Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Siloxanes and Silicones, Me 3,3,3-trifluoropropyl,
Me vinyl, hydroxy-terminated
Chemical Abstracts Service Registry Number
68952-02-3
Environment Canada
Health Canada
September 2011
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Characterization of Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential for Bioaccumulation
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix I: Relationship between viscosity, degree of polymerisation and molecular weight
- Appendix II: PBT Model Inputs Summary Table
- Appendix III: Available information on the acute mammalian toxicity of fluorinated siloxanes
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on siloxanes and silicones, Me 3,3,3-trifluoropropyl, Me vinyl, hydroxy-terminated (MVTFS), Chemical Abstracts Service Registry Number[1] 68952-02-3. This substance was identified as a high priority for screening assessment and included in the Challenge initiative under the Chemicals Management Plan because it had been found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
The substance, MVTFS, was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed for categorization of substances on the Domestic Substances List (DSL).
MVTFS was originally classified as an organic UVCB (Unknown or Variable Composition, Complex Reaction Products or Biological Material) during the DSL Categorization. Based on new information received, the substance is considered to be an organic siloxane polymer substance.
The substance is used in Canada primarily for the manufacturing of adhesives and synthetic rubber. It is not naturally produced in the environment. It is not reported to be manufactured in Canada; however, between 10 000 and 100 000 kg of the polymer were imported into the country in 2006.
Based on certain assumptions and reported use patterns in Canada, most of the substance is expected to end up in waste disposal sites. A small fraction is estimated to be released to wastewater, and to a lesser extent air and land, during the industrial use stage.
Based on the available information, it is determined that the form of MVTFS in commerce in Canada meets the Reduce Regulatory Requirement polymer criteria as specified in the New Substances Notification Regulations (Chemicals and Polymers). MVTFS is expected to be non-volatile and insoluble in water, with a specific gravity heavier than water. The substance is expected to display resistance to heat, some fluid and chemical attack, as well as to demonstrate a low glass transition temperature. The polymer is anticipated to exist in a rubber-like state at environmental temperatures and remain functional across a wide range of temperatures.
Based on the read-across data of the physical and chemical properties for its analogues, MVTFS is expected to be persistent in the environment. Based on consideration of recently identified information on the bioaccumulation of an analogous polymer, and taking into account the high molecular weight of MVTFS, the polymer is not likely to be bioavailable to environmental organisms and is not anticipated to have significant potential for bioaccumulation. It is therefore concluded that the substance meets the persistence criteria but does not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. In addition, identified experimental toxicity data for an analogous polymer and the model predictions on the hydrolysis products indicate that the polymer has a low potential to cause harm to organisms in the environment.
For this screening assessment, a conservative exposure scenario was developed in which an industrial operation (user of the polymer) discharges MVTFS into the aquatic environment. The predicted environmental concentration in water (PEC) for the polymer was well below the predicted no-effect concentration (PNEC) for aquatic organisms. Therefore, based on the information presented in this screening assessment, it is concluded that MVTFS is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Empirical health effects data were not identified for MVTFS. Based on limited health effects data for fluorosilicones which have similar structures, MVTFS is not considered to demonstrate high hazard potential.
Exposure of the general population to MVTFS through environmental media (air, drinking water and soil), or through food and beverages, is expected to be negligible. General population exposure from use of consumer products containing MVTFS is not expected. Accordingly, risk to human health from exposure to MVTFS in Canada is considered to be low. It is thus concluded that MVTFS is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Given the complexity associated with the polymer formulation and the potentially hazardous properties associated with low molecular weight polymers, there is concern that new activities for MVTFS which have not been identified or assessed under CEPA 1999 could lead to the substances meeting the criteria as set out in section 64 of the Act. Therefore, it is recommended that the DSL be amended to indicate that MVTFS meets the Reduced Regulatory Requirement (RRR) Polymer criteria. Should other forms of MVTFS, not meeting the Reduced Regulatory Requirement polymer criteria, be introduced on the Canadian market, those forms would be subject to the requirements of the New Substances Notification Regulations.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in theCanada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance siloxanes and silicones, Me 3,3,3-trifluoropropyl, Me vinyl, hydroxy-terminated (MVTFS) had been identified as a high priority for assessment of ecological risk during the DSL Categorization. The substance, based on model predictions, had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on September 26, 2009 (Canada 2009). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the properties and uses of the substance were received.
Although MVTFS was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
Screening assessments focus on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution[2].
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to May 2010 for the human health sections and December 2010 for the ecological sections of the document. Key studies were critically evaluated; modelling results may have been used to reach conclusions.
When available and relevant, information presented in hazard assessments from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological portion of this assessment has undergone external written peer review/consultation. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. Approaches used in the screening assessments under the Challenge have been reviewed by an independent Challenge Advisory Panel.
The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this document, the substance, siloxanes and silicones, Me 3,3,3-trifluoropropyl, Me vinyl, hydroxy-terminated, will be referred to as MVTFS, derived from the common name on the Domestic Substances List (DSL).
MVTFS was originally classified as an organic UVCB during the DSL Categorization. Based on new information received, the substance can be characterized by 1) the monomer units of methyl-trifluoropropyl-siloxy and methyl-vinyl-siloxy, and 2) two methyl groups and one hydroxyl group attached to the silicon atom functioning as the polymer terminating groups (Environment Canada 2010a).
According to results from gel permeation chromatography (GPC) analysis, MFTVS has been reported to have a number-average molecular weight (Mn) of approximately 358 000 g/mol (Environment Canada 2010a). Considerably smaller polymers (with a molecular weight as low as ~30 000 g/mol) have been detected in the polymer product, but at a very low concentration (< 1% by weight). Differences in molecular weights (MW) are primarily attributable to differences in the number of units. Therefore, MVTFS meets the definition of a polymer (OECD 1994), as summarized below:
- molecules characterized by the sequence of one or more types of monomer units;
- simple weight majority of molecules containing three or more monomer units that are covalently bound to one or more other monomer units or reactants; less than a simple weight majority of molecules of the same molecular weight; and
- molecules distributed over a range of molecular weights where the differences in the molecular weights are primarily attributable to differences in the number of units.
The identity of MVTFS is summarized in Table 1 below.
Table 1. Substance identity for MVTFS
| Chemical Abstracts Service Registry Number (CAS RN) | 68952-02-3 |
| DSL name | Siloxanes and Silicones, Me 3,3,3-trifluoropropyl, Me vinyl, hydroxy-terminated |
| National Chemical Inventories(NCI) names[1] | Siloxanes and Silicones, Me 3,3,3-trifluoropropyl, Me vinyl, hydroxy-terminated (TSCA, DSL, REACH, ECL, PICCS, ASIA-PAC, NZIoC); Siloxanes and silicones, methyl 3,3,3-trifluoropropyl, methyl vinyl, hydroxy terminated (AICS) |
| Other names | Not available |
| Chemical group (DSL Stream) |
Polymer |
| Major chemical class or use | Organosilicones |
| Major chemical sub-class | Siloxanes |
| Chemical formula | Not available |
| Representative chemical structure used to run the estimation model [2] | 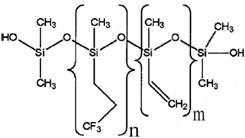 |
| SMILES[3] | Not available |
| Molecular mass | Number-average molecular weight: 358 000 g/mol (n=~2200, m=~20) Lowest molecular weight: 30 000 g/mol |
[2] This substance is an organic polymer; i.e., it is not a discrete chemical, and thus may be characterized by a representative structure.
[3] Simplified Molecular Input Line Entry System.
It is noted that the polymer may contain a small amount of impurities, i.e., residual monomers or by-products, which have much lower molecular weights. The quantities and the types of impurities vary, based on a number of factors, including different manufacturing processes and operations, reaction conditions, and use of catalysts and promoters. Based on the GPC analysis results, the smallest polymer has a molecular weight of 30 000 g/mol, indicating that MVTFS in commerce in Canada does not contain the low molecular weight impurities (Environment Canada 2010a). Therefore these impurities are not included in the assessment.
There have been very few experimental data reporting the physical and chemical properties of MVTFS.
According to a data submission, MVTFS in commerce in Canada has a high number-average molecular weight of approximately 358 000 g/mol (equivalent to approximately 2 200 repeating units of methyl-trifluoropropyl-siloxy and approximately 20 methyl-vinyl-siloxy, based on the molecular weight of the monomer units and the dominance of methyl-trifluoropropyl-siloxy in the polymer) (Environment Canada 2010a). The lowest molecular weight component is approximately 30 000 g/mol.
For the functional groups in the repeating unit, the trifluoropropyl group is anticipated to strongly bind to the silicon atom in the backbone of the polymer and release of this functional group from the repeating unit is unlikely (see discussion in the Environmental Persistence section related to degradation). The vinyl group in the repeating unit is somewhat reactive, but only exists in less than 1% of the total repeating unit. Given the above, the polymer meets Reduced Regulatory Requirement polymer criteria as specified in the New Substances Notification Regulations (Chemicals and Polymers).
Having a molecular weight much higher than the limit acceptable for the quantitative structure-activity relationship (QSAR) models, the polymer is outside the domains of applicability and no QSAR models can be used to assess the substance. Therefore, information on the analogous siloxane polymers, polymethyltrifluoropropylsiloxane (PMTFPS) and polydimethylsiloxanes (PDMS), has been used to fill knowledge gaps, and “read-across” data were considered when assessing MVTFS.
An analogue is a chemical which is structurally close to the substance under assessment and is therefore expected to have similarity in physical and chemical properties, environmental behaviour/fate, and/or toxicity. Where there are experimental data for a given parameter for an analogue substance, these can be used directly, or with adjustment, as an estimate of that parameter value for the substance under assessment.
Representative structures and available information of PMTFPS and PDMS are presented in Table 2 below.
Table 2. Physical and chemical properties of MVTFS, PMTFPS and PDMS
| Chemical | MVTFS | PMTFPS | PDMS |
|---|---|---|---|
| Representative structure | 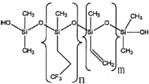 |
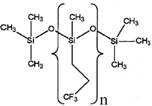 |
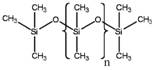 |
| Number-Average Molecular Weight (g/mol) | 358 000 | Not available | 1300 to 74000 |
| Smallest Molecular Weight (g/mol) | 30 000 | Not available | Not available |
| Physical State at the environmental temperatures | Rubber (read-across) |
Rubber | Liquid |
| Gravity (kg/m3) | 1,350 – 1650 (read-across) |
1,350 – 1,650 (experimental) |
960 (experimental) |
| Glass Transition Temperature (oC) | Low (read-across) |
-75 (experimental) |
Not applicable |
In general while PMTFPS is a closer structural analogue than PDMS, there are more relative data on the latter. As discussed below, PDMS has a much smaller molecular weight than MVTFS and remains in a liquid form at environmental temperatures, thus PDMS is more likely to be bioavailable than MVTFS, and makes a useful conservative choice of analogue for hazard characterization.
MVTFS vs. PMTFPS
Both MVTFS and PMTFPS contain the monomer unit of methyl-trifluoropropyl-siloxy in the polymer molecules. MVTFS also contains a small fraction (<1%) of the monomer unit of methyl-vinyl-siloxy in the backbone, and two methyl and a hydroxyl substituents in the terminating groups, while PMTFPS has three methyl substituents in the terminating groups. Due to the large molecular weight, the vinyl and the hydroxyl substituents in MVTFS are not anticipated to significantly affect the physical and chemical properties, i.e., vapour pressure, water solubility, octanol-water partition coefficient, or toxicity. The vinyl and hydroxyl substituents are present to permit crosslinking with other molecules, producing larger and less active molecules, which in turn makes MVTFS even less bioavailable and less potentially bioaccumulative.
Unlike a discrete non-polymeric substance, which can transform from a solid state to a liquid at its melting point, siloxane polymers with high molecular weights are usually characterized by the glass transition temperature (Tg), the temperature at which the mechanical behaviour of the polymer changes from rigid and brittle to tough and leathery. Fluoro-siloxane polymers are designed to remain functional over a wide temperature range (-60 to 200oC). For example, PMTFPS has a Tg at -75 oC and remains in a rubber-like state at room temperature. It also has a specific gravity of 1 350 – 1 650 kg/m3 (Drobny 2001).
Most high molecular weight siloxane polymers (>1000 g/mol) are non-volatile (the vapour pressure being <10-8 mm Hg) and demonstrate low water solubility (USEPA 2008). Furthermore, fluorinated organic substituents bonded to silicon are expected to impart unique properties to this specific group of polymers. PMTFPS is, for example, resistant to attack by fuels and oils, and is insoluble in common solvents such as chloroform, toluene or hexane (Kobayashi and Nishiumi 1993; Cai and Weber 2004). However the polymer has been reported to be soluble in polar solvents such as ketones and esters (Pagliani et al. 2008; Becerra et al. 1992).
Based on information for PMTFPS, MVTFS is expected to be heat-resistant, having a low Tg. The polymer is anticipated to be non-volatile and to have a density heavier than water. It would remain in a rubber-like state at environmental temperatures. The substance is expected to be insoluble in water and most common organic solvents.
MVTFS vs. PDMS
It should be noted that there are more differences in chemical structure between MVTFS and PDMS than between MVTFS and PMTFPS. In the monomer unit and the terminating group, PDMS only contains a methyl substituent attached to the silicon atoms in the backbone or the terminating group. However for MVTFS, there are different functional groups in both of two monomer units and the terminating group.
For the functional groups of trifluoropropyl and vinyl in the monomer units, the covalent bonds linking the side chains (vinyl and trifluoropropyl) to the silicon atoms in the backbone are expected to be very strong (Drobny 2001). There is experimental evidence indicating that MVTFS may be subject to the same degradation mechanism as PDMS, in which degradation is primarily due to the breakdown of the –Si-O-Si- backbone, instead of in the monomer units (Barrère et al. 2001; Veith and Cohen 1989). The hydroxyl group in the terminating unit may increase the water solubility for a polymer; however given the high molecular weight and the physical state of MVTFS, such effect is expected to be very minor. Therefore it is appropriate to use PDMS to predict the environmental persistence for MVTFS.
For siloxane polymers, there is a relationship between viscosity and the average molecular weight (Fendinger et al. 1997). Viscosity is a measure of how much a fluid resists flow when a force is applied to it. The viscosity of a polymer generally increases with its chain length (Fendinger et al. 1997; Wacker 1992), and the measure of viscosity, in the unit of cs[3] (centistokes), has been used to characterize the average molecular weight for a macromolecule (see Appendix I). When a silicone polymer has an average molecular weight of approximately 358 000 g/mol or more, it is anticipated to have elevated viscosity, low vapour pressure, low water solubility, and to exist in a rubber-like state at environmental temperatures.
PDMS, as referred in the assessment, has an average molecular weight up to 10 000 g/mol (equivalent to a viscosity of ~12 500 cs), which is much lower than the lowest molecular weight of MVTFS (30 000 g/mol). Under environmental conditions, PDMS remains in a liquid form, while MVTFS exhibits almost no mobility under the force of gravity. It can be assumed that if released to the environment, PDMS will be more bioavailable to organisms in water, soil and sediment. Therefore, use of analogue data for PDMS to assess the maximum potential bioaccumulation and ecological effects for MVTFS is considered to be a valid and conservative approach.
As no toxicity data exist for MVTFS and PMTFPS, and QSAR models are not applicable for high molecular weight compounds, toxicity data for PDMS are used to assess the ecological effects of MVTFS. The toxicity potential associated with the degradation products (especially the trifluoropropyl group) are also considered in the assessment. The vinyl and the hydroxyl end groups are functional groups that may be involved in crosslinking, and are not anticipated to demonstrate significant toxic effects.
MVTFS is not reported to be naturally produced in the environment.
Recent information was collected through surveys conducted for the 2005 and 2006 calendar years by means of Canada Gazette notices issued pursuant to section 71 of CEPA 1999 (Canada 2006). These notices required submission of data on the Canadian manufacture and import of MVTFS.In the notice for 2006, data were also required on the use quantity of the substance.
There was no manufacturing activity of MVTFS reported in either 2005 or 2006 in Canada. In 2005, more than 100 000 kg of MVTFS was imported into the country by fewer than four companies; while in 2006, between 10 000 – 100 000 kg of the polymer was imported by fewer than four companies. Based on the Declaration of Stakeholder Interest form associated with the section 71 survey for 2006, fewer than four companies reported a stakeholder interest for this substance.
The quantity reported to the Domestic Substances List (DSL) as being manufactured, imported or in commerce in Canada during the 1986 calendar year was between 200 000 kg and 2 000 000 kg.
Elsewhere, MVTFS has been reported to be in use in Denmark in 2001 and in Sweden in 2008; however, the amount used is confidential (SPIN 2006).
Information on uses for the 2005 and 2006 calendar years was also obtained in response to the CEPA 1999 section 71 notices (Canada 2006 and 2009).
In 2005, MVTFS was used in chemical product and preparation manufacturing, including rubber and aerospace product manufacturing.
In 2006, the substance was used in manufacturing rubber products and other chemical products. It was also used in moulding or casting in closed equipment, as well as in preparing adhesives and sealant substances.
MVTFS was not notified as an ingredient in cosmetic products in Canada (CNS 2010) and does not appear on the Cosmetic Ingredient Hotlist, Health Canada’s administrative list of ingredients that are intended to be prohibited or restricted for use in cosmetics in Canada (Health Canada 2009). MVTFS is not currently used in any pest control products registered for use in Canada as either an active ingredient or a formulant (PMRA 2007). MVTFS is not listed as an approved food additive under Division 16 of the Food and Drug Regulations (Canada 1978). MVTFS was not identified in food packaging applications or in incidental additives (April 2010 email from Food Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). MVTFS is not listed in the Drug Product Database (DPD), the Therapeutic Products Directorate's internal Non-Medicinal Ingredient Database, the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database as a medicinal or a non-medicinal ingredient present in final pharmaceutical products, natural health products or veterinary drugs (DPD 2010; NHPID 2010; LNHPD 2010; April 2010 email from Therapeutic Products Directorate, Health Canada to Risk Management Bureau, Health Canada; unreferenced).
The following DSL use codes have been identified for MVTFS during the DSL nomination period (1984 – 1986):
02 - Absorbent/adsorbent
36 - Polymer, component of formulation
37 - Polymer, cross-linking agent
92 - Rubber Products
Use of this substance other than as stated above has not been identified.
A method has been developed by Environment Canada to estimate a substance’s losses during different stages of its life cycle, including its fate after being incorporated into a finished product or article (Environment Canada 2008). This method consists of a life cycle analysis and a spreadsheet tool (Mass Flow Tool or MFT) that integrates information on the manufacturing, importation and use data available for the substance. Starting with an identified mass of the substance, each life cycle stage is subsequently evaluated until all of the mass is accounted for. Relevant factors are considered, uncertainties recognized and assumptions may be made during each stage, depending on information available. The estimated losses represent the complete mass balance over the life cycle of the substance and include releases to wastewater and other receiving compartments (land, air), chemical transformation, transfer to recycling activities and transfer to waste disposal sites (landfill, incineration). However, unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the method does not quantitatively account for releases to the environment from disposal. Ultimately, the estimated losses provide a first tier in the exposure analysis of a substance and help to estimate environmental releases and focus exposure characterization in the assessment.
In general, releases of a substance to the environment depend upon various losses from its manufacture, industrial use, and/or consumer/commercial use. These losses can be grouped into seven types: (1) discharge to wastewater; (2) emission to air; (3) loss to land; (4) chemical transformation; (5) disposal to landfill; (6) loss to incineration; and (7) disposal through recycling (i.e., recycling is deemed a loss and not considered further). These losses are estimated using regulatory survey data, industry data and data published by different organizations. The discharge to wastewater refers to raw wastewater prior to any treatment, whether it is on-site industrial wastewater treatment or off-site wastewater treatment. In a similar manner, the loss via chemical transformation refers to changes in a substance's identity that may occur within the manufacture, industrial use, and consumer/commercial use stages, but excludes those during waste management operations such as incineration and wastewater treatment. The loss to land includes unintentional transfer or leakage to soil or paved/unpaved surfaces during the substance’s use and service life (e.g., from the use of agricultural machinery or automobiles). The loss to land, however, does not include transfers subsequent to a substance’s use and service life (e.g., land application of biosolids and atmospheric deposition).
The losses estimated for MVTFS over its lifecycle (based on conservative assumptions) are presented in Table 3 (Environment Canada 2010b). The estimated losses of the substance are calculated based on the identified uses in 2006.
Table 3. Estimated Losses of MVTFS during Its Lifecycle
| Type of Loss | Proportion (%) | Pertinent Lifecycle Stages |
|---|---|---|
| Wastewater | 1.3 | Industrial use |
| Air emission | 0.2 | Industrial use |
| Land | < 0.1 | Industrial use |
| Chemical transformation | - | - |
| Landfill | 95.5 | Industrial use, consumer/commercial use |
| Incineration | 3.0 | Industrial use, consumer/commercial use |
| Recycling | - | - |
The above loss estimates indicate that MVTFS has a potential for release to the environment. Losses to landfill and incineration account for roughly 98.5% of the substance. Additionally, loss to wastewater can occur with potential subsequent loss to soil if biosolids are land-applied. Based on its high molecular weight, loss via air emission is expected to be minor for this polymer.
When the substance is unintentionally transferred to land, it may be washed into the sewer or transferred by rain to the area nearby. No recycling activity has been identified for the polymer. Finally, the potential for the substance to leach from landfill into groundwater is unlikely due to its very low water solubility.
There are no experimental data relating to the environmental fate of MVTFS. The assessment is thus based on the expected physical and chemical properties of analogous polymers.
Given the high molecular weight of MVTFS (number-average molecular weight of 358 000 g/mol and the lowest molecular weight of 30 000 g/mol) in commerce in Canada, the polymer is expected to have extremely low vapour pressure and therefore not anticipated to partition in air.
If released into water, MVTFS is expected to reside in suspended solids and sediment due to its very low water solubility, its specific gravity, and its existence in a rubber-like state at environmental temperatures. Volatilization from water surfaces is expected to be an unimportant fate process based upon the large molecular weight and the physical state for the polymer. Although the vinyl group in the repeating unit is somewhat reactive; there are less than 1% of the repeating units containing a vinyl group, therefore the impact on the environmental behaviour is negligible. Thus, if water is the receiving medium, MVTFS is expected to mainly partition into sediment, where some fraction may crosslink and form stable unreactive chemical species.
If released to soil, MVTFS is expected to be largely immobile and stay mainly in the medium, due to its large molecular size and insolubility in water. Volatilization from moist soil surfaces is likely to be an unimportant fate process due to the large molecular weight and the physical state of the polymer. This substance is not likely to volatilize from dry soil surfaces either. Therefore, if released to soil, MVTFS will mainly reside in this environmental compartment.
Environmental Persistence
No experimental degradation data for MVTFS have been identified.
Given that no QSAR models are applicable to predict the environmental persistence for the high molecular weight polymer, a simplified structure (containing 3 units of methyl-trifluoropropyl-siloxy) has been used in a QSAR model (CATABOL c2004-2008) to predict the potential for degradation in the monomer unit (release of trifluoropropyl group by the breakdown between the trifluoropropyl substitute and the silicon atom attached to in the backbone).
According to the CATABOL (c2004-2008) model estimates, trifluoroacetic acid and silanol are anticipated to be the intermediate products from the degradation of the simplified structure; however, probability of such degradation is less than 0.0001 (a value of 1.0 indicating 100% probability), indicating that the release of trifluoropropyl as a result of biodegradation within the monomer unit is highly unlikely, even for such a small molecule.
Studies have demonstrated that PMTFPS can degrade to cyclic oligomers under acidic and basic conditions by “backbiting” reactions (Barrère et al. 2001; Veith and Cohen 1989). In the laboratory environment, PMTFPS can degrade and generate a variety of cyclo-trifluoropropyl-siloxanes with ring sizes from 3 to above 20, when treated with the solvents (acetone and ethyl acetate, in which PMTFPS is soluble) and the non-solvent (methanol, in which PMTFPS is not soluble) (Kählig et al. 2009). The conditions employed in these tests are, however, expected to be quite different from those typically encountered in the environment. However, these studies indirectly confirmed that, when PMTFPS breaks apart during degradation, the preferred chemical reaction does not take place between the trifluoropropyl group and the silicon atom to which it is attached. Degradation of PMTFPS (and MVTFS) takes place in a manner similar to the environmental degradation of PDMS, where the molecule breaks in a random manner along the backbone (Fendinger et al. 1997). Therefore, information on PDMS will be used to assess the biodegradation potential of MVTFS in the environment.
Degradation and biodegradation of PDMS in the environment has been intensively reviewed (Graiver et al. 2003; Fendinger et al. 1997; ECETOC 1994).
Air
PDMS (molecular weight above 450 g/mol) is not volatile and is not expected to partition into the atmospheric compartment and is not expected to undergo further degradation in air (Graiver et al. 2003; ECETOC 1994). Using a substructure of MVTFS, which contains four methyl-trifluoropropyl and one methyl-vinyl repeating units (MW=877 g/mol), AOPWIN (2008, a sub-model in EPISUITE 2008) predicts a half-life of 3.2 hour due to hydroxyl radical reaction and a half-life of 6.5 days due to ozone reaction.
It is noted that MVTFS in commerce in Canada possesses a much higher molecular weight than PDMS; thus the substance is expected not to partition in air.
Water
There is no evidence indicating that the siloxane polymers would distribute or degrade rapidly in the aquatic environment (ECETOC 1994). PDMS was found not to degrade or decompose during the waste water treatment process (Graiver et al. 2003). Based on these findings (which are water-based), MVTFS is thus not anticipated to degrade rapidly in surface waters either, and the half-life is expected to be more than 182 days.
Soil
For degradation in soil, there have been a number of studies reporting the depolymerisation and rapid hydrolysis of PDMS resulting in the polymer being broken down to low molecular weight oligomers.
Buch and Ingebrigtson (1979) reported a half-life of approximately 30 days for PDMS (1 000 cs, equivalent to a molecular weight of ~15 000 g/mol) at a concentration of 6 000 mg/kg in a dry Iowa topsoil (cited in Fendinger et al. 1997). Such reaction was inhibited even with only 1% moisture content. The same study also reported an extensive loss of the polymer (50 cs) due to degradation over the 53-day test.
There are several studies of the environmental behaviour of PDMS in soil (summarized in Graiver et al. 2003). Results from these studies indicate that the polymer quickly depolymerizes by hydrolysis breaking the siloxane bonds and producing lower molecular weight oligomeric PDMS. Such soil-catalyzed hydrolytic degradation was found to be random, not specific to certain bonds or starting from the chain end. Furthermore, the hydrolysis catalyzed by clay minerals in soil does not occur in any of the other media (air, water or sediment) to a significant degree.
Lehmann et al. (1994a) and Carpenter et al. (1995) also studied degradation under more realistic soil conditions, by analyzing the degradation product extracted with either 0.01 M CaCl2or tetrahydrofuran. The results indicated that the degradation of PDMS (204 cs) took place in the soil, which was gradually dried from 12% to 3% moisture, faster than the degradation of the substance when the soil was maintained at constant moisture (12-13% moisture) in incubation chambers.
In another experiment, Lehmann et al. (1994b) further studied 7 soils with widely differing properties. PDMS (350 cs) was spiked into moist soil and allowed to gradually dry for 2 weeks. PDMS degraded extensively after 14 days to lower molecular weight materials. Although degradation took place when the soil was still moist, the degradation rates were greater in the most weather-dried soil. However, there is no correlation observed between PDMS degradation rate and the soil properties measured (i.e., pH, % organic matter, and % clay) other than moisture content.
The rate of depolymerisation by hydrolytic degradation in soil is fast but dependent upon several factors such as the moisture level and temperature. Lehmann et al. (1995) reported that the overall degradation rates of PDMS were slower in moist soil than in soil which was allowed to gradually dry for two weeks, indicating that continuous moisture could be an important factor, but moisture content is inversely related to the degradation rate (Lehmann et al. 1998b). In addition, the study results also demonstrated that warm temperatures favour the degradation rate during a yearly weather cycle.
In another study (Xu 1998), no PDMS was detected after 30 days of incubation in goethite and even faster degradation was observed when the polymer was incubated in Al-montmorillonite. For the impact of water content, the study reported a faster depolymerisation rate at the relative humidity (RH) of 32 % compared to a RH of 100%. Considering the field data from different types of soil in the study, the depolymerization half-life of PDMS is expected to be less than 3 weeks.
Given the evidence from the experimental studies, PDMS is anticipated to undergo clay-catalyzed hydrolysis rapidly in soil with a half-life of a few weeks. MVTFS is expected to degrade by the same mechanism and any breakdown of the polymer in soil to occur on the –Si-O-Si- backbone, but at a different degradation rate. The hydrolysis of MVTFS in soil may produce intermediates, i.e., oligomers of random length, and eventually would release methyl(3,3,3-trifluoropropyl)silanediol (CAS RN 660-78-6) and methylvinylsilanediol (CAS RN 3959-12-4) as the end products of the hydrolysis. Only very small quantities of dimethylsilanediol (DMSD, CAS RN 1066-42-8) could be produced, as DMSD precursors in MVTFS account for a tiny proportion of the molecule. Due to the high molecular weight and existence in a rubber-like state at environmental temperatures, it can be assumed that MVTFS will degrade more slowly than PDMS in soil due to a lower bioaccessability, however the half-life is not expected to be more than 182 days.
Sediment
In sediment, degradation of PDMS has also been observed, although the rate is apparently much slower than in soil, as the half-life is more than a year (Environment Canada 2010a). An experiment by Christensen (1994) examined the potential of PDMS to degrade in freshwater sediment. A concentration of 500 ppm of14C-labelled PDMS (350 cs) was added into sediment/water columns, and water soluble materials and the off-gas were monitored as the evidence of degradation. After 56 days, no detection of14CO2 or water soluble 14C was reported, indicating no degradation occurred during the test period.
In other longer incubation studies, degradation of PDMS in sediment was also evident (Fendinger et al. 1997). Following a year of incubation under aerobic conditions, 5-10% of the PDMS (350 cs) was hydrolyzed to DMSD and approximately 0.25% of the total14C had been oxidized to carbon dioxide (Carpenter et al. 1995). The rate and extent of DMSD formation and carbon dioxide production from sediment agree closely with the rates observed by Lehmann et al. (1994a, 1994b) in moist soil (3% DMSD and 0.13% carbon dioxide formed in 6 months). Given its high adsorption and immobility in sediment in combination with the water content of the substrate, it is anticipated that PDMS degrades very slowly in sediment and the half-life of the polymer in this environmental medium is expected to be longer than 365 days. The prediction can be extrapolated to the polymer under assessment such that MVTFS is not expected to degrade rapidly in sediment either, and the half-life in this environmental medium is also expected to be more than 365 days.
In general, MVTFS is expected to have lower water solubility and vapour pressure than PDMS. The difference in chemical structure is noted; however, the two polymers are expected to demonstrate the similar decomposing pattern that degradation takes place randomly along the –Si-O-Si- backbone.
Based on the model prediction on a substructure of PMTFPS, MVTFS is expected not to be persistent in air (half-life in air ≤ 2days). Based on data from experimental studies on PDMS, MVTFS is expected to degrade rapidly soil (half-life in soil ≤ 182 days). However the substance meets the persistence criteria in water and sediment (half-life in air ≥ 2days, half-life in water ≥ 182 days, and half-life in sediment ≥ 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000), and therefore concluded to be persistent in the environment.
There were no experimental data on the potential bioaccumulation for MVTFS.
The available QSAR models are not able to reliably predict a bioaccumulation factor (BAF) or bioconcentration factor (BCF) for MVTFS since its molecular weight is more than 1000 g/mol.
The molecular size of a substance is critical to determine whether it can physically pass through a biological membrane and be bioavailable to organisms (Gobas and Morrison 2000). Investigation by Dimitrov et al. (2002), Dimitrov et al. (2005) and the Baseline Bioaccumulation Model (BBM 2008) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum cross-sectional diameter (Dmax). The probability of passive diffusion lowers appreciably when cross-sectional diameter is > ~1.5 nm and more significantly for molecules having a cross-sectional diameter >1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion from a test set of about 1200 new and existing chemicals. They also observed that substances not having a very high bioconcentration potential often have a Dmax>2.0 nm and an effective diameter (Deff) >1.1 nm.
Due to its high molecular weight, MVTFS is beyond the limit for using the available models to calculate its cross-sectional diameter. However using a small PDMS containing 5 monomer units of dimethyl-siloxy (molecular weight of ~500 g/mol and a maximum diameter of 1.46 – 2.18 nm) as an indicator, MVTFS (minimum molecular weight of 30 000 g/mol) is anticipated to have a Dmax that is significantly larger than 2.0 nm. It is therefore expected that MVTFS, which is insoluble, will have very low uptake from water, and therefore not be bioavailable to aquatic organisms.
As mentioned previously in the Characterization of Physical and Chemical Properties section, PDMS has been selected as an analogue polymer for conservatively assessing characteristics of MVTFS.
Empirical data for bioaccumulation and bioconcentration of PDMS in aquatic organisms and other environmental species are summarized in Table 4a below.
Table 4a. Empirical data for bioaccumulation and bioconcentration of PDMS
| Polymer Character (Molecular Weight, Viscosity or Emulsion) |
Test Organism | Experimental Concentration (mg/L) and/or Exposure Source | Endpoint (BAF/BCF, L/kg) (BMF, dimensionless) |
Reference |
|---|---|---|---|---|
| 300 cs 15% emulsion |
Bluegill sunfish (Lepomis macrochirus) |
1.5 | BCF = 0.16 – 0.5 (after 2 weeks) |
Hobbs et al. 1975 |
| MD15-17M | Guppies (Poecilia reticulate) |
Saturation | BCF < 10 | Opperhuizen et al. 1987 |
| Approximately 32 mg/kg in dry food | BAF < 0.01 | |||
| Commercial PDMS MW (g/mol): 1200 6000 25000 56000 |
Carp sp. | Saturation solutions measured concentrations 1.330 0.486 0.135 0.060 |
BCF = 2.9 BCF = 7.1 BCF = 386 BCF = 1250 |
Watanabe et al. 1984a |
| 50 cs 35% emulsion |
Level 1: Phytoplankton (Tetraselmis sp.) Level 2: Mollusc (Mytilus edulis) |
70 Direct exposure from water Exposure from water and food |
BCF = 2.08 BMF = 0.12 |
Aubert et al. 1985 Guillemaut et al. 1987 |
| Level 1: Plankton (Tatreselmis sp.Artemia salina) Level 2: Fish (Carassius auratus) |
70 Direct exposure from water Exposure from water and food |
BCF = 1.9 BMF = 0.05 |
||
| Level 1: Annelid (Nereis diversicolor) Level 2: Fish (Scorpaena porcus) |
70 Direct exposure from water Exposure from water and food |
BCF = 0.036 BMF = 1.4 |
||
| Level 1: Annelid (Nereis diversicolor) Level 2: crab(Carcinus maenas) |
70 Direct exposure from water Exposure from water and food |
BCF = 0.036 BMF = 1.09 |
||
| 50 cs | Bullhead catfish (Ictalurus melas) |
Exposure from food | < detection level | Annelin 1979 |
Generally, PDMS with a variety of molecular weights (MW=1200 g/mol and above) demonstrates low bioaccumulation and bioconcentration in aquatic organisms and terrestrial species.
Opperhuizen et al. (1987) also conducted a bioaccumulation study on Poecilla reticulate (guppies) using PDMS (5 cs, MD15-17M). After 12 weeks of exposure, siloxane polymers were not detected in the whole fish.
In another study, bluegill sunfish (Lepomis macrochirus) were exposed to a PDMS with a molecular weight greater than 5 000 g/mol for 30-day (Hobbs et al. 1975). There was no relationship found between the levels of the polymer in tissue and duration or level of exposure. It was found, however, that the polymer was adsorbed to surfaces of the fish and not taken up into the cells.
In addition, there were studies of PDMS uptake by other species in other environmental media. In a 28-day study by Garvey et al. (1996), earthworms (Eisenia foetida) were exposed to 100 and 1 000 mg/kg PDMS in an artificial soil matrix. At the termination of the exposure phase, the earthworms from each replicate were transferred to exposure media that contained no PDMS for the 14-day depuration phase of the study. Samples were collected through the 28-day exposure phase; however there was no statistical difference in the tissue residue concentrations among samples collected at any test points. Furthermore, PDMS ingested by the earthworms and present in the gut was rapidly depurated during a following 14-day period.
Kukkonen and Landrum (1995) examined aquatic worms living in sediments and concluded that there was no bioaccumulation of PDMS observed in these worms.
Results from studies on other benthic and terrestrial organisms (Putt 1994; Putt and Mihaich 1996) also indicated very limited bioaccumulation potential for PDMS.
There is one study that reports a contradictory finding, in which the higher molecular weight PDMS materials bioaccumulated more than lower molecular weight compounds – based on tests using silver carp (Watanabe et al. 1984). The fact that the relationship between bioaccumulation and the molecular weight is the reverse of what would be expected could be the result of surface contamination, and no evidence was available to indicate whether whole fish or fillets were analysed. Thus these results are of uncertain quality.
Biomagnification or food-chain transfer of a PDMS (50 cs) emulsion in the marine environment has been studied (Aubert et al. 1985; Guillemaut et al. 1987). Organisms in the first level of the food chain were exposed to emulsified PDMS (50 cs) at a concentration of 70 mg/L in water. PDMS was either not bioconcentrated in water or the BCF was <2 in the first level of the food chain. The bioaccumulation between the two trophic levels was low, ranging between 0.05 and 1.4, indicating that the biomagnification of PDMS between two trophic levels is not significant in the aquatic food chain tested.
During degradation of MVTFS, the breakdown of the polymer is expected to primarily occur on the –Si-O-Si- backbone, and produce intermediates and oligomers of different lengths. Degradation may eventually release the monomers methyl(3,3,3-trifluoropropyl)silanediol (CAS RN 660-78-6) and methylvinylsilanediol (CAS RN 3959-12-4) as the end hydrolysis products. To characterize the potential for bioaccumulation after degradation, the monomers were assessed using a QSAR model (BCFBAF 2008) to predict the bioaccumulation potentials for the end hydrolysis products.
The modelled data are summarized in Table 4b below. They indicate that the end products of the hydrolysis are not anticipated to be bioaccumulative in aquatic organisms.
Table 4b. Modelled data for bioaccumulation of the hydrolysis products of MVTFS (using BCFBAF 2008)
| Chemical name and CAS RN | Bioaccumulation factor (BAF) (L/kg wet-wt) | Bioconcentration factor (BCF) (L/kg wet-wt) |
|---|---|---|
Methyl(3,3,3-trifluoropropyl)silanediol (660-78-6) |
1.80 | 3.16 |
Methylvinylsilanediol (3959-12-4) |
0.96 | 3.16 |
Based on the above experimental information on PDMS, as well as model predictions on the hydrolysis products for MVTFS, it is expected that, because of its large molecular size and low water solubility, MVTFS is not bioavailable to environmental organisms, and thus does not bioaccumulate in those organisms. Therefore it is concluded that MVTFS and its degradation products do not meet the bioaccumulation criteria (BAF or BCF ≥ 5000) as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
A - In the Aquatic Compartment
There were no experimental toxicity data identified for MVTFS.
It is noted that both PDMS and MVTFS do not contain any functional groups of concern for polymer ecotoxicity (e.g., amines, epoxides, etc.). The vinyl group is more reactive and not considered to present a significant toxic effect. According to the model prediction using CATABOL (c2004-2008), the trifluoropropyl unit is considered to strongly bind to silicone atom in the backbone, and the release of the functional group from the repeating unit is unlikely. Therefore, MVTFS is expected to possess a similar toxicity potential to PDMS, and information on the latter has thus been used to assess the toxicity of MVTFS.
There are a number of toxicity studies reporting effects of PDMS on aquatic organisms as summarized in Table 5a below. As mentioned in the previous section, PDMS (and MVTFS) are not taken up by aquatic organisms (Annelin and Frye 1989; Bruggeman et al. 1984), therefore systemic effects are not expected. Because PDMS is extremely hydrophobic and tends to adsorb strongly to solid particles, the polymer in emulsion form has been used in many studies to maintain constant and nominal exposure concentrations, which are higher than the water solubility of PDMS.
In general, PDMS has demonstrated low toxicity to fish. A chronic no observed effect concentration (NOEC) of 91 mg/L of PDMS (50 cs emulsion) in the sheepshead minnow was reported by Hill et al. (1984). In another study, Hill reported an LC50 at the exposure level of 350 mg/L of PDMS (50 cs emulsion) to plaice (Hill et al. 1980). The toxic effects of PDMS emulsions at high concentrations were shown to have been caused by the emulsifier used in the formulation (Hobbs et al. 1975; Aubert et al. 1985).
PDMS has also shown low toxicity to aquatic invertebrates (see Table 5a below). A 96-hour LC50 of 1 980 mg/L of PDMS (50 cs emulsion) has been reported from a study on the musselMytilus sp. (Hill et al. 1984). However, the adverse effects are somewhat difficult to interpret. PDMS fluids are insoluble in water and have a specific gravity less than 1 000 kg/m3. Therefore the polymer forms a surface film in which the aquatic crustacean tends to be caught. Such entrapment may occur and therefore interfere with the assessment of the chemical toxicity of the polymer.
Toxicity to aquatic microorganisms has also been studied and PDMS has been found not to be very hazardous to such organisms (see Table 5a).
It should be noted that the exposure concentrations of PDMS are well above the maximum solubility expected for the polymer in the aquatic toxicity studies, indicating that the polymer likely poses low hazard to aquatic organisms under environmental conditions. PDMS used in the toxicity studies were tested in emulsions and considerably more bioavailable than MVTFS would be. Meanwhile the molecular weights of PDMS reported in the toxicity studies are generally much smaller than the average molecular weights of MVTFS. Therefore the PDMS results are taken as useful and conservative surrogates for toxicity data on MVTFS.
Among a variety of aquatic species used in toxicity studies,Daphnia magna has been identified as the most sensitive species and there is a 48 hr LC50=73.4 mg/L reported by Hill et al. (1977), using a 35% PDMS emulsion (350 cs). For the purpose of characterizing the risk of MVTFS on aquatic organisms, the LC50=73.4 mg/L reported in this study has been used as the Critical Toxicity Value (CTV) to derive the predicted no effect concentration (PNEC) for risk characterization.
Table 5a. Toxicity data of PDMS on aquatic organisms
| Polymer Character -- (viscosity and/or emulsion) |
Test Organism | Type of Test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|---|
| Fish | |||||
| Not specified | Fish | Acute | LC50[1] | >1000 | Annelin and Humble 1978; Hobbs et al. 1975 |
| Not specified | Minnows (Phoxinus phoxinus) |
Semi-chronic | LT40[2] | 3000 (8 days) | Cabridenc 1978 |
| 100, 350, 12500 cs | Pomatoschistus minutus Gasterosteus aculeatus |
Acute (96 hours) |
LC0[3] | At saturation | Maggi and Alzieu 1977 |
| 50 cs 20% emulsion |
Plaice (Pleuronetes platessa) |
Acute (96 hours) |
EC[4] | 88 | Firmin 1984 |
| 50 cs 35% emulsion |
Scorpion fish Scorpaena porcus Carassius auratus |
Acute | LT50[5] | 1000 (12 hrs) 2000 (50 hrs) 10 000 (hrs) 700 (50 hours) 3500 (24 hours) |
Aubert et al. 1985 |
| 50 cs emulsion not specified |
Plaice (Pleuronectes platessa) |
Acute (96 hours) |
LC50 | 350 > 10 000 at the surface of the water (5 mg/L in water) |
Hill et al. 1980 Hill 1980 |
| 50 cs 35% emulsion |
Sheepshead minnow (Cyprinodon variegatus) | Chronic (33 days) |
NOEC[6]EC | 91 235 (minor effects observed) |
Hill et al. 1984 |
| 350 cs | Rainbow trout (Oncorhynchus mykiss) (Salmo gairdneri) |
Sub-chronic spiked food (28 days) |
NOEL[7] | 10 000 mg/kg bw/day | Mann et al. 1977 |
| 350 cs 30% emulsion |
Bluegill sunfish (Lepomis macrochirus) Rainbow trout (Salmo gairdneri) |
Acute (96 hours) |
LC50 Hypoactivity |
> 10 000 At both 1000 and 10000 |
Hobbs et al. 1975 |
| Aquatic invertebrate and molluscs | |||||
| 10 – 60000 cs 66% emulsion |
Daphnia | Acute | NOEC | >200 | Annelin et al. 1994 |
| 350 cs emulsion |
Daphnia magna | Acute (48 hrs) |
LC50 | 1000 | Spacie 1972 |
| 100, 350, and 12,500 cs | European oyster (Ostrea edulis) Mussel sp. (Mytilus edulis) Periwinkle (Littorina littorea) |
Acute (96 hours) |
LC0 | Saturation in sea water | Maggi and Alzieu 1977 |
| 50 cs 35% emulsion |
Mussel (Mytilus edulis) Crab (Artemia salina Carcinus maenas) |
Acute Semi-chronic (10 days) Semi-chronic |
LT50 LT50 LC0 LC50 Mortality |
3500 (96 hrs) 10000 (80 hrs) (emulsifier only) 700 7000 (9 days) 3500 (10 days) |
Aubert et al. 1985 |
| 50 cs 20% emulsion |
Mytilus sp. | Acute (96 hours) |
LC50 | 1980 | Hill et al. 1984 |
| 50 cs | Mytilus sp. | Acute (96 hours) |
LC50 | 1020 | Hill et al. 1984 |
| 100 cs 100 cs 30% emulsion 350 cs 30% emulsion |
Daphnia magna Daphnia magna Cockles (Prothaca spaminea) Mummichog (Fundulus heteroclitus) Shore crabs (Pachygrapsus crassipes) Brown shrimp (Penaeus oxtecus) |
Acute (48 hrs) Acute (48 hrs) Acute (96 hrs) |
TL50[8] TL50 TL50 |
44.5 (polymer formed a layer on the surface) 73.4[*] >1000 |
Hobbs et al. 1975 |
| Aquatic microorganisms (bacteria, fungi, and phytoplankton) | |||||
| 55 cs hydroxyl-endblock | Aerobic and anaerobic bacteria, algae, and protozoa | Chronic (24 weeks) |
NOEC | > 0.26 | Gettings and Lane 1982 |
| 100, 350, and 12,500 cs | Marine algae (diatoms and flagellates) | Semi-chronic (9 days) |
NOEC | Saturation in sea water | Maggi and Alzieu 1977 |
| 50 cs 35% emulsion |
Marine phytoplankton (Tetraselmis sp.) | Not specified | LOEC[9] | 350 | Maggi and Alzieu 1977 |
[2] LT40 – The concentration of a substance that is used in time-until-death to determine 40% lethality of the test organisms.
[3] LC0- The concentration of a substance that is estimated to have no lethal effect on the test organisms.
[4] EC - The concentration of a substance that is estimated to demonstrate toxic effect on the test organisms, however the percentage of affected test organisms can not be determined in the study.
[5] LT50 – The concentration of a substance that is used in time-until-death to determine 50% lethality of the test organisms in hours.
[6] NOEC – The No Observed Effect Concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls.
[7] NOEL – The No Observed Effect Level is the highest level of exposure in a toxicity test not causing a statistically significant effect in comparison to the controls.
[8] TL50 – The tolerance limit that corresponds to 50% survival of the test organisms.
[9] LOEC – The Lowest Observed Effect Concentration is the lowest concentration in a toxicity test causing a statistically significant effect in comparison to the controls.
[*] CTV – Critical Toxicity Value. It is further used to derive the predicted no effect concentration (PNEC).
For the hydrolysis products of MVTFS, no toxicity data has been found. ECOSAR has been used to predict the potential toxic effects associated with the hydrolysis products, and the estimates are summarized in Tables 5b and 5c below. The modelled predictions indicate that both hydrolysis products of MVTFS may demonstrate low to moderate toxicity effects in the aquatic organisms.
Table 5b. Modelled data for aquatic toxicity of methyl(3,3,3-trifluoropropyl) silanediol (CAS RN 660-78-6)
| Test organism | Type of test | Endpoint | Value (mg/L) |
|---|---|---|---|
| Fish | Acute (96 hours) | LC50[1] | 1038 |
| Acute (14 days) | 1032 | ||
| Fish (sea water) | Acute (96 hours) | LC50 | 1558 |
| Daphnid | Acute (48 hours) | LC50 | 504 |
| Green Algae | Acute (96 hours) | EC50[2] | 154 |
| Fish | Chronic (30 days) | ChV[3] | 101 |
| Daphnid | Chronic | ChV | 50 |
| Green Algae | Chronic | ChV | 50 |
| Mysid Shrimp | Acute (96 hours) | LC50 | 2126 |
| Fish (sea water) | Chronic | ChV | 85 |
| Mysid Shrimp (sea water) | Chronic | ChV | 248 |
[2] EC50- The concentration of a substance that is estimated to cause some effect on 50% of the test organisms.
[3] ChV - Chronic toxicity value.
Table 5c. Modelled data for aquatic toxicity of methylvinylsilanediol (CAS RN 3959-12-4)
| Test organism | Type of test | Endpoint | Value(mg/L) |
|---|---|---|---|
| Fish | Acute (96 hours) | LC50[1] | 5137 |
| Acute (14 days) | 5025 | ||
| Fish (sea water) | Acute (96 hours) | LC50 | 8347 |
| Daphnid | Acute (48 hours) | LC50 | 2139 |
| Green Algae | Acute (96 hours) | EC50[2] | 416 |
| Fish | Chronic (30 days) | ChV[3] | 466 |
| Daphnid | Chronic | ChV | 179 |
| Green Algae | Chronic | ChV | 113 |
| Mysid Shrimp | Acute (96 hours) | LC50 | 22,149 |
| Fish (sea water) | Chronic | ChV | 230 |
| Mysid Shrimp (sea water) | Chronic | ChV | 3517 |
[2] EC50- The concentration of a substance that is estimated to cause some effect on 50% of the test organisms.
[3] ChV - Chronic toxicity value.
Based on the above experimental information on PDMS and the model predictions on the hydrolysis products of MVTFS, it is concluded that MVTFS and its degradation products have low toxicity to aquatic organisms.
B - In Other Environmental Compartments
Given that the primary route of exposure of MVTFS in an aquatic environment would be through contact with the polymer absorbed to sediments, the toxic effects of the polymer on the sediment-dwelling organisms is also considered in this risk assessment. Furthermore, since biosolids from the treated sludge of wastewater systems can be used to augment agricultural soils and reclaim land, and while the proportion of loss to wastewater and hence to sludge is expected to be small (~1.3%), the potential impacts of the interaction of terrestrial organisms with sludge containing the polymers was also considered.
There are no experimental toxicity data for MVTFS in either sediment or soil. Therefore information on PDMS is used to assess the toxicity of MVTFS in these two environmental media.
Experimental data for toxicity of PDMS to sediment and soil organisms are summarized in Table 5d below. In summary, the polymer demonstrates low toxicity to biological species in these two environmental media.
Table 5d. Toxicity data for PDMS on sediment and soil organisms
| Polymer Character (viscosity and/or emulsion) |
Test Organism | Test Medium and Type |
Endpoint | Concentration Value (mg/kg, unless otherwise noted) | Reference |
|---|---|---|---|---|---|
| Sediment organisms | |||||
| 350 cs | Marine amphipod (Ampelisca abdita) | Sediment Semi-chronic (10 days) |
LC0[1] | >2300 | Putt and Mihaich 1996 |
| 350 cs | Freshwater amphipod (Hyallela azteca) | Sediment Chronic (28 days) |
EC0[2] | >2200 | Putt and Mihaich 1996 |
| PDMS (viscosity/ emulsion characteristics not noted) | Polychaete worm (Nereis diversicolor) | Sediment Chronic (28 days) |
EC0 | 1000 | Craig et al. 1984 |
| 50 cs | Annelids | Sediment Acute (96 hours) Chronic (28 days) |
EC0 | >10 000 | Craig and Caunter 1990 |
| 50 cs 35 % emulsion (sea water) |
Polychaete worm (Nereis diversicolor) | Sediment Semi-chronic (9 days) |
LC0 EC0 |
> 350 > 700 |
Aubert et al. 1985 |
| 350 cs | Benthic invertebrates (H. azteca and C. tentans) |
Sediment Semi-chronic (10 days) Life-cycle (28 days for H. azteca and 50-65 days forC. tentans) |
LC0 EC0 |
> 1000 | Henry et al. 2001 |
| Soil organisms | |||||
| 350 cs fluid |
Earthworm (Eisenia foetida) | Soil Chronic (21 days) |
EC0 | 1100 | Garvey et al. 1996 |
| 350 cs fluid |
Springtail (Folsomia candida) | Soil Chronic (21 days) |
NOEC[3] | 250 | Garvey et al. 1996 |
| Plant | |||||
| 10 cs 10% emulsion |
Conifer plantation | Soil Acute (right after the treatment) Chronic (1 year) |
NOEL[4] | 30 ml/m3 | Belt et al. 1977 |
| Territorial organisms | |||||
| 100 cs | Mallard duck (Anas platyrhynchos) Bobwhite quail (Colinus virginatus) |
Semi-chronic (5 days) |
LC0 | >5000 | Hobbs et al. 1975 (cited from ECETOC 1994) |
| 100 cs | White Leghorn chickens | Chronic (24 weeks) |
EC0 | >5000 | Hobbs et al. 1975 |
[2] EC0- The concentration of a substance that is estimated to have no adverse effect on the test organisms.
[3] NOEC – The No Observed Effect Concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls.
[4] NOEL – The No Observed Effect level is the highest level of exposure in a toxicity test not causing a statistically significant effect in comparison to the controls.
Given the likely degradation of MVTFS in soil, the toxicity of the hydrolysis products of the polymer has also been predicted using a QSAR model (ECOSAR 2008), and the results are summarized in Table 5f below. According to the model predictions, both hydrolysis products demonstrate low toxicity to earthworm.
Table 5f. Modelled data for toxicity on earthworm for the hydrolysis products of MVTFS (ECOSAR 2008)
| Chemical Names and CAS RN | Type of Test | Endpoint | Value (mg/L) |
|---|---|---|---|
| Methyl(3,3,3-trifluoropropyl)silanediol 660-78-6 |
Acute (14 days) | LC50[1] | 385 |
| Methylvinylsilanediol 3959-12-4 |
Acute (14 days) | LC50 | 296 |
Based on study results for PDMS and the model estimates for the hydrolysis products of MVTFS, it is expected that MVTFS does not have a significant potential for toxicity to either sediment or soil-dwelling organisms.
Ecological Exposure Assessment
A – Industrial Release
No data concerning concentrations of MVTFS in the environmental media in Canada have been identified. Environmental concentrations are therefore estimated based on the exposure analysis for PDMS with consideration of the quantities of MVTFS imported into Canada.
The Mass Flow Tool applied realistic worst-case assumptions for release of MVTFS to water (via wastewater effluent) from industrial use (Table 3). To address releases from industrial activities, a site-specific scenario was employed to estimate a conservative substance concentration in a watercourse receiving industrial effluents (Environment Canada 2010c). The scenario is designed to provide these estimates based on the amount of chemical processed and released, the number of processing days, wastewater treatment plant (WWTP) removal rate, and the size of the receiving watercourse.
The industrial-release in the site-specific scenario was based on loading data from sources such as industrial surveys and knowledge of the distribution of industrial discharges in the country, and calculates a predicted environmental concentration (PEC).
The aquatic exposure of MVTFS is expected if the substance is released from industrial use to a wastewater treatment plant that subsequently discharges its effluent to a receiving water body. The concentration of the substance in the receiving water near the discharge point of the wastewater treatment plant is used as the PEC in evaluating the aquatic risk of the substance. It can be calculated using the equation
Cwater-ind = [1000 × Q × L × (1 - R)] / [N × F × D]
where
Cwater-ind: aquatic concentration resulting from industrial releases, mg/L
Q: total substance quantity used annually at an industrial site, kg/yr
L: loss to wastewater, fraction
R: wastewater treatment plant removal rate, fraction
N: number of annual release days, d/yr
F: wastewater treatment plant effluent flow, m3/d
D: receiving water dilution factor, dimensionless
Based on the information obtained from the section 71 survey, one industrial facility has been identified as having the highest use quantity of the polymer (Environment Canada 2010a). The upper bound of a reporting range (1 000 to 10 000 kg/year), in which the site-specific use quantity of the polymer fits, was used to estimate the environmental concentration.
The loss to a local WWTP is estimated at 1.3% of the total quantity resulting from the cleaning of chemical containers and process equipment. A WWTP effluent flow of 56 747 m3/d has been identified for the site-specific scenario.
The scenario also assumes that 1) the release occurs 250 days per year, 2) the WWTP removal rate (adsorption to sludge) is 95% for the polymer (USEPA 2008), and 3) the down stream receiving water body flow is 144 460 m3/d with a dilution factor of 3.5.
Based on the above assumptions, the predicted environmental concentration (PEC) is calculated as 1.3 × 10-4 mg/L for the polymer (Environment Canada 2010c).
It is noted that the predominant pathway of MVTFS release to in the environment is through soil amendment with biosolids. Based on the substance’s high molecular weight and very low water solubility, and with consideration given to the toxicity data for the analogue (PDMS), it can be assumed that landfilled or incinerated sludge associated MVTFS is not expected to be dispersed in the environment. In soil, the substance is expected to be immobile and undergo rapid degradation, and consequently will result in no significant exposure to aquatic or terrestrial organisms.
B – Consumer Release
Consumer/commercial products containing MVTFS are estimated to be landfilled or incinerated, while the release to the atmospheric or aquatic compartment is expected to be insignificant and exposure in these environmental media is expected to be low.
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, toxicity, sources and fate of the substance.
Having a high molecular weight, MVTFS is expected not to partition in air. The polymer is expected to be persistent in water and sediment, and have a low bioaccumulation potential. The moderate volumes of MVTFS imported into Canada, along with information on its uses, indicate potential for release into the Canadian environment. Due to the high molecular weight, MVTFS is insoluble in water and remains in a rubber-like state at environmental temperatures. When released to water, it will eventually partition to sediment. The polymer is expected to have low potential for toxicity to aquatic organisms.
A risk quotient analysis, integrating conservative estimates of exposure with toxicity information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada. The site-specific industrial scenario presented above yielded a PEC of 1.3 × 10-4 mg/L (Environment Canada 2010c). A PNEC for MVTFS was derived from a 48 hrs LC50=73.4 mg/L (as the most sensitive valid experimental value for PDMS), by dividing this value by an assessment factor of 100 (to account for interspecies and intraspecies variability in sensitivity, extrapolation from an acute toxicity in a laboratory to chronic toxicity in the field, and the uncertainty associated with use of data on an analogue polymer) to give a value of 0.73 mg/L. The resulting risk quotient (PEC/PNEC) is approximately 1.7 × 10-4. Therefore harm to aquatic organisms is unlikely.
This information suggests that MVTFS does not have the potential to cause ecological harm to aquatic organisms in Canada.
When MVTFS is released into a water body, it is expected to partition into suspended particulate matter and to bottom sediments, where sediment-dwelling organisms would be exposed to the substance. However, no environmental monitoring data or toxicity data specific to sediment-dwelling organisms are available for this substance. Given the large molecular weight, the toxicity data for the analogue (PDMS) and the hydrolysis products of MVTFS, it is expected that MVTFS would demonstrate low toxicity to benthic species. Therefore considering the possible exposure to the polymer, harm to sediment-dwelling organisms from exposure to MVTFS in Canada is considered to be unlikely.
Uncertainties in Evaluation of Ecological Risk
One major uncertainty in the assessment is due to the complexity associated with the polymer. Unlike any discrete chemical, the polymer can only be described by a distribution of homologous structures with varying molecular weight. The average molecular weight, the content of any molecular weight species, and impurities vary depending on different polymer formulations. It is noted that the assessment has only addressed concerns on the polymer in commerce in Canada with consideration of the available information.
Another main area of uncertainty for the evaluation of MVTFS is associated with a lack of experimental data for physical and chemical properties, persistence, bioaccumulation and inherent toxicity. Due to the large molecular weight of the polymer, all QSAR models were considered to be not applicable for assessing the polymer. Polymer analogues have been identified and relevant information has been used for assessing MVTFS. Comparison of the chemical compositions in MVTFS and the analogues, suggests that the difference in the repeating units and the terminating units will not significantly influence their physical and chemical properties or ecotoxicity, but may have a bearing on the environmental fate in soils. There are also major differences in the molecular weights of the analogues, which are much smaller than MVTFS. The analogues are expected to be more bioavailable to organisms in the environment. Therefore it is considered to be valid and conservative to use such analogues for assessing MVTFS.
It is noted that the exposure concentrations of the analogous polymer (PDMS) in the toxicity studies are much higher than its expected water solubility in the aquatic environment. Results of toxicity studies using the polymer in emulsions indicate that the polymer likely poses low hazard to aquatic organisms under environmental conditions. Therefore the toxicity data on PDMS are considered as valid and conservative surrogates for assessing the effects of MVTFS on environmental organisms.
Another uncertainty is due to the lack of information on environmental concentrations in Canada of MVTFS. However, the majority of imported polymer is anticipated to end up in landfill at the waste disposal sites, and releases into the Canadian environment are expected to be low.
Metabolism of the polymer in environmental organisms is not fully understood. Although the analogue PDMS has demonstrated a rapid clearance from fish, it is not clear whether there is a definitive metabolism for siloxane polymers such as PDMS and MVTFS.
Given the use of this substance in other countries, it is possible that MVTFS is entering the Canadian market as a component of manufactured items or consumer products, in addition to those reported. However, information obtained from the section 71 survey and other information sources did not indicate that it was present in these types of products in Canada. Available information is currently not sufficient to derive a quantitative estimate that would help determine the importance of this source. However, it is anticipated that the life cycle stages and proportional losses resulting from use of these other products would not be significantly different from those considered and estimated in the assessment. Therefore, it is expected that the quantities of MVTFS released to the various environmental media would not be significant.
Although there is the possibility that other consumer/commercial products containing MVTFS may be imported into Canada in addition to those reported as a result of industry surveys conducted pursuant to Section 71 of CEPA 1999, no information is available on the quantity of such imports. It is anticipated that the life cycle stages and proportional losses resulting from use of these other products would not be significantly different from those considered and estimated above. However, the actual mass of the substance lost from each of the life cycle stages may be higher than the estimates provided above, if such information was available for consideration.
Exposure Assessment
Environmental Media
Empirical data on concentrations of MVTFS in environmental media in Canada were not identified. Physicochemical properties could not be predicted for MVTFS as it is a polymer of average molecular weight of 358 000 g/mol (equivalent to approximately 2 200 repeating units of methyl-trifluoropropyl-siloxy and approximately 20 repeating units of methyl-vinyl-siloxy) which is not within the domain of available QSAR models. Due to its large molecular weight, the polymer is expected to exhibit negligible volatility and water solubility. MVTFS is expected to exist in a chemically-resistant, rubber-like pure state at environmental temperatures, and exposure to the general population from environmental media is expected to be negligible.
Consumer Products
No consumer products containing or manufactured with MVTFS were identified. MVTFS was identified in a few industrial uses in 2006 in Canada; potential exposure to MVTFS from these uses is considered to be of occupational nature only (Environment Canada 2010a).
Health Effects Assessment
Empirical health effects data were not identified for MVTFS. Currently available quantitative or qualitative structure – activity relationship (QSAR) models are not considered to be suitable for predicting the toxicity of polymers.
Fluorosilicones (PMTFPS), which have similar structures to MVTFS (shown in Table 2 of the characterization of physical and chemical properties section) have been used in medical applications, such as intraocular tamponades (Heimann et al. 2008). Several studies have investigated the possible side effects of the medical uses of fluorosilicones. Intravitreous injection of fluorosilicones (1000, 5000 or 10 000 cs) into rabbit eyes for 2 to 6 months caused intravitreous inflammation, retinal edema, corneal edema, neovascularisation, and immune response (increased immunoglobulins and complement fractions), in some of the studies (Miyamoto et al. 1986; Gabel et al. 1987; Pastor et al. 1992; Versura et al. 2001). Keratopathy, defined as corneal thickening or edema, was also observed in some of the patients in clinical applications following intravitreous injection of fluorosilicone (Gremillion et al. 1990). It was proposed that ocular toxicity was induced by the low-molecular-weight components (LMWC) or impurities in the injected materials. It has been demonstrated that LMWC could enhance the growth of in vitro cultured rabbit fibroblasts and human retinal pigment epithelial cells (Sparrow et al. 1990; Friberg et al. 1991) and induce ocular inflammation and edema in rabbit eye following intravitreous injection (Nakamura et al. 1991).
There is no evidence that siloxane monomers or polymers may pose a genotoxicity potential (JACC 1994; Environment Canada, Health Canada 2008a, b, c).
The confidence in the health effects database is low as no substance specific experimental data were identified and the relevant health effects information regarding fluorinated and/or vinyl substituted siloxane monomers or polymers is very limited.
Characterization of Risk to Human Health
Empirical health effects data were not identified for MVTFS. Based on limited health effects data for fluorosilicones, which have similar structures, MVTFS is not considered to demonstrate high hazard potential.
Exposure of the general population to MVTFS through environmental media (air, drinking water and soil), or to food and beverages, is expected to be negligible. General population exposure from use of consumer products containing MVTFS is not expected. Accordingly, risk to human health from exposure to MVTFS in Canada is considered to be low.
Uncertainty in Evaluation of Risk to Human Health
Empirical data were not identified for concentrations of this substance in environmental media. However, consideration of physicochemical properties and uses notified under section 71 of CEPA 1999 indicates negligible exposure to the general population.
Due to the absence of health effects data on MVTFS, the human health assessment for MVTFS is based on limited health effects data on fluorinated siloxane polymers. Although the confidence in the determination of critical health effects for this substance is low, based on the physicochemical properties of siloxane polymers, i.e., large molecular size and weight, and low water solubility and vapour pressure, it is anticipated that this substance poses low bioavailbility, which limits any effective concentrations that may be associated with any potential adverse effects to be reached in the body.
Based on the information presented in this screening assessment, it is determined that the form of MVTFS in commerce in Canada meets the Reduced Regulatory Requirement polymer criteria as specified the New Substances Notification Regulations (Chemical and Polymers).
Based on the information available, it is concluded that MVTFS is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Additionally, MVTFS meets the criteria for persistence but does not meet the criteria for bioaccumulation as set out in the Persistence and Bioaccumulation Regulations(Canada 2000).
Based on the information available, it is concluded that MVTFS is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that MVTFS does not meet any of the criteria under section 64 of CEPA 1999.
Given the complexity associated with the polymer formulation and the potentially hazardous properties associated with low molecular weight polymers, there is concern that new activities for MVTFS which have not been identified or assessed under CEPA 1999 could lead to the substance meeting the criteria as set out in section 64 of the Act. Therefore, it is recommended that the DSL be amended to indicate that MVTFS meets the Reduced Regulatory Requirement polymer criteria. Should other forms of MVTFS not meeting the reduced regulatory requirements, be introduced on the Canadian market, those forms would be subject to the requirements of the New Substances Notification Regulations.
Annelin RB. 1979. Dow Corning internal report no. I-0005-0669. Cited in Fendinger et al. 1997.
Annelin RB, Frye CL. 1989. The piscine bioconcentration characteristics of cyclic and linear oligomeric permethylsiloxane. The Science of the Total Environment. 83:1.
Annelin RB, Humble DA. 1978. Dow Corning report I-0005-0596. Cited in Fendinger et al. 1997.
Annelin RB, Klein R, and Varaprath S. 1994. Dow Corning Corp Internal Report no. 1994-I-0000. Cited in Fendinger et al. 1997.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2008. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Aubert M, Aubert J, Augier H, and Guillemaut C. 1985. Study of the toxicity of some silicone compounds in relation to marine biological chains. Chemosphere. 14: 127-138.
Barrère M, Maitre C, Dourges MA, Hémery P. 2001. Anionic polymerization of 1,3,5-tris(trifluoropropylmethyl)cyclotrisiloxane (F3) in miniemulsion. Macromolecules. 34: 7276-80.
Battelle. 1992. Fate and effects of PDMS: Soil amendment sludge disposal. Report to Dow Corning Corporation, Battelle Study No SC 900090. May 8, 1992. Battelle, Columbus Lab., Ohio, USA. Cited in ECETOC 1994.
[BBM] Baseline Bioaccumulation Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division. [Model based on Dimitrov et al. 2005]. Available upon request.
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2008. Version 3.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Becerra MR, Fernandez-Sanchez E, Fernandez-Torres A, Garcia-Domiguez JA, Santiuste JM. 1992. Thermodynamic characterization by inverse gas chromatography of a 50% methyl, 50% trifluoropropyl polysiloxane. Macromolecules. 25:4665–70.
Belt GH, King JG, and Haupt HF. 1977. Augmenting summer stream flow by use of silicone antitranspirant. Cited by West KF, 1992. Water Resource Research. 13: 267-272. Cited in ECETOC 1994.
Buch RR and Ingebrigtson DN. 1979. Rearrangement of Polydimethylsiloxane Fluids
on Soil. Environmental Science and Technology. 13: 676.
Cabridenc R. 1978. Polydimethylsiloxanes, IRNS. Cited in TNO Report to EEC No U/83/208(605). Evaluation of the impact of Organo Silicone Compounds on the Aquatic Environment. TNO, The Hague, The Netherlands. Cited in ECETOC 1994.
Cai G, Weber WP. 2004. Synthesis of terminal Si–H irregular tetra-branched star
polysiloxanes. Pt-catalyzed hydrosilylation with unsaturated epoxides. Polysiloxane films by photo-acid catalyzed crosslinking. Polymer. 45: 2941–8.
Canada. 1978. Food and Drug Regulations, C.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette, Part III, Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, Vol. 134, No. 7.
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 11 Challenge substances. Canada Gazette, Part I, Vol. 143, No.39.
Carpenter JC, Cella JA, Dorn SB. 1995. Study of the degradation of polydimethylsiloxanes on soil. Environmental Science and Technology. 29: 864-868.
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. CATALOGIC.
Christensen K. 1994. Silicone Environmental Health and Safety Council Study Report, Document ID no. 47. Cited in Fendinger et al. 1997.
[CNS] Cosmetic Notification System [Proprietary Database]. 2010. Available from Health Canada, Cosmetics Division.
Craig NCD, Caunter JE, Eales GJ. 1984. ICI Let, Brixham, UK, prepared for Centre Europeen des Cilicones, Dow Corning report no. I-9039-0004. Cited in Fendinger et al. 1997.
Craig NCD, Caunter JE. 1990. The effects of polydimethylsiloxane (PDMS) in sediment on the polychaete worm Nereis diversicolor. Chemosphere. 21(6):751-759.
Dimitrov SD, Dimitrova NC, Walker JD, Veith GD, Mekenyan OG. 2002. Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size. Pure and Applied Chemistry. 74(10):1823-1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
Dow Corning Corporation. 1961. The results of range finding studies on several Dow Corning siloxane monomers, polymers and cyclic intermediates with letter dated 042094. Submission to Document Processing Center, Office of Toxic Substances. U.S. Environmental Protection Agency. Washington, DC. OTS0556497; Old Doc ID: 86940001043.
Dow Corning Corporation. 1972. Continuing chemical structure-biological activity-relationship of selected cyclotetrasiloxane on the make reproductive system. Submission to Document Processing Center, Office of Toxic Substances. U.S. Environmental Protection Agency. Washington, DC. OTS0572454; Old Doc ID: 86940000351.
[DPD] Drug Products Database [database on the Internet]. 2010. Ottawa (ON): Health Canada. [cited 2010 Apr]. Drug Product Database.
Drobny JG. 2001. Technology of fluoropolymers. CRC Press LLC, 2000 NW Corporate Blvd., Boca Raton, Florida 33431.
Eales GJ and Taylor D. 1983. A study of the movement of a polydimethylsiloxane fluid in sediment columns – Phase 2. Dow Corning Report I-9039-002. Cited in ECETOC 1994.
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 1994. Joint Assessment of Commodity Chemicals No. 26: Linear Polymethylsiloxanes (viscosity 10 – 100 000 centistokes) CAS No. 63148-62-9. European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, Belgium. JACC Reports.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2008. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Environment Canada. 2008. Guidance for Conducting Ecological Assessments under CEPA, 1999, Science Resource Technical Series, Technical Guidance Module: Mass Flow Tool. Working Document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2010a. Data for Batch 11 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain Batch 11 Challenge substances. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2010b. Assumptions, limitations and uncertainties of the Mass Flow Tool for MVTFS, CAS RN 68952-02-3. Internal draft document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2010c. Industrial release report: CAS RN 68952-02-3, 2010-05-19. Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada, Health Canada. 2008a. Screening Assessment for the Challenge: Octamethylcyclotetrasiloxane(D4): Chemical Abstracts Service Registry Number 556-67-2 [Internet]. Ottawa(ON): Environment Canada, Health Canada. [cited 2010 May].
Environment Canada, Health Canada. 2008b. Screening Assessment for the Challenge: Decamethylcyclopentasiloxane (D5): Chemical Abstracts Service Registry Number 541-02-6 [Internet]. Ottawa(ON): Environment Canada, Health Canada. [cited 2010 May].
Environment Canada, Health Canada. 2008c. Screening Assessment for the Challenge: Dodecamethylcyclohexasiloxane (D6): Chemical Abstracts Service Registry Number 540-97-6 [Internet]. Ottawa(ON): Environment Canada, Health Canada. [cited 2010 May].
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2008. Version 4.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Fendinger NJ, Lehmann RG, and Mihaich EM. 1997. Polydimethylsiloxane. Organosilicon Materials. The Handbook of Environmental Chemistry. Springer-Verlag Berlin Heidelberg.
Firmin R. 1984. Les composes organosiliciés et l’environnement aquatique. Mémoire, Sciences Environnement, Université Libre Bruxelles. Cited in TNO Report to EEC No U/83/208(605). Evaluation of the Impact of Organo Silicon Compouns on the Aquatic Environment. TNO, The Hague, The Netherlands. Cited in ECETOC 1994.
Friberg T.R., Verstraeten T.C., Wilcox D.K. 1991. Effects of emulsification, purity and fluorination silicone oil on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 32:2030-2034.
Gabel V.P., Kampik A., Gabel C.H., Spiegel D. 1987. Silicone oil with high specific gravity for intraocular use. Br J Ophthalmol 71:262-267.
Garvey N, Collins MK, Mihaich EM. 1996. Abs no. 256, SETAC 17th Annual Meeting, Washington, DC. Cited in Graiver et al. 2003.
Gettings RL and Lane TH. 1982. Dow Corning Corp, Internal Report no. I0005-10957. Cited in Fendinger et al. 1997 and ECETOC 1994.
Gobas FAPC and Morrison HA. 2000. Bioconcentration and biomagnificaiton in the aquatic environment. Handbook of property estimation methods for chemicals. Eds. Boethling RS, Mackay D. Lewis Publishers, Boca Raton, FL.
Graiver D, Farminer KW, Narayan R. 2003. A review of the fate and effects of silicones in the environment. Journal of Polymers and the Environment. 11 (4): 129-136.
Gremillion CM Jr., Peyman GA, Liu KR, Naguib KS. 1990. Fluorosilicone oil in the treatment of retinal detachment. Br J Ophthalmol 74:643-646.
Guillenaut G, Aubert J, Augier H. 1987. Study on organosilicon contamination and toxicity in relation to the marine biomass. Revue Internationale d’Océanographie Médicale. 85/86: 88 (cited in Fendinger et al. 1997).
Hayden JF, Barlow SA. 1972. Structure-activity relationships of organosiloxanes on the female reproductive system. Toxicol Appl Pharmacol 21:68-79.
Health Canada. 2009. The cosmetic ingredient hotlist – September 2009 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2010 May 14]. Cosmetics and Personal Care.
Heimann H., Stappler T., Wong D. 2008. Heavy tamponade 1: a review of indications, use, and complications. Eye 22: 1342-1359.
Henry KS, Wieland WH, Powell DE, Giesy JP. 2001. Laboratory analyses of the potential toxicity of sediment-associated polydimethylsiloxne to benthic macroinvertebrates. Environmental Toxicology and Chemistry. 20(11): 2611-2616.
Hill RW. 1980. Determination of acute toxicity of DC 561 and DC X2-3168 to mussel (Mytilus edulis), report DC 19039 (6 and 8). Cite in TNO Report to EEC No U/83/208(605). Evaluation of the Impact of Organo Silicone Compounds on the Aquatic Environment. TNO, The Hague, The Netherlands. Cited in ECETOC 1994.
Hill RW, Young BE, Eales GJ. 1980. Imperial Chemical Industries Ltd., Brixham, TQ58BA, England. Determination of acute toxicity of Dow Corning DC 561 to the plaice (Pleuronectes platessa). Report No. BL/B/2052, prepared for Dow Corning, (DC Report no. I-9030-5). Cited in Fendinger et al. 1997.
Hill RW, Caunter, JE, Eales GJ. 1984. Imperial Chemical Industries Ltd., Brixham, TQ58BA, England. Effect of an organosilicon formulation on embryos and larvae of the sheepshead minnow (Cyprinodon variegates). Report No. BL/B/2392A, prepared for Centre European des Silicones, 34 pp (DC Report no. I-9039-0003). Cited in Fendinger et al. 1997 and ECETOC 1994.
Hobbs EJ, Keplinger ML, and Calandra JC. 1975. Toxicity of polydimethylsiloxanes in certain environmental systems. Environmental Research. 10: 397-406.
[JACC] Joint Assessment of Commodity Chemicals No. 26. 1994. Linear Polymethylsiloxanes (viscosity 10 – 100 000 centistokes) CAS No. 63148-62-9. European Centre for Ecotoxicology and Toxicology of Chemicals. Brussels, Belgium. JACC Reports.
Kählig H, Zöllner P, Mayer-Helm BX. 2009. Characterization of degradation product of poly[(3,3,3-trifluoropropyl)methylsiloxane] by nuclear magnetic resonance spectroscopy, mass spectrometry and gas chromatography. Polymer Degradation and Stability. 94: 1254-1260.
Kobayashi H, Nishiumi W. 1993. Preparation and characterization of poly
(methyl-3,3,3-trifluoropropylsiloxanes-co-dimethylsiloxane). Makromol Chem. 194:1403–10.
Kukkonen J, Landrum PF. 1995. Effects of sediment-bound polydimethylsiloxane on the bioavailability and distribution of benzo[a]pyrene in lake sediment to Lumbriculus variegatus. Environmental Toxicology and Chemistry 14: 523-531.
Le Fevre R., Coulston F., Golberg L. 1972. Action of a copolymer of mixed phenylmethylcyclosiloxanes on reproduction in rats and rabbits. Toxicol Appl Pharmacol 21: 29-44.
Lehmann RG, Varaprath S, Frye CL. 1994a. Degradation of silicone polymers in soil. Environmental Toxicology and Chemistry. 13: 1061-1064.
Lehmann RG, Varaprath S, Frye CL. 1994b. Fate of silicone degradation products (silanols) in soil. Environmental Toxicology and Chemistry. 13: 1753-1759.
Lehmann RG, Varaprath S, Annelin RB, Arndt J. 1995. Degradation of silicone polymer in a variety of soils. Environmental Toxicology and Chemistry. 14: 1299-1305.
Lehmann RG, Miller JR, Xu S, Singh UB, Reece CF. 1998. Degradation of silicone polymer at different soil moistures. Environmental Science and Technology. 32: 1260-1264.
Levier RR. 1988. Permethylated siloxane insect toxicants. Silicon chemistry. COREY-GASPAR – Ellis Howood Publishers – Chap. 15 : 153-160. Cited in ECETOC 1994.
[LNHPD] Licensed Natural Health Products Database [database on the Internet]. 2010. Natural Health Products Directorate, Health Canada. [cited 2010 Apr].
Maggi P and Alzieu C. 1977. Etude de la nocivité d'huiles polydimethyl -siloxane à l'égard d'organismes marins. Science et Péche, Bulletin de l’Institut des Péches Maritimes. 269: 1-3. Cited in Stevens et al. 2001.
Mann H, Ollenschlager B, Reichenback-Klinke HH. 1977. Reichenbach-Klinke, Untersuchunger zur Wirkung von Siliconölen auf Regenbogenforellen. Fisch Umwelt. 3:19. Cited in Fendinger et al. 1997 and ECETOC 1994.
Miyamoto L, Refojo MF, Tolentino FI, Flournier SA. 1986. Fluorinate oils as experimental vitreous substitutes. Arch Ophthalmol 104:1053-1056.
Nakamura K, Refojo MF, Crabtree DV, Pastor J, Leong FL. 1991. Ocular toxicity of low-molecular-weight components of silicone and fluorosilicone oils. Invest Ophthalmol Vis Sci 32:3007-3020.
[NCI] National Chemical Inventories [database on CD-ROM]. 2009. Issue 2. Columbus (OH): American Chemical Society. [cited 2010 May]. National Chemical Inventories.
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2010. Natural Health Products Directorate, Health Canada. [cited 2010 Apr].
Nielson WR, Heinlein JP and Haigh WG. 1975. Acaridical and insectisidal activity of various silicone fluids. TSCA 8(c) Submission 87-8215084. Cited in ECETOC 1994.
Noll, W. 1968. Chemistry and Technology of Silicones. Academic Press, New York. Cited in USEPA 2008.
Opperhuizen A, Van Der Velde EW, Gobas FAPC, Liem DAK, Van Der Steen JMD, Hutzinger O. Relationship between bioconcentration in fish and steric factors of hydrophobic chemicals. 1985. Chemosphere. 14: 1871-1896.
OECD. 1994. Organization for Economic Co-operation and Development. Chemicals Group and Management Committee. Chairman’s Report, Third Meeting of OECD Experts on Polymers, Tokyo, 14-16. April 1993.
Pagliani M, Bravais E, Tonge L. 2008. 100% fluoro liquid silicone rubber: fuel and oil resistance achieved in an LSR. Rubber World. 237(5):22–5.
Pastor JC, Lopez MI, Saornil MA, Refojo MF. 1992. Intravitreal silicone and fluorosilicone oils: pathologic findings in rabbit eyes. Acta Ophthalml 70:651-656.
[PMRA] Pest Management Regulatory Agency. 2007. Regulatory Note REG 2007-04: PMRA list of formulants. Ottawa (ON): Health Canada, Pest Management Regulatory Agency. [cited 2010-05-14].
Putt AE. 1994. Polydimethylsiloxane (PDMS) – the subchronic toxicity to midge larvae (Chironomus tentans) under flow-through conditions. Springborn Laboratories, Inc., Wareham, MA, SLI Report no. 94-4-5235. Cited in Fendinger at al. 1997.
Putt AE, Mihaich EM. 1996. Effect of sediment-bound polydimethyl-siloxane (PDMS) to aquatic invertebrates. Abs no. 257, SETAC 17th Annual Meeting, Washington, DC. Cited in Stevens et al. 2001.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. Journal of Environmental Biology. 29(1):89-92.
Spacie A. 1972. Acute toxicity of SAG 10 and SAG 350 silicone antifoam to aquatic organisms. Internal Report, Union Carbide Chemicals Corp,. Danbury, USA. Cited in ECETOC 1994.
Sparrow JR, Ortiz R, Macleish PR, Chang D. 1990. Fibroblast behaviour at aqueous interfaces with perfluorocarbon, silicone and fluorosilicone liquids. Invest Ophthalmol Vis Sci 31:638-646.
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2006. Copenhagen (DK): Nordic Council of Ministers. [cited 2010 May]. SPIN - Substances in Preparation in Nordic Countries.
Stevens C, Powell DE, Mäkelä P, Karman C. 2001. Fate and effects of polydimethylsiloxane (PDMS) in marine environments. Marine Pollution Bulletin. 42 (7): 536-543.
USEPA. 2008. Interpretive assistance document for assessment of polymers. United States Environmental Protect Agency.
Veith CA, Cohen RE. 1989. Kinetic modelling and optimization of the trifluoropropylmethylsiloxane polymerization. Journal of Polymer Science, Part 1: Polym Chem. 27: 1241-58.
Versura P, Cellini M, Torreggiani A, Bernabini B, Rossi A, Moretti M, Caramazza R. 2001. The biocompatibility of silicone, fluorosilicone and perfluorocarbon liquids as vitreous tamponades. an ultrastructural and immunohistochemical study. Ophthalmologica. 215:276-83.
Wacker. 1992. Wacker-Broschüre: Silicone Fluids AK, Wacker Chemie AG, München, Germany. Cited in ECETOC 1994.
Watanabe N, Nakamura T, Watanabe E, Sato E, Ose Y. 1984a. Bioconcentration potential of polydimethylsiloxane (PDMS) fluids by fish. Science of the Total Environment. 38: 167.
Watanabe N, Yasuda Y, Kato K, Nakanura T, Funasaka R, Shimokawa K, Sato E, Ose Y. 1984b. Determination of trace amounts of siloxanes in water, sediments and fish tissues by inductively coupled plasma emission spectrometry. Science of the Total Environment. 34 (1-2): 169-176.
Xu S. 1998. Hydrolysis of poly(dimethylsiloxanes) on clay minerals as influenced by exchangeable cations and moisture. Environmental Science and Technology. 32(20): 3162–3168.
Appendix Ia: PDMS data (Wacker 1992 and Fendinger et al. 1997)
| Viscosity (cs) | Average Number of D-Units[*] | Number-Average Molecular Weight (g/mol) |
|---|---|---|
| 10 | 15 | 1 300 |
| 50 | 40 | 3 000 |
| 100 | 70 | 5 000 |
| 1 000 | 200 | 15 000 |
| 10 000 | 500 | 37 000 |
| 100 000 | 1 000 | 74 000 |
Appendix Ib: PDMS data from Noll (1968, cited in USEPA 2008)
| Viscosity (cs) | Average Number of D-Units | Number-Average Molecular Weight (g/mol) |
|---|---|---|
| 60 | 50 | 3 600 |
| 140 | 110 | 8 000 |
| 440 | 230 | 17 000 |
| 680 | 280 | 21 000 |
| 1 440 | 400 | 30 000 |
| 10 000 | 800 | 60 000 |
| 50 000 | 1 200 | 88 000 |
| 100 000 | 1 400 | 103 000 |
| 300 000 | 1 900 | 143 000 |
The siloxane polymer MVTFS has high molecular weight and been considered out of the domains of the available QSAR models. Therefore there is no model used in the assessment on MVTFS.
| Substance | Oral LD50 (rats) |
Eye irritation (rabbits) | Skin irritation (rabbits) | Single exposure of rats to saturated atmospheres | ||
|---|---|---|---|---|---|---|
| Temp (°C) | Exposure duration (hour) | Response | ||||
| Trifluoropropylmethyl siloxane polymer (250 cs)[1] | NR | NR | NR | 300 | 1.25 | Killed all |
| 250 | 1.25 | No death | ||||
| Trifluoropropylmethyl siloxane polymer (1000 cs) | NR | NR | NR | 375 | 1.25 | Killed all |
| 350 | 1.25 | No death | ||||
| Trifluoropropylmethyl siloxane, cyclic trimer[2] | NR | NR | NR | 150 | 1.25 | Killed ¾ |
| 100 | 7.0 | Killed all | ||||
| 100 | 4.0 | Killed all | ||||
| Trifluoropropylmethyl siloxane, cyclic trimer | LD50 about 180 mg/kg bw[3], with kidney pathology | Slight | Slight | Mist formed at 100 °C is quite toxic, 7 hour[2] exposure to vapour alone had essentially no effects. | ||
| Trifluoropropylmethyl siloxane, cyclic tetramer | NR | Slight | Slight | 100 | 7.0 | Essentially no response, no fog was formed |
| Trifluoropropylmethyl siloxane, cyclic pentamer | LD50 = 2000 mg/kg bw, no other effects | Slight | Slight | 100 | 7.0 | Essentially no response, no fog was formed |
[2] This substance has a different Dow Corning code number from the substance in the row below.
[3] The results cited are based on best interpretation where the reprint of the original document was difficult to read.
Footnotes
[2] A determination of whether one or more of the criteria of section 64 are met is based upon an assessment of potential risks to the environment and/or to human health associated with exposures in the general environment. For humans, this includes, but is not limited to, exposures from ambient and indoor air, drinking water, foodstuffs, and the use of consumer products. A conclusion under CEPA 1999 on the substances in the Chemicals Management Plan (CMP) Challenge Batches 1-12 is not relevant to, nor does it preclude, an assessment against the hazard criteria specified in the Controlled Products Regulations, which is part of regulatory framework for the Workplace Hazardous Materials Information System [WHMIS] for products intended for workplace use. Similarly, a conclusion based on the criteria contained in section 64 of CEPA 1999 does not preclude actions being taken under other sections of CEPA or other Acts.
[3] cs – centistokes, the unit for kinematic viscosity of a polymer. The viscosity of a polymer has been usually reported in scientific publications, which is used to characterize the molecular size and molecular weight of polymers.
Page details
- Date modified: