Assessment - Aldehydes group
Chemical Abstracts Service Registry Numbers:
- 100-52-7
- 124-13-0
- 124-19-6
- 1334-78-7
- 8024-06-4
Environment and Climate Change Canada
Health Canada
May 2024
Cat. No.: En84-375/2024E-PDF
ISBN 978-0-660-71120-1
Synopsis
Pursuant to section 77 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted an assessment of 5 substances referred to collectively under the Chemicals Management Plan as the Aldehydes Group. The 5 substances are listed in the table below along with their Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1), their Domestic Substances List (DSL) names and their common names.
| CAS RN | DSL name | Common name |
|---|---|---|
| 100-52-7 | Benzaldehyde | NA |
| 124-13-0 | Octanal | NA |
| 124-19-6 | Nonanal | NA |
| 1334-78-7 | Benzaldehyde, methyl- | Methylbenzaldehyde |
| 8024-06-4b | Oils, vanilla | Vanilla oils |
Abbreviation: NA, not available
a This CAS RN is a UVCB (Unknown or Variable composition, Complex reaction products or Biological materials).
Benzaldehyde, octanal, nonanal, and methylbenzaldehyde are reported to naturally occur in a variety of foods. Vanilla oils are also naturally occurring and are defined as the extractives and physically modified derivatives of Vanilla planifolia. All 5 substances in the Aldehydes Group were included in surveys issued pursuant to section 71 of CEPA. According to information submitted, octanal and methylbenzaldehyde were not imported or manufactured in Canada above the reporting threshold of 100 kg in 2011. Benzaldehyde, nonanal and vanilla oils were imported into Canada with quantities ranging from 123 kg to 9075 kg, while 3086 kg of benzaldehyde was manufactured in the same year. Reported uses include air care, cleaning and furnishing care, lubricants and greases, and personal care products.
In Canada, the substances in the Aldehydes Group have uses as ingredients in cosmetics, as formulants in pest control products, as non-medicinal ingredients in natural health products, and may be used as food flavouring agents and as components in the manufacture of certain food packaging materials. In addition, substances in the Aldehydes Group are present in various other products available to consumers, including air fresheners.
The ecological risks of the substances in the Aldehydes Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, the substances in the Aldehydes Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this assessment, there is low risk of harm to the environment from benzaldehyde, octanal, nonanal, methylbenzaldehyde and vanilla oils. It is concluded that benzaldehyde, octanal, nonanal, methylbenzaldehyde and vanilla oils do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to the general population of Canada, scenarios which result in the highest levels of exposure were used to characterize potential exposure of Canadians to the substances in the Aldehydes Group through the use of products available to consumers and from environmental media and food.
According to the available information, the general population is expected to be exposed to benzaldehyde from the use of various products available to consumers (such as body moisturizers and air fresheners), environmental media, its potential use as a food flavouring agent, and its natural occurrence in food. Based on laboratory studies, the critical health effects of benzaldehyde are liver toxicity when administered via the inhalation route and decreased survival rate when administered orally.
Exposure of the general population to octanal is expected from the use of various products available to consumers (such as natural health products), environmental media, its potential use as a food flavouring agent, and its natural occurrence in food. Potential health effects via the oral route were informed by read-across data from butanal, which was reported to cause stomach lesions. Potential health effects via the dermal route were informed by read-across data from nonanal.
Exposure of the general population to nonanal is expected from the use of various products available to consumers (such as air fresheners and spray sunscreens), environmental media, its potential use as a food flavouring agent, and its natural occurrence in food. Potential health effects via the oral route were informed by read-across data from butanal, which was reported to cause stomach lesions. When administered dermally, nonanal was found to cause skin irritation but no adverse systemic effects.
Due to a lack of data on the health effects of octanal and nonanal via the inhalation route, butanal and isobutanal were selected as analogues to support hazard characterization for inhalation exposures. These substances were found to act as respiratory irritants causing minimal to moderate nasal lesions in laboratory animals, but without any apparent systemic toxicity.
Exposure of the general population to methylbenzaldehyde is expected from its potential use as a food flavouring agent and from its natural occurrence in food. The critical health effect for this substance is reduced relative pituitary weight when administered orally.
According to comparisons of levels of exposure to benzaldehyde, octanal, nonanal and methylbenzaldehyde from environmental media, food, and/or from the use of products available to consumers with levels at which health effects occur, there are margins that are considered adequate to address uncertainties in the health effects and exposure datasets.
Exposure of the general population to vanilla oils is expected from its natural occurrence in food, from its potential use as a food flavouring agent, and from the use of various products available to consumers such as body moisturizers, lip balms, and bath products. No health effects information was available for vanilla oils; therefore, its major component, vanillin, was used to inform the health effects of vanilla oils. In several short-term and long-term repeated-dose studies, vanillin did not produce any adverse effects up to the limit dose and was negative for genotoxicity and carcinogenicity. There was no evidence of developmental or reproductive effects based on read-across to the analogue ethyl vanillin. Taking into account the available data, vanilla oils are considered to be of low hazard potential and therefore risk to human health is considered to be low.
The human health assessment took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. For substances in the Aldehydes Group, these subpopulations with potential for higher exposure, and those who may be more susceptible, were taken into account in the risk assessment outcomes.
Considering all the information presented in this assessment, it is concluded that benzaldehyde, octanal, nonanal, methylbenzaldehyde and vanilla oils do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that benzaldehyde, octanal, nonanal, methylbenzaldehyde and vanilla oils do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 77 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted an assessment on 5 of 6 substances, referred to collectively under the Chemicals Management Plan as the Aldehydes Group, to determine whether these 5 substances present or may present a risk to the environment or to human health. These 5 substances were identified as priorities for assessment as they met categorization criteria as described in ECCC, HC (modified 2017).
The remaining substance, benzaldehyde, 2-hydroxy-5-nonyl, oxime, branched (Chemical Abstracts Service Registry Number (CAS RN) 174333-80-3), was considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016), and was identified as being of low concern to both human health and the environment. As such, it is not further addressed in this report. Conclusions for this substance are provided in the Substances Identified as Being of Low Concern using the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC, HC 2018). The 5 substances addressed in this assessment will hereinafter be referred to as the Aldehydes Group.
The ecological risks of the substances in the Aldehydes Group were characterized using ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Benzaldehyde and vanilla oils have been identified in vaping products (MSDS 2010a, 2010b, 2012a, 2013, 2014, 2015a, 2016a). Vaping products (also known as electronic cigarettes) may represent an additional source of exposure to these substances. Benzaldehyde and vanilla oils are not proposed to be permitted as flavourants in vaping products in Canada (Canada 2021). The assessment of risk to the general population from this use, including risk relative to that associated with conventional cigarettes, and possible options to mitigate risk associated with these products are being addressed through a separate legislative framework (Health Canada [modified 2020]).
The substances in the Aldehydes Group and their analogues have been reviewed internationally through the Organisation for Economic Co-operation and Development (OECD), and Screening Information Data Set Initial Assessment Reports are available. These assessments undergo rigorous review (including peer review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change are active participants in these processes, and consider these assessments to be reliable. In addition, health effects for substances in the Aldehydes Group have been evaluated by the United States Environmental Protection Agency (US EPA), the European Food Safety Agency (EFSA), the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA), and the Australian Government Department of Health (AGDH). These assessments were used to inform the health effects characterization for certain substances in this assessment.
This assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to August 2020. Empirical data from key studies as well as results from models were used to reach these conclusions.
This assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portion of this assessment has undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Ms. Theresa Lopez, Ms. Jennifer Flippin, and Dr. Joan Garey at TetraTech. The ecological portion of this assessment is based on the ERC science approach document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this assessment (published on October 8, 2022) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information, if available, on subpopulations who may have greater susceptibility or greater exposure, vulnerable environments and cumulative effects, and by incorporating a weight-of-evidence approach and precaution.Footnote 2 This assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The CAS RNs, Domestic Substances List (DSL) names, and common names for the individual substances in the Aldehydes Group are presented in Table 2-1.
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 100-52-7 | Benzaldehyde |  C7H6O C7H6O |
106.12 |
| 124-13-0 | Octanal |  C8H16O C8H16O |
128.21 |
| 124-19-6 | Nonanal |  C9H18O C9H18O |
142.24 |
| 1334-78-7a | Benzaldehyde, methyl- (methylbenzaldehyde) |  C8H8O C8H8O |
121.16 |
|
8024-06-4b Major component: 121-33-5 |
Oils, vanilla (vanilla oils) Major component: vanillin |
N/A Major component:  C8H8O3 C8H8O3
|
N/A Major component: 152.15 |
Abbreviation: N/A, not applicable
a The chemical structure shown is the general structure, where the methyl group may be located at any remaining position on the phenyl ring.
b Substance is a UVCB (Unknown or Variable composition, Complex reaction products or Biological materials). These materials are derived from natural sources or complex reactions. A UVCB is not an intentional mixture of discrete substances and is considered a single substance. The complexity and variability of their compositions can make them difficult to fully and consistently characterize.
Methylbenzaldehyde was categorized as a discrete substance under CEPA (ECCC, HC [modified 2017]); however, it is recognized that this substance possesses some Unknown or Variable composition, Complex reaction products or Biological materials (UVCB)-type characteristics, as the methyl group may be located ortho, meta or para to the aldehyde functional group.
Vanilla oils are classified as a UVCB. Although vanilla oils may be sourced from Vanilla planifolia or Vanilla tahitensis, the National Chemicals Inventory (NCI 2020) defines vanilla oils as the extractives and physically modified derivatives of V. planifolia. In addition, the Personal Care Products Council’s Cosmetic Ingredient Identification Database (PCPC CIID) associates the extractives of V. planifolia with CAS RN 8024-06-4, while the extractives of V. tahitensis are associated with CAS RNs 94167-14-3 (V. tahitensis extract) and 953789-39-4 (V. tahitensis oils) (PCPC 2020). Thus, only vanilla oils from V. planifolia -- that is, CAS RN 8024-06-4 -- are considered in this assessment.
Vanilla oils are mostly produced from V. planifolia as an extract from macerated cured vanilla fruit (also known as vanilla beans or pods) by a percolation or oleoresin method, using either ethanol/water or ethanol only as an extraction solvent, where ethanol concentration is not less than 35% and the volatile aroma content comprises up to 4% of the total extract composition on a dry weight basis (CIR 2020). The volatile aroma content contains mostly phenolic compounds that evoke the distinct flavour and fragrance of vanilla, with vanillin being the most prominent, accounting for about 80% of the volatile aroma content and up to 3.6% of the total extract composition on a dry weight basis (Brunschwig et al. 2009; CIR 2020). Vanilla oils may also be produced by supercritical CO2 extraction which results in solvent-free extracts that have a vanillin composition as high as 97% of the volatile aroma content (Sinha et al. 2007). A compilation of volatile and semi-volatile compounds detected in V. planifolia fruit and/or extracts thereof identifies vanillin as the major component (Havkin-Frenkel and Belanger 2018). The fatty acid content of V. planifolia fruit extractives has been found to be up to 2.4% on a dry weight basis (Brunschwig et al. 2009). Compositional information on the non-volatile component of V. planifolia extractives is limited; the non-volatile constituents of vanilla fruit are tannins, polyphenols, resins and free amino acids (Ramachandra Rao and Ravishankar 2000). Vanilla oils may also be associated with vanilla seeds (which are available in powder form) or leaf cell extracts. The seed coats are mostly made up of acid-insoluble lignin polymers and cellulose, and the leaves mostly contain glucosides, chlorophyll and carotenoids (CIR 2020); however, the exact proportion of the volatile aroma content in these parts of the plant is unclear. Considering the low bioavailability of lignin when ingested by humans (Holloway et al. 1978), the lack of clear composition data on leaf cell extracts, and the well-characterized composition of vanilla fruit extracts, the extractives of the vanilla fruit, rather than the seed powder or leaf cell extracts of V. planifolia, are considered as the main source of vanilla oils for the purpose of this assessment. The substances of interest from a toxicological perspective (ecological and human health) are considered to be in the volatile aroma portion of the fruit extracts, wherein vanillin is the major component.
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ([Q]SAR) models, where appropriate, has been used to inform the ecological and human health assessments. Analogues were selected that were structurally similar to substances within this group and that had relevant empirical data that could be used to read across to substances with limited empirical data. Physical-chemical properties and toxicokinetics were also considered. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the ecological and human health assessments of the Aldehydes Group are further discussed in the relevant sections of this report.
Sodium benzoate was used as an analogue to inform the developmental and reproductive effects of benzaldehyde for the human health assessment. Sodium benzoate is structurally similar to benzaldehyde and is metabolized in the stomach to benzoic acid, which is one of the major metabolites of benzaldehyde.
To inform the hazard characterization for octanal and nonanal following inhalation exposure, butanal and isobutanal were selected as analogues for the human health assessment using a read-across approach. Both analogue substances are aliphatic aldehydes which are expected to share a common metabolic profile with octanal and nonanal.
Vanillin is considered the major component of vanilla oils that is of toxicological interest; it represents up to 3.6% of the total extract composition of V. planifolia on a dry weight basis and 80% of the volatile aroma content (Brunschwig et al. 2009; CIR 2020). Therefore, human health effects information on vanillin was used to inform the human health effects of vanilla oils. However, vanillin is associated with limited empirical toxicity data and therefore ethyl vanillin was used as an analogue in order to inform the hazard characterization of vanillin.
Information on the identity and chemical structure of the analogues used to inform this assessment is presented in Table 2-2. Further information can be found in Appendix A.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 532-32-1 | Benzoic acid, sodium salt (sodium benzoate) | ![c1(ccccc1)C(=O)[O-].[Na+]](/content/dam/eccc/images/pded/aldehydes/20220819-t22a.jpg) C7H5O2.Na C7H5O2.Na |
144.1 |
| 123-72-8 | Butanal (butyraldehyde) |  C4H8O C4H8O |
72.1 |
| 78-84-2 | Propanal, 2-methyl- (isobutanal) |  C4H8O C4H8O |
72.1 |
| 121-32-4 | Benzaldehyde, 3-ethoxy-4-hydroxy- (ethyl vanillin) |  C9H10O3 C9H10O3 |
166.18 |
3. Physical and chemical properties
A summary of physical and chemical property data of the substances in the Aldehydes Group is presented in Table 3-1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Benzaldehyde | Octanal | Nonanal | Methylbenzaldehydea | Vanilla oilsc | References |
|---|---|---|---|---|---|---|
| Physical state | Liquid | Liquid | Liquid | Liquid | NA | ECHA c2007-2019 |
| Melting point (°C) | -26 | -20 | -18.8 | -6 | NA | ECHA c2007-2019 |
| Vapour pressure (Pa) | 169 | 148 | 49 | 33 | NA | ECHA c2007-2019, PubChem 2004- |
| Henry’s law constant (Pa·m3/mol) | 2.7 | 52.1 | 74.4 | 5.1b | NA | ChemIDplus 1993-, PubChem 2004-, EPI Suite c2000-2012 |
| Water solubility (mg/L) | 6950 | 560 | 96 | 250 | NA | ECHA, HSDB 1983-, PubChem 2004- |
| Log Kow (dimensionless) | 1.48 | 3.50 | 3.40 | 2.25 | NA | ECHA c2007-2019 |
Abbreviations: NA, not available; Kow, octanol-water partition coefficient
a 4-Methylbenzaldehyde is used as the representative structure.
b This parameter was modelled using HENRYWIN v3.20 (EPI Suite c2000-2012).
c Refer to Appendix A for physical and chemical properties data for vanillin and ethyl vanillin, the major component and analogue, respectively, of this UVCB.
4. Sources and uses
Benzaldehyde occurs naturally, often in combined forms such as glycosides, in several plants and/or food items including oyster mushrooms, almonds, apricots, cherry and peach seeds, strawberry jam, cheeses, and black teas (Andersen 2006; Beltran-Garcia 1997; Burdock 2010; Opgrande et al. 2000). Octanal occurs naturally in various plants and/or food items including mandarins, key limes, milk, meats, and fruits (Burdock 2010; Chisholm et al. 2003a, 2003b; Verzera et al. 2000). Nonanal occurs naturally in essential oils including cinnamon oil, lemongrass oil, and citrus oil. It is also found in foods including milk, meats, and fruits (Burdock 2010). Methylbenzaldehyde occurs naturally in various food products including roasted nuts, tomatoes, cooked beef, and coffee (Burdock 2010). Vanilla oils are defined as the extractives and physically modified derivatives of V. planifolia (NCI 2020), which is a species of vanilla orchid, and are thus naturally occurring. Further, in the PCPC CIID (CIR 2020; PCPC 2020), the CAS RN 8024-06-4 for the vanilla oils substance is associated with V. planifolia flower extract, V. planifolia fruit, V. planifolia fruit extract, V. planifolia fruit oil, V. planifolia fruit water, V. planifolia leaf cell extract, V. planifolia seed, and V. planifolia seed powder. As such, all forms of extractives and other physically modified derivatives originating from V. planifolia were considered in the context of this assessment. The Food and Drug Regulations indicate that vanilla extract, vanilla essence, or vanilla flavour shall be the essence, extract or flavour prepared from the vanilla bean, the dried, cured fruit of V. planifolia, or V. tahitensis (Canada 1978). However, vanilla oils sourced from V. tahitensis are beyond the scope of this assessment as they are considered distinct from the substance represented by CAS RN 8024-06-4 and have their own corresponding CAS RNs (for example, CAS RNs 94167-14-3 and 953789-39-4).
The substances in the Aldehydes Group have been included in a survey issued pursuant to section 71 of CEPA for the 2011 reporting year (Environment Canada 2012). There were no reports of manufacture or import of octanal or methylbenzaldehyde into Canada above the reporting threshold of 100 kg. Table 4-1 presents a summary of information reported on the total manufacture and total import quantities for the Aldehydes Group.
| Common name | Total manufacturea (kg) | Total importsa (kg) |
|---|---|---|
| Benzaldehyde | 3086 | 9075 |
| Octanal | NRb | NRb |
| Nonanal | NRb | 3030 |
| Methylbenzaldehyde | NRb | NRb |
| Vanilla oils | NRb | 123 |
Abbreviation: NR, not reported
a Values reflect quantities reported in response to a CEPA section 71 survey (Environment Canada 2013). See survey for specific inclusions and exclusions (Schedules 2 and 3).
b No manufacturing and/or import quantities were reported for the substance above the reporting threshold of 100 kg for the 2011 reporting year.
Table 4-2 presents a summary of the major Canadian commercial and consumer uses of the substances in the Aldehydes Group according to information reported in response to a CEPA section 71 survey (Environment Canada 2012). Other uses were also reported but were identified as being confidential business information. These other uses, although not presented in this assessment, were taken into consideration in the risk assessment.
| Major usesa | Benzaldehyde | Octanal | Nonanal | Methylbenzaldehyde | Vanilla oils |
|---|---|---|---|---|---|
| Air care | Y | N | N | N | N |
| Apparel and footwear | Y | N | N | N | N |
| Automotive care | Y | N | N | N | N |
| Cleaning and furnishing care | Y | N | N | N | N |
| Laundry and dishwashing | Y | N | N | N | N |
| Lubricants and greases | Y | N | Y | N | N |
| Personal care | Y | Y | Y | N | Y |
| Pet care | Y | N | N | N | N |
Abbreviations: Y = yes this use was reported for this substance; N = no, this use was not reported for this substance or its use is considered confidential information
a Non-confidential uses reported in response to a CEPA section 71 survey (Environment Canada 2013). See survey for specific inclusions and exclusions (Schedules 2 and 3).
On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, 4 of the 5 substances in the Aldehydes Group have been notified to be present in cosmetics, including hair care (for example, hair conditioner), skin care (for example, body moisturizer), and lip care products containing benzaldehyde; lip balms containing octanal; lip balms containing nonanal; and skin care, lip balms, fragrance products, and non-fluorinated toothpastes containing vanilla oils. Methylbenzaldehyde has not been notified to be present in cosmetics (personal communication, emails from the Consumer and Hazardous Products Safety Directorate (CHPSD), Health Canada, to the Existing Substances Risk Assessment Bureau (ESRAB), Health Canada, 2018, 2020; unreferenced).
All substances in the Aldehydes Group, except methylbenzaldehyde, are present as formulants in currently registered pest control products in Canada. None of these substances are currently registered on the Pest Management Regulatory Agency’s (PMRA’s) List of Active Pesticide Ingredients (personal communication, emails from the PMRA, Health Canada, to the ESRAB, Health Canada, 2018; unreferenced).
Benzaldehyde may be used as a component in the formulation of a clarifying agent used in the manufacture of certain food packaging with direct food contact, and may also be a component in incidental additives used in food processing establishments (for example, surface cleaners, hand cleaners), with no expected food contact since the use of the cleaners is followed by a potable water rinse. Vanilla oils are naturally occurring in food (for example, vanilla beans) and are permitted in food as a flavouring preparation (as vanilla extract, vanilla essence, or vanilla flavour) as per the Food and Drug Regulations (Canada 1978; personal communication, emails from the Food Directorate (FD), Health Canada, to the ESRAB, Health Canada, 2018; unreferenced). No definitive information is available concerning the potential use of the other 4 substances in the Aldehydes Group as flavouring agents in foods sold in Canada. However, since these 4 substances are known to be used as food flavouring agents internationally, it is possible that they are present as such in foods sold in Canada (personal communication, emails from the FD, Health Canada, to the ESRAB, Health Canada, 2018; unreferenced).
Benzaldehyde is listed in the Natural Health Products Ingredients Database (NHPID) with a non-medicinal role for topical use up to 0.5% as a denaturant or fragrance ingredient, or for oral use as a flavour enhancer. Octanal, nonanal, and methylbenzaldehyde are listed in the NHPID with a non-medicinal role for oral use as a flavour enhancer. V. planifolia and related ingredients, such as vanilla, vanilla extract, V. planifolia essential oil, V. planifolia fruit, V. planifolia fruit extract, V. planifolia fruit oil, and vanilla powder are also listed with a medicinal or non-medicinal role in the NHPID. With the exception of methylbenzaldehyde, substances included in the Aldehydes Group are listed in the Licensed Natural Health Products Database as being present – mostly as non-medicinal ingredients – in natural health products (LNHPD [modified 2021]; NHPID [modified 2021]; personal communication, emails from the Natural and Non-prescription Health Products Directorate (NNHPD), Health Canada, to the ESRAB, Health Canada, 2018, 2019; unreferenced).
Benzaldehyde and vanilla oils are found as non-medicinal ingredients in various currently authorized marketed drug products (personal communication, emails from the Therapeutic Products Directorate, Health Canada, to the ESRAB, Health Canada, 2018, 2019; unreferenced).
Additional uses in Canada based on publicly available information were identified for some substances in the Aldehydes Group. Benzaldehyde was identified in air fresheners, stamp inks, and automotive cleaners (MSDS 2012b, 2016b, 2019), octanal was identified in air and dishwasher fresheners (MSDS 2008a, 2008b, 2010c, 2015b), and nonanal was identified in automotive air fresheners and spray sunscreens (MSDS 2008c, 2016c). Benzaldehyde and vanilla oils were also identified in flavoured
e-cigarette liquids (MSDS 2010a, 2010b, 2012a, 2013, 2014, 2015a, 2016a), and octanal and/or nonanal were also identified in tire-derived rubber flooring and granulates used in artificial turf infill in the United States (US) (CalRecycle 2010, 2011; Moretto 2007). In addition, benzaldehyde was identified as being both directly emitted and formed through secondary atmospheric reactions from gasoline emissions (OEHHA 2018).
Benzaldehyde, octanal, and nonanal were also measured in small-scale (0.05 m3) chamber tests conducted on building materials (for example, wooden panels, caulking, insulation) and products available to consumers (for example, incense sticks, air fresheners) (Won and Yang 2012; Won et al. 2013, 2014; Won 2015).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Aldehydes Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. Since vanilla oils are a UVCB substance and could not be suitably represented by a single chemical structure, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (for example, OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (Q)SAR or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of vanilla oils, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents, analyzing information submitted in response to a CEPA section 71 survey, making decisions on the basis of consideration of similar substances, and/or application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (that is, in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes 2 of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (that is, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Aldehydes Group and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the 5 substances in the Aldehydes Group are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Benzaldehyde | low | low | low |
| Octanal | low | low | low |
| Nonanal | low | low | low |
| Methylbenzaldehyde | low | low | low |
| Vanilla oils | high | low | low |
On the basis of low hazard and low exposure classifications according to information considered under ERC, benzaldehyde, octanal, nonanal and methylbenzaldehyde were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, vanilla oils were classified as having a low exposure potential. Vanilla oils were classified as having a high ecological hazard potential through a conservative manual classification which was applied due to uncertainties in the model outcomes for this substance. Vanilla oils were classified as having a low potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Potential exposures to substances in the Aldehydes Group from environmental media, food, and products available to consumers are presented in this section. As vanilla oils are considered to be of low hazard potential (see section 6.2), quantitative estimates of exposure to the general population were not derived for this substance. For benzaldehyde, octanal, nonanal, and methylbenzaldehyde, exposure scenarios resulting in the highest exposures for each age group were selected to characterize risk. Additional details regarding the exposure scenarios are summarized in Appendix B.
6.1.1 Environmental media
Neither methylbenzaldehyde nor vanilla oils were identified or measured in any environmental media in Canada or elsewhere. Since methylbenzaldehyde was not reported to be in commerce above the reporting threshold of the CEPA section 71 survey (Environment Canada 2013) and no products available to consumers were identified, exposure of the Canadian general population to methylbenzaldehyde from environment media is not expected.
Given that vanilla oils are considered to be of low hazard potential to human health, predicted environmental concentrations were not derived for this substance.
Air
Benzaldehyde has been measured in indoor and outdoor (ambient) air in Canada, while octanal and nonanal have been measured in Canadian indoor air and ambient air in the US. In the Canadian indoor air studies, air samples were collected inside and outside residential homes in cities across Canada (Health Canada, 2010a, 2010b, 2012, 2013; Li et al. 2019). The number of homes participating in each Canadian study ranged from 50 to approximately 3500. The detection frequencies of benzaldehyde, octanal, and nonanal ranged from 97% to 100%. An ambient air study conducted in the US analyzed constituents of atmospheric aerosols collected in the rural site of Niwot Ridge, Colorado (US EPA 2001). The measured Canadian air concentrations of these substances are provided in Table 6-1.
| Substance | Mean concentration (μg/m3) | 95th percentile concentration (μg/m3) | Media | Location | Reference |
|---|---|---|---|---|---|
| Benzaldehyde | 4.20 | 9.33 | Indoor air | Regina, SK | Health Canada 2010a |
| Benzaldehyde | 0.54 | 1.108 | Ambient air | Regina, SK | Health Canada 2010a |
| Benzaldehyde | 8.25 | 15.71 | Indoor air | Windsor, ON | Health Canada 2010b |
| Benzaldehyde | 0.49 | 1.206 | Ambient air | Windsor, ON | Health Canada 2010b |
| Benzaldehyde | 3.15 | 7.512 | Indoor air | Halifax, NS | Health Canada 2012 |
| Benzaldehyde | 0.40 | 0.82 | Ambient air | Halifax, NS | Health Canada 2012 |
| Benzaldehyde | 3.64 | 10.56 | Indoor air | Edmonton, AB | Health Canada 2013 |
| Benzaldehyde | 0.48 | 0.899 | Ambient air | Edmonton, AB | Health Canada 2013 |
| Benzaldehyde | 3.60 | 8.88 | Indoor air | 16 sites across Canada | Li et al. 2019 |
| Octanal | 4.06 | 11.0 | Indoor air | 16 sites across Canada | Li et al. 2019 |
| Nonanal | 10.50 | 30.6 | Indoor air | 16 sites across Canada | Li et al. 2019 |
Abbreviations: AB, Alberta; NS, Nova Scotia; ON, Ontario; SK, Saskatchewan
Estimated human intakes of benzaldehyde, octanal, and nonanal in air were derived using the highest measured 95th percentile concentrations from Table 6-1 where Canadian data was available. The resulting highest exposures relative to body weight were identified for toddlers (1 year of age). For indoor air, this corresponded to estimated daily exposures of 1.0 x 10-2, 7.0 x 10-3, and 1.9 x 10-2 mg/kg bw/day for benzaldehyde, octanal, and nonanal, respectively. For ambient air, this corresponded to estimated daily exposures of 1.1 x 10-4, 1.0 x 10-3, and 2.8 x 10-3 mg/kg bw/day for benzaldehyde, octanal, and nonanal, respectively. Canadian indoor air concentration was used as a surrogate for deriving ambient air exposure estimates for octanal and nonanal. See Appendix C for more details.
Benzaldehyde, octanal, and nonanal have also been reported to be emitted from building materials in small-scale (0.05 m3) chamber studies (Won and Yang 2012; Won et al. 2013, 2014; Won 2015). This source of exposure is considered to be addressed by the characterization of exposure from other scenarios in Table 6-5 (that is, air fresheners and spray sunscreen) which result in concentrations likely higher than those emitted from building materials.
Water
The substances in the Aldehydes Group have not been identified or measured in Canadian water samples. Given the limited use and industrial activity for octanal and nonanal based on information submitted in response to a CEPA section 71 survey (Environment Canada 2013), exposure to these 2 substances via water is not expected. Benzaldehyde has been measured in US ground and surface water up to 10 μg/L and 5.1 μg/L, respectively (NWQMC 2019). However, given the absence of Canadian surface or drinking water monitoring data, concentration of benzaldehyde in surface water was estimated using the level III fugacity model ChemCAN v6.00 (ChemCAN 2003) and used as a surrogate for drinking water. The resulting predicted environmental concentration of benzaldehyde in surface water was 7.9 x 10-4 μg/L. Daily exposure to benzaldehyde from drinking water for the age group with the highest exposure relative to body weight (formula-fed infants, 0 to 0.5 months) was estimated to be 1.0 x 10-7 mg/kg bw/day. See Appendix C for more details.
Soil
There were no measured soil concentration data for any of the substances in Aldehydes Group. Given the limited use and industrial activity for octanal and nonanal based on information submitted in response to a CEPA section 71 survey (Environment Canada 2013), exposure to these 2 substances via soil is not expected. Using the level III fugacity model ChemCAN v6.00 (ChemCAN 2003), the concentration of benzaldehyde in soil was predicted to be 2.8 x 10-2 μg/kg. In consideration of this value, potential daily exposure to benzaldehyde from soil is expected to be negligible. See Appendix C for more details.
6.1.2 Food
Benzaldehyde may be used as a component in the formulation of a clarifying agent used in the manufacture of certain food packaging with direct food contact; however, dietary exposure from this use is considered to be negligible (personal communication, emails from the FD, Health Canada to the ESRAB, Health Canada, 2018; unreferenced).
The JECFA evaluated benzaldehyde, methylbenzaldehyde, nonanal and octanal for use as food flavouring agents, and estimated the per capita intakes for the US population of these substances based on annual production volumes reported by the food industry (WHO 1999, 2002).
Vanilla oils are permitted in food as a flavouring preparation (as vanilla extract, vanilla essence, or vanilla flavour) as per the Food and Drug Regulations (Canada 1978; personal communication, emails from the FD, Health Canada, to the ESRAB, Health Canada, 2018; unreferenced). Given that vanilla oils are considered to be of low hazard potential, dietary intakes were not estimated for this substance.
In the absence of data on the actual use, if any, of benzaldehyde, methylbenzaldehyde, nonanal, or octanal as flavouring agents in foods sold in Canada, the per capita intake estimates for the US population were used as estimates of possible Canadian dietary exposure to these substances from this use in food (Table 6-2; personal communication, emails from the FD, Health Canada, to the ESRAB, Health Canada, 2019; unreferenced).
| Substance | Intake (μg per day) | Exposure estimate (μg/kg bw/day)a | Reference |
|---|---|---|---|
| Benzaldehyde | 36 000 | 600 | WHO 2002 |
| Methylbenzaldehyde | 1100 | 18 | WHO 2002 |
| Nonanal | 90 | 1.5 | WHO 1999 |
| Octanal | 17 | 0.29 | WHO 1999 |
a Exposure based on a 60 kg person (WHO 1999, 2002; Burdock 2010). In the absence of age group-specific exposure estimates, these exposures were assumed to be the same across all relevant age groups (1 year old and older). The bodyweight adjusted intakes using a 60 kg bodyweight is considered to be sufficiently conservative to represent the entire population 1 year of age and older (personal communication, emails from the FD, Health Canada, to the ESRAB, Health Canada, 2019 and 2020; unreferenced).
Benzaldehyde, nonanal, and octanal have also been identified to occur naturally in various food items (VCF 1992-2019; Burdock 2010). Based on production volumes and consumption ratios derived from US consumption data (Stofberg and Grundschober 1987), any potential exposures to benzaldehyde from its natural occurrence in food are expected to be less than those from its use as a food flavouring agent (personal communication, emails from the FD, Health Canada, to the ESRAB, Health Canada, 2019; unreferenced) and therefore were not estimated. According to JECFA’s review of octanal and nonanal as food flavouring agents (WHO 1999), the dietary exposure to these substances in the US from their natural occurrence in foods is expected to exceed the exposure to these substances from their use as food flavouring agents based on consumption ratios of 8.6 and 1900 for octanal and nonanal, respectively (Stofberg and Grundschober 1987), indicating their predominant natural occurrence in food (personal communication, emails from the FD, Health Canada, to the ESRAB, Health Canada, 2018; unreferenced). Due to the significant uncertainty that would result from estimating dietary exposure from naturally occurring octanal and nonanal in foods (for example, as a result of the presence of the substances in low concentrations in hundreds of foods and variability in data on concentrations in food and Canadian consumption patterns, etc.), derivation of dietary exposures from this source was not considered to be meaningful; thus, these exposures were not quantified.
Methylbenzaldehyde was identified to occur naturally but in a limited number of foods with little information on its concentrations. Therefore, the estimated intakes from its use as a food flavouring agent was considered as the main source of dietary exposure for this substance (personal communication, emails from the FD, Health Canada, to the ESRAB, Health Canada, 2019; unreferenced).
Vanilla oils are also naturally occurring in food (for example, vanilla beans). Although dietary intakes were not estimated for this substance, it has been noted that dietary exposure to vanilla oils from its natural occurrence in vanilla beans used as ingredients in foods is expected to be minor compared to exposure from food flavouring uses of these substances (personal communication, emails from the FD, Health Canada, to the ESRAB, Health Canada, 2020; unreferenced).
6.1.3 Products available to consumers
Potential exposures of the Canadian general population to the substances in the Aldehydes Group from products available to consumers were evaluated. Product scenarios that resulted in the highest levels of potential exposure for each substance by the oral, dermal, and inhalation routes are presented in Tables 6-3, 6-4, and 6-5, respectively. For octanal, exposure by the dermal route from identified products available to consumers was expected to be minimal and hence was not quantified. The estimated daily exposures from these product scenarios were found to be higher compared to potential exposures from the use of other products that are expected to occur on a per event or intermittent basis. As such, only daily exposures are presented.
Potential exposures were estimated based on conservative assumptions and using default values from sentinel exposure scenarios; see Appendix B for further details.
| Substance | Product scenario | Concentration | Age group | Daily systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Benzaldehyde | Lip balm | 3%a | Toddler (aged 2 to 3 years) | 0.044 |
| Octanal | Vitamin and mineral supplement tablet | 0.03%b | Child (aged 9 to 13 years) | 0.023 |
| Nonanal | Vitamin and mineral supplement tablet | 0.03%b | Child (aged 9 to 13 years) | 0.023 |
a Concentrations are on the basis of notifications submitted under the Cosmetic Regulations to Health Canada (personal communication, emails from the CHPSD, Health Canada, to the ESRAB, Health Canada, 2018 and 2020; unreferenced).
b Octanal and nonanal, together, contribute up to 1% of the flavouring mixture which in turn contributes 95 mg per 3052 mg tablet (personal communication, emails from the NNHPD, Health Canada, to the ESRAB, Health Canada, 2018; unreferenced).
| Substance | Product scenario | Concentration | Age group | Daily systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Benzaldehyde | Body moisturizer | 0.83%a | Toddler (2 to 3 years)b | 1.8c |
| Nonanal | Spray sunscreen | 1% (MSDS 2016c) | Toddler (2 to 3 years) | 2.3c |
a Concentrations are on the basis of notifications submitted under the Cosmetic Regulations to Health Canada (personal communication, emails from the CHPSD, Health Canada, to the ESRAB, Health Canada, 2018 and 2020; unreferenced).
b Based on available product information, use on infants is not expected.
c Dermal absorption was considered to be equivalent to oral absorption.
| Substance | Product scenario | Concentration (reference) | Age group | Daily systemic exposure (mg/kg bw/day)a | Mean event concentration (mg/m3) | Time-weighted average concentration (mg/m3) |
|---|---|---|---|---|---|---|
| Benzaldehyde | Air freshener | 50% (MSDS 2019) | Toddler (1 year) | 0.88 | N/A | N/A |
| Octanal | Air freshener | 5% (MSDS 2015b) | Toddler (1 year)b | 0.073 | 0.1 | N/A |
| Nonanal | Spray sunscreen | 1% (MSDS 2016c) | Teen (14 to 18 years)b | 0.11 | 4.4 | 0.03c |
Abbreviation: N/A, not applicable
a 100% absorption from the inhalation route is assumed for systemic exposure.
b Age group is applicable only to the daily exposure on a mg/kg bw/day basis. Air concentrations are applicable to all age groups.
c Ten-minute time-weighted average (TWA) concentration was derived to match up with the adjusted exposure duration of the critical effects study used to characterize risk which represents continuous exposure. 10-minute TWA = Mean event concentration x exposure duration / 24 hours.
No products available to consumers containing methylbenzaldehyde were identified. Therefore, exposure of the Canadian general population to methylbenzaldehyde from the use of such products is not expected.
For vanilla oils, exposure via the oral or dermal routes may result from the use of related ingredients as non-medicinal in natural health products intended for oral or topical use, respectively. Exposures via these routes may also result from the use of such ingredients in cosmetics (for example, body moisturizers, hair care products, toothpastes, and deodorants). As vanilla oils are considered to be of low hazard potential, quantitative estimates of these potential exposures were not derived.
6.2 Health effects assessment
6.2.1 Benzaldehyde
Benzaldehyde has been previously evaluated by the AGDH (2016), EFSA (2005, 2012), the US EPA (2010, 2015), the OECD (2002) and JECFA (WHO 1967, 1996, 2002). JECFA established an acceptable daily intake (ADI) of 0 to 5 mg/kg bw (expressed as benzoic acid equivalents) when used as a flavouring agent and did not identify any safety concern at current levels of intake for that specific use. A Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) registration dossier is also available (ECHA c2007-2019).
Toxicokinetics
Benzaldehyde is readily absorbed by the respiratory and gastrointestinal tracts, where only 1.2% remains in the respiratory tract 1.5 minutes after inhalation and almost 80% is excreted in the urine after oral ingestion (AGDH 2016). It can also be absorbed through intact skin and is rapidly cleared after reaching peak concentration with a half-life of around 10 minutes (US EPA 2015; AGDH 2016). When inhaled, a half-life of 8 minutes in the blood was determined (AGDH 2016). It is metabolized in the liver into mainly benzoic acid (free and glucuronic conjugates) and hippuric acid. In addition to other benzoyl or benzyl glucuronic conjugates, it may also be excreted unchanged. The formation of hippuric acid relies on the conjugation of benzoic acid with glycine, which is a rate-limiting step (WHO 1997). When administered orally or by inhalation, excretion of benzaldehyde metabolites occurs mainly via urine (OECD 2002).
Repeated-dose toxicity
In a short-term whole-body inhalation study, Sprague-Dawley rats (14/sex/concentration) were exposed to 0, 500, 750, or 1000 ppm (0, 2170, 3260, or 4341 mg/m3) benzaldehyde 6 hours/day for 14 consecutive days (Laham et al. 1991). Observed health effects included significant increases in absolute and relative liver weights in the females in all test groups and a significant decrease in body weight gain in males of all test groups. In addition, females in all test groups exhibited a significant increase in serum aspartate aminotransferase (AST) levels (49% to 152%) and a significant decrease in serum albumin (8% to 11%), total protein (5% to 8%), and cholinesterase levels (26% to 35%). Females in the 750 ppm group also exhibited a significant increase in serum alanine aminotransferase levels (34%). Males showed a significant increase in serum AST levels only (31% to 58%). Other biochemical changes were observed in all test groups; however, these were considered within the normal historical range of the animal colonies used by the study authors, and thus no biological significance was attributed to them. Although liver weight and serum biochemistry parameters were altered, there were no accompanying histopathological changes in the liver, suggesting that the liver effects may be adaptive. The most significant histopathological finding was mild goblet cell metaplasia observed in the respiratory epithelium lining the nasal septum in males, which did not differ in severity between the 500 and 1000 ppm groups, along with less pronounced changes in nasal tissue morphology in females. Mortality and clinical signs of toxicity (abnormal gait, aggression, tremors, seizures) were observed at the highest concentration tested. Based on the observed nasal tissue injury, reduced serum cholinesterase levels, and changes in liver weight and enzymes, a lowest observed adverse effect concentration (LOAEC) at 500 ppm (2170 mg/m3), the lowest concentration tested, is determined to account for portal-of-entry and systemic effects.
In a sub-chronic inhalation study with limited details (OECD 2002), rats were exposed to benzaldehyde at concentrations ranging from 6 to 26 mg/m3 for 5 hours/day, for 4 months. Changes in blood parameters and body weight occurred at 26 mg/m3 which reversed after cessation of treatment.
In 2 short-term oral studies (Kluwe et al. 1983; NTP 1990), B6C3F1 mice (5/sex/dose) were given 0, 200, 400, 800, 1600, or 3200 mg/kg bw/day benzaldehyde, while Fischer 344 rats (5/sex/dose) were given 0, 100, 200, 400, 800, or 1600 mg/kg bw/day benzaldehyde dissolved in corn oil by gavage for 5 days/week for 16 days, followed by a two-day recovery period. Significant decreases in body weights of males (14%) and females (11%) occurred in rats exposed to 800 mg/kg bw/day, but body weight changes in other groups were within 10% of control. A similar effect was not observed in mice. At 800 mg/kg bw/day, 2 rats from each sex and one male mouse died, while all rats and mice died within 2 or 3 days after exposure to 1600 mg/kg bw/day or 3200 mg/kg bw/day, respectively. Incidences of hyperexcitability, tremors and inactivity throughout the study at 800 and 1600 mg/kg bw/day in rats were reported for one study (Kluwe et al. 1983) but not the other (NTP 1990). A no observed adverse effect level (NOAEL) of 400 mg/kg bw/day is determined based on decreased survival rate in rats and mice, and decreased body weight and increased incidence of clinical signs in rats at 800 mg/kg bw/day and higher doses.
In sub-chronic oral studies (Kluwe et al. 1983; NTP 1990), B6C3F1 mice (10/sex/dose) were given 0, 75, 150, 300, 600 or 1200 mg/kg bw/day benzaldehyde, while Fischer 344 rats (10 for each dose and sex) were given 0, 50, 100, 200, 400 or 800 mg/kg bw/day benzaldehyde in corn oil by oral gavage for 5 days/week for 13 weeks. At 1200 mg/kg bw/day, 1 female and 9 male mice died within the first 4 weeks of treatment, with mild to moderate renal tubule degeneration observed in the males. In male and female rats exposed to 800 mg/kg bw/day, a significant increase in the incidence of mild epithelial hyperplasia or hyperkeratosis of the forestomach, degeneration and/or necrosis of the liver and kidneys, and necrotic and degenerative lesions of the cerebellar and hippocampal regions of the brain was observed. In addition, 6 male and 4 female rats died at 800 mg/kg bw/day, with reports of reduced absolute and relative (to brain) weights of the thymus and testes of male rats, and increased liver, thymus, kidney and heart weights in female rats. Mild epithelial hyperplasia and hyperkeratosis of the forestomach was reported for 2 male rats at 400 mg/kg bw/day in one study (Kluwe et al. 1983), which was not reported at this dose level in the other study (NTP 1990). Based on these results, a NOAEL of 400 mg/kg bw/day is determined in rats based on the incidence of various tissue lesions and decreased survival rate at 800 mg/kg bw/day (which is the highest dose tested in rats), and a NOAEL of 600 mg/kg bw/day is determined in mice based on decreased survival rate and renal tubule degeneration at the next dose level of 1200 mg/kg bw/day (which is the highest dose tested in mice). The US EPA identified a no observed effect level (NOEL) of 200 mg/kg bw/day based on hyperplasia and hyperkeratosis of the forestomach observed at 400 mg/kg bw/day in rats and kidney effects at 600 mg/kg bw/day in mice (Kluwe et al. 1983) for the purpose of deriving a chronic oral reference dose (US EPA 1988, 2015). However, it was derived before the 13-week and 2-year National Toxicology Program studies were available (described below) (NTP 1990).
In 2 long-term diet studies (Hagan et al. 1967), Osborne-Mendel rats (5/sex/dose, 10/sex in the control group) were given benzaldehyde at 0, 1000, or 10 000 ppm (0, 70, or 870 mg/kg bw/day for males; 0, 77, or 950 mg/kg bw/day for females, as estimated by US EPA 2015) for 16 to 28 weeks. No adverse effects were reported for any of the examined parameters at any of the tested doses.
No dermal repeated-dose toxicity studies on benzaldehyde were identified.
Genotoxicity and carcinogenicity
Benzaldehyde had equivocal results for mutagenicity. Several bacterial reverse mutation assays were negative, with and without metabolic activation (US EPA 2015). One study (US EPA 2015) using base-specific tester strains of Salmonella typhimurium showed a positive result in strain TA7005 only, in the presence of metabolic activation. This strain detects G:C to A:T mutations. In 2 mutagenicity studies using L5178Y TK+/- mouse lymphoma cells, one reported positive results at near-cytotoxic concentrations (US EPA 2015), while the other reported negative results at similar concentrations (US EPA 2015).
There are conflicting reports on the ability of benzaldehyde to cause DNA damage in vitro. Chromosomal aberrations were observed in Chinese hamster B241 cells (with and without metabolic activation) and lung cells (only with metabolic activation), but not in Chinese hamster ovary cells (US EPA 2015). Positive results for sister chromatid exchange assays were reported in Chinese hamster lung cells and human lymphocytes in the absence of metabolic activation (US EPA 2015). Benzaldehyde caused DNA damage as evidenced by increased tail momentums and length of DNA comet assays using human lymphocytes and Drosophila melanogaster hemocytes (US EPA 2015). Using Bacillus subtilis strains in DNA repair assays, benzaldehyde showed either negative or equivocal results of DNA damage (US EPA 2015).
Overall, the AGDH (2016) and EFSA (2005) concluded that there were no safety concerns with respect to the genotoxic potential of benzaldehyde.
In an oral carcinogenicity study (NTP 1990) in Fischer 344 rats and B6C3F1 mice (50/sex/dose), benzaldehyde dissolved in corn oil was administered by oral gavage at various doses (0, 200, or 400 mg/kg bw/day for male and female mice and male rats; 0, 300, or 600 mg/kg bw/day for female rats) for 103 or 104 weeks. Only body weights, gross necropsy and histopathological evaluations were performed. Male rats showed a significantly decreased survival rate at 400 mg/kg bw/day in the second year of the study (21/50 animals survived at study termination compared to 37/50 in the controls; survival days in the high dose were 608 compared to 698 in controls). Male rats in the 400 mg/kg bw/day dose group exhibited a significant increase in pre-neoplastic lesions in the form of pancreatic hyperplasia (12/48 compared to 6/49 in the controls) and a significant increase in pancreatic adenomas (7/48 compared to 3/49), which were considered within the historical range (0/49 to 11/50) and not treatment-related by the study authors. Female mice exhibited significantly increased focal hyperplasia and squamous cell papillomas of the forestomach at both doses, showing a statistically significant dose-dependent trend, while male mice showed similar effects at the highest dose only that were not statistically significant. In all cases, progression to carcinoma was not detected. The authors concluded that there was no clear evidence of carcinogenicity in rats, while there was some evidence of carcinogenicity in mice. However, the development of forestomach proliferative lesions is known to occur due to injury from the gavage method of administration, particularly with the use of high concentrations of corn oil (10 mL/kg), which can be cytotoxic. Considering the localization of the proliferative lesions to the forestomach and the lack of progression to carcinomas, the carcinogenic potential of benzaldehyde is considered to be unclear by the study authors. The US EPA (2015) derived a benchmark dose lower confidence limit for 10% extra risk (BMDL10) based on the forestomach squamous cell papilloma incidence in female mice. However, the AGDH (2016), JECFA (WHO 1996) and EFSA (2005) regarded this effect as not relevant or not treatment-related, and determined there were no concerns with regard to the carcinogenic potential of benzaldehyde from oral exposure. In line with the AGDH (2016), JECFA (WHO 1996) and EFSA (2005), benzaldehyde is not considered to have carcinogenic potential. The critical health endpoint from this study was based on a lower survival rate in male rats at 400 mg/kg bw/day, and thus a NOAEL of 200 mg/kg bw/day was identified for systemic toxicity.
Reproductive and developmental toxicity
In a non-guideline, one-generation reproductive toxicity study (OECD 2002; ECHA c2007-2019), pregnant rats (10/dose) were administered benzaldehyde 0 or 5 mg/kg bw/day by oral gavage every second day from 75 days before mating and through 2 pregnancy cycles, for a total of 32 weeks. Examined parameters included the number of pregnant females, number of born pups, pup body weight at days 7 and 21 post-partum, and pup viability. No adverse effects were reported by the study authors in either the dams or the pups. However, it is likely the study did not administer a high enough dose of the substance.
No other reproductive or developmental toxicity studies on benzaldehyde were identified.
Information on the toxicity of sodium benzoate as an analogue substance was also used to inform the assessment of developmental and reproductive effects of benzaldehyde. Sodium benzoate is ionized in the stomach into benzoic acid, which is one of the main metabolites of benzaldehyde. Sodium benzoate was used by JECFA (WHO 1996) and EFSA (2012) to characterize the developmental toxicity of benzyl derivatives, including benzaldehyde.
In one developmental toxicity study (ECHA C2007-2019), pregnant Wistar rats were fed a diet containing sodium benzoate. No adverse developmental effects were noted at doses up to 1306 mg/kg bw/day, which exceeds the limit dose of 1000 mg/kg bw/day. In a second study (ECHA C2007-2019), no adverse developmental effects were observed at up to the highest dose tested for each species (175 mg/kg bw/day for Wistar rats and CD-1 mice, 300 mg/kg bw/day for Golden hamsters, and 250 mg/kg bw/day for Dutch belted rabbits).
AGDH (2016), JECFA (WHO 1996) and EFSA (2012) determined that benzaldehyde does not show potential to be a reproductive or developmental toxicant.
6.2.2 Octanal
Octanal has been evaluated by EFSA (2013, 2017) and JECFA (WHO 1967, 1979, 1981, 1984, 1999). JECFA established an ADI of 0 to 0.1 mg/kg bw when used as a flavouring agent and did not identify any safety concern at current levels of intake for that specific use. A REACH registration dossier for octanal is also available (ECHA c2007-2019).
Toxicokinetics
Limited toxicokinetics data are available for this substance. Octanal is expected to rapidly oxidize into octanoic acid, possibly via the enzyme aldehyde dehydrogenase (ECHA c2007-2019), which is then metabolized via the fatty acid and tricarboxylic acid pathways (WHO 1999).
Repeated-dose toxicity
In a sub-chronic feeding study, male and female weanling rats (12/sex/dose) were fed a mixture of 6 aliphatic aldehydes for 12 weeks, providing a daily intake of 13 mg/kg bw/day of octanal (total mixture intake = 112 mg/kg bw/day). At this single-dose level of 13 mg/kg bw/day, no adverse effects were observed in any of the examined parameters: appearance, behaviour, growth food intake, sugar or albumin urine levels, blood haemoglobin, liver and kidney weights, and gross pathology (WHO 1979).
No dermal or inhalation repeated-dose toxicity studies were identified.
To support hazard characterization for dermal and inhalation exposures, and to supplement the limited health effects information for oral exposures, nonanal, butanal and isobutanal were selected as analogues. The analogue substances are aliphatic aldehydes which are expected to share a common metabolic profile with octanal. Butanal and isobutanal have been evaluated by the OECD (1996a, 1996b) and have REACH registration dossiers (ECHA c2007-2019).
In a short-term dermal study on nonanal in New Zealand rabbits (Biodynamics Inc. 1981; ECHA c2007-2019), no systemic effects were reported at the single dose level of 500 mg/kg bw/day. Refer to the repeated-dose toxicity section of nonanal (section 6.2.3) for a description of this study.
In short-term oral studies (ECHA c2007-2019), F344 rats and B6C3F1 mice (10/sex/dose) were given 0, 156, 313, 625, 1250, or 2500 mg/kg bw/day butanal by gavage for 14 days. Reported effects include nasal and stomach lesions at 625 mg/kg bw/day and above in rats, and at 1250 mg/kg bw/day and above in mice. In addition, mortality was observed at the same dose levels; however, the rats appeared more sensitive. The authors determined a NOAEL of 313 mg/kg bw/day in rats and a NOAEL of 625 mg/kg bw/day in mice, based on local lesions in nasal and stomach tissues and increased mortality at higher doses.
In a sub-chronic oral study (ECHA c2007-2019; OECD 1996a), F344 rats and B6C3F1 mice (10/sex/dose) were given 0, 75, 150, 300, 600, or 1200 mg/kg bw/day butanal by gavage for 13 weeks. Reported effects include nasal lesions at all doses in rats and at 300 mg/kg bw/day and above in mice, stomach lesions at 600 mg/kg bw/day and above in rats and at 1200 mg/kg bw/day in mice, and decreased body weight gain at 1200 mg/kg bw/day in rats and mice, as well as mortality. A lowest observed adverse effect level (LOAEL) of 75 mg/kg bw/day for rats and a NOAEL of 150 mg/kg bw/day for mice were determined by the authors based on nasal lesions occurring at the lowest dose tested for rats and at 300 mg/kg bw/day for mice.
In a sub-chronic inhalation study (OECD 1996a), Sprague-Dawley rats (20/sex/concentration) and male Beagle dogs (4/concentration) were exposed to butanal at 0, 117, 462, or 1852 ppm (0, 345, 1362, or 5461 mg/m3) for 6 hours/day, 5 days/week for 13 to 14 weeks. In rats, goblet cell hyperplasia of the nasal epithelium, mild to severe rhinitis and squamous cell metaplasia of the respiratory epithelium were observed in all treatment groups with the incidence and severity decreasing with decreasing concentration. Atrophy of goblet cells occurred mainly in the highest concentration group. In dogs, similar effects were observed; goblet cell hyperplasia of the nasal mucosa occurred in the low- and mid-concentration groups, in addition to marked rhinitis, mucosal cell hyperplasia, inflammation and squamous metaplasia at the highest concentration. No systemic or organ-specific toxicity was observed. Based on these observations, a LOAEC of 117 ppm (345 mg/m3), which is the lowest tested concentration, is determined based on portal-of-entry effects.
In another sub-chronic inhalation study (OECD 1996a), Fischer 344 rats (15/sex/concentration) were exposed to butanal at 0, 1, 10, or 51 ppm (0, 2.9, 29, or 150 mg/m3) for 6 hours/day, 5 days/week for 13 weeks. No local or systemic adverse effects were observed up to the highest concentration tested of 150 mg/m3.
In a 2-year chronic inhalation study (OECD 1996b), Fischer 344 rats (50/sex/concentration) and B6C3F1 mice (50/sex/concentration) were exposed to isobutanal at 0, 500, 1000, or 2000 ppm (0, 1474, 2949, or 5898 mg/m3) for 6 hours/day, 5 days/week. Mild to moderate squamous metaplasia of the respiratory epithelium was observed in female rats at 500 ppm, and in male and female rats and mice exposed to 1000 or 2000 ppm. Rhinitis and minimal to mild degeneration of the olfactory epithelium were observed in rats and mice exposed to 1000 or 2000 ppm. In addition, survival of male mice exposed to 2000 ppm was lower than control, while female mice exposed to 1000 or 2000 ppm had lower mean body weights of female mice in the second year of the study compared to control. As such, a LOAEC of 500 ppm (1474 mg/m3) is determined based on portal-of-entry effects in female rats.
Genotoxicity and carcinogenicity
Octanal was negative for mutagenicity in a bacterial reverse mutation assay in the presence and absence of metabolic activation (Florin 1980).
No carcinogenicity studies for octanal were identified; therefore, data from a 2-year inhalation study using the analogue isobutanal was used for read-across (OECD 1996b). Fischer 344 rats (50/sex/concentration) and B6C3F1 mice (50/sex/concentration) were exposed at 0, 500, 1000, or 2000 ppm (0, 1474, 2949, or 5898 mg/m3) for 6 hours/day, 5 days/week. No neoplastic lesions were observed in either species. Non-neoplastic lesions included mild to moderate squamous metaplasia of the respiratory epithelium in both species at all concentrations. No carcinogenic activity was identified at up to 2000 ppm (5898 mg/m3), the highest concentration tested (OECD 1996b).
Reproductive and developmental toxicity
No reproductive or developmental toxicity studies were identified for octanal; therefore, data from isobutanal were used in a read-across approach. In a repeated-dose inhalation toxicity study (OECD 1996b) male and female Fischer 344 rats and B6C3F1 mice were exposed to isobutanal up to 4000 ppm (11 796 mg/m3; in rats) or 2000 ppm (5898 mg/m3; in mice) for 6 hours/day, 5 days/week for 13 weeks; no adverse effects were observed in the reproductive parameters that were examined (sperm cytology and male reproductive organ weights). Male rats in the highest concentration group had decreased body weight and body weight gains. Observations of decreased sperm motility in rats were deemed non-treatment related by the study authors due to a variable dose-response relationship. No adverse effects on sperm density, morphology or testis weight were observed in rats or mice, although a decrease in the absolute weight of the right cauda epididymis and the absolute and relative weight of the right epididymis in rats exposed to 4000 ppm was observed.
In a developmental study (OECD 1996b), pregnant Wistar rats (25/concentration) were exposed to isobutanal at 0, 1000, 2500 or 4000 ppm (0, 1474, 7372 or 11 796 mg/m3) for 6 hours/day from gestational day (GD) 6 to 15. Decreased body weight gain and lesions of the nasal mucosa were observed in the dams at 2500 and 4000 ppm. No adverse effects were observed in the offspring. Therefore, a maternal toxicity no observed adverse effect concentration (NOAEC) of 1000 ppm (1474 mg/m3) was determined, and no foetal toxicity was observed at concentrations up to 4000 ppm (11 796 mg/m3), the highest concentration tested.
6.2.3 Nonanal
Nonanal has been evaluated by EFSA (2013, 2017) and JECFA (WHO 1967, 1979, 1984, 1999, 2002). JECFA established an ADI of 0 to 0.1 mg/kg bw when used as a flavouring agent and did not identify any safety concern at current levels of intake for that specific use. A REACH registration dossier for nonanal is also available (ECHA c2007-2019).
Toxicokinetics
Limited toxicokinetics data are available. Nonanal is expected to rapidly oxidize into nonanoic acid, which is then metabolized via the fatty acid and tricarboxylic acid pathways (WHO 1999).
Repeated-dose toxicity
In a short-term dermal study (Biodynamics Inc. 1981; ECHA c2007-2019), male and female New Zealand rabbits (5/sex/dose) were treated with 0 or 500 mg/kg bw/day nonanal dissolved in mineral oil for 5 days a week for 2 weeks. Nonanal was applied unoccluded to abraded and intact skin. While some minor body weight and food consumption changes occurred during treatment, these effects fully reversed during the recovery period. Histopathology examination revealed skin irritation at the site of application in the form of epidermal necrosis, hyperplasia and hyperkeratosis during the second week of exposure. However, the skin appeared healed by the end of the recovery period. No other effects were reported by the study authors. As such, while there were local, reversible effects, no systemic effects were observed at the single dose level of 500 mg/kg bw/day.
In a sub-chronic feeding study, male and female weanling rats (12/sex) were fed a mixture of 6 aliphatic aldehydes for 12 weeks, providing a daily intake of 29 mg/kg bw/day of nonanal (total mixture intake = 112 mg/kg bw/day). At this single-dose level of 29 mg/kg bw/day, no adverse effects were observed in any of the examined parameters: appearance, behaviour, growth food intake, sugar or albumin urine levels, blood haemoglobin, liver and kidney weights, and gross pathology (WHO 1979).
No repeated-dose inhalation toxicity studies were identified.
To support hazard characterization for inhalation exposures, and to supplement the limited health effects information for oral exposures, butanal and isobutanal were selected as analogues to inform the health effects for nonanal that are specific to these routes using a read-across approach. Both analogue substances are aliphatic aldehydes which are expected to share a common mode of action and toxicokinetics profile with nonanal. Both substances have been evaluated by the OECD (1996a, 1996b).
Refer to the repeated-dose toxicity section for octanal (section 6.2.2) for a description of the oral and inhalation toxicity studies of butanal and isobutanal.
Genotoxicity and carcinogenicity
Results for nonanal mutagenicity are equivocal. In 4 Ames assays, nonanal did not induce reverse mutations in several strains of Salmonella typhimurium (TA97, TA98, TA100, TA102, TA104, TA1535, TA1537 or TA1538), with and without metabolic activation (Marnett et al. 1985; Florin et al. 1980; Jagannath et al. 1980; Mortelmans et al. 1986). A thymidine kinase forward mutation assay in mouse lymphoma cells gave negative results without metabolic activation, or weakly positive results for mutagenicity with metabolic activation, although this occurred at cytotoxic concentrations (Myhr et al. 1981). In another thymidine kinase forward mutation assay in V79 Chinese hamster cells, nonanal induced mutagenicity without metabolic activation (Brambilla et al. 1989).
Results for nonanal clastogenicity are equivocal. Nonanal did not induce an increase in unscheduled DNA synthesis in rat or human hepatocytes (Martelli et al. 1994). In several experiments conducted in the same lab, nonanal induced sister chromatid exchange, but did not induce an increase in mitotic index, chromosomal aberrations or micronuclei in rat hepatocytes (Esterbauer et al. 1990; Eckl 1993).
No carcinogenicity studies for nonanal were identified. A 2-year carcinogenicity inhalation study for isobutanal is used to inform nonanal’s carcinogenic potential using a read-across approach. Refer to the carcinogenicity section for octanal (section 6.2.2) for a description of this study on isobutanal.
Reproductive and developmental toxicity
No reproductive or developmental toxicity studies were identified for nonanal.
Refer to the reproductive and developmental toxicity section for octanal (section 6.2.2) for a description of the studies used to describe the potential reproductive and developmental toxicity of isobutanal, which are also used to inform these endpoints for nonanal.
6.2.4 Methylbenzaldehyde
As noted in section 2, methylbenzaldehyde exists as a mixture of the ortho, para, and meta isomers. It has been evaluated by EFSA (2005, 2009) and JECFA (WHO 2002); although no ADI was established, JECFA did not identify any safety concern at current levels of intake for methylbenzaldehyde when used as a flavouring agent.
Toxicokinetics
All 3 isomers of methylbenzaldehyde are oxidized in vivo to their corresponding acids (BIBRA 1990; WHO 2002), possibly by hepatic microsomal enzymes (Watanabe et al. 1995).
Repeated-dose toxicity
In a sub-chronic study (Brantom et al. 1972), rats (15/sex/dose) were administered methylbenzaldehyde (meta and para isomers in equal proportions) dissolved in corn oil by oral gavage at 0, 50, 250, or 500 mg/kg bw/day for 13 weeks. The only significant effect was reduced relative weight (14% less than controls) of the pituitary in females of the highest dose tested at week 13; however, no associated histopathological effects were observed. Some changes in the weight of the small intestines were noted; however, the study authors did not consider them treatment-related. The study authors assigned a NOAEL of 250 mg/kg bw/day based on changes in relative pituitary weight in the females at 500 mg/kg bw/day.
In another sub-chronic study (Oser et al. 1965), FDRL rats (15/sex/dose) were given methylbenzaldehyde (proportions of isomers not specified) dissolved in cottonseed oil in the diet at 0, 36 (males), or 43 (females) mg/kg bw/day for 90 days. No effects were reported by the study authors in males or females in any of the examined parameters.
No dermal or inhalation repeated-dose toxicity studies were identified.
Genotoxicity and carcinogenicity
Methylbenzaldehyde (proportions of isomers not specified) was negative for mutagenicity in several reverse and forward mutation assays, with and without metabolic activation (Aeschbacher et al. 1989; Florin et al. 1980; Heck et al. 1989; Marnett et al. 1989; Zeiger et al.1988). In addition, methylbenzaldehyde did not induce unscheduled DNA synthesis in rat hepatocytes (Heck et al. 1989).
No carcinogenicity studies were identified for methylbenzaldehyde.
Reproductive and developmental toxicity
No reproductive or developmental toxicity studies were identified for methylbenzaldehyde.
6.2.5 Vanilla oils
Limited data are available regarding the toxicity of vanilla oils per se. As described in section 2, vanilla oils are defined as the extractives and physically modified derivatives of V. planifolia (NCI 2020), and the PCPC CIID associates the CAS RN 8024-06-4 with vanilla oils sourced from V. planifolia flower extract, V. planifolia fruit, V. planifolia fruit extract, V. planifolia fruit oil, V. planifolia fruit water, V. planifolia leaf cell extract, V. planifolia seed, and V. planifolia seed powder. However, the extractives of V. planifolia fruit were determined to be the main source of vanilla oils for the purpose of this assessment, and the substances of interest from a toxicological perspective are assumed to be in the volatile aroma portion therein. The volatile aroma content of the extract comprises 4% of the total extract composition (ethanol and water comprising the remaining portion), with vanillin being the most abundant substance in this volatile portion of the UVCB, making up approximately 80% (or up to 3.6% of the total extract composition) on a dry weight basis (Brunschwig et al. 2009; CIR 2020). As such, vanillin is considered to be the major component of toxicological interest, and information on the health effects of vanillin was used to assess vanilla oils.
Vanillin has been evaluated by the US EPA (2010), OECD (1996c), EFSA (2005) and JECFA (WHO 1967, 2002). JECFA established an ADI of 0 to 10 mg/kg bw when used as a flavouring agent and did not identify any safety concerns with current levels of intake for that specific use. A REACH dossier is also available (ECHA c2007-2019).
Vanillin
Toxicokinetics
Vanillin is metabolized in vivo into both free and conjugated forms of primarily vanillic acid, as well as catechol. Other metabolites include conjugated forms of both vanillin and vanillyl alcohol, and trace amounts of formaldehyde (OECD 1996c). Conjugates were mostly glucuronides, sulphates and glycines (OECD 1996). Vanillin metabolites are almost completely excreted through urine within 24 hours in rats, rabbits and humans, demonstrating a short half-life (OECD 1996c).
Repeated-dose toxicity
In several sub-chronic and long-term studies, vanillin did not produce any adverse effects up to the highest doses tested via the oral and dermal routes in rats, mice and dogs (OECD 1996c). Some of these studies are described below as examples; they do not constitute the entire health effects database.
In a 90-day oral study (OECD 1996c), rats (10/sex/dose) administered vanillin at 3000, 10 000, or 50 000 ppm (150, 500, or 2500 mg/kg bw/day) in the diet showed some mild adverse effects at the mid-dose and growth depression as well as enlargement of the liver, spleen and kidney at the highest dose tested. No information about the use of control groups was reported and the nature of the mild adverse effects at 500 mg/kg bw/day was not discussed.
In several long-term oral studies by the same lab (Hagan et al. 1967), Osborne-Mendel rats (5 to 12/sex/dose) were administered vanillin in the diet at doses ranging from 1000 to 20 000 ppm (50 to 1000 mg/kg bw/day) for 16 to 104 weeks. No adverse effects on growth, haematology or organ tissue were observed at any dose when compared to control. In a 1-year diet study in male Osborne-Mendel rats (5/dose) administered vanillin up to 50 000 ppm (2500 mg/kg bw/day), similar results were reported (Hagan et al. 1967).
In a feeding study (OECD 1996c), dogs (1/sex/dose) were given vanillin at 0, 25, or 100 mg/kg bw/day in a capsule 5 days a week for 26 to 27 weeks. No adverse effects were reported by the study authors on growth, behaviour, haematology, biochemistry, urinalysis and organ tissues at any dose.
In a 26-week study (OECD 1996c), male rats (10/dose) were fed vanillin at 0, 1000, 5000, or 10 000 ppm (0, 50, 250, or 500 mg/kg bw/day) in the diet for 26 weeks. No adverse effects were reported on growth or histopathology of examined tissues at any dose.
No inhalation toxicity studies on vanillin were identified.
Genotoxicity and carcinogenicity
Vanillin was negative for genotoxicity in several in vitro assays using bacterial or mammalian cells, in the presence and absence of metabolic activation (OECD 1996c). Some evidence of DNA damage was observed in sister chromatid exchange assays in human lymphocytes; however, in vivo experiments in mice showed no evidence of micronuclei up to 500 mg/kg bw (OECD 1996c).
In a 2-year diet study, Osborne-Mendel rats (12/sex/dose) were administered vanillin at 0, 5000, 10 000, or 20 000 ppm (0, 250, 500, or 1000 mg/kg bw/day). No evidence of carcinogenicity was reported in any of the examined tissues (heart, liver, kidneys, spleen, testes, bone marrow, muscle) (Hagan et al. 1967).
Several other repeated-dose oral studies in rats, ranging from 16 weeks to one year of dietary exposure, showed no evidence of tumour formation or carcinogenic potential at doses up to 2500 mg/kg bw/day (Hagan et al. 1967).
Mice (20/dose) were exposed dermally to vanillin dissolved in acetone at 0 or 3000 mg/kg bw/day, 3 times a week for 3 weeks, for a total of 10 applications. No evidence of carcinogenicity was observed in the lungs or the skin (the only organs examined) (OECD 1996c), although there were study limitations (for example, study duration, dosing frequency, organs examined).
Reproductive and developmental toxicity
An unpublished report of reproductive and developmental toxicity of vanillin (EFSA 2005) shows that pregnant rats (10/dose) given vanillin at 0, 125, 250 or 500 mg/kg bw/day by oral gavage from one week before mating until post-natal day 4 did not exhibit any adverse reproductive or developmental effects up to the highest dose tested. However, this study lacked proper reporting.
No adverse effects on testis weight or tissue were reported upon examination in several repeated-dose oral studies in rats, ranging from 16 weeks to one year of dietary exposure (OECD 1996c).
In 2 non-guideline developmental toxicity studies, no maternal or foetal toxicity was reported when vanillin was administered by oral gavage or intraperitoneal injection in single doses of up to 500 mg/kg bw on GD 10 or 11 (OECD 1996c; Imanishi et al. 1990).
Due to the lack of sufficient quality data on the potential reproductive and developmental toxicity of this substance, a read-across approach using ethyl vanillin as an analogue was used to inform these endpoints. Ethyl vanillin is structurally similar to vanillin, the major component of vanilla oils, and has similar physical-chemical properties and a similar toxicokinetic profile (EFSA 2005). It has been reviewed by EFSA (2005) and JECFA (WHO 2002). A REACH registration dossier is also available (ECHA c2007-2019).
In a reproduction and developmental toxicity screening test (ECHA c2007-2019), ethyl vanillin dissolved in propylene glycol was administered to Wistar rats (10/sex/dose) by oral gavage at 0, 250, 500, or 1000 mg/kg bw/day. Males were treated for 31 days, including during pre-mating, mating and up to a day before necropsy, while females were additionally treated during gestation and at least 13 days after parturition, up to the day before necropsy. In adults, 5 females in the 1000 mg/kg bw/day group showed transient treatment-related clinical signs during the pre-mating period consisting of decreased activity and/or abnormal breathing, piloerection and cold body. Decreased activity was also reported for males of the same group. These effects occurred mainly during the first week of the pre-mating period. Mean body weight gain of male and female adults was lower than controls at 1000 mg/kg bw/day during the pre-mating and gestation periods. No other systemic treatment-related effects were observed in any of the other examined parameters. No treatment-related adverse effects on reproductive or developmental parameters were reported up to the highest dose tested. Thus, the authors derived a parental systemic NOAEL of 500 mg/kg bw/day for decreased body weight gain in adult rats, and no reproductive or developmental toxicity was observed at up to 1000 mg/kg bw/day, the highest dose tested.
In a prenatal developmental toxicity study (ECHA c2007-2019), pregnant Wistar rats (22/dose) were administered ethyl vanillin dissolved in propylene glycol by oral gavage at 0, 250, 500, or 1000 mg/kg bw/day from GD 6 to 20. Dams exposed to 1000 mg/kg bw/day showed transient but adverse clinical signs such as irregular and/or rapid breathing, decreased activity and wheezing shortly after the first or second dosing. Mean body weight gain was transiently lower in dams exposed to 1000 mg/kg bw/day from GD 6 to 9 compared to control, which was accompanied by a concurrent transient decrease in food consumption from GD 6 to 12. However, mean body weight gains and food consumption across the treatment period (GD 6 to 21) were comparable. Incidences of external, soft-tissue and skeletal malformations were observed in 7 of 240 foetuses examined across 6 litters from dams exposed to the limit dose of 1000 mg/kg bw/day. These malformations include abnormalities of the heart, cranium, tail, mandible and sternebra. Overall, the authors determined a maternal and an embryo-fetal developmental NOAEL of 500 mg/kg bw/day on the basis of observations at the limit dose.
While this developmental toxicity study showed a small incidence of malformed foetuses from dams exposed to 1000 mg/kg bw/day ethyl vanillin, these effects occurred in conjunction with maternal toxicity and at the limit dose. In addition, in another developmental study conducted with ethyl vanillin in Wistar rats, no external malformations were observed at similar doses, and no developmental effects were observed in the offspring of several studies using relatively high doses of vanillin. Thus, ethyl vanillin and vanillin are not considered to be teratogenic.
Vanilla oils are therefore considered to have a low hazard potential (Health Canada 2017), given that no adverse effects were observed following oral or dermal exposure to vanillin and ethyl vanillin up to the limit dose in rats and mice, including reproductive, genotoxic or mutagenic effects. The available information also indicates a low potential for the developmental toxicity of vanillin and ethyl vanillin.
6.3 Characterization of risk to human health
Tables 6-6 to 6-9 provide all relevant exposure and hazard values for the substances in the Aldehydes Group, as well as resultant margins of exposure (MOEs), for the determination of risk for sentinel scenarios for the oral, dermal, and inhalation routes, as applicable.
| Exposure scenario | Estimated exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Environmental media and food (food flavouring usea), all routes, daily, toddler (1 year old) | 0.61 mg/kg bw/day | NOAEL = 200 mg/kg bw/day (2-year oral study in rats) | Decreased survival rate in male rats at 400 mg/kg bw/day | 328d |
| Body moisturizer, dermal, daily, toddler (2 to 3 years old) | 1.8 mg/kg bw/dayb | NOAEL = 200 mg/kg bw/day (2-year oral study in rats) |
Decreased survival rate in male rats at 400 mg/kg bw/day | 111d |
| Air freshener, inhalation, daily, toddler (1 year old) | 0.88 mg/kg bw/day | LOAELadj = 512c mg/kg bw/day (14-day inhalation study in rats) |
Liver weight and enzyme changes and morphological changes in nasal tissues in rats at adjusted dose of 512c mg/kg bw/day | 582e |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level; LOAELadj, adjusted lowest observed adverse effect level
a Intakes from uses as food flavouring ingredient are per capita intakes representing the entire population 1 year of age and older.
b Dermal absorption was considered to be equivalent to oral absorption.
c Critical effect levels are calculated based on converting no observed adverse effect concentrations (NOAECs) or lowest observed adverse effect concentrations (LOAECs) from inhalation toxicity studies into internal doses that account for animal inhalation rate (m3/day), body weight (kg), and time adjustment factors (hours of exposure/24; days of exposure in a week/7), unless specified otherwise. Animal inhalation rates were determined using the equation provided in Bide et al. (2000). Animal body weights were derived from the study reports if available; a default value as presented in Meek et al. (1994) was used otherwise.
d Target MOE = 100 (x10 for interspecies variation; x10 for intraspecies variation)
e Target MOE = 300 (x10 for interspecies variation; x10 for intraspecies variation; x3 for the use of a LOAEL)
A NOAEL of 200 mg/kg bw/day, based on a lower survival rate of male rats (compared to controls) at the next dose of 400 mg/kg bw/day, was considered to be the most relevant endpoint for the characterization of risk to human health from daily exposures to benzaldehyde via the oral and dermal routes. It should be noted that the decreased survival rate was observed towards the end of the 2-year study duration and in male rats only, whereas female rats and male and female mice did not show the same effect. Moreover, the estimate of dermal exposure to benzaldehyde from the use of body moisturizers is considered to be conservative as it is based on the assumption that 100% of benzaldehyde in the applied cream is absorbed through the skin. As such, the resulting MOEs for the oral and dermal routes are considered adequate.
An adjusted LOAEL of 512 mg/kg bw/day from a short-term inhalation study was selected as the most relevant endpoint to characterize the risk from benzaldehyde exposure from air fresheners. Considering the short half-life of benzaldehyde via the inhalation route and the mild and reversible nature of the portal-of-entry nasal tissue irritation that was observed in the study, systemic effects were considered to be most relevant for characterization of human health risk via inhalation. Effects included changes in absolute and relative liver weight and changes in serum parameters and liver enzymes; however, there were no accompanying histopathological changes in the liver. In addition, the margin between the estimate of inhalation exposure and the lowest relevant endpoint for systemic oral exposure (NOAEL of 200 mg/kg bw/day from a 2-year oral study in rats) would be above 200. Based on the above information, the resulting MOE is considered adequate for the inhalation route.
| Exposure scenario | Estimated exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Vitamin and mineral supplement tablet, oral, daily, child (9 to 13 years old) | 0.023 mg/kg bw/day | NOAEL = 300 mg/kg bw/day (13-week gavage study in rats, read-across from butanal) | Stomach lesions at 600 mg/kg bw/day | 13000b |
| Environmental media (air), inhalation, daily, all age groups | 0.011 mg/m3 | NOAECadj = 27a mg/m3 (13-week inhalation study in rats, read-across from butanal) |
Goblet cell hyperplasia and squamous epithelial metaplasia of nasal tissue in rats and dogs exposed for 13 weeks at an adjusted concentration of 62a mg/m3 | 2450b |
| Air freshener, inhalation, daily, all age groups | 0.1 mg/m3 | NOAECadj = 27a mg/m3 (13-week inhalation study in rats, read-across from butanal) |
Goblet cell hyperplasia and squamous epithelial metaplasia of nasal tissue in rats and dogs exposed for 13 weeks at an adjusted concentration of 62a mg/m3 | 270b |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level; NOAECadj, adjusted no observed adverse effect concentration
a The NOAEC of 150 mg/m3 and LOAEC of 345 mg/m3 from the butanal 13-week inhalation studies were adjusted for comparison with a 24-hour time-weighted average octanal exposure concentration from continuous air intake estimates by factoring in the equivalent duration and days of exposure from the study (150 x 6/24 hours x 5/7 days = 27 mg/m3 or 345 x 6/24 hours x 5/7 days = 62 mg/m3).
b Target MOE = 100 (x10 for interspecies variation; x10 for intraspecies variation)
In characterizing the risk from octanal exposure via the oral route, it was considered that there was a lack of adverse effects in rats fed a mixture of aliphatic aldehydes, which included 13 mg/kg bw/day of the substance, for 12 weeks. However, due to limitations in this study (for example, single dose, mixture of compounds), a read-across approach is applied. EFSA (2013) relied on an 11-week drinking water study in rats using acetaldehyde as an analogue to read-across to a large category of aliphatic aldehydes, which included octanal, but butanal is a more suitable analogue as it is more similar to octanal than acetaldehyde. Data suggests that butanal elicits local toxicity when given orally to rats and mice in the nasal (from 75 mg/kg bw/day) and stomach (from 600 mg/kg bw/day) tissues. The effects in the nasal and stomach tissues are likely due to butanal’s solvent-like properties (OECD 1996a). Since octanal has a much lower vapour pressure (148 Pa) compared to butanal (1.44 × 104 Pa), it is unlikely that octanal will elicit nasal lesions when taken orally as observed in the butanal oral studies. As such, a NOAEL of 300 mg/kg bw/day, based on stomach lesions at 600 mg/kg bw/day, was selected. The resulting MOE is considered adequate to address the risk from oral exposure to octanal from its use as a non-medicinal ingredient in a vitamin and mineral supplement tablet licensed as a natural health product.
Oral exposure to octanal is expected through the diet from its potential use as a food flavouring agent and its natural occurrence in foods. Dietary exposure from its natural occurrence in foods is expected to exceed that from its use as a food flavouring agent. Quantitative estimates of exposure from naturally occurring octanal in foods were not considered to be meaningful and thus not derived. Considering that no adverse effects were identified from oral intake of octanal, as well as the NOAEL of 300 mg/kg bw/day that was identified in rats and mice for the analogue butanal, dietary intake of octanal from natural occurrence in food is not expected to be a concern for human health. As such, derivation of a margin of exposure from this source was not considered to be meaningful. Dietary intake of octanal from its potential use as a food flavouring agent results in lower exposure via the oral route than from its use as a non-medicinal ingredient in a vitamin and mineral supplement tablet licensed as a natural health product as presented in Table 6-7, above.
For the inhalation route, an adjusted NOAEC of 27 mg/m3 (converted from a NOAEC of 150 mg/m3) was selected based on lack of effects in rats exposed to the analogue butanal by inhalation for 13 weeks. Another butanal inhalation study showed portal-of-entry effects in the nasal epithelium of rats and dogs exposed for 13 weeks at 345 mg/m3, the lowest tested concentration in that study. Considering that no adverse portal-of-entry or systemic effects were seen in rats up to 150 mg/m3 of butanal and the reversible nature of the portal-of-entry effects observed at 345 mg/m3, the resulting MOEs are considered adequate to address the risk from inhalation exposure to octanal.
| Exposure scenario | Estimated exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Vitamin and mineral supplement tablet, oral, daily, child (9 to 13 years old) | 0.023 mg/kg bw/day | NOAEL = 300 mg/kg bw/day (13-week gavage study in rats, read-across from butanal) | Stomach lesions at 600 mg/kg bw/day | 13000c |
| Spray sunscreen, dermal, daily (2 to 3 years old) | 2.3 mg/kg bw/day | NOAEL = 500 mg/kg bw/day (2-week dermal study in rabbits) | No observed systemic effects | 217c |
| Environmental media (air), inhalation, daily, all age groups | 0.03 mg/m3 | NOAECadj = 27b mg/m3 (13-week inhalation study in rats, read-across from butanal) | Goblet cell hyperplasia and squamous epithelial metaplasia of nasal tissue in rats and dogs exposed for 13 weeks at an adjusted concentration of 62b mg/m3 | 900c |
| Spray sunscreen, inhalation daily, all age groups | 0.03 mg/m3 a | NOAECadj = 27b mg/m3 (13-week inhalation study in rats, read-across from butanal) | Goblet cell hyperplasia and squamous epithelial metaplasia of nasal tissue in rats and dogs exposed for 13 weeks at an adjusted concentration of 62b mg/m3 | 900c |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level; NOAECadj, adjusted no observed adverse effect concentration
a Ten-minute time-weighted average (TWA) concentration was derived to match up with the exposure duration of the critical effects study used to characterize risk which represents continuous exposure. 10-minute TWA = Mean event concentration x exposure duration / 24 hours.
b The NOAEC of 150 mg/m3 and LOAEC of 345 mg/m3 from the butanal 13-week inhalation studies were adjusted for comparison with a 24-hour TWA nonanal exposure concentration from continuous air intake estimates by factoring in the equivalent duration and days of exposure from the study (150 x 6/24 hours x 5/7 days = 27 mg/m3 or 345 x 6/24 hours x 5/7 days = 62 mg/m3).
c Target MOE = 100 (x10 for interspecies variation; x10 for intraspecies variation)
In characterizing the risk from nonanal exposure via the oral route, there was a lack of adverse effects in rats fed a mixture of aliphatic aldehydes, including 29 mg/kg bw/day of the substance, for 12 weeks. However, similar to the approach taken for octanal, butanal is used as an analogue due to limitations in the mixture study. Since nonanal has a much lower vapour pressure (49 Pa) compared to butanal (1.44 × 104 Pa), it is unlikely that nonanal will elicit nasal lesions as observed in the butanal oral studies. As such, a NOAEL of 300 mg/kg bw/day, based on stomach lesions at 600 mg/kg bw/day, was selected. The resulting MOE is considered adequate to address the risk from oral exposure to nonanal from its use as a non-medicinal ingredient in a vitamin and mineral supplement tablet licensed as a natural health product (Table 6-8).
Oral exposure to nonanal is expected through the diet from its potential use as a food flavouring agent and its natural occurrence in foods. Dietary exposure from its natural occurrence in foods is expected to exceed that from its use as a food flavouring agent. Quantitative estimates of exposure from naturally occurring nonanal in foods were not considered to be meaningful and thus not derived. Considering that no adverse effects were identified from oral intake of nonanal as well as the NOAEL of 300 mg/kg bw/day that was identified in rats and mice for the analogue butanal, dietary intake of nonanal from natural occurrence in food is not expected to be a concern for human health. As such, derivation of a margin of exposure from this source was not considered to be meaningful. Dietary intake of nonanal from its use as a food flavouring agent results in lower exposure via the oral route than from its use as a non-medicinal ingredient in a vitamin and mineral supplement tablet licensed as a natural health product as presented in Table 6-8, above.
For the dermal route, a NOAEL of 500 mg/kg bw/day was selected based on a two-week dermal study of nonanal in rabbits, where there was a lack of systemic toxicity. The resulting MOE is considered adequate to address the risk from dermal exposure to nonanal.
For the inhalation route, an adjusted NOAEC of 27 mg/m3 (converted from a NOAEC of 150 mg/m3) was selected based on lack of effects in rats exposed to the analogue butanal by inhalation for 13 weeks. Another butanal inhalation study showed portal-of-entry effects in the nasal epithelium of rats and dogs exposed for 13 weeks at 345 mg/m3, the lowest tested concentration in that study. Considering that no adverse portal-of-entry or systemic effects were seen in rats up to 150 mg/m3 of butanal and the reversible nature of the portal-of-entry effects observed at 345 mg/m3, the resulting MOEs are considered adequate to address the risk from inhalation exposure to nonanal.
| Exposure scenario | Estimated exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Food (food flavouring usea), oral, daily, toddler (1 year old and older) | 0.02 mg/kg bw/day | NOAEL = 250 mg/kg bw/day (13-week oral study in rats) | Reduced relative pituitary weight in female rats at 500 mg/kg bw/day | 12500b |
Abbreviations: MOE, margin of exposure; NOAEL, no observed adverse effect level
a Intakes from uses as food flavouring ingredient are per capita intakes representing the entire population 1 year of age and older.
b Target MOE = 100 (x10 for interspecies variation; x10 for intraspecies variation)
A NOAEL of 250 mg/kg bw/day was selected as the most relevant endpoint to characterize the risk from methylbenzaldehyde exposure, based on reduced relative pituitary weight in female rats given the substance orally for 13 weeks. The resulting MOE is considered adequate to address the risk from exposure via food.
Vanilla oils are considered to have a low hazard potential. As such, quantitative exposure estimates were not derived for vanilla oils and the risk to human health is considered to be low (Health Canada 2017).
The human health assessment took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. For substances in the Aldehydes Group, these subpopulations with potential for higher exposure, and those who may be more susceptible, were taken into account in the risk assessment outcomes.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| As Canadian occurrence data were not available, food flavouring use exposure estimates were based on US population intakes. | +/- |
| There are no sub-chronic or chronic inhalation repeated-dose toxicity studies for benzaldehyde. | +/- |
| There are no chronic oral or inhalation repeated-dose toxicity studies for octanal, nonanal or methylbenzaldehyde. | +/- |
| There are no reproductive or developmental toxicity studies for methylbenzaldehyde. | +/- |
| There are no substance-specific studies on toxicological endpoints for vanilla oils (as the UVCB). | +/- |
| Selection of vanilla fruit extracts as the main source of vanilla oils while they may not be representative of all possible extraction and physical modification methods that would result in vanilla oils. There is therefore some remaining uncertainty in the selection of vanillin as the major component of toxicological interest. | +/- |
| There are no available studies for the inhalation route for vanillin or ethyl vanillin. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure/risk; +/- = unknown potential to cause over- or underestimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this assessment, there is low risk of harm to the environment from benzaldehyde, octanal, nonanal, methylbenzaldehyde and vanilla oils. It is concluded that benzaldehyde, octanal, nonanal, methylbenzaldehyde and vanilla oils do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this assessment, it is concluded that benzaldehyde, octanal, nonanal, methylbenzaldehyde and vanilla oils do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that benzaldehyde, octanal, nonanal, methylbenzaldehyde and vanilla oils do not meet any of the criteria set out in section 64 of CEPA.
References
Aeschbacher H, Wolleb U, Löliger J, Spadone J, Liardon R. 1989. Contribution of coffee aroma constituents to the mutagenicity of coffee. Food Chem Toxicol, 27(4):227-32.
[AGDH] Australian Government Department of Health. 2016. Tier II Human Health Assessments Inventory Multi-Tiered Assessment and Prioritisation (IMAP) Framework [PDF]. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme. [accessed 2024 March 12].
Andersen A. 2006. Final report on the safety assessment of benzaldehyde. Int J Toxicol. 25(Suppl 1):11–27.
Beltran-Garcia MJ, Estarron-Espinosa M, Ogura T. 1997. Volatile Compounds Secreted by the Oyster Mushroom (Pleurotus ostreatus) and Their Antibacterial Activities. J Agric Food Chem. 45(10):4049-4052.
BIBRA. 1990. Toxicity profile: Tolualdehydes. TNO BIBRA International Ltd.
Bide RW, Armour SJ, Yee E. 2000. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol. 20(4):273-290.
Biodynamics Inc. 1981. A 28-day dermal toxicity study in rabbits (final report) on nonanal with attachments and cover letter dated 112291. Dallas (TX): Hoechst Celanese Corp. Report No. OTS0534473. Available from: NTIS, Springfield, VA, USA.
Brambilla G, Cajelli E, Canonero R, Martelli A, Marinari U. 1989. Mutagenicity in V79 Chinese hamster cells of n-alkanals produced by lipid peroxidation. Mutagenesis. 4(4):277-279.
Brantom P, Gaunt I, Grasso P, Lansdown A, Gangolli S. 1972. Short-term toxicity of tolualdehyde in rats. Food Cosmet Toxicol. 10(5):637-647.
Brunschwig C, Collard FX, Bianchini JP, Raharivelomanana P. 2009. Evaluation of chemical variability of cured vanilla beans (Vanilla tahitensis and Vanilla planifolia). Nat Prod Commun. 4(10):1393-1400.
Burdock GA. 2010. Fenaroli’s handbook of flavor ingredients. 6th ed. Orlando (FL): Burdock Group.
CalRecycle. 2010. Safety Study of Artificial Turf Containing Crumb Rubber Infill Made from Recycled Tires: Rubber Infill Made from Recycled Tires: Measurements of Chemicals and Particulates in the Air, Bacteria in the Turf, and Skin Abrasions Caused by Contact with the Surface [Internet]. Publication # DRRR-2010-009. Sacramento (CA): California Department of Resources Recycling and Recovery (CalRecycle).
CalRecycle. 2011. Tire-derived Rubber Flooring Chemical Emissions Study: Laboratory Study Report. [Internet]. Publication # DRRR-2011-002. Sacramento (CA): California Department of Resources Recycling and Recovery (CalRecycle).
Canada. [1978]. Food and Drug Regulations. C.R.C., c.870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2021. Order amending schedules 2 and 3 to the Tobacco and Vaping Products Act (flavours). Canada Gazette Part 1, vol. 155, no. 25.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2019 Sept 23].
Chisholm MG, Jell JA, Cass Jr DM. 2003a. Characterization of the major odorants found in the peel oil of Citrus reticulata Blanco cv. Clementine using gas chromatography–olfactometry. Flavour Fragr J. 18:275-281.
Chisholm MG, Wilson MA, Gaskey GM. 2003b. John Wiley & Sons, Ltd. Characterization of aroma volatiles in key lime essential oils (Citrus aurantifolia Swingle). Flavour Fragr J. 18:106-115.c
[CIR] Cosmetic Ingredient Review. 2020. Safety Assessment of Vanilla-Derived Ingredients as Used in Cosmetics [PDF]. Washington (DC): CIR. [accessed 2020 Aug 20].
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2019 Registered substances database; search results for CAS RN 100-52-7, 121-33-5, 124-13-0, 124-19-6, 1334-78-7, 8024-06-4. Helsinki (FI): ECHA. [accessed Feb 2019].
Eckl PM, Ortner A, Esterbauer H. 1993. Genotoxic properties of 4-hydroxyalkenals and analogous aldehydes. Mutat Res. 290(2):183-192.
[EFSA] European Food Safety Authority. 2005. Flavouring group evaluation 52 (FGE.52): Consideration of hydroxy- and alkoxy-substituted benzyl derivatives evaluated by JECFA (57th meeting) structurally related to benzyl alcohols, benzaldehydes, a related acetal, benzoic acids, and related esters evaluated by EFSA in FGE.20. EFSA Journal. 637:1-69.
[EFSA] European Food Safety Authority. 2009. Scientific opinion. Flavouring group evaluation 54, revision 1 (FGE.54Rev1): Consideration of benzyl derivatives evaluated by JECFA (57th meeting) structurally related to benzyl alcohols, benzaldehydes, a related acetal, benzoic acids and related esters evaluated by EFSA in FGE.20Rev1 (2009). EFSA Journal. 1025:1-73.
[EFSA] European Food Safety Authority. 2012. Scientific opinion on flavouring group evaluation 20, revision 4 (FGE.20Rev4): Benzyl alcohols, benzaldehydes, a related acetal, benzoic acids, and related esters from chemical groups 23 and 301. Parma, Italy: European Food Safety Authority. Report No. 2994.
[EFSA] European Food Safety Authority. 2013. Scientific Opinion on the safety and efficacy of straight‐chain primary aliphatic alcohols/aldehydes/acids, acetals and esters with esters containing saturated alcohols and acetals containing saturated aldehydes (chemical group 1) when used as flavourings for all animal species. EFSA Journal. 11(4): 3169.
[EFSA] European Food Safety Authority. 2017. Scientific opinion of flavouring group evaluation 502 (FGE.502): Grill flavour ‘Grillin’ 5078’. EFSA Journal. 15(9).
Environment Canada. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Esterbauer H, Eckl P, Ortner A. 1990. Possible mutagens derived from lipids and lipid precursors. Mutat Res. 238(3):223-233.
Florin I, Rutberg L, Curvall M, Enzell CR. 1980. Screening of tabacco smoke constituents for mutagenicity using the ames' test. Toxicology. 15(3):219-232.
Hagan E, Hansen W, Fitzhugh O, Jenner P, Jones W, Taylor JM, Long EL, Nelson A, Brouwer J. 1967. Food flavourings and compounds of related structure. II. subacute and chronic toxicity. Food Cosmet Toxicol. 5:141-157.
Havkin-Frenkel D, Belanger FC. 2018. Handbook of Vanilla Science and Technology. 2nd ed. Hoboken (NJ): Wiley & Sons Ltd.
Health Canada. 2010a. Regina Indoor Air Quality Study (2007): Data Summary for Volatile Organic Compound Sampling. Ottawa (ON): Government of Canada.
Health Canada. 2010b. Windsor Exposure Assessment Study (2005-2006): Data Summary for Volatile Organic Compound Sampling. Ottawa (ON): Government of Canada.
Health Canada. 2012. Halifax Indoor Air Quality Study (2009): Volatile Organic Compounds (VOC) Data Summary. Ottawa (ON): Government of Canada.
Health Canada. 2013. Edmonton Indoor Air Quality Study (2010). Volatile Organic Compounds (VOC) Data Summary. Ottawa (ON): Government of Canada.
Health Canada. [modified 2015 Dec 14]. Cosmetic Ingredient Hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Government of Canada.
Health Canada. 2015. Food consumption table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa (ON): Government of Canada.
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Health Canada. 2017. Science Approach Document. Science approach document for substances with low human health hazard potential. Ottawa (ON): Government of Canada.
Holloway WD, Tasman-Jones C, Lee SP. 1978. Digestion of certain fractions of dietary fiber in humans. Am J Clin Nutr. 31(6):927-930.
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): National Library of Medicine (US). [accessed Feb 2019].
Imanishi H, Sasaki Y, Matsumoto K, Watanabe M, Ohta T, Shirasu Y, Tutikawa K. 1990. Suppression of 6-TG-resistant mutations in V79 cells and recessive spot formations in mice by vanillin. Mutat Res. 243(2):151-158.
Jagannath DR. 1980. Mutagenic evaluation of C-192 in the Ames salmonella/microsome plate test. Project no. 20988. Unpublished Report [accessed 2019 September 23].
Kluwe W, Montgomery C, Giles H, Prejean J. 1983. Encephalopathy in rats and nephropathy in rats and mice after subchronic oral exposure to benzaldehyde. Food Chem Toxicol. 21(3):245-250.
Laham S, Broxup B, Robinet M, Potvin M, Schrader K. 1991. Subacute inhalation toxicity of benzaldehyde in the sprague-dawley rat. Am Ind Hyg Assoc J. 52(12):503-510.
Li Y, Cakmak S, Zhu J. 2019. Profiles and monthly variations of selected volatile organic compounds in indoor air in Canadian homes: Results of Canadian national indoor air survey 2012-2013. Environ Int. 126:134-144.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2021 Sep 08]. Ottawa (ON): Government of Canada. [accessed 2022 Feb 22].
Lucas CD, Putnam JM, Hallagan JB & the Flavor and Extract Manufacturers’ Association of the United States Flavor Ingredients Committee. 1999. 1995 Poundage and Technical Effects Update Survey, Washington (DC): Flavor and Extract Manufacturers’ Association of the United States.
Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, Ames BN. 1985. Naturally occurring carbonyl compounds are mutagens salmonella tester strain TA104. Mutat Res. 148(1-2):25-34.
Martelli A, Canonero R, Cavanna M, Ceradello M, Marinari UM. 1994. Cytotoxic and genotoxic effects of five n-alkanals in primary cultures of rat and human hepatocytes. Mutat Res. 323(3):121-126.
Meek ME, Newhook R, Liteplo RG, Armstrong VC. 1994. Approach to assessment of risk to human health for priority substances under the Canadian Environmental Protection Act. J Environ Sci Health C. 12(2):105-134.
Moretto R. 2007. Environmental and health evaluation of the use of elastomer granulates (virgin and from used tyres) as filling in third-generation artificial turf [Internet] [PDF]. ADEME/ALIAPUR/FIELDTURF TARKETT.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests: II. results from the testing of 270 chemicals. Environ Mutagen. 8(S7 S7):56-119.
[MSDS] Material Safety Data Sheet. 2008a. Airwick air freshener liquid bottle (wick). Winplus North America. [accessed 2016 Jan 29].
[MSDS] Material Safety Data Sheet. 2008b. Airwick air freshener vent membrane. Winplus North America. [accessed 2016 Jan 29].
[MSDS] Material Safety Data Sheet. 2008c. Type-s oval air freshener, white, summer breeze. Givaudan France Fragrances SAS. [accessed 2018 Dec 27].
[MSDS] Material Safety Data Sheet. 2010a. Menthol flavor e-liquid. Changning Dekang Biotechnology Co., Ltd. [accessed 2019 Oct 23].
[MSDS] Material Safety Data Sheet. 2010b. DK-Tab flavor e-liquid. Changning Dekang Biotechnology Co., Ltd. [accessed 2019 Oct 23].
[MSDS] Material Safety Data Sheet. 2010c. Finish dishwasher freshener odor stop fresh. Reckitt Benckiser Inc. [accessed 2016 Jan 29].
[MSDS] Material Safety Data Sheet. 2012a. NJOY updated assessment 2/9/12. NJOY. [accessed 2019 Oct 23].
[MSDS] Material Safety Data Sheet. 2012b. Stamp pad ink. Horizon Group. [accessed 2019 Aug 22].
[MSDS] Material Safety Data Sheet. 2013. Desert ship flavor e-liquid. Hangsen Holding Co., Ltd. [accessed 2019 Oct 23].
[MSDS] Material Safety Data Sheet. 2014. Cherry 1.8%. Tobacco Technology, Inc. [accessed 2019 Oct 23].
[MSDS] Material Safety Data Sheet. 2015a. Chocolate flavor e-liquid. Hangsen Holding Co., Ltd
[MSDS] Material Safety Data Sheet. 2015b. Febreze Noticeables Air Freshener - Gain Island Fresh (1 of 2). Procter and Gamble. [accessed 2019 Oct 23].
[MSDS] Material Safety Data Sheet. 2016a. Cherry Crush. Alternative Ingredients, Inc. [accessed 2019 Oct 23].
[MSDS] Material Safety Data Sheet. 2016b. R-O PRPK 24TRIM/24HEADLIGT WN BIL. Rustom-Oleum Consumber Brands Canada. [accessed 2019 Aug 22].
[MSDS] Material Safety Data Sheet. 2016c. Very emollient sunscreen kid spray broad spectrum SPF 40. The Hain Celestial Group, In. Alba Botanica. [accessed 2019 Oct 23].
[MSDS] Material Safety Data Sheet. 2019. California Scents Spillproof Coronado Cherry. Energizer Manufacturing Inc. [accessed 2019 Sep 30].
Myhr B. 1981. Mutagenic an evaluation of nonanal in the mouse lymphoma forward mutation assay. LBI Project No. 20989. Unpublished Report [accessed 2019 September 23].
[NCI] National Chemical Inventories Global [database]. [modified 2020]. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [accessed 2020 Sep 28].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2021 Dec 07]. Ottawa (ON): Government of Canada. [accessed 2022 Feb 22].
[NTP] National Toxicology Program (US). 1990. TOXICOLOGY AND CARCINOGENESIS STUDIES OF BENZALDEHYDE IN F344/N RATS AND B6C3F1 MICE (GAVAGE STUDIES). Research Triangle Park, NC: US Department of Health and Human Services, National Toxicology Program. Report No. 378.
[NWQMC] National Water Quality Monitoring Council. 2019. Water quality portal. USGS, US EPA, and NWQMC: United States. [accessed 2019 Aug 26].
[OECD] Organisation for Economic Co-operation and Development. 1996a. SIDS initial assessment report: butyraldehyde: CAS No. 123-72-8. Paris, France: SIAM [SIDS Initial Assessment Meeting] 5. [accessed 2019 September 23].
[OECD] Organisation for Economic Co-operation and Development. 1996b. SIDS initial assessment report: isobutanal: CAS No. 78-84-2. Paris, France: SIAM [SIDS Initial Assessment Meeting] 5. [accessed 2019 September 23].
[OECD] Organisation for Economic Co-operation and Development. 1996c. SIDS initial assessment report: vanillin: CAS No. 121-33-5. Paris, France: SIAM [SIDS Initial Assessment Meeting] 5. [accessed 2019 September 23].
[OECD] Organisation for Economic Co-operation and Development. 2002. SIDS initial assessment report: benzaldehyde: CAS No. 100-52-7. Paris, France: SIAM [SIDS Initial Assessment Meeting] 2. [accessed 2019 September 23].
[OECD] Organisation for Economic Co-operation and Development. 2009. Emission scenario document on coating industry (paints, lacquers and varnishes) [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 22; Report No.: ENV/JM/MONO(2009)24, JT03267833).
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris, France: Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[OEHHA] Office of Environmental Health Hazard Assessment. 2018. Gasoline-related air pollutants in California – trends in exposure and health risk 1996 to 2014 [PDF]. California Environmental Protection Agency, Sacramento, SA. [accessed Dec 2019].
Opgrande JL, Dobratz CJ, Brown E, Liang J, Conn GS, Shelton FJ, With J. 2000. Benzaldehyde. Kirk‐Othmer Encyclopedia of Chemical Technology. Online version. New York (NY): John Wiley and Sons, Inc.
Oser B, Carson S, Oser M. 1965. Toxicological tests on flavouring matters. Food Cosmet Toxicol. 3:563-9.
[PCPC] Personal Care Products Council. 2020. Cosmetic Ingredient Identification Database (International Cosmetic Ingredient Dictionary (INCI)). [accessed 2020 Sep 9].
PubChem [database]. 2004- . Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [accessed Feb 2019].
Ramachandra Rao S, Ravishankar GA. 2000. Vanilla flavour: production by conventional and biotechnological routes. J Sci Food Agric. 80(3):289-304.
Sinha A, Sharma U, Sharma N. 2007. A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. Int J Food Sci Nutr. 59(4):299-326.
Stofberg J, Grundschober F. 1987. Consumption ratio and food predominance of flavoring materials. Perfumer an Florist. 12(4):27-68.
[US EPA] United States Environmental Protection Agency. 1988. Integrated Risk Information System (IRIS). Chemical Assessment Summary for benzaldehyde (CAS RN 100-52-7). Washington (DC): National Center for Environmental Assessment.
[US EPA] United States Environmental Protection Agency. 2001. Air quality criteria for particulate matter, volume I. Washington (DC): Office of Research and Development, US EPA. (EPA 600/P-99/002aB).
[US EPA] United States Environmental Protection Agency. 2010. Benzyl derivatives category screening level hazard characterization. Washington (DC): US EPA.
[US EPA] United States Environmental Protection Agency. 2011. Exposure Factors Handbook 2011 Edition (Final). Washington (DC): US EPA. EPA/600/R-09/052F.
[US EPA] United States Environmental Protection Agency. 2015. Provisional peer-reviewed toxicity values for benzaldehyde (CASRN 100-52-7). Washington (DC): US EPA. Report No. EPA/690/R-15/001F.
[US FDA]. United States Food and Drug Administration. 2019. Code of Federal Regulations. Title 21: Volume 2. Sec. 169.175 Vanilla extract. PART 169 – food dressings and flavorings. [21CFR169.175]. Washington (DC): US FDA.
[VCF] Volatile Compounds in Foods [database]. 1992-2019. Version 16.6.1. Zeist (NL): Triskelion. [updated continually; accessed 2019 May 31].
Verzera A, Trozzi A, Cotroneo A, Lorenzo D, Dellacassa E. 2000. Uruguayan Essential Oil. 12. Composition of Nova and Satsuma Mandarin Oils. J Agric Food Chem. 48(7):2903-2909.
Watanabe K, Matsunaga T, Yamamoto I, Yashimura H. 1995. Oxidation of tolualdehydes to toluic acids catalyzed by cytochrome P450-dependent aldehyde oxygenase in the mouse liver. Drug Metab Dispos. 23(2):261-5.
Won D. 2015. VOC emissions from evaporative sources in residential garages. Ottawa (ON): NRC-CNRC. 221 p. Report No.: A1-000342-03.
Won D, Nong G, Yang W, Collins W. 2014. Material emissions testing: VOCs from wood, paint, and insulation materials. Ottawa (ON): NRC-CNRC. 154 p. Report No.: A1-000342.2
Won D, Nong G, Yang W, Schleibinger H. 2013. Material emissions data: 52 building materials tested for 124 compounds. Ottawa (ON): NRC-CNRC. 286 p. Report No.: A1-000342.
Won D, Yang W. 2012. Material emission information from 105 building materials and consumer products. Ottawa (ON): NRC-CNRC. 151 p. Report No.: B3341.1.
[WHO] World Health Organization. 1967. Toxicological evaluation og some flavouring substances and non-nutritive sweetening agents. Geneva: World Health Organization. Report No. 44A.
[WHO] World Health Organization. 1979. Octanal (WHO food additives series 14). Geneva: World Health Organization. Report No. 482.
[WHO] World Health Organization. 1981. Evaluation of certain food additives. twenty-fifth report of the joint FAO/WHO expert committee on food additives. Geneva: World Health Organization. Report No. 669.
[WHO] World Health Organization. 1984. Evaluation of certain food additives and contaminants. twenty-eighth report of the joint FAO/WHO expert committee on food additives. Geneva: World Health Organization. Report No. 710.
[WHO] World Health Organization. 1996. Evaluation of certain food additives and contaminants. forty-sixth report of the joint FAO/ WHO expert committee on food additives. Geneva: World Health Organization. Report No. WHO Technical Report Series 868.
[WHO] World Health Organization. 1999. Evaluation of certain food additives and contaminants. forty-ninth report of the joint FAO/WHO expert committee on food additives. Geneva: World Health Organization. Report No. 884.
[WHO] World Health Organization. 2002. Evaluation of certain food additives and contaminants. fifty-seventh report of the joint FAO/WHO expert committee on food additives. Geneva: World Health Organization. Report No. WHO Technical Report Series 909.
Zeiger E, Andersn B, Haworth S, Lawlor T, Mortelmans K. 1992. Salmonella mutagenicity tests: V. results from the testing of 311 chemicals. Environ Mol Mutagen. 19(S21):2-141.
Appendix A. Summary tables of read-across for health effects endpoints
| Property | Benzaldehyde (target) | Sodium benzoate (analogue) |
|---|---|---|
| Structure |  |
![c1(ccccc1)C(=O)[O-].[Na+]](/content/dam/eccc/images/pded/aldehydes/20220819-a1b.jpg) |
| Physical state | Liquid | Solid |
| Melting point (°C)a | -26 | 436 |
| Vapour pressure (Pa)a,b | 15 | 4.89 x 10-7 |
| Henry’s law constant (Pa·m3/mol)b | 2.7 | NA |
| Water solubility (mg/L)a | 6950 | 5.56 x 105 |
| Log Kow (dimensionless)a | 1.48 | 1.88 |
| Genotoxicity | Negative | N/A |
| Short-term oral | NOAEL = 400 mg/kg bw/day (2-week oral study in mice and rats) | N/A |
| Short-term inhalation | LOAEC = 2170 mg/m3 LOAELadj = 512 mg/kg bw/day (14-day inhalation study in rats) |
N/A |
| Short-term dermal | N/A | N/A |
| Sub-chronic oral | NOAEL = 400-600 mg/kg bw/day (13-week oral study in mice and rats) | N/A |
| Sub-chronic inhalation | N/A | N/A |
| Sub-chronic dermal | N/A | N/A |
| Chronic oral | NOAEL = 200 mg/kg bw/day (2-year oral study in mice and rats) | N/A |
| Chronic inhalation | N/A | N/A |
| Chronic dermal | N/A | N/A |
| Carcinogenicity | NOAEL = 400-600 mg/kg bw/day (2-year oral study in mice and rats; no carcinogenic effects) | N/A |
| Reproductive and/or developmental toxicity | NOAEL = 1306 mg/kg bw/day (diet developmental study in rats) (read-across from sodium benzoate) | NOAEL = 1306 mg/kg bw/day (diet developmental study in rats) |
Abbreviations: N/A, not applicable; NA, not available
a ECHA c2007-2019
b ChemIDplus 1993-
| Property | Octanal (target 1) | Nonanal (target 2) | Butanal (analogue 1) | Isobutanal (analogue 2) |
|---|---|---|---|---|
| Structure |  |
 |
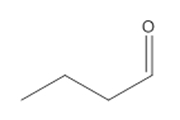 |
 |
| Physical state | Liquid | Liquid | Liquid | Liquid |
| Melting point (°C)a,b | -20 | -18.8 | -99 | -65.9 |
| Vapour pressure (Pa)a | 148 | 49 | 1.44 × 104 | 2.31 × 104 |
| Henry’s law constant (Pa·m3/mol)b | 52.1 | 74.4 | 11.7 | 18.2 |
| Water solubility (mg/L)a | 560 | 96 | 5 × 105 | 6 × 105 |
| Log Kow (dimensionless)a | 3.50 | 3.40 | 1.3 | 0.77 |
| Genotoxicity | Negative | Equivocal | N/A | N/A |
| Short-term oral | NOAEL = 313 mg/kg bw/day (14-day gavage study in rats) (read-across from butanal) | NOAEL = 313 mg/kg bw/day (14-day gavage study in rats) (read-across from butanal) | NOAEL = 313 mg/kg bw/day (14-day gavage study in rats) | N/A |
| Short-term inhalation | NA | NA | N/A | N/A |
| Short-term dermal | NOAEL = 500 mg/kg bw/day (2-week dermal study in rats) (read-across from nonanal) | NOAEL = 500 mg/kg bw/day (2-week dermal study in rats) | N/A | N/A |
| Sub-chronic oral | NOAEL = 13 mg/kg bw/day (12-week diet study in rats) | NOAEL = 29 mg/kg bw/day (12-week diet study in rats) | NOAEL = 300 mg/kg bw/day (13-week gavage study in rats) | N/A |
| Sub-chronic inhalation | NOAEC = 150 mg/m3; NOAECadj = 27a mg/m3 (13-week inhalation study in rats) (read-across from butanal) | NOAEC = 150 mg/m3; NOAECadj = 27a mg/m3 (13-week inhalation study in rats) (read-across from butanal) | NOAEC = 150 mg/m3; NOAECadj = 27a mg/m3 (13-week inhalation study in rats) | N/A |
| Sub-chronic dermal | NA | NA | N/A | N/A |
| Chronic oral | NA | NA | N/A | N/A |
| Chronic inhalation | LOAEC = 1474 mg/m3 (2-year inhalation study in rats and mice; respiratory epithelial damage in female rats) (read-across from isobutanal) | LOAEC = 1474 mg/m3 (2-year inhalation study in rats and mice; respiratory epithelial damage in female rats) (read-across from isobutanal) | N/A | LOAEC = 1474 mg/m3 (2-year inhalation study in rats and mice; respiratory epithelial damage in female rats) |
| Chronic dermal | NA | NA | N/A | N/A |
| Carcinogenicity | NOAEC = 5898 mg/m3 (2-year inhalation carcinogenicity study in rats and mice; no carcinogenic effects) (read-across from isobutanal) | NOAEC = 5898 mg/m3 (2-year inhalation carcinogenicity study in rats and mice; no carcinogenic effects) (read-across from isobutanal) | N/A | NOAEC = 5898 mg/m3 (2-year inhalation carcinogenicity study in rats and mice; no carcinogenic effects) |
| Reproductive and/or developmental toxicity | NOAEC = 11 796 mg/m3 (inhalation reproductive and developmental studies in rats; no adverse fetal effects) (read-across from isobutanal) | NOAEC = 11 796 mg/m3 (inhalation reproductive and developmental studies in rats; no adverse fetal effects) (read-across from isobutanal) | N/A | NOAEC = 11 796 mg/m3 (inhalation reproductive and developmental studies in rats; no adverse fetal effects) |
Abbreviations: LOAEC, lowest observed adverse effect concentration; N/A, not applicable; NA, not available; NOAEC , no observed adverse effect concentrations; NOAEL, no observed adverse effect level
a ECHA c2007-2019
b ChemIDplus 1993-
| Property | Vanilla oils | Vanillin (target) | Ethyl vanillin (analogue) |
|---|---|---|---|
| Structure | N/A |  |
 |
| Physical state | N/Ac | Solid | Solid |
| Melting point (°C)a | N/Ac | 80 | 65 |
| Vapour pressure (Pa)a | N/Ac | 0.3 | 0.03 |
| Henry’s law constant (Pa·m3/mol)b | N/Ac | 2.18 × 10-4 | 1.11 × 10-5 |
| Water solubility (mg/L)a | N/Ac | 9 × 103 | 2.8 × 103 |
| Log Kow (dimensionless)a | N/Ac | 1.21 | 1.58 |
| Genotoxicity | Negative (read-across from vanillin) | Negative | N/A |
| Short-term oral | NA | N/A | N/A |
| Short-term inhalation | NA | N/A | N/A |
| Short-term dermal | NA | N/A | N/A |
| Sub-chronic oral | NOAEL = 1000 mg/kg bw/day (16-week diet study in rats; no adverse effects) (read-across from vanillin) | NOAEL = 1000 mg/kg bw/day (16-week diet study in rats; no adverse effects) | N/A |
| Sub-chronic inhalation | NA | N/A | N/A |
| Sub-chronic dermal | NA | N/A | N/A |
| Chronic oral | NOAEL = 2500 mg/kg bw/day (1-year diet study in rats; no adverse effects) (read-across from vanillin) | NOAEL = 2500 mg/kg bw/day (1-year diet study in rats; no adverse effects) | N/A |
| Chronic inhalation | NA | N/A | N/A |
| Chronic dermal | NA | N/A | N/A |
| Carcinogenicity | NOAEL = 2500 mg/kg bw/day (1-year diet study in rats; no carcinogenic effects) (read-across from vanillin) | NOAEL = 2500 mg/kg bw/day (1-year diet study in rats; no carcinogenic effects) | N/A |
| Reproductive and/or developmental toxicity | NOAEL = 1000 mg/kg bw/day (oral reproductive and developmental studies in rats; no treatment related fetal adverse effects) (read-across from ethyl vanillin) | NOAEL = 1000 mg/kg bw/day (oral reproductive and developmental studies in rats; no treatment related fetal adverse effects) (read-across from ethyl vanillin) | NOAEL = 1000 mg/kg bw/day (oral reproductive and developmental studies in rats; no treatment related fetal adverse effects) |
Abbreviations: N/A, not applicable; NA, not available; NOAEL, no observed adverse effect level.
a ECHA c2007-2019
b ChemIDplus 1993-
c Substance is a UVCB
Appendix B. Estimated human exposures to substances in the Aldehydes Group
Human exposure estimates from the use of products available to consumers were estimated using ConsExpo Web (2018). Dermal absorption of 100% was assumed for daily systemic exposures in the absence of dermal absorption data; 100% absorption from the inhalation route was also assumed. The parameters used in the estimation of oral, inhalation, and dermal exposures are described in Table B-2. Unless specified, the defaults from the relevant ConsExpo Fact Sheet for the scenario were used.
Inhalation rates and body weights of the users are specified in Health Canada (2015) and are summarized in Table B-1.
| Age group | Inhalation rate (m3/day) | Body weight (kg) |
|---|---|---|
| 19 years or above | 15.1 | 74 |
| 14 to 18 years | 15.9 | 62 |
| 9 to 13 years | 13.9 | 42 |
| 4 to 8 years | 11.1 | 23 |
| 2 to 3 years | 9.2 | 15 |
| 1 year | 8.0 | 11 |
| 6 to 11 months | 5.4 | 9.1 |
| 0 to 5 months | 3.7 | 6.3 |
| Exposure scenario | Model parameters and assumptions |
|---|---|
| Air freshener, benzaldehyde and octanal, inhalation, daily |
For inhalation exposure: Release product (g/h) = initial product amount (g) / product exhaustion durationa (h) Model: Exposure to vapour – instantaneous release |
| Body moisturizer, benzaldehyde, dermal, daily, toddler (2-3 years) |
For dermal exposure: Product amount × Weight fraction × Frequency / Body weight × Unit conversion |
| Lip balm, benzaldehyde, oral, daily, toddler (aged 2-3 years) |
For oral exposure: Product amount × Weight fraction × Frequency / Body weight × Unit conversion |
| Vitamin and mineral supplement tablet, octanal and nonanal, oral, daily, child (9-13 years) |
For oral exposure: Product amount × Weight fraction × Frequency / Body weight × Unit conversion |
| Spray sunscreen, nonanal, dermal and inhalation, daily, infant (6-11 months) and teen (14-18 years) |
For inhalation exposure (14-18 years): For dermal exposure (6-11 months): Product amount × Weight fraction × Frequency / Body weight × Unit conversion |
a Based on advertised product lifetime.
b Based on facial surface area as the product is marketed as a “wake-up” spray product and it is expected that the product will be sprayed towards the face.
Appendix C. Estimates of daily intake by various age groups within the general population of Canada
| Route of exposure | 0 to 5 monthsa (human milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Ambient airk | 8.85×10-2 | 8.85×10-2 | 8.95×10-2 | 1.10×10-1 | 9.25×10-2 | 7.28×10-2 | 4.99×10-2 | 3.87×10-2 | 3.08×10-2 |
| Indoor airl | 8.07 | 8.07 | 8.16 | 1.00×101 | 8.43 | 6.63 | 4.55 | 3.53 | 2.80 |
| Drinking waterm | N/A | 1.04×10-4 | 6.63×10-5 | 2.59×10-5 | 2.26×10-5 | 1.82×10-5 | 1.39×10-5 | 1.39×10-5 | 1.63×10-5 |
| Soiln | N/A | N/A | 2.25×10-8 | 2.24×10-8 | 1.16×10-8 | 1.06×10-8 | 4.60×10-9 | 6.32×10-10 | 6.05×10-10 |
| Food and beverageso | N/A | N/A | N/A | 6.00×102 | 6.00×102 | 6.00×102 | 6.00×102 | 6.00×102 | 6.00×102 |
| Total intake | 8.16 | 8.16 | 8.25 | 6.10×102 | 6.09×102 | 6.07×102 | 6.05×102 | 6.04×102 | 6.03×102 |
Abbreviation: N/A, not applicable.
a Assumed to weigh 6.3 kg (Health Canada 2015), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for human milk-fed infants, assumed to consume 0.744 L of human milk per day (Health Canada 2018), and human milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For human milk-fed infants, assumed to consume 0.632 L of human milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k The 95th percentile ambient Canadian air concentration (1.21 µg/m3) was used for deriving upper-bounding estimates of daily intake for ambient air exposure as a conservative estimate (Health Canada 2010a). Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
l The 95th percentile indoor air concentration from Canadian homes (15.71 µg/m3) was used for deriving upper-bounding estimates of daily intake for indoor air exposure as a conservative estimate (Health Canada 2010b). Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
m No monitoring data of water Canada were identified. Based on an estimate of 7.9x10-4 µg/L on the basis of a 100% release scenario to water from ChemCAN v6.00 where the simulations conservatively assumed that total quantities were released into a single region of Canada, that is, the Ontario Mixed-Wood Plain region, at a 100% emission factor and assuming 0% removal for wastewater treatment processes (for water releases).
n No monitoring data of soil in Canada were identified. Based on an estimate of 0.03 ng/g on the basis of a 100% release scenario to soil from ChemCAN v6.00 where the simulations conservatively assumed that total quantities were released into a single region of Canada, that is, the Ontario Mixed-Wood Plain region.
o No definitive information is available concerning the potential use of benzaldehyde as a food flavouring agent in Canada. The JECFA per capita intake estimate for the United States (based on annual production volumes reported by the food industry in poundage surveys) was used to derive daily intakes of benzaldehyde as a food flavouring agent (see section 6.1) In the absence of age group-specific intake estimates, exposures based on a 60 kg person (WHO 2002) were applied to all relevant age groups (1 year of age and older). The bodyweight adjusted intake using a 60 kg bodyweight is considered to be sufficiently conservative to represent the entire population 1 year of age and older (personal communication, emails from FD, Health Canada, to ESRAB, Health Canada, 2019; unreferenced).
| Route of exposure | 0 to 5 monthsa (human milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Ambient airk | 8.08× 10-1 | 8.08× 10-1 | 8.16× 10-1 | 1.00 | 8.43× 10-1 | 6.64× 10-1 | 4.55× 10-1 | 3.53× 10-1 | 2.81× 10-1 |
| Indoor airl | 5.65 | 5.65 | 5.71 | 7.00 | 5.90 | 4.65 | 3.19 | 2.47 | 1.96 |
| Food and beveragesm | N/A | N/A | N/A | 2.90× 10-1 | 2.90× 10-1 | 2.90× 10-1 | 2.90× 10-1 | 2.90× 10-1 | 2.90× 10-1 |
| Total intake | 6.46 | 6.46 | 6.53 | 8.29 | 7.04 | 5.60 | 3.93 | 3.11 | 2.53 |
Abbreviations: N/A, not applicable.
a Assumed to weigh 6.3 kg (Health Canada 2015), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for human milk-fed infants, assumed to consume 0.744 L of human milk per day (Health Canada 2018), and human milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For human milk-fed infants, assumed to consume 0.632 L of human milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k The 95th percentile indoor air concentration from Canadian homes (11.0 µg/m3) was used as a surrogate for deriving upper-bounding estimates of daily intake for ambient air exposure as a conservative estimate (Li et al. 2019). Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
l The 95th percentile indoor air concentration from Canadian homes (11.0 µg/m3) was used for deriving upper-bounding estimates of daily intake for indoor air exposure as a conservative estimate (Li et al. 2019). Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
m No definitive information is available concerning the potential use of octanal as a food flavouring agent in Canada. The JECFA per capita intake estimate for the United States (based on annual production volumes reported by the food industry in poundage surveys) was used to derive daily intakes of octanal as a food flavouring agent (see section 6.1). In the absence of age group-specific intake estimates, exposures based on a 60 kg person (WHO 1999) were applied to all relevant age groups. The bodyweight adjusted intake using a 60 kg bodyweight is considered to be sufficiently conservative to represent the entire population 1 year of age and older (personal communication, emails from FD, Health Canada, to ESRAB, Health Canada, 2019; unreferenced).
| Route of exposure | 0 to 5 monthsa (human milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Ambient airk | 2.25 | 2.25 | 2.27 | 2.78 | 2.35 | 1.85 | 1.27 | 9.81×10-1 | 7.81×10-1 |
| Indoor airl | 1.57×101 | 1.57×101 | 1.59×101 | 1.95×101 | 1.64×101 | 1.29×101 | 8.86 | 6.87 | 5.46 |
| Food and beveragesm | N/A | N/A | N/A | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Total intake | 1.80×101 | 1.80×101 | 1.82×101 | 2.38×101 | 2.03×101 | 1.63×101 | 1.16×101 | 9.35 | 7.74 |
Abbreviation: N/A, not applicable.
a Assumed to weigh 6.3 kg (Health Canada 2015), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for human milk-fed infants, assumed to consume 0.744 L of human milk per day (Health Canada 2018), and human milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For human milk-fed infants, assumed to consume 0.632 L of human milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k The 95th percentile indoor air concentration from Canadian homes (30.6 µg/m3) was used as a surrogate for deriving upper-bounding estimates of daily intake for ambient air exposure as a conservative estimate (Li et al. 2019). Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
l The 95th percentile indoor air concentration from Canadian homes (30.6 µg/m3) was used for deriving upper-bounding estimates of daily intake for indoor air exposure as a conservative estimate (Li et al. 2019). Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
m No definitive information is available concerning the potential use of nonanal as a food flavouring agent in Canada. The JECFA per capita intake estimate for the United States (based on annual production volumes reported by the food industry in poundage surveys) was used to derive daily intakes of nonanal as a food flavouring agent (see section 6.1). In the absence of age group-specific intake estimates, exposures based on a 60 kg person (WHO 1999) were applied to all relevant age groups. The bodyweight adjusted intake using a 60 kg bodyweight is considered to be sufficiently conservative to represent the entire population 1 year of age and older (personal communication, emails from FD, Health Canada, to ESRAB, Health Canada, 2019; unreferenced).
| Route of exposure | 0 to 5 monthsa (human milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Food and beveragesk | N/A | N/A | N/A | 1.80×101 | 1.80×101 | 1.80×101 | 1.80×101 | 1.80×101 | 1.80×101 |
| Total intake | N/A | N/A | N/A | 1.80×101 | 1.80×101 | 1.80×101 | 1.80×101 | 1.80×101 | 1.80×101 |
Abbreviation: N/A, not applicable.
a Assumed to weigh 6.3 kg (Health Canada 2015), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for human milk-fed infants, assumed to consume 0.744 L of human milk per day (Health Canada 2018), and human milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For human milk-fed infants, assumed to consume 0.632 L of human milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k No definitive information is available concerning the potential use of methylbenzaldehyde as a food flavouring agent in Canada. The JECFA per capita intake estimate for the United States (based on annual production volumes reported by the food industry in poundage surveys) was used to derive daily intakes of methylbenzaldehyde as a food flavouring agent (see section 6.1). In the absence of age group-specific intake estimates, exposures based on a 60 kg person (WHO 2002) were applied to all relevant age groups. The bodyweight adjusted intake using a 60 kg bodyweight is considered to be sufficiently conservative to represent the entire population 1 year of age and older (personal communication, emails from FD, Health Canada, to ESRAB, Health Canada, 2019; unreferenced).
Page details
- Date modified:
