 16 d | 0.0007 | ECOSAR 2004 |
| Green algae | Chronic | ChV | 96 h | 0.003 | ECOSAR 2004 |
The modelled results for D5 using ECOSAR are below the water solubility limit of 0.017 mg/L, which would suggest acute or chronic effects at aqueous saturation. However, the reliability of the predicted toxicity values is considered to be low. The log Kow for D5 (8.03) is narrowly higher than the recommended ECOSAR log Kow cut off (8.0) for prediction of chronic toxicity using the neutral organic structure-activity relationships and the lack of empirical siloxane data in the model's training set limit their consideration in this assessment. Also, the reliability of acute and chronic toxicity predictions at and below the microgram per litre range becomes increasingly uncertain given that these predictions rely solely on log Kow and the empirical ability to quantify effects in this range is highly variable. The model predicted aquatic toxicity data were, therefore, not considered further.
The empirical data suggest that D5 does not exhibit adverse effects on fish and Daphnia at concentrations at or below its solubility limit (0.017 mg/L). However, caution should be exercised in relation to these results. As mentioned in trout and Daphnia toxicity studies conducted with the analogue D4, mortality caused by D4 was not observed until the test organisms were exposed to the substance for 7-14 days, at which time the D4 concentration in fish tissue reached steady state and a body burden sufficient to cause toxicity (Sousa et al. 1995). With D5, there is uncertainty as to whether tissue concentrations achieved steady-state within 14 days during the BCF study with fathead minnows (Drottar 2005). This calls into question whether the 14-day toxicity test duration with D5 and trout (Springborn Laboratories 2000) was sufficiently long to achieve a toxic body burden in the fish tissue and, thus, sufficiently long to determine toxicity in the study. Another observation is that D4-related mortality was only observed in small rainbow trout (≤ 1 g) and not larger trout or other fish species, indicating that D4 could be more toxic to sensitive aquatic organisms and/or aquatic organisms at sensitive early-life stages. D5, however, was only tested with larger trout (~2.3 g), and no early-life stage tests were conducted. Due to the structural similarity between D4 and D5, it is possible that D5 toxicity may have been observed had smaller trout been used. Therefore, questions regarding the sufficiency of the test duration, as well as a lack of empirical data comparable to that in which D4 toxicity was noted (i.e., comparable fish size), as well as a lack of empirical data for early-life stage fish contributes to the uncertainty in the toxicity of D5 to aquatic organisms.
B - In Other Environmental Compartments
In the Sediment Compartment
New empirical sediment toxicity data were received for D5 and have been accepted for this screening assessment report.
A full life-cycle toxicity test with D5 in sediments was conducted using midge (Chironomus riparius) in a series of 10-day and 28-day exposures under static conditions (Springborn Smithers Laboratories 2003b). Midge larvae were exposed to measured concentrations of sediment-incorporated radio-labelled 14C-D5 (99.37% radiochemical purity) ranging from 13 to 580 mg/kg (mean measured concentration from test days 0 and 10) and 12 to 570 mg/kg (mean measured concentration from test days 0, 10 and 28). Artificial sediment was used, composed of 79% sand, 4% silt and 17% clay. Tests were conducted with sediments of 2.0% organic carbon content. The measured pH of the sediment was 6.6-7.6 and the measured temperature was 19-22°C. The midge survival rates determined after 10 days of exposure were 100%, 97%, 98%, 97% and 27%, respectively, for treatment levels of 13, 30, 73, 180 and 580 mg/kg. The average wet weights determined after 10 days of exposure were 5.59, 5.65, 4.77, 4.44 and 1.36 mg/midge, respectively for treatment levels of 13, 30, 73, 180 and 580 mg/kg. The 10-day median lethal concentration (LC50) for midge survival was calculated to be 450 mg/kg dry weight and the median effects concentration (EC50) for midge growth was calculated to be 410 mg/kg dry weight (Table 8c). The emergence and development rates of midge larvae were determined after 28 days of exposure. The mean percent emergence in the 470 mg/kg treatment level was significantly lower (18%) than that in the control (87%). The mean development rates of male midges at the 180 and 570 mg/kg treatment levels were significantly decreased compared to the control. The decrease in mean development rates among females and combined males/females were only significant at the 570 mg/kg treatment level. The 28-day no-observed-effect concentration (NOEC) for midge larvae development is 69 mg/kg and the lowest-observed-effect concentration (LOEC) for midge larvae development is 180 mg/kg (Table 10c).
| Test organism | Type of test | Endpoint[1] | Value (mg/kg dry weight) | Reference |
|---|---|---|---|---|
| [1] LC50: the lowest concentration causing 50% mortality; EC50: the lowest concentration causing sublethal effects in 50% of the population; NOEC: no-observed-effect concentration; LOEC: lowest-observed-effect concentration | ||||
| Midge Chironomus riparius |
Acute | LC50 | 450 | Springborn Smithers Laboratories 2003b |
| Midge Chironomus riparius |
Acute | EC50 | 410 | Springborn Smithers Laboratories 2003b |
| Midge Chironomus riparius |
Chronic | Male development NOEC | 69 | Springborn Smithers Laboratories 2003b |
| Midge Chironomus riparius |
Chronic | Male development LOEC | 180 | Springborn Smithers Laboratories 2003b |
| Midge Chironomus riparius |
Chronic | Female development NOEC | 180 | Springborn Smithers Laboratories 2003b |
| Midge Chironomus riparius |
Chronic | Male/female development NOEC | 180 | Springborn Smithers Laboratories 2003b |
| Midge Chironomus riparius |
Chronic | Emergence NOEC | 180 | Springborn Smithers Laboratories 2003b |
| Oligochaete Lumbriculus variegates | Chronic | NOEC | 1272 | Krueger et al. 2007 |
| Midge Chironomus riparius |
Chronic | LC50 | 257 | Krueger et al. 2008 |
| Midge Chironomus riparius |
Chronic | Development NOEC | 70 | Krueger et al. 2008 |
| Midge Chironomus riparius |
Chronic | Development LOEC | 160 | Krueger et al. 2008 |
| Midge Chironomus riparius |
Chronic | Emergence NOEC | 160 | Krueger et al. 2008 |
| Midge Chironomus riparius |
Chronic | Emergence LOEC | 248 | Krueger et al. 2008 |
A recent chronic toxicity study (Krueger et al. 2007) with the freshwater oligochaete Lumbriculus variegates (blackworm) was also critically reviewed and accepted for this screening assessment. Adult blackworms were exposed to measured concentrations of sediment-incorporated D5 (99.19% purity) ranging from 24 mg/kg to 1272 mg/kg (mean measured concentration from test days 0, 7 and 28) for 28 days under flow-through conditions. Formulated sediment was used, composed of 13% peat, 10% kaolin clay and 77% industrial quartz sand. The measured pH of the sediment was 7.9-8.3 and the measured temperature was 22.7-23.4°C (OECD protocol 20+/-2). The organic carbon content of the sediment was 3.7% based on peat as the sole source of organic carbon. Since blackworms reproduced during the 28-day test and it was hard to distinguish adults from young organisms, the total number of organisms present at test termination was used as the measurement endpoint. The mean number of worms was 33, 30, 31, 21, 19 and 26, respectively for treatment levels of 24, 46, 94, 226, 495 and 1272 mg/kg dry weight. No mortality was observed throughout the test. It was concluded that the mean numbers were not significantly different from that of control (26). No effects were observed for the growth of the surviving worms. The observed NOEC was thus determined to be 1272 mg/kg.
Another prolonged sediment toxicity study on midges, Chironomus riparius, was conducted using spiked sediment (Krueger et al. 2008). The midges were exposed to mean measured concentrations of D5 ranging from 35 mg/kg to 759 mg/kg for 28 days at 20°C. The organic carbon content of the formulated sediment was 3.2%. The overlaying water was renewed partially every week due to the high ammonia measured in the test chamber. The observed NOEC for percent survival and emergence ratio was determined to be 160 mg/kg (measured). The calculated LC50 value for survival was 257 mg/kg. Midges exposed to 160 mg/kg of D5 showed statistically significant reduction in development. The NOEC and LOEC for midge development were determined to be 70 mg/kg and 160 mg/kg, respectively.
In the Soil Compartment
No effects studies for soil organisms were found for D5 or its analogues.
In the Terrestrial Compartment
No ecological studies were identified for terrestrial wildlife. Laboratory studies on mammals are discussed under the "Potential to Cause Harm to Human Health" section in this screening assessment.
Ecological Exposure Assessment
In Air
In Canada, preliminary environmental measurement of volatile methyl siloxanes, including D5, were conducted in the Great Lakes region during February and March of 2006 (personal communication, Environment Canada, Canadian Centre for Inland Waters, 2007, unreferenced). Eighteen outdoor air samples were collected from rural and urban areas in Ontario and D5 was present in almost all the samples at concentration levels of < 1 µg/m3, with the exception of one relatively high D5 concentration, reported to be ~20.5 µg/m3, in the Toronto urban area. This result is in agreement with what has been reported in other jurisdictions (Table 11a).
It is, however, possible that the detection of D5 in ambient air is in part a result of sample contamination. Volatile cyclosiloxanes are present in a wide variety of commercial products, and both Canadian and Nordic monitoring programs have reported problems of high levels of cyclosiloxanes in sample blanks. The methodology for measuring and analyzing air concentrations at ng/m3 to the low µg/m3level is still under development. Very few duplicate measurements are available for outdoor air monitoring and the few that are available exhibit poor reproducibility. It has been hypothesized that the particulate phase may be important to consider and that this could influence results for duplicates (personal communication, Environment Canada, Canadian Centre for Inland Waters, 2007, unreferenced).
| Medium | Location; year | Concentration | Reference |
|---|---|---|---|
| [1] Outdoor samples (n=24) were collected in Nordic countries. The detection limit for D5 was 0.02 µg/m3. [2] Personal communication, Environment Canada, Canadian Centre for Inland Waters, 2007, unreferenced |
|||
| Air | Great Lakes region, Canada; February and March 2006 | < 1-20.5 µg/m3 | see footnote 2 |
| Air | Nordic countries[1]; 2004-2005 | 0.05-19 µg/m3 | Norden 2005 |
In Water
In Canada, water from a total of nine sewage treatment plants (STPs), including conventional secondary and tertiary water treatment plants and lagoons, in large urban centres in southwestern Ontario was sampled in October 2005 and winter 2005 (personal communication, Environment Canada, Canadian Centre for Inland Waters, 2007, unreferenced). D5 was detected at concentrations of 0.49–227.72 µg/L and 1.002.29 µg/L, respectively, in the influents and effluents. Seasonal differences in D5 concentrations in influents in STPs were also noted; most influent concentrations increased from 0.49–57.33 µg/L in the fall to 8.04–227.72 µg/L in the winter. Seasonal differences in D5 concentrations in effluents in STPs were also observed but these were not as significant as those for influents (from 1.00–1.30 in the fall to 1.65–2.29 in the winter).
Similar monitoring results have been reported in other jurisdictions (Table 11b). In the United States, D5 has been qualitatively detected in drinking water systems (Lucas 1984, as cited in US EPA 1992). In Europe, water samples were also collected from influents, effluents and downstream of sewage treatment plants including some associated with silicone manufacturing facilities (Boehmer and Gerhards 2003; see Table 11b of this assessment). Higher concentrations of D5 were detected in the influents and effluents of plants associated with silicone industries. Most water samples downstream of silicone industries contained non-detectable levels of D5 with one exception where the concentration reported was 0.4 µg/L.
| Medium | Location; year | Concentration | Reference |
|---|---|---|---|
| [1] A total of 28 sampling sites excluding STP influents and effluents [2] 7 STP influent sampling sites [3] 12 STP effluent sampling sites [4] A total of 12 marine water samples collected at 2 locations [5] 7 STP influent samples collected [6] 4 STP effluent samples collected [7] A total of 5 samples collected at 2 locations [8] A total of 8 samples collected at 3 locations [9] Personal communication, Environment Canada, Canadian Centre for Inland Waters, 2007, unreferenced d.l. = detection limit |
|||
| STP influents | Southwestern Ontario, Canada; October 2005 | 0.49-227.72 µg/L | See footnote 9 |
| STP effluents | Southwestern Ontario, Canada; October 2005 | 1.00-2.29 µg/L | See footnote 9 |
| Drinking water | United States | Qualitatively detected | Lucas 1984, as cited in USEPA 1992 |
| Water | Background and urban sites,[1] Nordic countries | < 0.07 (d.l.) | Norden 2005, NILU 2007 |
| Water | Germany[4]; 2000-2001 | < 0.04 µg/L (d.l.) | Boehmer and Gerhards 2003 |
| STP influents | Nordic countries[2] | 0.33-26 µg/L | Norden 2005, NILU 2007 |
| STP effluents | Nordic countries[3] | < 0.063-1.0 µg/L | Norden 2005, NILU 2007 |
| STP influents | Germany[5]; 2000-2001 | 1.3-50.1 µg/L | Boehmer and Gerhards 2003 |
| STP effluents | Germany[6]; 2000-2001 | 0.1-1.0 µg/L | Boehmer and Gerhards 2003 |
| STP influents | Wastewater treatment plants (WWTPs) associated with silicone industries in Germany, France and the United Kingdom (UK)[7]; 2001 | 365-3694 µg/L | Boehmer and Gerhards 2003 |
| STP effluents | WWTPs associated with silicone industries in Germany, France and the UK[8]; 2001 | < 0.02 (d.l.) - 26.7 µg/L | Boehmer and Gerhards 2003 |
| Water | Downstream of WWTPs associated with silicone industries in Germany, France and the UK[8]; 2001 | < 0.02 (d.l.) - 0.4 µg/L | Boehmer and Gerhards 2003 |
In Sediments
In Canada, surface sediments and sediment cores were collected from Lake Ontario in July 2006 and analyzed for D4, D5 and D6 (Powell and Kozerski 2007). Surface sediments consisting of the upper 5 cm of sediment were collected from Toronto Harbour and the Kingston Basin. Sediment cores, which were sectioned into strata 5 mm thick, were collected from the Rochester, Mississauga, and Niagara basins. The surface sediments from Toronto Harbour and Kingston Basin contain moderate total organic carbon (TOC = 2.1-2.4% dw), while sediment cores contain high TOC (4-5% dw). Loss-on-ignition analysis of sediments also demonstrated lower water contents in surface sediments (55-70% ww) than in sediment cores (80-89%). Sediments in Toronto Harbour and the four sedimentary basins are known to be contaminated with a variety of organic compounds that enter the lake through direct discharges of treated wastewater, flow from the upper Great Lakes (Erie, Huron and Michigan) and the Niagara River, and atmospheric deposition. Surface sediments from Toronto Harbour contained the highest concentration of D5, at 0.78 µg/g dry weight. In contrast, concentrations of the cyclic siloxane materials in the surface sediments and sediment cores from the four sedimentary basins were all less than the analytical method detection limit, which was 0.017 µg/g for D5. Similar monitoring results have been reported in other jurisdictions, where D5 was detected in surface sediments from urban areas and point sources (Table 11c).
A preliminary monitoring study of a remote ecosystem was conducted in Lake Opeongo, the largest lake in Algonquin Provincial Park, Ontario, Canada. The lake is relatively remote from potential sources of cVMS from sewage and runoff (Powell 2008). Preliminary analysis of surface sediment and sediment core samples found no D5, with a limit of detection of 15.9 ng.
The sediment monitoring results from Lake Opeongo and the Lake Ontario area suggest that D5 contamination is more likely to be found near urban centres and point sources.
| Medium | Location; year | Concentration | Reference |
|---|---|---|---|
| [1] A total of 30 sediment sampling sites [2] A total of 11 sampling matrix at 9 locations [3] A total of 12 marine sediment samples from 2 areas [4] Background corrected mass as reported in the preliminary study dw = dry weight, d.l. = detection limit, q.l. = quantification limit |
|||
| Surface sediments | Toronto Harbour, Canada; July 2006 | 0.78 µg/g dw | Powell and Kozerski 2007 |
| Surface sediments | Kingston Basin, Canada; July 2006 | <0.017 µg/g dw (d.l.) | Powell and Kozerski 2007 |
| Sediment cores | Rochester, Mississauga, and Niagara basins, Canada; July 2006 | <0.017 µg/g dw (d.l.) | Powell and Kozerski 2007 |
| Surface sediments | Lake Opeongo, Algonquin Provincial Park, Ontario, Canada; October 2007 | 15.9 ng (d.l.) [4] | Powell 2008 |
| Sediment cores | Lake Opeongo, Algonquin Provincial Park, Ontario, Canada; October 2007 | 15.9 ng (d.l.) [4] | Powell 2008 |
| Sediments | Nordic countries[1] | < d.l. (varied from sample to sample) - 0.92 µg/g dw | Norden 2005, NILU 2007 |
| River sediments | Germany[2] | < 0.003 (q.l.) - 0.042 µg/g dw | Boehmer and Gerhards 2003 |
| Marine sediments | Germany[3] | 0.033-0.28 µg/g dw | Boehmer and Gerhards 2003 |
In Soil
D5 may enter soil from land application of sewage sludge. No monitoring data for D5 in sewage sludge are available for Canada. In Europe, D5 is present in sewage sludge at levels ranging from the low mg/kg dry weight level up to 130 mg/kg dry weight (Norden 2005, Kaj et al. 2005, NILU 2007).
No monitoring data are available for D5 in Canadian soil. D5 concentrations in two soil samples from the Faroe Islands were below detection limit (< 5 ng/g dw) (Norden 2005).
In Biota
A preliminary monitoring study of a remote ecosystem was conducted in Lake Opeongo, the largest lake in Algonquin Provincial Park, Ontario, Canada. The lake is relatively remote from potential sources of cVMS from sewage and runoff (Powell 2008). Preliminary analysis of zooplankton samples found no D5. Bulk zooplankton samples were pooled into a single sample for each of the two locations from the lake without being sorted into species. The limit of detection was 15.9 ng (background corrected mass).
In Europe, D5 was the predominant cyclosiloxane in fish livers and marine mammals in the Nordic screening program. The substance was detected in both freshwater and marine fish from sampling sites in urban areas and near STPs, at concentrations in the range of < 5-84 ng/g wet weight, except for one sample of cod liver (9 livers pooled) collected at a location near a city centre in Norway that had an extremely high concentration of D5 (2200 ng/g ww). The follow-up environmental monitoring program conducted by the Norwegian government confirmed the originally reported levels of D5 in cod livers (NILU 2007; see Table 11d of this assessment). D5 was also found in common mussels, flounder livers and fillets, and in cod stomach contents from Norway in the same monitoring program. The concentrations varied with species, gender, and age. D5 was also detected in the blubber of seals and pilot whales at concentrations ranging from <5 to 24 ng/g ww (Norden 2005). D5 was also detected in fish samples in Germany at concentrations ranging from 0.15 to 2.6 mg/kg (SEHSC 2005a). D5 was not detected in fish muscle samples in Sweden (Kaj et al. 2005).
The presence of D5 in European biota indicates that despite the low detected concentrations or even non-detection of the substance in or near fish habitats, D5 is available in the environment for biota to take up and accumulate.
| Organism | Location; year | Concentration | Reference |
|---|---|---|---|
| [1] A total of 11 sampling matrices for marine fish [2] A total of 10 sampling matrices for freshwater fish [3] A total of 7 sampling matrices for marine mammals [4] A total of 17 sampling matrices for seabird eggs [5] A total of 3 sampling matrices for mussel [6] A total of 2 sampling matrices for flounder [7] A total of 3 sampling matrices for cod [8] A total of 5 fish matrces were sampled from the Rhine River; a Danish salmon obtained from an unspecified location showed no detectable D5 ww = wet weight, d.l. = detection limit, q.l. = quantification limit |
|||
| Zooplankton | Lake Opeongo, Algonquin Provincial Park, Ontario, Canada; October 2007 | < 15.9 ng (d.l.) | Powell 2008 |
| Marine fish liver | Nordic countries[1]; 2002-2004 |
< 5 (d.l.) - 2200 ng/g ww | Norden 2005 |
| Freshwater fish liver | Nordic countries[2]; 2002 | < 5 (d.l.) - 84 ng/g ww | Norden 2005 |
| Marine mammals | Nordic countries[3]; 2002 | < 5 (d.l.) - 24 ng/g ww | Norden 2005 |
| Seabird eggs | Nordic countries[4]; 2000-2005 |
< 5 ng/g ww (d.l.) | Norden 2005 |
| Common mussels | Norway[5]; 2006 | 3.3-8.7 ng/g ww | NILU 2007 |
| Flounder livers | Norway[6]; 2006 | 27.1 ng/g ww | NILU 2007 |
| Flounder fillets | Norway[6]; 2006 | 3.4 ng/g ww | NILU 2007 |
| Cod stomach contents | Norway[7]; 2006 | 22.9-149.3 ng/g ww | NILU 2007 |
| Cod livers | Norway[7]; 2006 | 1490.8-1978.5 ng/g ww | NILU 2007 |
| Fish | Rhine River,[8] Germany | 150-2600 ng/g ww | SEHSC 2005b |
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under subsection 76.1 of CEPA 1999. Particular consideration was given to risk quotient analysis, persistence, bioaccumulation potential, toxicity, sources and fate in the environment.
Based on the information available, D5 has been determined to be highly persistent in water and potentially bioaccumulative in aquatic organisms. A quantitative risk quotient evaluation of exposure and of ecological effects was therefore conducted as part of the weight-of-evidence evaluation of D5's potential to cause harm.
In the aquatic compartment, results of two acceptable experimental chronic toxicity studies with Daphnia magnaand rainbow trout indicated that D5 caused no significant adverse effects at concentrations of up to 0.015 mg/L, a concentration that is very close to the water solubility limit of the substance (~0.017 mg/L). No application factor was applied to the chronic NOEC of 0.015 mg/L. The calculated predicted no effect concentration (PNEC) is therefore 0.015 mg/L. It is considered that D5 exposure to aquatic organisms may reach the level above its laboratory-measured water solubility under realistic environmental conditions.
A risk quotient (RQ) analysis, integrating level of exposure with a toxicity threshold, was performed for D5. In order to address the potential risk of D5 on a national scale in Canada, a distribution profiling risk quotients in water at multiple release sites where the substance can be released by industry or by consumers (i.e., municipal sewage treatment plants) was determined. This type of analysis provides a line of evidence for the risk assessment of a substance when the full range of geographic locations of the industrial and consumer releases of the substance cannot be fully established.
Specifically, when a substance is used by a number of industry sectors but the actual facilities involved cannot be identified, the aquatic exposure can be estimated for all sites where facilities related to these sectors are located. In addition to this, information on potential releases from consumer use can be integrated into the calculations. A predicted environmental concentration (PEC) for the water compartment is determined based on the use quantities identified from the section 71 survey submissions and estimates of releases from individual industrial sites and from consumers. The receiving water is either a watercourse or a lake, and a dilution factor based on the size of the receiving water–up to a maximum of 10–is used to estimate the PEC. The risk quotient at each site is then determined for the water column. The distribution indicates not only the proportion or number of threshold-exceeding sites, but also the magnitude of the exceedence at each of these sites. Further details on the approach are provided in Environment Canada (2008b).
The consumer releases used a database of approximately 1000 municipal discharge sites accounting for about 2/3 of the Canadian population. The industrial release analysis was done for 64 sites relating to 87 industrial facilities identified by NAICS code as possible users of D5. Under these scenarios, a total of 65 (~6.8%) of all evaluated municipal discharge sites across Canada showed a risk to aquatic organisms, with RQs exceeding 1 (Figure 1). The equation and inputs used to calculate the PEC in the receiving watercourses are described in Environment Canada (2008c).

Figure 1. Aquatic risk distribution for D5 (Environment Canada 2008b, 2008c)
Although a risk quotient analysis was conducted for D5, the empirical ecotoxicity evidence suggests that the threshold at which adverse effects in pelagic biota is expected to occur has not been observed in available toxicity tests. Therefore, the RQs calculated in the above scenario are essentially "unbounded" and may not represent "real" observable effects expected at the above sites.
The high logKow for D5 would suggest that it is in the class of "super-hydrophobic" chemicals which are often associated with low bioavailability to pelagic and benthic biota. However, because the logKow for D5 is not higher than ~8.0 and a threshold for effects in benthic biota was observed, some degree of bioavailability is expected in the environment, especially for longer term exposures. Laboratory bioconcentration data, albeit optimized, as well as evidence of detection in biota sampled from the field suggest that the potential for body burdens reaching critical internal levels may not be mitigated by the expected bioavailability of D5, especially near STP outfalls.
The physical-chemical property, bioconcentration and ecotoxicity profiles for D5 do not provide a consensus basis for a weight of evidence. There are some uncertainties as to whether D5 poses the potential to cause ecological harm. D5 is imported into Canada in significant quantities and is considered persistent in the environment. Based on the information available, the quantities of D5 imported and used in Canada have increased significantly since the DSL nomination (Environment Canada 1988). Given the trend of increasing use, its environmental release and potential for environmental exposure may increase.
Therefore, considering the above, a reasonable level of precaution is required and as such it is concluded that D5 may have the potential to cause ecological harm when released to the Canadian environment, particularly for long term exposures near discharge zones.
Uncertainties in Evaluation of Ecological Risk
D5 is one of the major components in CAS RN 69430-24-6. It is also present in PDMS at up to 3%. The Challenge to industry and other stakeholders issued by the Government of Canada (Canada 2007) did not survey CAS RN 69430-24-6 (cyclomethicone, the mixture) or PDMS. Even though there is evidence that some companies did report individual cVMS in the mixture under the survey, the quantities of these substances imported into Canada and their uses in 2006 are not completely known, and their releases into the Canadian environment are not considered fully in this screening assessment report.
Several factors contribute to the uncertainty of the assessment of toxicity in the aquatic compartment. These include a lack of toxicity studies using fish of comparable size as those for which toxicity was demonstrated with the similar cVMS, D4; a lack of toxicity studies with fish early-life stages; a lack of testing with exposures somewhat above the solubility limit; and concerns that the exposure duration was insufficient for the achievement of steady-state tissue concentrations. For the risk distribution analysis, the NOEC was derived from a chronic Daphnia magna toxicity study of D5, a concentration that is very close to its water solubility limit (~ 0.017 mg/L). This NOEC was at the highest concentration tested (unbounded), and thus no application factor was applied to extrapolate from laboratory data to the field due to a lack of observed significant adverse effects at this concentration. Since there is likely variability in water solubility under realistic environmental conditions, and it is possible that D5 attached to suspended organic matter coming from STPs (which potentially contain higher concentration of D5 than its water solubility (see Table 9b)) may be ingested by aquatic organisms, it is possible that chronic adverse effects may occur. Since no release information from industrial operations was available for the risk distribution analysis, it is assumed that releases to wastewater were uniformly distributed among 64 industrial sites evaluated. In reality, certain industrial sites may use higher quantities of D5 than others, resulting in higher releases to the municipal discharging sites associated with these industrial sites and therefore a higher risk than predicted. The distribution concentrations in the analysis applied instantaneous dilution of the effluent from sewage treatment plants (STP) into the receiving water. However, under realistic environmental conditions, instantaneous dilution may not be achieved over a certain distance from the discharge point, and the area near the discharge point of an STP may present a higher risk than predicted.
Sediment is an important media of concern for D5. The D4 sediment degradation studies were used as read-across values for D5 and those studies are not without uncertainties. Extrapolation of half-lives at low temperature in sediment based on hydrolysis data may also contribute to the overall uncertainty in sediment persistence. Limited data for bioaccumulation potential in this compartment also contributes to the overall ecological assessment uncertainty.
The available bioconcentration data and biomagnification factor and sediment accumulation values for D5 are conflicting. There is a lack of field data on bioaccumulation potential via the food web and in non-aquatic organisms.
Model predictions were also included in this screening assessment as a line of evidence. There are some uncertainties associated with predicted values, as few siloxanes or chemicals with high log Kow values (> 8) have been studied and included in the models. The predicted inherent toxicity for aquatic organisms may have an additional source of uncertainty when these concentrations exceed the solubility of the chemical in water. Given that concentrations for both the toxicity and water solubility under realistic environmental conditions are often uncertain (vary from optimal laboratory conditions), toxicity values that exceed solubility estimates by up to a factor of 1000 were accepted during categorization.
Environmental monitoring data in Canada and elsewhere are limited. Sample contamination is a potential problem in environmental monitoring due to D5's widespread uses. Data on environmental concentrations of D5 in biota and surface water in Canada are lacking and few environmental concentrations have been reported outside of urban areas in Canada. Consequently, monitoring data from European countries have been presented in this report. However, monitoring has been identified as a key component in the Chemicals Management Plan in Canada and D5 is being considered for environmental monitoring under the Plan. Environmental monitoring will contribute to a better understanding of the environmental presence and "true" environmental accumulation potential of the substance in the environment.
Exposure Assessment
The data on levels of D5 found in environmental media including ambient air near and away from point sources, surface waters, sediments, sewage sludge and biota are described in this report in the section entitled “Ecological Exposure Assessment.” Unpublished data from Canada include measurements taken of biogas at landfills (personal communication, Environment Canada, Environmental Technology Centre, 2008, unreferenced); air near and away from point sources (personal communication, Environment Canada, Canadian Centre for Inland Waters, 2007, unreferenced); air, influent and effluent water at wastewater treatment plants (personal communication, Environment Canada, Canadian Centre for Inland Waters, 2007, unreferenced); and Great Lakes sediment (Powell and Kozerski 2007). Many analyses of volatile siloxanes have been confounded by sample contamination during collection and analysis, resulting in siloxanes being detected in blanks at levels comparable in some cases to those in samples taken near point sources. A survey of volatile organic chemicals, including siloxanes, in residential air was conducted from 2002 to 2004 in homes in Syracuse, New York, U.S.A. (NYIEQ 2005). Results of extensive sampling and measurement of siloxanes in environmental media in Scandinavia have been published by the Nordic Council of Ministers and the Swedish Environmental Research Institute (Norden 2005, Kaj et al. 2005). Data from these reports were considered reliable and were used to produce the upperbounding estimates of exposure to siloxanes in air, water and soil by the general population in Canada.
The upper-bounding estimates of daily intake of D5 for six age groups in the Canadian population are shown in Appendix 1. The estimates of intake from environmental media and diet range from 68.7 µg/kg-body weight/day (µg/kg-bw/day )for adults aged 60 years and older to 208 µg/kg-bw/day for children aged 6 months to 4 years. The most significant contribution to daily intake from environmental media is inhalation of indoor air, based on a study of approximately 130 homes in Syracuse, New York, in which D5 was detected in 85% of homes. The maximum concentration of D5 in indoor air measured in homes in this study was 1560 µg/m3(mean 136 µg/m3) (NYIEQ 2005). To characterize a more representative upper-bounding estimate, the 90thpercentile was estimated, based on available data. An approximate 90th percentile concentration of 393 µg/m3was derived (email from Health Canada, Biostatistics Division, January 2008, unreferenced) and this value was used in the intake table (Appendix 1).
Confidence in the upper-bounding estimate of exposure to D5 through environmental media and diet is moderate. No Canadian data were used, but data from studies in Scandinavia and the United States were available for ambient and indoor air, water and soil. The derivation of the 90th percentile from the indoor air study was based on available data and an assumption of normal distribution, although the available data indicate the data may not be normally distributed. The use of a regulatory limit for dimethylpolysiloxane in one quarter of dairy products and one half of processed food may overestimate the dietary contribution to total exposure, but the estimated contribution from all food to exposure of the general population from environmental media and diet is less than one percent of the contribution from air.
Using ConsExpo 4.1, software developed to estimate exposure to consumer products, the potential systemic dose of D5 through the use of personal care products was estimated for women that use skin care products, hair care products and antiperspirants (RIVM 2006). Manufacturers of personal care products are required to notify Health Canada of the concentration, within broad ranges, of siloxanes, including D5 and polydimethylcyclosiloxanes, termed cyclomethicone in personal care products.[1] Health Canada has been notified of approximately 3000 cosmetic products that contain D5 as the sole siloxane or in mixtures with other specified siloxanes , and that department has also been notified of approximately 6000 cosmetic products that contain cyclomethicone or cyclomethicone mixtures (CNS 2007). The data on the concentration of D5 in personal care products were obtained principally from the information provided by Canadian industry (Environment Canada 2007, CNS 2007) and were supplemented by information from other sources noted in Appendix 2. Market share data were not used to determine the concentration of D5 in the dominant products in each use category. In cases where the concentration for a product category reported in response to a notice published under section 71 of CEPA 1999 was higher than concentrations reported in the CNS database, a lower concentration of D5 for that product category, consistent with the range reported to the CNS, was used in estimating the systemic dose (Environment Canada 2007, CNS 2007).
Based on experimental observations that 80-91% of D5 evaporated from skin in 24 hours (Jovanovic et al. 2008), it was assumed that 80% of a product left on the skin evaporated during use and was therefore not available for dermal absorption. A distinction was made between products that are washed off and those that are left on the body.
For the purpose of modelling the absorption of personal care products, it was decided to use the same absorption rates for exposure by inhalation and ingestion as those established for D4, consistent with a conservative approach to estimating dose. For dermal absorption, the figure of 0.17% established by Jovanovic et al. (2008) experimentally for rat skin was used. Other assumptions are noted in Appendix 2.
The results of a sample calculation for the application of body lotion are shown in Appendix 2, and a summary of the estimated systemic dose arising from the use of personal care products by women is shown in Table 1. For adult women, the upper-bounding estimate of daily systemic dose from the modelled personal care products, aggregated over inhalation, dermal and oral exposure, is 0.17 mg/kg-bw/day. In the screening assessment of octamethylcyclotetrasiloxane (D4) it was shown that the systemic dose received by women from the use of personal care products was higher than the dose received by men and this is expected to be the case for D5.
An exposure assessment for use of D5, including personal care product uses, was submitted to the Government of Canada under the Challenge Program (SEHSC 2008a). The methodology is different from that shown in Appendix 2, Table 1, as a Monte Carlo probabilistic analysis was conducted and aggregate general population exposures from all sources (including personal care products) and routes (inhalation, dermal, ingestion) were derived. The contribution of the use of personal care products to total exposure via separate exposure routes (inhalation, dermal, oral) was characterized and then summed to arrive at an aggregated exposure estimate. Independent review of the submitted probabilistic assessment showed that this assessment evaluated exposure to both user and non-user groups (see Appendix 4). The data were re-analyzed based on just user groups to allow comparison with the deterministic exposure assessment in this screening assessment. Based on user groups only, the probabilistic exposure values for adult females (most highly exposed adult group) were 10-16 times lower than the deterministic values shown in Appendix 2, Table 1. Note that due to the requirement for detailed analysis and validation of probabilistic exposure assessments, such assessments are normally outside scope for conducting the exposure component of a screening assessment.
Based on user groups only, the probabilistic exposure values for children aged 0-6 months (most highly exposed children's group) were in the range of 0.016-0.032 mg/kg bw/day (see Appendix 4). A comparison with a deterministic exposure assessment for children was not possible due to the lack of sufficient product use data required for modelling children's exposure in a deterministic exposure assessment. When the children's probabilistic exposure values are compared with the adult female deterministic exposure values, they are 5-10 times lower.
Other types of consumer products such as surface coatings, caulking and cleaners were deemed to contribute significantly less to daily exposure through daily use and were not further considered in the modelling of daily dose through consumer exposure scenarios. Both personal care products and other consumer products such as surface coatings, caulking and cleaners contribute to the concentration of D5 in indoor and ambient air and thus exposure by inhalation. The contribution of all consumer products to total exposure of non-occupationally exposed individuals is estimated via indoor air in the multi-media environmental exposure model discussed in the preceding text of this section.
Confidence in the estimate of systemic dose of D5 through the use of personal care products is low. All estimates were made by the use of models and the use pattern data were not from Canadian studies. The extent of use of D5 in personal care products and the concentration of D5 in products on the market currently may be lower than used in estimating the systemic dose reported above and in Appendix 2 (Environment Canada 2007, SEHSC 2007a). Consequently, these values are expected to be overestimates of exposure to D5 from use of personal care products.
Health Effects Assessment
Appendix 3 contains a summary of the available health effects information for decamethylcyclopentasiloxane (D5).
No international agency has classified D5 for carcinogenicity, genotoxicity or reproductive/developmental toxicity. Only one national review on the health effects of cyclosiloxanes was identified to date, that of the Danish Environmental Protection Agency (EPA). They reviewed health effects for D4 and D5 (Lassen et al. 2005).
Based on the assessment of the Danish EPA, a potential effect for repeat-dose toxicity is carcinogenicity. This was based on uterine tumours observed in a two-year rat inhalation study (Lassen et al. 2005). The US EPA, in a fact sheet on D5 in dry-cleaning applications, also noted this endpoint and indicated that they will determine whether it is appropriate to conduct a risk assessment for D5 once a mode-of-action analysis is completed (US EPA 2005). No further information on D5 was identified from the US EPA.
In a chronic toxicity and carcinogenicity study, rats were exposed to vapour concentrations of 0, 10, 40 or 160 ppm D5 for 6 h/day, 5 days/week for 24 months. In the highest dose group, there was a statistically significant increase in uterine tumours (endometrial adenocarcinomas) observed in female rats in the highest exposure group and an increased incidence of hyaline inclusions in the nasal respiratory/olfactory epithelium of both sexes. Also, a subgroup exposed to the same concentrations for 12 months with a 12-month recovery period showed an increased incidence of hyaline inclusions in the nasal respiratory/olfactory epithelium of both sexes at 40 and 160 ppm (Dow Corning 2005). Decamethylcyclopentasiloxane (D5) was not genotoxic in several in vitro and in vivo assays (see Appendix 3). The limited genotoxicity results suggest that the tumours observed in the chronic toxicity/carcinogenicity study could be due to threshold effects.
Although a thorough analysis of the mode of action of decamethylcyclopentasiloxane is beyond the scope of this screening level assessment, it is recognized that D5 may possibly act as a dopamine agonist, thus contributing to the observed tumourigenic effects in female rats (SEHSC 2008a). Although the Silicones Environmental, Health and Safety Council (SEHSC 2008a) state that this mode of action is not relevant to humans, this position has not been adopted by other regulatory agencies due to lack of a thorough mode-of-action analysis by these agencies (such as the Danish EPA assessment).
In the Danish EPA assessment, the lung was identified as the primary target organ (Lassen et al. 2005). The lowest concentration at which lung effects were observed was 450 mg/m3 (30 ppm) based on a significant increased incidence of pulmonary vascular mineralization as observed in an inhalation reproduction study (Siddiqui et al. 2007; see below). However, other respiratory tract effects were observed at lower doses. The lowest-observed-effect concentration (LOEC) for repeated inhalation exposure to D5 was 10 ppm (150 mg/m3) based on nasal cavity effects (increased incidence and severity of goblet cell proliferation in level 1 of nasal cavity in both sexes) in a 28-day rat study (Burns-Naas et al. 1998a). At higher concentrations (≥ 380 mg/m3, ≥ 25 ppm) in short- and long-term rat studies, additional effects included increased liver and lung weights, reversible changes in thymus weight, further effects in the nasal cavity, lung effects, and clinical chemistry effects (Burns-Naas et al. 1998a, 1998b; TNO 1984). A LOEC of 700 mg/m3 (46 ppm) was determined in a 3-month rat study based on increased liver weight (TNO 1984) and a LOEC of 600 mg/m3 (40 ppm) was determined in a 2-year rat study based on an increased incidence of hyaline inclusions in the nasal respiratory/olfactory epithelium (Dow Corning 2005).
For repeated-dose oral exposures, the lowest-observed-effect level (LOEL) was 5 mg/kg-bw/day based on increased liver enzyme activities (CYP2B1/2 and EROD) in a 4-day rat study (Zhang et al. 2000). At higher doses (≥ 20 mg/kg-bw/day) in short-term oral rat studies, other effects included increased liver weights (Zhang et al. 2000, Jager and Hartmann 1991).
Liver effects were observed in both oral and inhalation studies using D5. For example, there was a significant increase in liver weight in a 3-month oral rat study (Jager and Hartmann 1991) and increased liver weight in a 3-month inhalation rat study (LOEC determined to be 700 mg/m3; Burns-Naas 1998b), thus indicating a common effect based on both oral and inhalation exposures of the same duration. Although the Danish EPA (Lassen et al. 2005) did not identify liver effects as critical for D5, it did state that "D5 has an enzyme induction profile similar to that of D4" and that the liver was a target organ for D4 exposure. D4 was described as an inducer of hepatocellular enzymes, and thus based on similarity of the liver enzyme induction profile for D4 and D5, it is considered that the liver is a target organ for D5 oral and inhalation exposures. Zhang (2000) noted an increased induction of the liver enzyme, CYP3A1/2, by D5 that was greater than the induction caused by phenobarbital in a 4-day oral rat study, and concluded that, although similar to a phenobarbital type of induction caused by the CYP2B enzyme series, there may be important mechanistic differences in the induction caused by D5. However, for D4, Falany and Li (2005) also noted an increased induction of liver enzyme, CYP3A1/2, in 8-day rat studies but suggested that this was part of the phenobarbital-type induction due to related induction of the CYP2B and PROD enzymes. Consequently, it was not considered appropriate to determine adverse effect levels based on enzyme induction alone.
In a 2-generation study, Sprague-Dawley rats were exposed by whole-body vapour inhalation to 0, 450, 1050 or 2400 mg/m3 (0, 30, 70, or 160 ppm) D5 for 6 h/day. Reproductive (number of days between pairing and mating, mating and fertility indices, gestation length, and parturition) and spermatogenic parameters, ovarian primordial follicle counts and number of corpora lutea in the F0 and F1 parental animals in all exposure groups were not significantly different from the control. The total number of F1 and F2 pups born, mean live litter size, sex ratio of litters, pup body weight and postnatal pup survival were not affected by exposure to D5. However, a significant increase in the incidence of pulmonary vascular mineralization was observed in all F0 and F1 animals at 450 mg/m3 (30 ppm) and above. Also, significantly increased incidences of minimal alveolar histiocytosis were observed at the high concentration in F0 and F1 females (Siddiqui et al. 2007).
Estrogenic, androgenic and progestagenic activities were evaluated in several in vitro and in vivobioassays. In vivo rat studies (16 hours/day for 3 days inhalation exposure to 160 ppm D5) showed no increase in uterine wet or blotted weights and no increase in male reproductive organ weights. In addition, D5 did not bind to human estrogen receptors a and ß or progesterone receptors and was negative in ERa and PRß reporter gene assays (Quinn et al. 2007).
It is uncertain whether liver weight increases due to treatment with D5 are adaptive or adverse. As per a US EPA Health Effects Division guidance document (2002), observations of increased liver weight or hepatocellular hypertrophy should be associated with significantly increased or decreased serum levels of at least two of the liver enzymes–alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase or gamma-glutamyl transferase–before the changes are ascribed to liver toxicity. In the 1-month rat inhalation study, at 2400 mg/m3 (160 ppm), relative liver weight was significantly increased in both sexes and serum alkaline phosphatase was decreased in females, even after a 14-day recovery period (as well as increased lung weight and focal alveolar macrophage accumulation in the lung in both sexes) (Burns-Naas et al. 1998a). As observed in the 3-month rat inhalation study, there were significant increases in liver/brain weight ratios and in gamma glutamyl transferase (γ-GT) in female rats at 700 mg/m3 (46 ppm) and higher (Burns-Naas et al. 1998b). And as observed in a one-year rat inhalation study, absolute and relative liver (liver/body and liver/brain) weights and gamma glutamyl transferase (γ-GT) were significantly increased in females at 2400 mg/m3 (160 ppm) (Dow Corning 2005).
However, data on changes in levels of these liver enzymes following oral administration were not located. As shown in Appendix 3, liver weights were increased at 100, 330 and 1000 mg/kg-bw/day of D5 in a 3-month oral rat study (Jager and Hartmann 1991). Although no other effects were observed, 100 mg/kg-bw/day is considered to be the critical effect level for D5 based on the critical oral LOELs chosen for the similar compounds, D4 and D6. For D4, an oral LOEL of 100 mg/kg-bw/day was determined based on decreased serum estradiol in the 7-day mouse studies and decreased body weights and relative liver weights in fetuses in 8-day rat studies (D4 administered to pregnant females). For D6, an oral LOEL of 100 mg/kg-bw/day was determined based on increased liver weights and periportal lipidosis in the liver of females and follicular cell hypertrophy of the thyroid in both sexes of rats in a 28-day oral study.
Toxicokinetic studies in rats indicated that 0.17% of topically applied 14C-D5 was absorbed across the skin, approximately 30% of the absorbed D5 reached the systemic compartment (Jovanovic et al. 2008), and it was metabolized and eliminated in urine (Varaprath et al.2003). In a 6-hr nose-only inhalation study in rats, 1-2% of 14C-D5 was retained in the body, 69-80% of the absorbed D5 was deposited in tissues, and the primary route of elimination was expired air (Tobin et al. 2008)
The confidence in the toxicity database is low to moderate as there was information to address effects that may be of concern and identify critical endpoints based on inhalation exposures, as well as some relevant supporting information. However, there was a lack of dermal and/or oral studies for several endpoints (subchronic, chronic toxicity/carcinogenicity, reproductive and developmental studies).
Characterization of Risk to Human Health
Based principally on the weight-of-evidence assessment of the Danish EPA, a potential effect for repeated-dose toxicity is carcinogenicity, as observed in a 2-year rat study (Lassen et al. 2005). As stated above, the lack of genotoxic effects for D5 based on limited genotoxicity data suggests that the uterine tumours observed in the chronic toxicity/carcinogenicity study could be due to threshold effects. It is also noted that these tumours were observed at higher exposure levels than the effects identified for the lung and liver as described below.
The Danish EPA also identified the lung as a target organ for D5 exposures. Thus, the critical target organ is considered to be the lung and the critical effect level for repeated-dose toxicity is considered to be 450 mg/m3 (30 ppm) via the inhalation route based on a significant increased incidence of pulmonary vascular mineralization as observed in both generations in the rat reproduction study (Siddiqui et al. 2007). Although a lower effect level was determined in another repeated-dose study (150 mg/m3 in a 28-day rat study), the effect observed (increased incidence and severity of goblet cell proliferation in level 1 of the nasal cavity) was not considered to be critical because the 2-year rat inhalation study showed no effects at this same lower concentration, but at the next higher concentration (600 mg/m3), effects were observed in the nasal cavity (and the nasal cavity was not identified as a target organ by the Danish EPA).
Comparison of the critical effect level for repeated-dose effects via inhalation (450 mg/m3) and the upper-bounding exposure estimate (90th percentile from indoor air survey) via inhalation for decamethylcyclopentasiloxane (393 µg/m3) results in a margin of exposure of approximately 1150. Use of the 90th percentile rather than the maximum concentration from the indoor air survey (1560 µg/m3) to derive the margin of exposure was considered an appropriate refinement to the risk characterization as use of the 90th percentile is considered to result in upper-bounding exposure estimates. Thus, the margin of exposure for repeated-dose effects via inhalation and exposure via environmental air concentrations to the general population is adequate to account for uncertainties in the databases on exposure and effects. This margin of exposure also provides protection against potential carcinogenic effects as the endometrial tumours in the chronic/carcinogenicity study were observed at a much higher dose, 2400 mg/m3.
The Danish EPA assessment did not distinguish between exposure routes. Thus, it is considered prudent to establish a critical effect level for oral exposure, as a limited amount of oral toxicity data was available. The critical effect level for repeated-dose toxicity is considered to be 100 mg/kg-bw/day via the oral route. This is based on increased liver weights in a 90-day rat study, as well as the extrapolation of critical effects and levels from oral data on the similar compounds D4 and D6 to D5 (see the "Health Effects Assessment" section).
Due to similar effects in the liver via the oral and inhalation routes, it is considered appropriate to compare oral critical effects with exposure intake estimates which aggregate the three main routes of exposure (oral, inhalation, dermal). Comparison of the critical effect level for repeated dosing via the oral route (100 mg/kg-bw/day) and the upper-bounding estimate of daily intake of D5 by the general population in Canada results in a margin of exposure of approximately 17 200. This is based on adjusting the inhalation contribution to daily intake by an inhalation absorption value of 2%, resulting in a systemic exposure of 5.8 µg/kg-bw/day. This margin of exposure is considered adequate to account for uncertainties in the database on exposure and effects.
The systemic dose of 0.16 mg/kg-bw/day from use of personal care products incorporates absorption factors for the dermal, inhalation and oral routes of exposure (Table 1, Appendix 2). To calculate the equivalent oral dose, this systemic dose of 0.16 mg/kg-bw/day was corrected by applying the reciprocal of the oral factor, resulting in an equivalent systemic dose of 0.21 mg/kg-bw/day. Using this calculated upper-bounding estimate of 0.21 mg/kg-bw/day, a comparison with the critical effect level for repeated dosing via the oral route (100 mg/kg-bw/day) resulted in a margin of exposure of approximately 480 from use of personal care products. However, it is considered that the exposure estimates presented above are overestimates of actual exposure based on an independent review of the submitted probabilistic assessment and information indicating that the percentage of personal care products containing D5 on the Canadian market may be lower than assumed in deriving exposure estimates. Based on values derived from the independent review of the probabilistic exposure assessment, it appears that the margin of exposure from use of personal care products would be at least 10 times higher for adults and at least 5 times higher for children from that shown above (i.e. > 2000). On the basis of the above considerations, including consideration for the extent of its database, D5 is considered not to meet the criteria under paragraph 64(c) of CEPA 1999.
Uncertainties in Evaluation of Risk to Human Health
The scope of this screening assessment does not take into consideration a full analysis of the mechanism of action of decamethylcyclopentasiloxane and it does not take into account possible differences between humans and experimental species in sensitivity to effects induced by this substance. There is uncertainty surrounding the mechanism of carcinogenicity following exposure via the inhalation route. There is also uncertainty as to the mechanism of action resulting in liver effects following exposure via the inhalation or oral routes.
Potential adverse effects of D5 via the oral route were based on using D4 and D6 as analogues and there is uncertainty on the boundaries/limits for using D4 and D6 to extrapolate effects for D5.
Although physiologically based pharmacokinetic modelling of dermal absorption data have been published (Reddy et al.2007), only experimental data on absorption were used in this assessment.
There is uncertainty regarding the estimation of exposure and systemic dose because of the use of modelling and a lack of Canadian data. There is uncertainty associated with the use of models and the choice of variables related to the use of consumer products including quantity and frequency of use, absorbed fraction and environmental parameters.
The cumulative exposures of the cyclosiloxanes in polydimethylsiloxanes (PDMS) are not considered in this assessment. However, D4 and D6 are considered in separate assessments.
Based on the information presented in this screening assessment on the potential of D5 to cause ecological harm, it is concluded that decamethylcyclopentasiloxane is entering or may be entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity.
Based on the available information on its potential to cause harm to human health, it is concluded that decamethylcyclopentasiloxane is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that decamethylcyclopentasiloxane meets the definition of toxic as set out in paragraph 64a of CEPA 1999. It is concluded that D5 meets the criteria for persistence as set out in the Persistence and Bioaccumulation Regulations. However, it is not possible to conclude that D5 meets the criterion for bioaccumulation, considering the conflicting evidence presented in this screening assessment report.
The conclusion in this screening assessment is based on the available information at this time and acknowledges that there are uncertainties associated with this assessment. Research on cVMS is currently being conducted to help address these uncertainties, but some of this research has not been completed at this time. In the context of the Challenge program, any new information provided after the final screening assessment may be considered during the risk management phase.
Monitoring has also been identified as a key component in the Chemicals Management Plan in Canada and D5 is being considered for environmental monitoring under the Plan. Field level data will contribute to a better understanding of the distribution of D5 in the environment and its bioaccumulation potential in relevant food webs.
Allen RB, Kochs P, Chandra G. 1997. Industrial organic materials, their environmental entry and predicted fate. In: Organosilicon Materials, Hutzinger O. editor, Handbook of Environmental Chemistry. Berlin: Springer-Verlag. P.1-25.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb. Sci. 22(3): 337-345.
Arnot JA, Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev. 14(4): 257-297.
Arnot JA, Mackay D, Bonnell M. 2008a. Estimating Metabolic Biotransformation Rates in Fish from Laboratory Data. Environ. Toxicol. Chem. 27(2): 341–351.
Arnot JA, MacKay D, Parkerton T, Bonnell M. 2008b. A database of fish biotransformation rate constants. Environ Sci Technol (in press).
Atkinson R. 1989. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organics compounds. Journal of Physical and Chemical Reference Data. Monograph No. 1.
Atkinson R. 1991. Kinetics of the gas-phase reactions of a series of organosilicon compounds with OH and NO3radicals and O3 at 297 ± 2 K. Environmental Science and Technology. 25(5): 863-866.
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing Long-Range Transport Potential of Persistent Organic Pollutants. Environ Sci Technol 34(4): 699-703.
Bidleman TF. 2008. Review of the Dow-Corning Health & Environmental Sciences Technical Reports: "Hydrolysis of Octamethylcyclopentasiloxane (D4)" and "Hydrolysis of Decamethylcyclopentasiloxane (D5)". Centre for Atmospheric Research Experiments (Egbert, ON.). Science and Technology Branch, Environment Canada.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Boehmer T, Gerhards R. 2003. Decamethylcyclopentasiloxane (D5) a compilation of environmental data. Centre Européen des Silicones (CES).
Bruggeman WA, Weber-Fung D, Opperhuizen A, Van Der Steen J, Wijbenga A, Hutzinger O. 1984. Absorption and retention of polydimethylsiloxanes (silicones) in fish: preliminary experiments. Toxicological and Environmental Chemistry. 7:287-296.
Burns-Naas LA, Mast RW, Klykken PC, McCay JA, White KL, Jr., Mann PC, Naas DJ. 1998a. Toxicology and humoral immunity assessment of decamethylcyclopentasiloxane (D5) following a 1-month whole body inhalation exposure in Fischer 344 rats. Toxicol Sci 43(1):28-38.
Burns-Naas LA, Mast RW, Meeks RG, Mann PC, Thevenaz P. 1998b. Inhalation toxicology of decamethylcyclopentasiloxane (D5) following a 3-month nose-only exposure in Fischer 344 rats. Toxicol Sci 43(2):230-40.
Canada. 1999. Canadian Environmental Protection Act, 1999. S. C., 1999 c.33. Canada Gazette, Part III, Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, Vol. 134, No. 7.
Canada. Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49.
Canada. Dept. of the Environment, Dept. of Health. 2007. Canadian Environmental Protection Act, 1999: Notice of second release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, Vol. 141. No. 19.
[CATABOL] [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Prof. Assen Zlatorov University. Laboratory of Mathematical Chemistry. [2008 February 4]. OASIS Software.
[CNS] Cosmetic Notification System [proprietary database]. 2007. Ottawa (ON): Health Canada. [cited 2008 Jan.]
David M, Fendinger N, Hand V. 2000. Determination of Henry's Law Constants for organosilicones in actual and simulated wastewater. Environ Sci Technol 34: 4554-4559.
Domoradzki J. 2008a. Refinement in the determination of the BMF value for D4 from a fish feeding study in rainbow trout. Midland (MI) Dow Corning Corporation, Health and Environmental Sciences.
Domoradzki J. 2008b. Refinement in the determination of the BMF value for D5 from a fish feeding study in rainbow trout. Midland (MI) Dow Corning Corporation, Health and Environmental Sciences.
Dow Corning. 1990a. A 14-day subchronic oral gavage study with D5 in rats. Report no 1990-I0000-35074. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2007. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2008].
Dow Corning. 1990b. A 28-day subchronic oral gavage feasibility study of various low molecular weight silicone oligomers in rats. Report no. 1990-I0000-35105. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Dow Corning. 1990c. A 28-day dermal toxicity study of decamethylcyclopentasiloxane (D5) in rats. Report no. 1990-I0000-35172-11, Dow Corning Corporation, March 12, 1990. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Dow Corning. 1990d. A 90-day inhalation study of decamethylcyclopentasiloxane (D5) in rats. Report no. 1990-I0000-35190, Dow Corning Corporation, March 19, 1990. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Dow Corning. 1994. 4-Hour acute inhalation toxicity study with decamethylcyclopentasiloxane in rats. Report no 1994-I0000-39167. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Dow Corning Corporation. 1998. An oral gavage study to compare the absorption potential of 14C-D4 in Fischer rats when delivered in various carriers. Report No. 1998-10000-44815. [cited in SCCP 2005, ref. 35].
Dow Corning. 2004a. Analysis of the genotoxic potential of decamethylcyclopentasiloxane (D5) in fischer-344 rats following whole body vapor inhalation for 7 days. Report nr 2003-I0000-53252. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Dow Corning. 2004b. In vitro chromosome aberration test in Chinese hamster V79 cells with decamethylcyclopentasiloxane (D5). Report nr 2003-I0000-52921. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Dow Corning. 2004c. Salmonella Typhimurium and Escherichia Coli reverse mutation assay with decamethylcyclopentasiloxane (D5). Report no 2003-I0000-52921. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Dow Corning (Corporation). 2005. Decamethylcyclopentasiloxane (D5): A 24-month combined chronic toxicity and carcinogenicity whole body vapor inhalation study in Fischer 344 rats. Study No. 9346, Report Number: 2003-I0000-53252.
[DPD] Drug Product Database [database on the internet]. 2007. Ottawa (ON): Health Canada. [cited 2008 Jan.]
Drottar K. 2007. 14C-Decamethylcyclopentasiloxane (14C-D5): Dietary bioaccumulation in the rainbow trout (Oncorhynchus mykiss) under flow-through conditions. Dow Corning Report No. 2007-I0000-57314.
Drottar K. 2006. 14C-Octamethylcyclotetrasiloxane (14C-D4): Dietary bioaccumulation in the rainbow trout (Oncorhynchus mykiss) under flow-through conditions. Dow Corning Report No. 2007-I0000-57314.
Drottar KR. 2005. 14C- Decamethylcyclohexasiloxane (14C-D5): Bioconcentration in the fathead minnow (Pimephales promelas) under flow-through test conditions. Dow Corning Corporation. Health and Safety Council. Study Number 9882-102.
Durham J. 2006. Hydrolysis of octamethylcyclotetrasiloxane (D5). Silicones Environment, Health and Safety Council. Study Number 10040-102.
Durham J, Kozerski G. 2005. Hydrolysis of octamethylcyclotetrasiloxane (D4). Silicones Environment, Health and Safety Council. Study Number 10000-102.
[ECB] European Chemicals Bureau. 2007. ESIS (European Chemical Substances Information System), Version 4.60. [cited 2007 Dec.]
[ECOSAR] Ecological Structural Activity Relationships [Internet]. [2004] Version 0.99g. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Environment Canada. 1988. Data relating to the Domestic Substance List (DSL) 1984-1986, collected under CEPA, 1988, s. 25(1). Based on: Reporting for the Domestic Substance List [guide] 1988 Data prepared by: Environment Canada.
Environment Canada. 2007. Data for Batch 2 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain Batch 2 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2008a. Assumptions, limitations and uncertainties of the mass flow tool for decamethylcyclopentasiloxane CAS RN 541-02-6. Existing Substances Division, Environment Canada, Gatineau (QC). Internal draft document available on request.
Environment Canada. 2008b. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Overview of Aquatic Risk Distribution Methodology. Working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008c. Aquatic risk distribution summary for decamethylcyclopentasiloxane, CAS RN 541-02-6. 2008-09-08. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, The Canadian Environmental Modelling Centre and Chemistry - EQC Model.
Fackler PH, Dionne E, Hartley DA, Hamelink JL. 1995. Bioconcentration by fish of a highly volatile silicone compound in a totally enclosed aquatic exposure system. Environmental Toxicology and Chemistry 14(10):1649-1656.
Falany CN, Li G. 2005. Effects of age and pregnancy on cytochrome P450 induction by octamethyltetracyclosiloxane in female Sprague-Dawley rats. J Biochem Mol Toxicol. 19(2):129-138.
Fenner K, Scheringer M, MacLeod M, Matthies M, McKone TE, Stroebe M, Beyer A, Bonnell M, Le Gall A, Klasmeier J et al. 2005. Comparing estimates of persistence and long-range transport potential among multimedia models. Environmental Science and Technology 39:1932-1942.
Flaningam OL. 1986. Vapor pressure of poly (dimethylsiloxane) oligomers. J Chem Eng Data 31:266-272.
[GEMStat] Global Water Quality Data and Statistics [database on the internet] Burlington (OM) United Nations. Global Environment Monitoring System (GEMS) Water Programme.. [cited 2008 September]. About GEMStat.org.
Gobas FAPC, Kelly BC, Arnot JA. 2003. Quantitative Structure Activity Relationship for predicting the bioaccumulation of POPs in terrestrial food-webs. QSAR. 22 : 329-335.¸
Green Earth Cleaning. 2008.
Han X, Nabb DL, Mingoia RT, Yang C-H. 2007. Determination of xenobiotic intrinsic clearance in freshly isolated hepatocytes from rainbow trout (Oncorhynchus mykiss) and rat and its application in bioaccumulation assessment. Environ. Sci. Technol. 41: 3269 -3276.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate. Available upon request.
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Herner AV, Flassbeck D, Gruemping R. 2002. Organosilicon Compounds in the Environment. In: Craig PJ, editor, Organometallic Compounds in the Environment. 2nd ed. New York. John Wiley & Sons, Ltd. p. 324.
Hu T-M, Layton WL. 2001. Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPS PharmSci. 3(4) Article 29 cited 2008 Oct].
Huntingdon Research Center. 1979. Twenty-one day repeated dermal in the rabbit of material SF-1202. Project no. 792048. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Hurd CB. 1946. Studies on siloxanes. 1. The specific volume and viscosity in relation to temperature and constitution. J Am Chem Soc 68(3):364.
Isquith A, Matheson D, Slesinski R. 1988. Genotoxicity studies on selected organosilicon compounds: in vitro assays. Food Chem Toxicol 26(3):255-61.
Jager R, Hartmann E. 1991. Subchronische toxikologische untersuchungen an ratten (magensondenapplikation uber13 Wochen oral gavage study with D5 in rats. Report nr 20204. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Jovanovic ML, McMahaon JM, McNett DA, Tobin JM, Plotzke KP. 2008. In vitro and in vivo percutaneous absorption of 14C-octmethylcyclotetrasiloxane (14C-D4) and 14C-decamethylcyclopentasiloxane (14C-D5). Regul Toxicol Pharmacol 50: 239-248.
Kaj L, Andersson J, Palm Cousins A, Remberger M, Ekheden Y, Dusan B, and Bror-ström-Lundén E. 2005. Results from the Swedish National Screening programme 2004: Subreport 4: Siloxanes. IVL.
Klasmeier J, Matthies M, MacLeod M, Fenner K, Scheringer M, Stroebe M, Le Gall AC, McKone TE, van de Meent D, Wania F. 2006. Application of multimedia models for screening assessment of Long-Range Transport Potential and overall persistence, Environmental Science and Technology. 40(1) : 53–60.
Kochetkov A, Smith JS, Ravikrishna R, Valsaraj KT, Thibodeaux LJ. 2001. Air-water partition constants for volatile methyl siloxanes. Environmental Toxicology and Chemistry. 20(10):2184–2188.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Kozerski G. 2007. Determination of the 1-Octanol/Water Partition Coefficient of Decamethylcyclopentasiloxane (D5) by the Slow-Stirring Method Using Gas Chromatography and Mass Spectrometry. Silicones Environmental, Health, and Safety Council (SEHSC).
Kozerski G. 2008. SEHSC response to Dr. Bidleman's review on hydrolysis studies of D4 and D5. Dow Corning Corporation. July 2008.
Kramp F, Volz-Thomas A. 1997. On the budget of OH radicals and ozone in an urban plume from the decay of C5-C8 hydrocarbons and NOx. Journal of Atmospheric Chemistry. 28(1-3):263-282.
Krötlinger F. 1988. Subakute toxikolische Untersuchungen an Kanninchen. Bayer AG. Report no. R 4374, April 13, 1988. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Krueger HO, Thomas ST, Kendall TZ. 2007. D5: a prolonged sediment toxicity test with Lumbriculus variegatus using spiked sediment. Wildlife International, LTD. Project Number 583A-108. Centre Européen des Silicones (CES).
Krueger HO, Thomas ST, Kendall TZ. 2008. D5: a prolonged sediment toxicity test with Chironomus riparius using spiked sediment. Wildlife International, LTD. Project Number 570A-108. Silicones Environmental, Health, and Safety Council (SEHSC).
Lassen C, Hansen CL, Mikkelson SJ, Maag J. 2005. Siloxanes - consumption, toxicity and alternatives. Danish Ministry of the Environment, Environmental Protection Agency (Danish EPA). Environmental Project No. 1031.
Litton Bionetics, Inc. 1978. Project No. 20893; Mutagenicity evaluation of decamethylcyclopentasiloxane (Me2SiO)5, Final Report April, 1978. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Löser E. 1984. Untersuchungen zur akuten oralen toxizitat an mannlichen und weblichen Wistar ratten. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Lucas SV. 1984. GC/MS analysis of organics in drinking water concentrates and advanced waste treatment concentrates. Vol. 1. Analysis results for 17 drinking water and 16 advanced waste treatment and 3 process blank concentrate. EPA-600/1-84-020A. (NTIS P85-128221). Columbus, (OH):. Columbus Labs. Health Effects Research Laboratory. p. 45, 46, 147, 150.
Maxim LD. 1998. D4, D5, and D6 Exposure in the Manufacture and Use of Personal Care Products: An Interim Assessment. Dow Corning Corporation.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Navea JG, Stanier CO, Young MA, Grassian VH. 2007. A laboratory and modeling study at the University of Iowa designed to better understand the atmospheric fate of D4 and D5. Technical annual report (August 2006-July 2007). The University of Iowa, Department of Chemistry, and Chemical and Biochemical Engineering, Iowa City, (IA).
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Columbus (OH): American Chemical Society. [cited 2006 Oct 11]. National Chemical Inventories (NCI) on CD.
[NHW] National Health and Welfare. 1990. Present patterns and trends in infant feeding in Canada. Department of National Health and Welfare, Ottawa. [cited in Health Canada 1998].
Nichols JW, Fitzsimmons PN, Burkhard LP. 2007. In vitro – in vivo extrapolation of quantitative hepatic biotransformation data for fish. II. Modeled effects on chemical bioaccumulation. Environ. Toxicol. Chem. 26(6): 1304-1319.
[NILU] Norsk institutt for luftforskning. 2007. Siloxanes in the Environment of the Inner Oslofjord. Repport No. 986/2007. Kjeller(NO) Norvegian Institute of Air Research.
[NMI] Non-Medicinal Ingredients [proprietary database]. 2007. Ottawa (ON): Health Canada. [cited 2008 Jan.]
[NOAA] National Oceanic and Atmospheric Administration. 2008. NOAA CoastWatch Great Lakes Program. NOAA Great Lakes Environmental Research Laboratory [cited 2008 Sep].
Norden. 2005. Siloxanes in the Nordic Environment. TemaNord 2005:593. Copenhagen (NO), Nordic Council of Ministers.
[NYIEQ] New York Indoor Environmental Quality Center. 2005. Indoor Environmental Quality: Assessing and Mitigating the Impact of Exposure to Multiple Indoor Contaminants. Project No. R828605-01.
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission Scenario Document on Plastics Additives [Internet]. Paris (FR): OECD Environmental Directorate, Environmental Health and Safety Division. [cited 2004 Sep].
[OECD] Organisation for Economic Co-operation and Development. 2006. Draft Emission Scenario Document on Transport and Storage of Chemicals. Prepared by the Environment Agency (UK). Available on request from: Environment Canada, Existing Substances Division, Ottawa, K1A 0H3.
[OECD] Organisation for Economic Co-operation and Development. 2007. Manual for investigation of HPV chemicals. OECD Secretariat, July 2007. [cited 2008 Jan.]. Manual for the Assessment of Chemicals.
Pauluhn J. 1984. Untersuchungen zur akuten inhalationstoxizität. Bayer AG. Report no. 13142, December 18, 1984. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of o decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2006. Syracuse (NY): Syracuse Research Corporation. [cited 2006 Mar] Interactive PhysProp Database Demo.
[PMRA] Pest Management Regulatory Agency. 2007. Regulatory Note REG 2007-04: PMRA list of formulants [Internet]. Ottawa (ON): Health Canada, Pest Management Regulatory Agency. [cited 2008 Sep].
Powell D, Kozerski G. 2007. Cyclic methylsiloxane (cVMS) materials in surface sediments and cores for Lake Ontario. Centre Europeen des Silicones (CES). Draft Report.
Powell DE. 2008. Interim update on cyclic methylsiloxane (cVMS) materials in surface sediment, cores, and zooplankton for Lake Opeongo, Ontario, Canada. Centre Europeen des Silicones (CES). July 14, 2008.
Quinn AL, Regan JM, Tobin JM, Marinik BJ, McMahon JM, McNett DA, Sushynski CM, Crofoot SD, Jean PA,Plotzke KP. 2007. In vitro and in vivo evaluation of the estrogenic, androgenic, and progestagenic potential of two cyclic siloxanes. Toxicol Sci 96(1):145-53.
RCC Group. 1995. One-month repeated dose inhalation toxicity with D5 in rats. Report no. 1995-I0000-40185, March 13, 1995. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. February 13, 2007].
Reddy MB, Looney RJ, Utell MJ, Plotzke KP, Andersen ME. 2007. Modeling of human dermal absorption of octamethylcyclotetrasiloxane (D4) and decamethylcyclopentasiloxane (D5). Toxicol Sci 99(2): 422-431.
Ren X, Harder H, Martinez M, Lesher RL, Oliger A, Shirley T, Adams J, Simpas JB, Brune WH. 2003. HOx concentrations and OH reactivity observations in New York City during PMTACS-NY2001. Atmospheric Environment 37:3627-3637.
Ren X, Brune WH, Mao J, Mitchell MJ, Lesher RL, Simpas JB, Metcalf AR, Schwab JJ, Cai C, Li Y et al. 2006. Behaviour of OH and HO2 in the winter atmosphere in New York City. Atmospheric Environment 40:S252-S263.
Rivett AC, Martin D, Gray DJ, Price CS, Nickless G, Simmonds PG, O'Doherty SJ, Greally BR, Knights A, Shallcross DE. 2003. The role of volatile organic compounds in the polluted urban atmosphere of Bristol, UK. Atmos Chem Phys Discuss 3:769-796.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2006. Consumer Exposure (ConsExpo) Model - Version 4.1 [Internet]. Version 4.1. The Netherlands: The National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu).
[SCCP] Scientific Committee on Consumer Products. 2005. Opinion on octamethylcyclotetrasiloxane (D4) cyclomethicone (INCI name). (Adopted by the SCCP during the 6th plenary meeting of 13th December 2005). European Commission, Health & Consumer Protection Directorate-General (Directorate C - Public Health and Risk Assessment). SCCP/0893/05.
Scheringer M, MacLeod M, Wegmann F. 2006. The OECD POV and LRTP Screening Tool, Version 2.0. Distributed at OECD/UNEP Workshop on “Application of Multimedia Models for Identification of Persistent Organic Pollutants”, Ottawa, Canada, May 31 to June 3, 2006.
[SEHSC] Silicones Environmental, Health and Safety Council. 2005a. IUCLID Dataset for CAS No. 541-05-9. Submitted by Silicones Environmental, Health and Safety Council, Herndon, VA. September, 2005.
[SEHSC] Silicones Environmental, Health and Safety Council. 2005b. Degussa Corporation/Goldschmidt GmbH: TSCA 8(e): Notification of Substantial Risk; Detection of decamethylcyclopentasiloxane and octamethylcyclotetrasiloxane in the tissue of fish from the Rhine River in Germany, October 10, 2005. Data submitted to Environment Canada by the SEHSC, Herndon, VA, December 20, 2005. Environment Canada submission # s70-2006-007.
[SEHSC] Silicones Environmental, Health and Safety Council. 2007a. SEHSC presentations to EC/HC on October 2nd, 2007
[SEHSC] Silicones Environmental, Health and Safety Council. 2007b. SEHSC communication to EC/HC on CAS No. 69430-24-6, 2007
[SEHSC] Silicones Environmental, Health and Safety Council. 2008a. Additional toxicity and exposure information for a screening health assessment of decamethylcyclopentasiloxane (D5), CAS No. 541-02-6. November 13, 2007 (Updated February 13, 2008).
[SEHSC] Silicones Environmental, Health and Safety Council. 2008b. Silicone industry comments on Health and Environment Canada's Draft Screening Assessment of D5: Special pattern of cVMS environmental release and its effects on their half-lives in the atmosphere. July 16, 2008.
[SEHSC] Silicones Environmental, Health and Safety Council. 2008c. Silicone industry comments on Health and Environment Canada's Draft Screening Assessment of D5. July 16, 2008.
Sible V. 2006. Determination of n-octanol/water partition coefficient of 14C-decamethylcyclopentasiloxane (14C-D5) by Liquid Scintillation Counting. SEHSC Report Study No. 9803-102. August 2, 2006.
Siddiqui WH, Stump DG, Reynolds VL, Plotzke KP, Holson JF, Meeks RG. 2007. A two-generation reproductive toxicity study of decamethylcyclopentasiloxane (D5) in rats exposed by whole-body vapor inhalation. Reprod Toxicol 23(2): 216-225.
Sousa JV, McNamara PC, Putt AE, Machado MW, Surprenant DC, Hamelink JL, Kent DJ, Silberhorn EM, Hobson JF. 1995. Effects of octamethylcyclotetrasiloxane (OMCTS) on freshwater and marine organisms. Environmental Toxicology and Chemistry 14(10):1639-1647.
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2007. Copenhagen (DK): Nordic Council of Ministers. [cited 2007 Feb].
Springborn Laboratories. 2000. Decamethylcyclopentasiloxane - 14-day prolonged acute toxicity to rainbow trout (Oncorhynchus mykiss) under flow-through conditions. Report No. 12023.6125. Silicones Environmental, Health and Safety Council (SEHSC).
Springborn Laboratories. 2001. Decamethylcyclopentasiloxane (D5) - toxicity to the freshwater green alga, Pseudokirchneriella subcapitata. Report No. 12023.6126. Silicones Environmental, Health and Safety Council (SEHSC).
Springborn Smithers Laboratories. 2002. Decamethylcyclopentasiloxane - acute toxicity to daphnids (Daphnia magna) under static conditions. Report No. 12023.6129. Silicones Environmental, Health and Safety Council (SEHSC).
Springborn Smithers Laboratories. 2003a. Decamethylcyclopentasiloxane (D5) - full life-cycle toxicity with water fleas (Daphnia magna) under static-renewal conditions. Report No. 12023.6141. Silicones Environmental, Health and Safety Council (SEHSC).
Springborn Smithers Laboratories. 2003b. Decamethylcyclopentasiloxane (D5) - the full life-cycle toxicity to midge (Chironomous riparius) under static conditions. Report No. 12023.6140. Silicones Environmental, Health and Safety Council (SEHSC).
Springborn Smithers Laboratories. 2004. Cyclopentasiloxane, decamethyl-(D5) and cyclotrisiloxane, hexamethyl-(D3) – determination of the biodegradability of a test substance. Study No. 12023.6142. Silicones Environmental, Health and Safety Council (SEHSC).
Springer T. 2007. Decamethylcyclopentasiloxane (D5): a 96-hour study of the elimination and metabolism of orally gavaged 14C-D5 in rainbow trout (Oncorhynchus mykiss). HES Study Number: 10218-101. Centre Européen des Silicones (CES).
[TaPL3] Long Range Transport and Persistence Level III model [Internet]. 2000. Version 2.10. Peterborough (ON): Trent University, The Canadian Environmental Modelling Centre and Chemistry - TaPL3.
TNO. 1984. Sub-acute inhalation toxicity study of silicone oil KF995 in rats. Report nr V84.389/231262. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of octamethylcyclotetrasiloxane (D4), CAS No. 556-67-2. February 13, 2007].
Tobin JM, McNett DA, Durham JA, Plotzke KP. 2008. Disposition of decamethylcyclopentasiloxane in Fischer 344 rats following single or repeated inhalation exposure to 14C-decamethylcyclopentasiloxane (14C-D5). Inhalation Toxicology 20: 513-531.
[US EPA] United States Environmental Protection Agency. 1992. Thirtieth report of the Interagency Agency Testing committee to the Administrator, receipt of report and request for comments regarding Priority Testing List of chemicals. July 9, 1992. Federal Register. 57(132):30603-30618.
[US EPA] United States Environmental Protection Agency. 1994. 1994a. Federal Register Environmental Documents - Air Quality: Revision to Definition of Volatile Organic Compounds - Exclusion of Volatile Methyl Siloxanes and Parachlorobenzotrifluoride [cited 2008 Feb].
[US EPA] United States Environmental Protection Agency. 2002. Hepatocellular hypertrophy: HED Guidance Document #G2002.01. Unpublished document. Washington (DC) US EPA, Health Effects Division, Toxicology Science Advisory Council. October 21, 2002
[US EPA] United States Environmental Protection Agency. 2005. Siloxane D5 in Drycleaning Applications - Fact Sheet.
[US EPA] United States Environmental Protection Agency. 2007. High Production Volume (HPV) - Sponsored Chemicals, September 2007. [cited 2008 Feb].
Varaprath S, Frye CL, Hamelink J. 1996. Aqueous solubility of permethylsiloxanes (silicones), Short Communication. Environmental Toxicology and Chemistry. 15(8):1263-1265.
Varaprath S, McMahon JM, Plotzke KP. 2003. Metabolites of hexamethyldisiloxane and decamethylcyclopentasiloxane in Fischer 344 rat urine--a comparison of a linear and a cyclic siloxane. Drug Metab Dispos 31(2):206-14.
Wania F. 2003. Assessing the potential of persistent organic chemicals for long-range transport and accumulation in polar regions. Environ Sci Technol. 37(7): 1344-1351.
Wania F. 2006. Potential of degradable organic chemicals for absolute and relative enrichment in the Arctic. Environ Sci Technol. 40(2): 569-577.
WIL Research Laboratories Inc. 1996. An inhalation range finding reproductive toxicity study of D5 in the rat. Report no. 1996-I0000-41336, August 27, 1996. [cited in SEHCS (Silicones Environmental, Health and Safety Council). 2008. Additional toxicity and exposure information for a screening health assessment of octamethylcyclotetrasiloxane (D4), CAS No. 556-67-2. February 13, 2007].
Will R, Löchner U, Masahiro Y. 2007. CEH Marketing Research Report Siloxanes. Menlo Park (CA). SRI Consulting.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41 Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Xu S, Lehmann RG, Miller JR, Chandra G. 1998. Degradation of polydimethylsiloxanes (silicones) as influenced by clay minerals Environ Sci Technol 32: 1199-1206.
Xu S. 1999. Fate of cyclic methylsiloxanes in soils. 1. The degradation pathway. Environmental Science and Technology 33(22): 603-608.
Xu S, Chandra G. 1999. Fate of cyclic methylsiloxanes in soils. 2. Rates of degradation and volatilization. Environmental Science and Technology 33:4034-4039.
Xu S. 2006. 1-Octanol/air partitioning coefficients of octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), and dodecamethylcyclohexasiloxane (D6) at different temperatures. Centre Européen des Silicones (CES). CES Report December 27, 2006.
Xu S, Kropscott G. 2007. Simultaneous determination of partition coefficients for octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane. Draft Report. Dow Corning non-regulated technical report. DCC study # 10336-101.
Xu S, Miller JA. 2008. Aerobic transformation of octamethylcyclotetrasiloxane (D4) in water/sediment system. Centre Europeen des Silicones (CES). Interim report.
Zhang J, Falany JL, Xie X, Falany CN. 2000. Induction of rat hepatic drug metabolizing enzymes by dimethylcyclosiloxanes. Chem Biol Interact 124(2):133-47.
Appendix of the Screening Assessment
- Appendix 1: Upper-bounding Estimates of Daily Intake of D5 by the General Population in Canada (µg/kg-bw/day by Various Age Groups)
- Appendix 2: Consumer Exposure Modelling
- Appendix 3: Summary of Health Effects Information for Decamethylcyclopentasiloxane (D5)
- Appendix 4: Review of D5 Probabilistic Exposure Assessment
- Appendix 5: Multimedia Modelling Input Parameters for D5 in the Ecological Screening Assessment
Footnote
Page details
- Date modified: