In-Depth Analysis: Human Respiratory Disease Associated with Avian Influenza A(H5N6)
Table of contents
- Risk to Canada
- Background
- Virological information
- Animal infections and environmental detections
- Exposure
- Global epidemiology
- Clinical course
- Public health actions
- Links and resources
- References
Eight (8) human cases of avian influenza A(H5N6) were reported in January 2022, all from China. Including these cases, a total of 66 laboratory-confirmed human cases of avian influenza A(H5N6), including at least 29 deaths, have been reported globally since 2014. Further information can be found below.
Risk to Canada
The goal of this in-depth analysis is to summarize the current available information on the epidemiology of human infection with avian influenza A(H5N6) [A(H5N6)].
Since the emergence of A(H5N6) in the human population in 2014 and as of February 1st, 2022, 66 human cases have been reported from the People's Republic of China (China) and Lao People's Democratic Republic (Lao PDR). No cases of A(H5N6) have been reported in Canadian residents. Although the current available information suggests this virus does not have the ability to transmit easily among humans, additional sporadic human cases of A(H5N6) are expected to be reported in regions where A(H5N6) continues to circulate in wild and domestic birds.
Risk assessments for avian influenza A(H5N6) have been undertaken by several institutions, including the World Health Organization (WHO), the United States (US) Centers for Disease Control and Prevention (CDC) [using the Influenza Risk Assessment Tool (Tool)], and the United Kingdom (UK). A risk assessment for A(H5N6) has not been completed for Canada.
Guidance related to avian influenzas like A(H5N6), including the Public Health Agency of Canada (PHAC)'s Severe Acute Respiratory Infection (SARI) Case Report Form, laboratory guidance for SARI investigations, epidemiologic guidance for SARI investigations, biosecurity guidance for H5/H7/H9 influenzas, surveillance guidance for avian influenza in humans [specific to A(H7N9) but can be applied to A(H5N6)], and the Canadian Pandemic Influenza Planning (CPIP) Guidance are available online.
Background
Avian influenza viruses (AIVs) can affect several species of domestic or wild birds. Based on their pathogenicity, AIVs are classified as either low pathogenicity avian influenza (LPAI) or highly pathogenic avian influenza (HPAI). Over the past two (2) decades, AIVs have become enzootic in domestic poultry populations in many Asian countries Footnote 1. Today, China is recognized as a hotspot for the emergence, transmission, and dissemination of AIVs due to their widespread persistence, the nature and growth of the poultry production industry, live poultry trading, and the mixing of host species in live bird markets (LBMs) Footnote 2Footnote 3Footnote 4.
Avian influenza A(H5N6) is a HPAI virus that can cause severe disease and high mortality in infected poultry Footnote 3. Outbreaks of A(H5N6) were reported in birds in Laos, China, and Vietnam prior to the report of the first human case in China in 2014 Footnote 5. Human cases of A(H5N6) have continued to be reported since, with a marked increase in detections in 2021 [Figure 1]. Although it is possible that this increase coincides with heightened surveillance and diagnostic systems resulting from the COVID-19 pandemic, other factors like the spread of AIVs in poultry populations likely also play a role in the increased number of cases Footnote 6.

Figure 1. Epidemiologic curve of human avian influenza A(H5N6) infections, by year of illness onset or report date, 2014-January 31, 2022 (n=66). - Text Equivalent
| Illness Onset Year | Reported Number of Human Avian Influenza A(H5N6) Cases |
|---|---|
| 2014 | 3 |
| 2015 | 6 |
| 2016 | 9 |
| 2017 | 1 |
| 2018 | 4 |
| 2019 | 1 |
| 2020 | 5 |
| 2021 | 35 |
| 2022 | 2 |
Note: Illness onset data were unavailable for three (3) cases; for these cases, report date data were utilized to create this figure. The cases included in this figure are cases reported up until January 31, 2022. Source: the Centre for Immunization and Respiratory Infectious Diseases (CIRID)'s International Monitoring and Assessment Tool (IMAT).
Further understanding of both the human and animal epidemiology of this disease is essential to characterize the risk of emergence and human health impact posed by this novel influenza virus. This in-depth analysis is based on a review of published literature, supplemented with a selected search of official organizational materials published by the WHO, US CDC, Hong Kong's Centre for Health Protection (Hong Kong CHP), and the World Organisation for Animal Health (OIE). Data from these sources were collated, verified, recorded in IMAT, and cleaned to conduct epidemiologic analyses on human cases of A(H5N6) reported globally.
Virological Information
Avian influenza A(H5N6) is a variant HPAI A(H5) virus belonging to novel clade 2.3.4.4 Footnote 7 Footnote 8. Genetic analysis of avian and human isolates from 2014-2021 confirm this virus is a reassortant AIV with hemagglutinin (HA) from clade 2.3.4.4 A(H5) viruses, and neuraminidase (NA) from H5N6, H6N6, H3N6, or H10N6 viruses. The internal genes are either from 2.3.4.4 A(H5) clade viruses, 2.3.2.1 A(H5) clade viruses, or H3N2, H3N8, H4N2, H6N6, H7N9 and H9N2 viruses [Figure 2] Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17Footnote 18Footnote 19Footnote 20Footnote 21. The source species for the genes of these human isolates include chicken, goose, swan, duck, gulls, and humans Footnote 16Footnote 17Footnote 18Footnote 19Footnote 20Footnote 21. Every A(H5N6) virus that was sequenced from human cases in China with an illness onset date after February 2021 (and prior to November 29, 2021) belonged to the 2.3.4.4b genetic clade, although one viral sequence from earlier in the year belonged to a different clade that had been commonly detected in birds up to that pointFootnote 6.
Genetic signature analyses of A(H5N6) isolates indicate preferable binding to avian-like receptorsFootnote 3Footnote 4Footnote 9Footnote 22. Nevertheless, certain sequences also indicate associations with mammalian adaptations, such as increased human cell receptor binding Footnote 3Footnote 4Footnote 6Footnote 9Footnote 22. The diversity of AIVs circulating in China, along with continued interaction between host species, allows for continued reassortment of these viruses [Figure 2].

Figure 2. Schematic of genetic source of select human isolates of avian influenza A(H5N6) analyzed from 2014-2021. - Text Equivalent
Figure 2 is a hypothesis of genetic lineages for some human avian influenza A(H5N6) isolates from 2014-2020.
In 2014, potential genetic lineages of two (2) human avian influenza A(H5N6) isolates were described. The first isolate's genes may be derived from H6N6 and clade 2.3.4.4 H5 viruses. The second isolate's genes may be derived from clade 2.3.2.1 H5, H6N6, and clade 2.3.4.4 H5 viruses.
In 2015, potential genetic lineages of two (2) human avian influenza A(H5N6) isolates were described. The first isolate's genes may be derived from H6N6 and clade 2.3.4.4 H5 viruses. The second isolate's genes may be derived from H9N2/H7N9, H6N6, and clade 2.3.4.4 H5 viruses.
In 2016, potential genetic lineages of four (4) human avian influenza A(H5N6) isolates were described. The first isolate's genes may be derived from H6N6 and clade 2.3.4.4 H5 viruses. The second isolate's genes may be derived from H9N2/H7N9, H6N6, and clade 2.3.4.4 H5 viruses. The third isolate's genes may be derived from H6N6 and clade 2.3.4.4 H5 viruses. The fourth isolate's genes may be derived from H10N6, H9N2, H3N6, and clade 2.3.4.4 H5 viruses.
In 2017, potential genetic lineages of one (1) human avian influenza A(H5N6) isolate was described. The isolate's genes may be derived from H6N6 and clade 2.3.4.4 H5 viruses.
In 2018, potential genetic lineages of one (1) human avian influenza A(H5N6) isolate was described. The isolate's genes may be derived from H3N8, H3N2, H6N6, and calde 2.3.4.4 H5 viruses.
In 2019, potential genetic lineages of one (1) human avian influenza A(H5N6) isolate was described. The isolate's genes may be derived from clade 2.3.4.4 H5 viruses.
In 2020, potential genetic lineages of one (1) human avian influenza A(H5N6) isolate was described. The isolate's genes may be derived from H9N2 and clade 2.3.4.4 H5 viruses.
In 2021, potential genetic lineages of two (2) human avian influenza A(H5N6) isolates were described. The first isolate's genes may be derived from H3N2 and clade 2.3.4.4 H5 viruses. The second isolate's genes may be derived from H4N2 and clade 2.3.4.4 H5 viruses.
Note: This figure, a hypothesis of genetic lineages for some human avian influenza A(H5N6) isolates, was completed using data from peer-reviewed published literature Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15Footnote 16Footnote 17Footnote 18Footnote 19Footnote 20Footnote 21. It is not a comprehensive diagram and may contain incomplete information. Viruses are represented by circular shaped structures. The horizontal bars within the viruses in the diagram signify viral genes. From top to bottom, the eight (8) genes in each virus are: PB2, PB1, PA, HA, NP, NA, MP, and NS.
Animal Infections and Environmental Detections
In 2014, HPAI A(H5N6) was detected in domestic and wild bird populations in Asia, from China, Vietnam, and Laos. Since then, widespread avian outbreaks have continued to be reported in bird populations worldwide, with detections reported to the OIE from 21 different countries in Asia, Europe, and Africa to date [Figure 3] Footnote 23.

Figure 3. Summary of animal outbreaks of avian influenza A(H5N6) reported to OIE, 2014-2021. - Text Equivalent
Figure 3 outlines which Asian, European, and African countries reported animal outbreaks of avian influenza A(H5N6) to the OIE from 2014-2021. The outbreaks were reported in domestic, wild, or both animal populations.
In 2014, China reported domestic and wild animal outbreaks, Lao PDR reported domestic animal outbreaks, and Vietnam reported domestic animal outbreaks. In 2015, China reported doemestic and wild animal outbreaks, Hong Kong reported wild animal outbreaks, Lao PDR reported domestic animal outbreaks, and Vietnam reported domestic animal outbreaks. In 2016, China reported domestic and wild animal outbreaks, Hong Kong reported domestic and wild animal outbreaks, Japan reported domestic and wild animal outbreaks, Korea reported domestic animal outbreaks, Myanmar reported domestic animal outbreaks, and Vietname reported domestic animal outbreaks. In 2017, China reported domestic and wild animal outbreaks, Hong Kong reported wild animal outbreaks, Japan reported domestic and wild animal outbreaks, Korea reported domestic and wild animal outbreaks, Philippines reported domestic animal outbreaks, Taiwan reported domestic and wild animal outbreaks, Vietname reported domestic animal outbreaks, Greece reported domestic animal outbreaks, the Netherlands reported domestic and wild animal outbreaks, and Switzerland reported wild animal oubreaks. In 2018, domestic and wild animal outbreaks were reported by Hong Kong, Japan, Germany, the Netherlands, Sweden, and the United Kingdom. In 2018, domestic animal outbreaks were reported by China, Korea, Philippines, and Vietnam. In 2018, wild animal outbreaks were reported by Iran, Denmark, Finland, Irland, and Slovakia. In 2019, domestic and wild animal outbreaks were reported by China. In 2019, domestic animal outbreaks were reported by Cambodia, Vietnam, and Nigeria. In 2019, wild animal outbreaks were reported by Denmark. In 2020, domestic and wild animal outbreaks were reported by China. In 2020, domestic animal outbreaks were reported by Philippines, Vietnam, and Nigeria. In 2021, domestic and wild animal outbreaks were reported by China. In 2021, domestic animal outbreaks were reported by Vietnam.
Note: This figure was created with data extracted from the OIE World Animal Health Information System (WAHIS) on December 16, 2021.
A(H5N6) has been detected in multiple environments. For instance, environmental samples of live poultry farming and trading places (including LBMs) revealed positive A(H5N6) swabs from chopping boards, sewage (including water used to clean slaughtered poultry, processing tools, and cages)/fecal samples, and poultry feeding/drinking sites Footnote 17Footnote 18Footnote 19Footnote 24Footnote 25. This virus has also been detected in residences (e.g. positive samples from backyard poultry, environments) of cases with domestic poultry or contact with birds Footnote 26.
Exposure
Modes of Transmission
Avian influenza A(H5N6) primarily infects birds, but also infects mammals including humans. This virus is transmitted from bird-to-bird through secretions and droppingsFootnote 27. Asymptomatic transmission is also a possibility as some species of wild birds, such as ducks, can carry the virus and infect other birds without developing illness. Although avian influenza A(H5N6) rarely infects humans, transmission may occur through contact with contaminated environments or infected birds Footnote 27Footnote 28. To date, there is no evidence of sustained human-to-human transmission.
Exposure Sources
Live Poultry Feeding and Trading Markets (LPFTMs) are considered a risk factor for human cases of various AIVs, including HPAI A(H5N6) Footnote 24. Exposure to birds through these environments may increase risk of A(H5N6) infection through direct or indirect contact with infected poultry Footnote 29. In fact, every case with known exposure information (53/53; 100%) reported indirect or direct contact with birds prior to illness onset, through LBMs, poultry workers, slain and cooked poultry, and/or domestic/backyard poultry Footnote 26. Epidemiological investigations have revealed positive H5 results from the backyards of several cases in China who kept domestic poultry or had wild birds frequent their residences. Confirmed human A(H5N6) cases have also been linked to local LPFTMs through genetic analysis and comparison of viral case and environmental samples [Figure 4] Footnote 19Footnote 21Footnote 24Footnote 26. Workers with occupational exposures, such as poultry sellers, are at higher risk of positive serology and investigators have observed positive A(H5N6) serology specimens from poultry workers in the past (note: this does not constitute a positive case). They also noted an elevated risk exists for those aged ≥55 years and individuals exposed to sick or dead poultry Footnote 30. However, the risk of animal-to-human transmission is low in the presence of personal protective equipment (PPE) and other biosecurity and preventive measures such as antiviral prophylaxis after potential exposure Footnote 31.

Figure 4. Schematic of the transmission of avian influenza A(H5N6) from wild birds to humans. - Text Equivalent
Figure 4 is a schematic of the transmission pathways of avian influenza A(H5N6) from wild birds to humans. It demonstrates the role free range poultry farming, the interface between wild birds and domestic poultry, wild aquatic birds, live poultry markets, the interface between domestic poultry and humans, the interface between poultry traders/humanms, and the interface between domestic exposures and humans have on avian influenza A(H5N6) transmission.
Note: This figure, a schematic of AIV A(H5N6) transmission from wild birds to humans, was completed using data from peer-reviewed published literature Footnote 19Footnote 21Footnote 24Footnote 26. It is not a comprehensive diagram and may contain incomplete information.
Global Epidemiology
Case Characteristics
While avian influenza A(H5N6) outbreaks in birds have been observed worldwide, avian-to-human transmission has only been detected in two (2) Asian countries. Since 2014, 66 human cases of A(H5N6) have been reported globally, all from China and Lao PDR. The median age of these cases was 50.5 years, with an age range of 1-81 years. Nine (9) (9/66, 14%) reported cases were children <18 years of age [Table 1]. Half (33/66; 50%) of the cases were males [Table 1]. At least 29 cases have died [case fatality rate (CFR): 44%] and of the cases with unknown outcome but available disposition data, 84% (21/25) were in critical or severe condition at the time of last report. All cases with known exposure data (53/53; 100%) reported contact with birds prior to illness onset. Of the 22 cases with reported occupational background, 82% (18/22) had obvious associations with agricultural work and/or poultry exposure as farmers (15/22; 68%) or dealers with contact with Live Bird Markets (LBMs) (3/21; 14%).

Table 1. Descriptive Characteristics of Cases by Sex and Age Groups, 2014 - January 31, 2022 - Text Equivalent
| Variable | Sex | Age Group | ||
|---|---|---|---|---|
| Males (n=33) | Females (n=33) | Children (<18 years old) (n=9) | Adults (≥18 years old) (n=57) | |
| Median Age (range) | 53 (3-75) | 47 (1-81) | 4 (1-11) | 52 (22-81) |
| Proportion Males (%) | N/A | N/A | 22 | 54 |
| Proportion Hospitalized (%) | 97 | 91 | 56 | 100 |
| Hospitalization Delay (Days) | 3 | 3.5 | 3 | 3 |
| Case Fatality Rate (CFR) (%) | 67 | 59 | 38 | 69 |
Note: 29 males, 32 females, 9 children, and 52 adults had available hospitalization data, with 28 males, 29 females, 5 children, and 52 adults reporting history of hospitalization. Hospitalization delay refers to the median number of days from symptom onset to hospitalization, for hospitalized cases that also had available symptom onset and hospitalization date data (23 males, 26 females, 4 children, and 45 adults). Fifteen males, 22 females, 8 children, and 29 adults had final outcome data that were used to determine CFRs. Missing outcome information may affect the CFR of reported groups. This table contains data on cases reported from 2014 to January 31, 2022.
Geographic Distribution
Approximately 98% (65/66) of human avian influenza A(H5N6) infections have been reported from China. One (1) case was reported from a bordering country, Lao PDR, in March 2021. Within China, the cases to date have been detected in 14 different regions: Hunan Province (14 cases), Guangdong Province (13 cases), Guangxi Zhuang Autonomous Region (12 cases), Sichuan Province (10 cases), Chongqing Municipality (3 cases), Anhui Province (2 cases), Jiangsu Province (2 cases), Yunnan Province (2 cases), Beijing Municipality (1 case), Guizhou Province (1 case), Hubei Province (1 case), Jiangxi Province (1 case), Fujian Province (1 case), and Zhejiang Province (2 cases) [Figure 5].

Figure 5. Spatial distribution of human cases of avian influenza A(H5N6) in China and Lao PDR, 2014-January 31, 2022. - Text Equivalent
Figure 5 is a map of China and Lao PDR demonstrating the spatial distribution of human cases of avian influenza A(H5N6) in China and Lao PDR from 2014 - January 31, 2022. Prior to 2021, human cases of A(H5N6) were reported from: Hunan Province, Sichuan Province, Guangdong Province, Yunnan Province, Jiangxi Province, Hubei Province, Anhui Province, Guangxi Zhuang. Autonomous Region, Fujian Province, Jiangsu Province, and Beijing Municipality.
From 2021 onwards, human cases of A(H5N6) were reported from: Chongqing Municipality, Guizhou Province, Anhui Province, Guangxi Zhuang Autonomous Region, Sichuan Province, Hunan Province, Guangdong Province, Zhejiang Province, and Luang Prabang Province.
Note: The cases included in this figure are cases reported up until January 31, 2022. Source: CIRID's IMAT.
The majority of these cases have been reported in south or southeast China, coinciding with a high density and popularity of LBMs coupled with free-range farming practices in this region, as well as an abundance of water resources that serve as habitats for AIV hosts Footnote 30. However, one (1) case was reported in northern China for the first time in 2019 [Figure 6] Footnote 20. This case, a 59-year-old female, developed illness after cooking poultry Footnote 20. One (1) case from Lao PDR was detected in a child from Luang Prabang Province, who also reported history of poultry exposure.
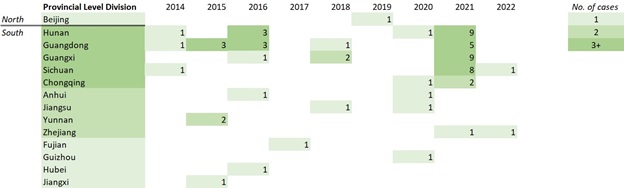
Figure 6. Geographic spread of human cases of avian influenza A(H5N6) in China by year of illness onset or report date, 2014-January 31, 2022. - Text Equivalent
Figure 6 outlines human cases of avian influenza A(H5N6) detected in provincial level divisions in China by year of illness onset (2014 - January 31, 2022). In 2014, Hunan, Guangdong, and Chongqing reported one (1) case each. In 2015, Guangdong reported three (3) cases and Yunnan reported two (2) cases. In 2016, Hunan and Guangdong reported three (3) cases each and Guangxi, Anhui, and Hubei reported one (1) case each. In 2017, Fujian reported one (1) case. In 2018, Guangdong and Jiangsu reported one (1) case each and Guangxi reported two (2) cases. In 2019, Beijing reported one (1) case. In 2020, Hunan, Chongqing, Anhui, Jiangsu, and Guizhou reported one (1) case each. In 2021, Hunan and uangxi reported nine (9) cases each, Sichuan reported eight (8) cases, Guangdong reported five (5) cases, Chongqing reported two (2) cases, and Zhejian reported one (1) case. In 2022, Sichuan and Zhejiang reported one (1) case each. Overall, Hunan, Guangdong, Guangxi, Sichuan, and Chongqing have all reported over three (3) cases, Anhui, Jiangsu, Yunnan, and Zhejiang have all reported two (2) cases, and Beijing, Fujian, Guizhou, Hubei, and Jiangxi reported one (1) case.
Note: Illness onset data were unavailable for three (3) cases; for these cases, report date was used to create this figure. The cases included in this figure are cases reported up until January 31, 2022. Source: CIRID's IMAT.
Timeline of Detections
The first report of human A(H5N6) infection was made on May 5, 2014, when China reported one (1) fatal A(H5N6) case in a poultry dealer from Sichuan Province. However, researchers retrospectively identified an A(H5N6) infection in a child who developed symptoms in February 2014, making her the first known human A(H5N6) case Footnote 32. This case was identified in Hunan Province, the region that has reported the highest number of cases (14/66; 21%) [Figure 6].
A marked increase in case incidence occurred in 2021, with nearly half of all cases (32/66; 48%) reported during this year [Figure 1]. Thirty-five cases (35/66; 53%) had symptom onset in 2021. These cases were reported from six (6) different regions in China [Figure 5] and A(H5N6) infected a resident in a different country (Lao PDR) for the first time, exhibiting greater geographic distribution than previous years. However, similar to prior cases, the cases with illness onset in 2021 had a median age of 54 years (age range: 3-75) and a comparable sex distribution (21/35; 60% males). Outcome data were available for 13 of the cases from 2021, of which ten (10/13; 77%) died. Human cases of A(H5N6) continue to be reported into 2022, with two (2) cases with illness onset in January 2022 notified as of January 31, 2022.
Although it has been postulated that an increase in cases may be observed in the winter and autumn, coinciding with influenza A seasonality in humans and avian migratory pathways, no seasonality was observed in the cases reported to date [Figure 7] Footnote 17. Cases are detected throughout the course of the year with a slight decrease in illnesses in the spring.
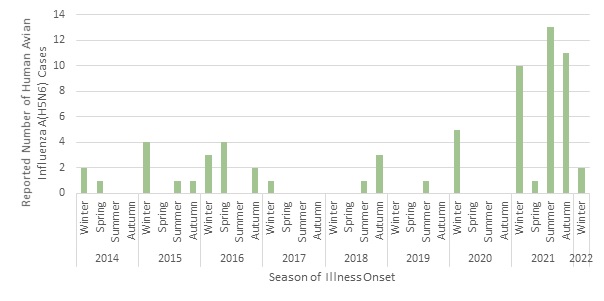
Figure 7. Epidemiologic curve of human avian influenza A(H5N6) infections, by season of illness onset or report date, 2014-January 31, 2022 (n=66). - Text Equivalent
| Season of Illness Onset | Reported Number of Human Avian Influenza A(H5N6) Cases |
|---|---|
| 2014 Winter | 2 |
| 2014 Spring | 1 |
| 2014 Summer | 0 |
| 2014 Autumn | 0 |
| 2015 Winter | 4 |
| 2015 Spring | 0 |
| 2015 Summer | 1 |
| 2015 Autumn | 1 |
| 2016 Winter | 3 |
| 2016 Spring | 4 |
| 2016 Summer | 0 |
| 2016 Autumn | 2 |
| 2017 Winter | 1 |
| 2017 Spring | 0 |
| 2017 Summer | 0 |
| 2017 Autumn | 0 |
| 2018 Winter | 0 |
| 2018 Spring | 0 |
| 2018 Summer | 1 |
| 2018 Autumn | 3 |
| 2019 Winter | 0 |
| 2019 Spring | 0 |
| 2019 Summer | 1 |
| 2019 Autumn | 0 |
| 2020 Winter | 5 |
| 2020 Spring | 0 |
| 2020 Summer | 0 |
| 2020 Autumn | 0 |
| 2021 Winter | 10 |
| 2021 Spring | 1 |
| 2021 Summer | 13 |
| 2021 Autumn | 11 |
| 2022 Winter | 2 |
Note: Illness onset data were unavailable for three (3) cases; for these cases, report date data were utilized to create this figure. Winter includes the months of December, January, and February; spring includes March, April, and May; summer includes June, July, and August; and autumn includes September, October, and November. The cases included in this figure are cases reported up until January 31, 2022 so Winter 2022 only includes data from one month (January 2022) instead of three (3). Source: CIRID's IMAT.
Clinical Course
Signs and Symptoms
Similar to other human infections with HPAI H5 viruses, the clinical manifestation of human cases of avian influenza A(H5N6) often begins with fever, upper respiratory tract symptoms, and myalgia. This manifestation rapidly progresses to lower respiratory tract illness, resulting in pneumonia, multiple organ failure, acute respiratory distress syndrome (ARDS), and oftentimes death Footnote 33. The diagram below [Figure 8] outlines the clinical course of cases detected to date.

Figure 8. Clinical course of human avian influenza A(H5N6) infections, 2014-January 31, 2022 (n=66). - Text Equivalent
Figure 8 outlines the clinical course of infection for human cases of avian influenza A(H5N6) reported up until January 31, 2022. Following an unspecified exposure period, all cases had illness onset or report date on day 1. Case 1 experienced illness onset on day 1 (2014-02-16) and outcome on day 4. Case 2 experienced illness onset on day 1 (2014-04-13), hospitalization and specimen collection on day 8, ICU admission on day 9, and outcome on day 11. Case 3 experienced illness onset on day 1 (2014-12-23). Case 4 experienced illness onset on day 1 (2015-01-27), hospitalization on day 8, specimen collection on day 10, and outcome on day 11. Case 5 experienced illness onset on day 1 (2015-07-06), hospitalization on day 4, and outcome on day 5. Case 6 experienced illness onset on day 1 (2015-10-20), hospitalization on day 9, and outcome on day 14. Case 7 experienced illness onset on day 1 (2015-12-24), hospitalization on day 4, and outcome on day 7. Case 8 experienced illness onset on day 1 (2015-12-22) and hospitalization on day 7. Case 9 experienced illness onset on day 1 (2016-01-01), hospitalization on day 4, and discharge on day 10. Case 10 experienced illness onset on day 1 (2015-12-12), hospitalization on day 8 and outcome on day 10. Case 11 experienced illness onset on day 1 (2016-01-08. Case 12 experienced illness onset on day 1 (2016-02-20) and hospitalization on day 3. Case 13 experienced illness onset on day 1 (2016-04-09) and hospitalization on day 4. Case 14 experienced illness onset on day 1 (2016-04-11) and hospitalization on day 2. Case 15 experienced illness onset on day 1 (2016-04-24) and hospitalization on day 4. Case 16 experienced illness onset on day 1 (2016-05-23) and hospitalization on day 6. Case 17 experienced illness onset and hospitalization on day 1 (2016-11-18) and outcome on day 3. Case 18 experienced illness onset on day 1 (2016-11-08) and hospitalization on day 11. Case 19 experienced illness onset on day 1 (2017-12-19). Case 20 experienced illness onset on day 1 (2018-08-01). Case 21 experienced illness onset on day 1 (2018-09-25). Case 22 experienced illness onset on day 1 (2018-10-18), hospitalization on day 4, and outcome on day 10. Case 23 experienced illness onset on day 1 (2018-10-29), hospitalization on day 6, and ICU admission on day 9. Case 24 experienced illness onset on day 1 (2019-08-06), hospitalization on day 6, and specimen collection on day 11. Case 25 experienced illness onset on day 1 (2020-11-16), outcome on day 12, and specimen collection on day 13. Case 26 experienced illness onset on day 1 (2020-12-08), hospitalization on day 6, and specimen collection on day 10. Case 27 experienced illness onset on day 1 (2020-12-18). Case 28 experienced illness onset on day 1 (2020-11-21). Case 29 experienced illness onset on day 1 (2020-12-22) and outcome on day 7. Case 30 experienced illness onset on day 1 (2021-02-16), specimen collection on day 12, and outcome on day 15. Case 31 experienced illness onset on day 1 (2021-02-28) and outcome on day 9. Case 32 experienced illness onset on day 1 (2021-05-13) and hospitalization on day 4. Case 33 experienced illness onset on day 1 (2021-06-30). Case 34 experienced illness onset on day 1 (2021-06-22) and hospitalization on day 14. Case 35 experienced illness onset on day 1 (2021-06-23). Case 36 experienced illness onse ton day 1 (2021-06-25), hospitalization on day 8, and outcome on day 10. Case 37 experienced illness onset on day 1 (2021-07-06). Case 38 experienced illness onset and hospitalization on day 1 (2021-07-13). Case 39 experienced illness onset on day 1 (2021-07-26) and hospitalization on day 5. Case 40 experienced illness onset and hospitalization on day 1. Case 41 experienced illness onset on day 1 (2021-07-31). Case 42 experienced illness onset and hospitalization on day 1 (2021-08-17). Case 43 experienced illness onset on day 1 (2021-08-25). Case 44 experienced illness onset on day 1 (2021-09-08) and hospitalization on day 2. Case 45 experienced illness onset on day 1 (2021-09-13) and hospitalization on day 6. Case 46 experienced illness onset on day 1 (2021-08-14) and hospitalization on day 6. Case 47 experienced illness onset on day 1 (2021-09-26). Case 48 experienced illness onset on day 1 (2021-09-26). Case 49 experienced illness onset on day 1 (2021-09-26) and hospitalization on day 2. Case 50 experienced illness onset on day 1 (2021-10-03) and hospitalization on day 11. Case 51 experienced illness onset on day 1 (2021-10-20) and hospitalization on day 2. Case 52 experienced illness onset on day 1 (2021-08-29) and hospitalization on day 3. Case 53 experienced illness onset on day 1 (2021-11-17), hospitalization on day 3, and outcome on day 7. Case 54 experienced illness onset on day 1 (2021-12-03) and hospitalization on day 5. Case 55 experienced illness onset on day 1 (2021-11-15) and hospitalization on day 2. Case 56 experienced illness onset on day 1 (2021-11-22) and hospitalization on day 4. Case 57 experienced illness onset on day 1 (2021-11-24) and hospitalization on day 6. Case 58 experienced illness onset on day 1 (2021-12-04). Case 59 experienced illness onset on day 1 (2021-12-04) and hospitalization on day 5. Case 60 experienced illness onset on day 1 (2021-12-04), hospitalization on day 4, and outcome on day 12. Case 61 experienced illness onse ton day 1 (2021-12-04) and hospitalization on day 9. Case 62 experienced illness onset on day 1 (2021-12-04) and hospitalization on day 4. Case 63 experienced illness onset on day 1 (2021-12-04) and hospitalization on day 5. Case 64 experienced illness onset and hospitalization on day 1 (2021-12-04). Case 65 experienced illness onset on day 1 (2021-12-04) and hospitalization on day 2. Case 66 experienced illness onset on day 1 (2021-12-04) and hospitalization on day 4.
Note: illness onset data were unavailable for three (3) cases; for these cases, report date data were utilized to determine Day 1 of case clinical course. Hospitalization, ICU admission, discharge, and outcome (death or survival) dates were unavailable for several cases Footnote 26. Source: CIRID's IMAT.
Disease Severity
The case fatality rate (CFR) for human infections with A(H5N6) to date is 44% (29/66). Among the 61 cases with available hospitalization data, 93% (57/61) required hospital admittance, further highlighting the severity of this disease. However, this percentage should be interpreted with caution given data can be subject to selection bias. It is possible that cases are tested more often when they are severe and/or hospitalized, leading to a higher proportion of hospitalizations since the denominator might not capture most mild or asymptomatic cases. Conversely, it is also possible that there is undercounting of community deaths, e.g., where people do not present to hospital at all and are not tested for avian influenza infections. Research suggests mild or asymptomatic illness is uncommon with A(H5N6) and less likely as compared with other AIVs Footnote 30. Intensive care unit (ICU) admission details were too sparse to draw conclusions.
Outcome data were only available for 56% of the cases (37/66). Of these individuals, 38% (14/37) survived their infection, while 62% (23/37) died. When comparing details of fatal cases to non-fatal cases, fatal cases were older, had a higher proportion of hospitalized cases, and had a greater delay in hospitalization [Table 2]. Researchers propose longer delays in antiviral administration may also contribute to increased mortality, and have emphasized the importance of timely influenza testing, antiviral treatment, and increased healthcare professional awareness to mitigate negative outcomes Footnote 25. Of the fatal cases with known comorbidity information, half (3/6; 50%) reported the presence of comorbidities, as opposed to one-third (1/3; 33%) of the non-fatal cases. No large difference was observed in the male to female ratio of fatal and non-fatal cases [Table 2].

Table 2. Clinical outcomes of laboratory-confirmed human cases of avian influenza A(H5N6), 2014-January 31, 2022 (n=37) - Text Equivalent
| Outcome of Illness | Number of Cases | Median Age (Age Range) | Male:Female Ratio | Proportion Hospitalized (%) | Median Time between Symptom Onset to Hospitalization (Days) |
|---|---|---|---|---|---|
| Alive | 14 | 30.5 (1-65) | 1:1.8 | 71 | 3 |
| Deceased | 23 | 49 (3-81) | 1:1.3 | 100 | 5 |
Note: 20/23 (87%) fatal cases and 7/14 (50%) non-fatal cases had available symptom onset and hospitalization date data. One (1) non-fatal case that had missing symptom onset data but available hospitalization data had available report date data. The median time from symptom onset to hospitalization (days) was calculated for these 20 fatal and 8 (7 with symptom onset date data; 1 with report date data) non-fatal cases. The cases included in this figure are cases reported up until January 31, 2022. Source: CIRID's IMAT.
Since the earliest human A(H5N6) cases were detected in 2014, no data are yet available on the long-term clinical effects of A(H5N6) infection in humans.
Public Health Actions
Therapeutics and Vaccines
Antiviral administration may also help treat human cases of A(H5N6), but researchers have emphasized that antivirals be administered in a timely manner to reduce adverse outcomes Footnote 25.
The WHO coordinates the preparation of candidate vaccine viruses (CVVs) that may be used by vaccine manufacturers to produce influenza vaccines. As part of the global strategy for pandemic preparedness, the WHO has selected A(H5N6) clade 2.3.4.4 virus for development as a CVV. Two A(H5N6) clade 2.3.4.4 CVVs have already been developed (2.3.4.4e A/duck/Hyogo/1/2016 and 2.3.4.4a A/Sichuan/26221/2014) and five (5) others are pending (34) (6). However, considering the ever-changing genetic profile of HPAI A(H5N6), it is important to update CVVs frequently Footnote 19.
Public Health Measures
Adhering to public health measures like regular thorough handwashing, staying home if you feel sick, and minimizing contact with wild, sick, and/or dead birds and contaminated and/or high-risk environments (e.g. LBMs) may protect you and others from A(H5N6) infection. The WHO recommends avoiding contact with high-risk environments like LPMs or contaminated surfaces Footnote 26. For those who cannot avoid contacting birds, e.g. poultry farmers or others with occupational exposure, the use of personal protective equipment (PPE) may help prevent influenza infection Footnote 35. During outbreaks or following exposure to infected birds, research also shows biosecurity measures like antiviral prophylaxis could prevent infections Footnote 35. Seasonal influenza vaccination may also help prevent co-infections of novel and seasonal influenza, thereby reducing the risk of reassortants.
At a national level, Canada has engaged in several pandemic preparedness activities that mitigate the likelihood and impact of novel influenza [e.g. A(H5N6)] infections in humans. For instance, PHAC has issued planning guidance for the health sector in the case of an influenza pandemic. PHAC can also employ its National Emergency Strategic Stockpile (NESS), which contains supplies like medical equipment and pharmaceuticals to provide provincial and/or territorial regions relief and support in the event of emergencies like influenza outbreaks.
Surveillance
In addition to the public health measures outlined above, global surveillance is recommended by the WHO to detect virological, epidemiological, and clinical changes associated with viruses like A(H5N6) that can affect human and animal health. As AIV A(H5N6) continues to circulate in bird populations and contaminate various environments, more detections of sporadic human cases of A(H5N6) are expected. Timely information sharing of these cases under the International Health Regulations (2005) remains key for human A(H5N6) infection risk assessment and mitigation Footnote 26.
Links and Resources
- PHAC's FluWatch surveillance
- PHAC's Avian Influenza Travel Health Notice
- PHAC's National Emergency Strategic Stockpile
- PHAC's Protocol for Microbiological Investigations of Severe Acute Respiratory Infections (SARI)
- PHAC's SARI Case Definition
- PHAC's SARI Case Report Form
- PHAC's biosecurity guidance for H5/H7/H9 influenzas
- PHAC's surveillance guidance for avian influenza (specifically H7N9, but applicable to other HPAI like H5N6) in humans
- PHAC's CPIP: Planning Guidance for the Health Sector
- PHAC's Avian Influenza (Bird Flu)
- Canadian Food Inspection Agency (CFIA)'s Avian Influenza (bird flu)
- US CDC's Information on Avian Influenza
- North American Plan for Animal and Pandemic Influenza (NAPAPI)
- OIE's Avian Influenza
- Food and Agriculture Organization of the United Nations (FAO)'s Avian Influenza
- WHO's Surveillance - Avian influenza
- WHO's Risk assessment summaries of influenza at the human-animal interface
References
Footnotes
- Footnote 1
-
Xing G, Gu J, Yan L, Lei J, Lai A, Su S, et al. Human infections by avian influenza virus H5N6: Increasing risk by dynamic reassortment? Infec Genet Evol 2016;42:46-48.
- Footnote 2
-
Li X., Yang J., Liu B., Jia Y., Guo J., Gao X., et al. Co-circulation of H5N6, H3N2, H3N8, and Emergence of Novel Reassortant H3N6 in a Local Community in Hunan Province in China. Sci Rep 2016;6:25549.
- Footnote 3
-
Bi Y., Liu H., Xiong C., Liu D, Shi W., Li M., et al. Novel avian influenza A (H5N6) viruses isolated in migratory waterfowl before the first human case reported in China, 2014. Sci Rep 2016;6:29888.
- Footnote 4
-
Su S., Bi Y., Wong G., Gray G.C., Gao G.F., Li S. Epidemiology, evolution, and recent outbreaks of avian influenza virus in China. J Virol 2015;89(17):8671-8676.
- Footnote 5
-
World Organisation for Animal Health. Update on Avian Influenza - OIE. 2017; Available at: http://www.oie.int/animal-health-in-the-world/update-on-avian-influenza/2016/. Accessed January/10, 2017.
- Footnote 6
-
World Health Organization. Assessment of risk associated with highly pathogenic avian influenza A(H5N6) virus. 2021.
- Footnote 7
-
Ma M.-J., Chen S.-H., Wang G.-L., Zhao T., Qian Y.-H., Wu M.-N., et al. Novel highly pathogenic avian H5 influenza a viruses in live poultry markets, Wuxi City, China, 2013-2014. Open Forum Infect Dis 2016;3(2):ofw054.
- Footnote 8
-
Zhang Z, Li R, Jiang L, Xiong C, Chen Y, Zhao G, et al. The complexity of human infected AIV H5N6 isolated from China. BMC Infect Dis 2016;16(1).
- Footnote 9
-
Bi Y., Chen Q., Wang Q., Chen J., Jin T., Wong G., et al. Genesis, Evolution and Prevalence of H5N6 Avian Influenza Viruses in China. Cell Host and Microbe 2016;20(6):810-821.
- Footnote 10
-
Shen HanQin, Wu BoLiang, Chen YiMin, Bi YingZuo, Xie Q. Influenza A(H5N6) virus reassortant, southern China, 2014. Emerging Infectious Diseases 2015;21(7):1261-1262. 11 ref.
- Footnote 11
-
Wong FY, Phommachanh P, Kalpravidh W, Chanthavisouk C, Gilbert J, Bingham J, et al. Reassortant highly pathogenic infuenza a(h5n6) virus in laos. Emerg Infect Dis 2015;21(3):511-516.
- Footnote 12
-
Wu H, Lu R, Peng X, Xu L, Cheng L, Lu X, et al. Novel reassortant highly pathogenic H5N6 avian influenza viruses in poultry in China. Infec Genet Evol 2015;31:64-67.
- Footnote 13
-
Jiao P, Cui J, Song Y, Song H, Zhao Z, Wu S, et al. New reassortant H5N6 highly pathogenic avian influenza viruses in Southern China, 2014. Front Microbiol 2016;7(MAY).
- Footnote 14
-
Yuan R, Wang Z, Kang Y, Wu J, Zou L, Liang L, et al. Continuing reassortant of H5N6 subtype highly pathogenic avian influenza virus in Guangdong. Front Microbiol 2016;7(APR).
- Footnote 15
-
Shen Y-, Ke C-, Li Q, Yuan R-, Xiang D, Jia W-, et al. Novel reassortant avian influenza a(H5N6) viruses in Humans, Guangdong, China, 2015. Emerg Infect Dis 2016;22(8):1507-1509.
- Footnote 16
-
He J., Liu B.-Y., Gong L., Chen Z., Chen X.-L., Hou S., et al. Genetic characterization of the first detected human case of avian influenza A (H5N6) in Anhui Province, East China. Sci Rep 2018;8(1):15282.
- Footnote 17
-
Chen P, Xie J, Lin Q, Zhao L, Zhang Y, Chen H, et al. A study of the relationship between human infection with avian influenza a (H5N6) and environmental avian influenza viruses in Fujian, China. BMC Infect Dis 2019 20190902;19(1):762.
- Footnote 18
-
Dong Z, Ya X, Wang D, Liu C, Shen Q, Xia Y. Genetic Characterization of a Novel Reassortant H5N6 Avian Influenza Virus Identified from a 10-Year-Old Girl. Jpn J Infect Dis 2020 20191031;73(1):36-43.
- Footnote 19
-
Xiao C, Xu J, Lan Y, Huang Z, Zhou L, Guo Y, et al. Five Independent Cases of Human Infection with Avian Influenza H5N6 - Sichuan Province, China, 2021. China CDC wkly 2021;3(36):751-756.
- Footnote 20
-
Yang L, Zhao X, Li X, Bo H, Li D, Liu J, et al. Case report for human infection with a highly pathogenic avian influenza A(H5N6) virus in Beijing, China 2019. Biosafety and Health 2020;2(1):49-52.
- Footnote 21
-
Yu J, Hou S, Feng Y, Bu G, Chen Q, Meng Z, et al. Emergence of a young case infected with avian influenza A (H5N6) in Anhui Province, East China during the COVID-19 pandemic. J Med Virol 2021 20210714;93(10):5998-6007.
- Footnote 22
-
Sun H, Pu J, Wei Y, Sun Y, Hu J, Liu L, et al. Highly Pathogenic Avian Influenza H5N6 Viruses Exhibit Enhanced Affinity for Human Type Sialic Acid Receptor and In-Contact Transmission in Model Ferrets. J Virol 2016 Jul 15;90(14):6235-6243.
- Footnote 23
-
World Organisation for Animal Health. World Animal Health Information System (WAHIS). 2021.
- Footnote 24
-
Zhang R, Lei Z, Liu C, Zhu Y, Chen J, Yao D, et al. Live poultry feeding and trading network and the transmission of avian influenza A(H5N6) virus in a large city in China, 2014-2015. Int J Infect Dis 2021 20210514;108:72-80.
- Footnote 25
-
Chen L, Huo X, Qi X, Liu C, Huang H, Yu H, et al. A fatal paediatric case infected with reassortant avian influenza A(H5N6) virus in Eastern China. Transbound Emerg Dis 2020 20200405.
- Footnote 26
-
World Health Organization. Posts: Influenza due to identified avian or animal influenza virus . 2018-2021.
- Footnote 27
-
CDC (Centers for Disease Control and Prevention). Information on Avian Influenza. March 21, 2019; Available at: https://www.cdc.gov/flu/avianflu/index.htm.
- Footnote 28
-
Government of Canada. Avian Influenza (Bird Flu). 2008; Available at: https://www.canada.ca/en/health-canada/services/healthy-living/your-health/diseases/avian-influenza-bird-flu.html.
- Footnote 29
-
Ryu S, Kim C-, Kim K, Woo SH, Chun BC. Serosurveillance of avian influenza A/H5N6 virus infection in poultry farmers, Gyeonggi Province, Republic of Korea, 2016-2017. Int J Infect Dis 2018;75:49-51.
- Footnote 30
-
Quan C, Wang Q, Zhang J, Zhao M, Dai Q, Huang T, et al. Avian Influenza A Viruses among Occupationally Exposed Populations, China, 2014-2016. Emerg Infect Dis 2019;25(12):2215-2225.
- Footnote 31
-
Ryu S, Lim J-, Cowling BJ, Chun BC. Low risk of avian influenza A (H5N6) transmission to depopulation workers in Korea. Influ Other Respir Viruses 2018;12(3):412-415.
- Footnote 32
-
Zhang R, Chen T, Ou X, Liu R, Yang Y, Ye W, et al. Clinical, epidemiological and virological characteristics of the first detected human case of avian influenza A(H5N6) virus. Infec Genet Evol 2016;40:236-242.
- Footnote 33
-
Bi Y, Tan S, Yang Y, Wong G, Zhao M, Zhang Q, et al. Clinical and Immunological Characteristics of Human Infections With H5N6 Avian Influenza Virus. Clin Infect Dis 2019;68(7):1100-1109.
- Footnote 34
-
World Health Organization. Summary of status of development and availability of A(H5) non-A(H5N1) candidate vaccine viruses and potency testing reagents. 2021; Available at: https://cdn.who.int/media/docs/default-source/influenza/cvvs/cvv-zoonotic---southern-hemisphere-2022/h5-non-h5n1_20210930.pdf?sfvrsn=cb6745e5_8&download=true.
- Footnote 35
-
Ryu S, Lim J-, Cowling BJ, Chun BC. Low risk of avian influenza A (H5N6) transmission to depopulation workers in Korea; 29236360. Influenza and other Respiratory Viruses 2018;12(3):412-415.
Page details
- Date modified: