Update on the use of pneumococcal vaccines in adults 65 years of age and older – A Public Health Perspective
An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI)
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada (hereafter referred to as the PHAC) with ongoing and timely medical, scientific, and public health advice relating to immunization. PHAC acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Table of contents
Summary of information contained in this NACI statement
The following highlights key information for immunization providers. Please refer to the remainder of the Statement for details.
1. What
Streptococcus pneumoniae (S. pneumoniae) is a bacterium that can cause many types of diseases including invasive pneumococcal disease (IPD), and community-acquired pneumonia (CAP).
For the prevention of diseases caused by S. pneumoniae in adults, two types of vaccines are available in Canada: pneumococcal 23-valent polysaccharide (PNEU-P-23) vaccine containing 23 pneumococcal serotypes and pneumococcal 13-valent conjugate (PNEU-C-13) vaccine containing 13 pneumococcal serotypes.
NACI has been tasked with providing a recommendation from a public health perspective on the use of pneumococcal vaccines in adults who are 65 years of age and older, following the implementation of routine childhood pneumococcal vaccine programs in Canada.

Download the alternative format
(PDF format, 956 KB, 93 pages)
Organization: Public Health Agency of Canada
Published: November 2018
Cat.: HP40-221/2018E-PDF
ISBN: 978-0-660-27296-2
Pub.: 180186
Related Topics
2. Who
Information in this statement is intended for provinces and territories (P/Ts) making decisions for publicly funded, routine, immunization programs for adults who are 65 years of age and older without risk factors increasing their risk of IPD. These recommendations supplement the recent NACI recommendations on this topic that were issued for individual-level decision making in 2016.
3. How
For routine, publicly funded, immunization programs for adults 65 years of age without other risk factors increasing their risk of IPD, NACI does not recommend the inclusion of PNEU-C-13 vaccine at its current price. One dose of PNEU-P-23 vaccine is recommended for all adults 65 years of age and older, regardless of risk factors or previous pneumococcal vaccination. Individual-level recommendations for PNEU-C-13 vaccine have been discussed in the 2016 NACI recommendations.
4. Why
The programmatic recommendation for adults age 65 years and older is based on the epidemiology of circulating S. pneumoniae serotypes causing IPD and CAP in Canada and the evidence of changing incidence of pneumococcal disease following the implementation of childhood PNEU C vaccination programs. Although there is clinical trial evidence for PNEU-C-13 vaccine efficacy in older adults for preventing IPD and CAP, currently within the Canadian context, such a publicly funded program would not significantly decrease the disease burden in a cost-effective manner.
I. Introduction
The objective of this Statement Update is to provide evidence and recommendations, from a public health perspective, for the use of pneumococcal vaccines for the prevention of community-acquired pneumonia (CAP) and invasive pneumococcal disease (IPD) in adults 65 years of age and older, without other risk factors increasing their risk of IPD.
This statement:
Updates the epidemiology of pneumococcal disease in those 65 years of age and older in Canada with regards to serotypes included in PNEU-P-23 and PNEU-C-13 using the most recently available national surveillance data (2015);
- Provides an update to the review of the literature on the use of PNEU-P-23 in adults;
- Provides an overview of the available literature on the changes observed internationally following the implementation of childhood PNEU-C programs on the general population of adults;
- Provides an economic analysis of pneumococcal vaccination for adults over 65 years of age; and
- Provides updated programmatic, population-level, recommendations for the use of pneumococcal vaccines in adults who are 65 years of age and older without other risk factors increasing their risk of IPD.
PNEU-P-23 vaccine is recommended for use in Canada for the prevention of IPD in adults who are 65 years of age and older. Since July 2015, PNEU-C-13 vaccine has been authorized for the prevention of IPD and CAP caused by the serotypes included in the vaccine, for all adults 18 years of age and older.
In 2016, in addition to recommending the use of PNEU-P-23 vaccine for all adults 65 years of age and older, NACI has recommended the use of PNEU-C-13 vaccine to individuals desiring additional protection against strains contained in the vaccine.(1) In addition to the individual-level recommendations that were developed in consideration of the existing evidence on vaccine safety, immunogenicity and efficacy, as part of its expanded mandate, NACI has also been tasked to provide a public health-level recommendation based on a comprehensive evaluation of programmatic factors including vaccine program cost-effectiveness and the impact on disease burden. The intent of the public-health level guidance is to support P/Ts in developing programmatic recommendations concerning the inclusion of PNEU-C-13 vaccine into the existing pneumococcal programs for adults 65 years of age and older. Previously published NACI Statements are available on the Government of Canada website.
National Vaccine Coverage Goals and Disease Reduction Targets have been set and endorsed by the Public Health Network council. For IPD, in individuals over the age of 65 years, a disease reduction target to less than 23.5 cases per 100,000 population per year (P-Y) by 2025 has been set(2).
II. Methods
NACI reviewed the key questions for the literature review, as proposed by the Pneumococcal Working Group (PWG), including such considerations as the burden of disease to be prevented and the target population(s), safety, immunogenicity, efficacy, effectiveness of the vaccine(s), vaccine schedules, indirect effects of concurrent vaccination program, and other aspects of the overall immunization strategy. Knowledge review and synthesis of studies published until April 10, 2017 was performed by two graduate students and technical advisors at PHAC, and supervised by the NACI PWG Chair.
The national surveillance data on IPD came from the Canadian Notifiable Disease Surveillance System (CNDSS). Data from 8 jurisdictions (BC, AB, SK, ON, QC, PEI, YK and NU) representing 90% of the Canadian population were used to perform the analyses. All cases were presumed to meet the national case definition for IPD. CNDSS data are limited by the lack of information on cases' risk factors, including immuno-competence and vaccination status; other data limitations are provided on the Government of Canada website.
The CNDSS cases and the National Microbiology Laboratory (NML) specimen data are not linked and have therefore been reported separately. To enhance comparability, only the NML serotype data for specimens submitted by 8 jurisdictions that provide national line level surveillance data (namely BC, AB, SK, ON, QC, PEI, YK and NU) were analyzed. The exception is the antimicrobial resistance data, for which specimens from all jurisdictions have been reported. The NML data are limited by reporting differences between jurisdictions and the availability of bacterial isolates submitted for testing; other NML data limitations are provided on the Government of Canada website. The following overall limitations of existing surveillance programs in Canada are noted:
- Nationally representative data are not currently available on the burden of all-cause CAP and vaccine type (VT) CAP in Canada;
- National surveillance data on vaccination status or additional risk factors (e.g. comorbidities) are not available for identified cases of IPD and VT IPD in Canada;
- Missing data are present within both the CNDSS and NML datasets.
The systematic review and meta-analysis by Kraicer-Melamed et al., 2016 (3, 4) was developed in consultation with the PWG and was used as the evidence base for decision-making by NACI. Since the publication of the systematic review by Kraicer-Melamed et al., a similar systematic review and meta-analysis was published by Falkenhorst et al., 2017(5), to examine the efficacy or effectiveness of PPV23 to prevent IPD and pneumococcal pneumonia (PP) in individuals aged 60 years and older. Further to this, a systematic review and meta-analysis by Htar et al., 2017, also assessed the vaccine efficacy of PNEU-P-23 and PNEU-C-13 against CAP. For comparative purposes, all systematic reviews and meta-analyses were appraised by the PWG using the AMSTAR measurement tool for the assessment of the methodological quality of systematic reviews. (6-8)
A narrative literature review on the indirect effects of routinely administered pneumococcal infant immunization programs on the incidence of pneumococcal disease in adults was also performed.
To guide protection against pneumococcal disease at the population level, recommendations were made in consideration of the Erickson-De Wals framework for immunization programs in Canada.(9) Following thorough review of the evidence and consultation at the NACI meetings of September 27-28, 2017 and February 7-8, 2018, the committee voted on specific recommendations. The description of relevant considerations, rationale for specific decisions, and knowledge gaps are described in the text.
III. Epidemiology
S. pneumoniae can spread from person to person via droplets from the nose or mouth, by sneezing or coughing. Although asymptomatic upper respiratory tract colonization is common, infection with S. pneumoniae can cause many types of diseases, with IPD and non-invasive pneumococcal community acquired pneumonia (NIpCAP) being the most common in adults. IPD is a severe form of infection that occurs when the bacteria invade normally sterile sites, such as the bloodstream or central nervous system, leading to bacteremia and meningitis. Certain conditions predispose individuals to diseases that are caused by S. pneumoniae, including sickle-cell disease and other hemoglobinopathies, chronic renal failure, chronic liver disease, immunosuppression, anatomic or functional asplenia, cerebrospinal fluid leaks, diabetes mellitus, and HIV infection. There are currently over 90 serotypes recognized worldwide, 15 of which are known to cause the majority of pneumococcal disease. (10-12)
National surveillance data on cases meeting the national IPD case definition are routinely collected through CNDSS. The NML provides data for isolates submitted by provincial and territorial public health laboratories including Laboratoire de santé publique du Québec (LSPQ), the Alberta Provincial Laboratory for Public Health (ProvLab) and the Toronto Invasive Bacterial Diseases Network (TIBDN). Though national laboratory and epidemiologic data are not linked at the case level, it is estimated that approximately 75% of specimens from cases reported through CNDSS are provided to NML for testing. Information about IPD and CAP in hospitalized adults 65 years of age and older, including vaccination history and immune status, is collected through the Serious Outcomes Surveillance (SOS) Network of the Canadian Immunization Research Network (CIRN).
Detailed epidemiological information on IPD in Canada is provided on the Government of Canada website.
III.1 Disease distribution by age group
Following the initial NACI recommendation in 1989, all Canadian provinces and territories have implemented PNEU-P-23 vaccination programs for adults who are 65 years of age and older. In addition, all Canadian provinces and territories have implemented routine childhood PNEU-C vaccination programs between 2002 and 2006 (Table 1), with the majority of P/Ts currently using a 3-dose PNEU-C-13 vaccination schedule.(13-27) PNEU-C-13 vaccine has been recommended by NACI as a preferred product for childhood programs since 2010, and was included in all provincial and territorial (P/T) pediatric vaccination programs by 2011.
| P/T | Year of routine PNEU-C-7 program introduction | Year of routine PNEU-C-10 program introduction | Year of routine PNEU-C-13 program introduction |
|---|---|---|---|
| BC | September 2003 | N/A | June 2010 |
| AB | September 2002 | N/A | July 2010 |
| SK | April 2005 | N/A | July 2010 |
| MB | October 2004 | N/A | July 2010 |
| ON | January 2005 | December 2009 | November 2010 |
| QC | December 2004 | June 2009 | January 2011 |
| NL | March 2005 | October 2009 | September 2010 |
| NB | April 2005 | N/A | July 2010 |
| NS | January 2005 | N/A | July 2010 |
| PE | June 2003 | N/A | September 2010 |
| YT | June 2005 | N/A | May 2011 |
| NT | January 2006 | September 2009 | September 2010 |
| NU | April 2002 | N/A | September 2010 |
Since the introduction of pediatric PNEU-C programs in Canada, a 2.6-fold decrease in IPD incidence was observed in children less than 5-years-old between 2002 and 2006 (from 39.1 to 15.3 cases per 100,000 population), followed by an additional 1.6-fold reduction between 2010 and 2015 (17.7 to 10.8 cases per 100,000 population). Among older age groups, the impact of routine pediatric immunization programs on IPD has been more modest, with reductions in PNEU-C-13 serotypes being offset by an increase in non-vaccine serotypes and serotypes that are unique to the PNEU-P-23 vaccine (Figure 1, Figure 3).

Figure 1.
Figure 1 shows incidence rate of IPD (vertical axis) in cases per 100,000 population with respect to year (horizontal axis) in shown yearly increments from years 2001 to 2015. Text boxes provide additional timeline information concerning Health Canada's PNEU-C-7 NOC issuance (2001), implementation (2002-2006) and PNEU-C-13 implementation (2010-2012). Data points are based on Canadian Notifiable Disease Surveillance System data.
Surveillance results are plotted in distinct individual colors, differentiating between age brackets. Age brackets are represented as: < 5 (purple), 5 to 17 (light blue); 18 to 49 (teal); 50 to 64 (green) and > 65 (red).
The age bracket with the highest incidence rate variance is shown to be the < 5 bracket. This plot exhibits concave properties during its first five years (2001-2005) with an incidence rate of ~29 cases per 100,000 population (2001), increasing to a global maximum of ~42 cases per 100,000 population (2003). The incidence rate then decreases steadily down to a local minimum of ~16 cases per 100,000 population (2006), bouncing back to a local maximum of ~21 cases per 100,000 population (2009), to finally progressively decrease toward its global minimum of ~11 cases per 100,000 population.
Both 50 to 64 and > 65 age brackets exhibit similar trends, although the > 65 bracket's incidence rate hovers ~12.5 cases per 100,000 population higher than the 50-64 age bracket. The 50-64 age bracket display a slow but steady increasing trend from ~6 to ~14 cases per 100,000 population (2001 to 2007), stabilizing around ~12.5 cases per 100,000 population during the following years. The > 65 age bracket display a somewhat steeper increase in incidence rate from ~13 to ~25 cases per 100,000 population (2001 to 2004), stabilizing around ~25 cases per 100,000 population during the following years.
The two lowest incidence age brackets are shown to be the 5 to 17 and 18 to 49 brackets, which exhibit rates < 5 cases per 100,000 population, with the exception of years 2006 to 2008, where the 18 to 49 age bracket displayed slightly higher incidenceincidence rate (~6 cases per 100,000 population).
In adults 65 years of age and older, the annual incidence rate of IPD has decreased non-significantly from 25.1 cases per 100,000 population (pop.) in 2011 to 23.6 cases per 100,000 pop. in 2015 (p>0.05). In this age group, the largest decrease has been observed in adults 85 years of age and older, while in other age groups incidence has remained unchanged. (Figure 2)

Figure 2.
Figure 2 shows incidence rate of IPD (vertical axis) in cases per 100,000 population with respect to year (horizontal axis) in shown yearly increments from year 2011 to 2015 Data points are based on Canadian Notifiable Disease Surveillance System data.
Surveillance results are plotted in distinct individual colors, differentiating between age brackets. Age brackets are represented as: 65-74 (yellow), 75 to 84 (green) and 85+ (red). In 85 years and older age group, the largest decrease has been observed (~50 to ~42 cases per 100,000 population), while other age groups display incidence rate stability: ~18.5 cases per 100,000 population among 65-74 age bracket; ~28 cases per 100,000 population among 85+ age bracket.
III.2 Disease distribution by serotype
Based on the data from isolates submitted to NML, the proportion of PNEU-C-13 vaccine serotypes has been decreasing in all age groups since the introduction of routine pediatric programs.(28) When comparing the number of specimens containing PNEU-C-13 vaccine serotypes in 2011 with the number in 2015, a reduction of approximately 30% has been observed in adults 65 years of age and older (Figure 3).

Figure 3.
| YEAR | PNEU-C-13 (Number; % of total) | PNEU-P-23* (Number; % of total) | NVT (Number; % of total) | Total (Number) |
|---|---|---|---|---|
| 2011 | 357; 43% | 196; 24% | 268; 33% | 821 |
| 2012 | 327; 40% | 240; 29% | 250; 30% | 818 |
| 2013 | 300; 34% | 309; 35% | 285; 32% | 895 |
| 2014 | 230; 27% | 297; 35% | 326; 38% | 855 |
| 2015 | 245; 27% | 309; 34% | 340; 38% | 896 |
| Total | 1459; 34% | 1351; 31% | 1469; 34% | 4285 |
*excluding serotypes contained in PNEU-C-13 XXX
| YEAR | PNEU-C-13 | PNEU-P-23* | NVT | Total |
|---|---|---|---|---|
| 2011 | 357 | 196 | 268 | 821 |
| 2012 | 327 | 240 | 250 | 818 |
| 2013 | 300 | 309 | 285 | 895 |
| 2014 | 230 | 297 | 326 | 855 |
| 2015 | 245 | 309 | 340 | 896 |
| Total | 1459 | 1351 | 1469 | 4285 |
During the same period, there has been an approximately 50% observed increase in the number of specimens with unique serotypes contained in PNEU-P-23 and a 25% increase in NVT serotypes. Detailed information about IPD specimens by serotype for adults 65 to 74 years of age is provided in Table 3.
| Vaccine product | Serotype | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|
| PNEU-C-13 | 19A | 116 | 107 | 98 | 67 | 67 |
| 3 | 88 | 85 | 86 | 76 | 89 | |
| 7F | 85 | 63 | 63 | 46 | 26 | |
| 19F | 15 | 8 | 9 | 11 | 19 | |
| 4 | 11 | 17 | 12 | 6 | 11 | |
| 6A | 14 | 14 | 7 | 8 | 6 | |
| 14 | 7 | 7 | 6 | 4 | 7 | |
| 6B | 7 | 7 | 8 | 3 | 4 | |
| 23F | 4 | 6 | 5 | 7 | 7 | |
| 18C | 2 | 5 | 4 | 2 | 3 | |
| 9V | 7 | 4 | 2 | 0 | 2 | |
| 1 | 1 | 2 | 0 | 0 | 2 | |
| 5 | 0 | 2 | 0 | 0 | 2 | |
| PNEU-P-23 (*excluding serotypes contained in PNEU-C-13) |
22F | 84 | 106 | 116 | 111 | 103 |
| 11A | 23 | 30 | 33 | 39 | 48 | |
| 9N | 22 | 15 | 40 | 30 | 30 | |
| 8 | 15 | 22 | 35 | 25 | 32 | |
| 15B | 10 | 11 | 16 | 14 | 27 | |
| 12F | 15 | 9 | 13 | 14 | 19 | |
| 20 | 4 | 8 | 16 | 21 | 20 | |
| 10A | 6 | 13 | 13 | 11 | 15 | |
| 17F | 7 | 9 | 3 | 12 | 5 | |
| 2 | 0 | 0 | 0 | 0 | 0 | |
| NVT | 15A | 36 | 48 | 54 | 58 | 73 |
| 23A | 44 | 44 | 41 | 51 | 50 | |
| 6C | 52 | 45 | 46 | 39 | 35 | |
| 16F | 12 | 26 | 24 | 35 | 22 | |
| 23B | 18 | 19 | 16 | 31 | 26 | |
| 35B | 18 | 13 | 29 | 21 | 29 | |
| 35F | 13 | 14 | 14 | 24 | 26 | |
| 31 | 10 | 9 | 12 | 20 | 14 | |
| 38 | 14 | 6 | 18 | 7 | 14 | |
| 15C | 9 | 11 | 8 | 8 | 6 | |
| 34 | 12 | 4 | 5 | 3 | 13 | |
| Others† | 30 | 11 | 18 | 29 | 32 |
In adults 65 years of age and older, among serotypes that are contained in the conjugate vaccines, the most prevalent were those unique to the PNEU-C-13 vaccine (i.e. 3, 7F and 19A). With the exception of serotype 3 (ST3), a declining trend for serotypes contained in the PNEU-C-13 vaccine has been observed since 2011. (Table 3, Figure 4). Similar trends have also been reported by other international jurisdictions that have implemented routine pediatric PNEU-C vaccine programs. (29-35)

Figure 4.
| YEAR | 19A | 3 | 7F | Others |
|---|---|---|---|---|
| 2011 | 32% | 25% | 24% | 19% |
| 2012 | 33% | 26% | 19% | 22% |
| 2013 | 33% | 29% | 21% | 18% |
| 2014 | 29% | 33% | 20% | 18% |
| 2015 | 27% | 36% | 11% | 26% |
III.3 Disease distribution by antimicrobial resistance (AMR)
Following the introduction of PNEU-C vaccine programs in Canada, an overall decline in AMR pneumococci has been observed concomitant with the decline in PNEU-C vaccine contained serotypes, including the multi-drug resistant serotype 19A.(12, 36) Between 2011 and 2014, resistance of S. pneumoniae to penicillin decreased from 12% to 9% and resistance to clindamycin declined from 7% to 4%. Over the same period, resistance to three or more classes of antimicrobials has also declined from 8% to 5%. Resistance to clarithromycin, which can be used in community-acquired pneumonia (CAP), decreased from 25% in 2012/13 to 22% in 2014. Resistance to doxycycline and trimethoprim/sulfamethoxazole has remained relatively stable around 8% and 6%, respectively (Figure 5). All isolates tested between 2011 and 2014 have shown susceptibility to vancomycin, ertapenem, linezolid, and tigecycline.

Figure 5.
Figure 5 (clustered bar graph) shows the percentage of antimicrobial resistance (AMR) from isolates of S. pneumonia submitted to NML from 2011 (dark blue), 2012 (orange); 2013 (green); 2014 (purple) and 2015 (teal). The different clusters are identified as such: AUG = amoxicillin/clavulanic acid; PENm = penicillin using the parenteral meningitis CLSI interpretive standard;; PENn = penicillin using the parenteral non-meningitis interpretive standard; PENo = penicillin using the oral penicillin V interpretive standard; LEV = levofloxacin; MOX = moxifloxacin; AXOm = ceftriaxone using the parenteral meningitis interpretive standard; AXOn = ceftriaxone using the parenteral non-meningitis interpretative standard; FURo = cefuroxime using the oral interpretative standard; FURp = cefuroxime using the parenteral interpretative standard; ETP = ertapenem; IMI = imipenem; MER = meropenem; CLA = clarithromycin; CLI = clindamycin; CHL = chloramphenicol; DOX = doxycycline; SXT = trimethoprim/sulfamethoxazole.
Between 2011 and 2014, resistance of S. pneumoniae to penicillin decreased from 12% to 9% and resistance to clindamycin declined from 7% to 4%. Over the same period, resistance to three or more classes of antimicrobials has also declined from 8% to 5%. Resistance to clarithromycin, which can be used in community-acquired pneumonia (CAP), decreased from 25% in 2012/13 to 22% in 2014. Resistance to doxycycline and trimethoprim/sulfamethoxazole has remained relatively stable around 8% and 6%, respectively. All isolates tested between 2011 and 2014 have shown susceptibility to vancomycin, ertapenem, linezolid, and tigecycline.
*Data from NML. The data is limited by reporting differences between jurisdictions; variability in sample sizes amongst the smaller jurisdictions that result in small counts representing large relative proportions; and the availability of bacterial isolates submitted for testing; other data limitations of NML are provided at https://www.canada.ca/en/public-health/services/publications/drugs-health-products/national-laboratory-surveillance-invasive-streptococcal-disease-canada-annual-summary-2014.html.
III.4 CAP
Using SOS Network data from five provinces, Leblanc et al.(37) reported on CAP incidence in hospitalized adults from December 2010 to December 2013. CAP caused by S. pneumoniae was identified through sputum culture, commercial pan-pneumococcal urine antigen detection (UAD) or a serotype-specific UAD. Over the course of the study, 14.3% (549/3851) of all-cause CAP was found to be caused by S. pneumoniae when any of the diagnostic tests were used. Among serotypable specimens (384/549), 70.1% (269/384) were serotypes included in the PNEU-C-13 vaccine. Of all S. pneumoniae CAP captured during the study period, there was an observed decline in the proportion of PNEU-C-13 serotypes from 72.9% in 2011 to 63.5% in 2013. In adults 65 years of age and older, the proportion of all-cause CAP that was caused by serotypes contained in PNEU-C-13 vaccine decreased from 15.5% in 2011 to 10.8% in 2013. Among individuals included in the study, approximately a third had an immunocompromising condition.
III.5 Immunization coverage
In 2014, national immunization coverage for PNEU-P-23 among immunocompetent adults 65 years of age and older was estimated to be 36.5% (95% CI: 32.7 - 40.3).(38)
For 2015, national immunization coverage for PNEU-C in children at the age of 2 years was estimated to be 80.3% (95% CI: 75.1 - 84.7).(39)
IV. Vaccine
IV.1 Preparations authorized for use in Canada
Two preparations of pneumococcal vaccine for use in adults are available in Canada and are described in past NACI statements.(1, 40-42)
PNEU-P-23 (Pneumovax®23) is a sterile solution of 23 highly purified capsular polysaccharides (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F). PNEU-P-23 is available as a 3 ml single-dose vial containing 0.5 ml dose of liquid vaccine and a 1.5 ml prefilled syringe containing 0.5 ml dose of liquid vaccine.
PNEU-C-13 (Prevnar®13) is a sterile solution of polysaccharide capsular antigen of 13 serotypes of S. pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F). The antigens are individually conjugated to a diphtheria CRM197 protein carrier. The CRM197 protein carrier is adsorbed on aluminum phosphate as an adjuvant. Each dose of vaccine contains 4.4 mcg of the 6B polysaccharide, and 2.2 mcg each of the remaining polysaccharides. PNEU-C-13 is marketed in a single dose, prefilled 1ml syringe containing 0.5mL of vaccine.
A comprehensive list of vaccine contents is available online within the Canadian Immunization Guide.
IV.2a Efficacy - Direct
PNEU-P-23 vaccine
IPD
A Cochrane review of studies published up to June 2012 identified 5 RCTs that recruited 27,886 otherwise healthy adults living in high-income countries. The study authors reported evidence of protective efficacy against IPD for <2 to 6 years following immunization, with a vaccine efficacy of 80% odds ratio (OR: 0.20; 95% CI 0.10 to 0.39; random-effects model, I2 = 0%).(7) An additional trial conducted by Hönkanen et al.in Finland estimated PNEU-P-23's efficacy in preventing IPD among individuals 65 years of age and older to be 60% risk ratio (RR: 0.40; 95% CI: 0.10 - 1.90).(98)
CAP
A Cochrane review of 6 RCTs published up to June 2012 and involving 29,186 adults from high-income countries found a pooled estimated vaccine efficacy against all cause pneumonia of 29% (OR: 0.71; 95% CI 0.45 to 1.12; I2=93). The systematic review analysis conducted by Kraicer-Melamed et al.(4, 44) was similarly not able to find conclusive evidence for vaccine efficacy of PNEU-P-23 in preventing CAP, reporting a pooled vaccine efficacy of three trials to be -10% (95% CI: -36% - 12).
PNEU-C-13 vaccine
Vaccine efficacy was reported for a mean follow-up time of 3.97 years(45). For overall VT CAP, vaccine efficacy was estimated to be 45.6% (95% CI: 21.8, 62.5) and for non-bacteremic VT CAP 45.0% (95% CI: 14.2, 65.3). For VT IPD vaccine efficacy was estimated to be 75.0% (95% CI: 41.4, 90.8). Please refer to the previous NACI statement for additional information on PNEU-C-13 efficacy in the general population of adults who are 65 years of age and older.(1)
IV.2b Effectiveness - Direct and Indirect
The effectiveness of PNEU-P-23 and PNEU-C-13 vaccines in preventing pneumococcal disease (IPD and CAP) was evaluated on the basis of data obtained from published systematic reviews(4, 5, 7, 8, 44) and a review of studies summarized in a previous NACI statement.(46) The following table (Appendix A) summarizes the AMSTAR score for each systematic review assessed.
Several differences in study inclusion and exclusion criteria resulted in different summary estimates for PNEU-P-23 vaccine effectiveness against IPD. Most notably, Kraicer-Melamed et al.(4) excluded studies of patients with significant underlying immunocompromising medical conditions or residents of nursing homes or assisted living settings as these were not considered a reasonable representation of disease transmission and health outcomes for the general population. Kraicer-Melamed et al. did not exclude studies with observational designs, and they did not include populations <50 years old. Inclusion and exclusion criteria for the referenced systematic reviews are detailed in Appendix B.
Direct effects
PNEU-P-23 vaccine
IPD
The review of data from eight cohort and four case-control studies conducted by Kraicer-Melamed et al. estimated pooled vaccine effectiveness for IPD to be 50% (95% CI: 21%-69%) for cohort studies and 54% (95% CI: 32%-69%) for case-control studies.(4) Stratification by method of diagnosis, time since vaccination (2 weeks up to 5 years), and quality indicated that estimates were largely unaffected by these additional factors.
In addition, a Cochrane review(47-51) of 5 non-RCT studies that included adults over 55 years of age reported a vaccine effectiveness of 68% for all IPD (OR: 0.32; 95% CI 0.22 to 0.47; random-effects model; I2 =18%, P = 0.30).(47-51) However, the pooled analysis included data from trials that used polysaccharide vaccines of lower valency (i.e. 14-valent vaccines) or vaccines for which valency was not specified.
The literature search also found three recently conducted systematic reviews. A meta-analysis by Falkenhorst et al.(5) reported PNEU-P-23's vaccine effectiveness against all IPD of 58% (OR: 0.42; 95% CI: 0.28 to 0.62; I2 =11%) based on data from 5 cohort studies and of 45% (OR: 0.55; 95% CI: 0.35 to 0.85 I2 =0%) when two cohort studies with high risk of bias were excluded. For case-control studies vaccine effectiveness was reported at 59% (OR: 0.41; 95% CI: 0.26 to 0.65; I2=60%) for all pneumococcus and 73% (OR: 0.27; 95% CI: 0.16 to 0.44; I2 =0%) for VT IPD based on pooled data from 3 and 2 studies, respectively. Data from four indirect cohort studies was also analyzed by the study authors, who estimated vaccine effectiveness against VT IPD at 37% (OR: 0.63; 95%CI 0.55 to 0.73; I2 =0%).
CAP
The literature search identified three recently published systematic reviews. The analysis conducted by Kraicer-Melamed et al.(3) provided pooled vaccine effectiveness estimates for all cause CAP. Based on data from nine cohort studies(48, 49, 52-58) and seven case-control studies(47, 59-64), pooled vaccine effectiveness for all-cause CAP was estimated at 17% (95% CI: -26%-45%) and 7% (95% CI: -10%-21%), respectively. Additional stratifications by method of diagnosis, time since vaccination, and study quality did not affect the overall conclusion of no statistically significant effect of PNEU-P-23 vaccination on the prevention of CAP.
A study by Falkenhorst et al. provided a pooled estimate for vaccine effectiveness in preventing pneumococcal pneumonia(PP) based on two cohort studies to be 48% [95% CI: 25-63%, I2 = 0%]) and one case-control study with a vaccine effectiveness against PP of 53% (95% CI 33-68%).(5) Htar et al.(65) also conducted a systematic review of 33 observational studies that reported vaccine effectiveness results on the protection for any clinically relevant outcome other than IPD. Depending on the conducted analysis, in adults 65 years of age and older, the study authors reported wide ranges of vaccine effectiveness (-143% to 60%). Presence of pediatric PNEU-C vaccine programs and time since vaccination were found to significantly (p<0.01) influence PNEU-P-23 vaccine effectiveness, with diversity of study populations, circulation of S. pneumoniae serotypes and case definition further explaining very high between-study heterogeneity (I2 = 99.24%, p < 0.01). The reported meta-analyzed vaccine effectiveness estimate for any-CAP requiring hospitalization in the general population was 10.2% (95%CI: -12.6; 33.0) and -6.31 (95% CI: -15.78; 3.17, I2 = 60%) in countries with a national childhood PNEU-C immunization program. In the stratified meta-analysis by maximum time since vaccination, vaccine effectiveness was 32.6% (95%CI: -5.9; 71.1, I2 = 99%) and 2.4% (95%CI: -5.4; 10.1, I2 = 65%) when the time since vaccination was less than 60 months and 60 months or more, respectively.
PNEU-C-13 vaccine
There were no publications identified describing direct effectiveness of PNEU-C-13 at the time of the literature search. With the ACIP's recommendation to use PNEU-C-13 in all adults aged 65 years and older, vaccine effectiveness data should become available in the future as the effect of vaccine in that population is analysed. (1)
Indirect effects of routinely administered infant immunization programs with PNEU-C vaccine on pneumococcal disease
There is evidence that the use of conjugate vaccines in children can generate herd effects in adults. As demonstrated through the evaluation of mass meningococcal vaccine programs, such an effect has been observed for meningococcal conjugate vaccines, but not for polysaccharide vaccines.
To better understand prospective changes in IPD, VT IPD, CAP, and VT CAP in individuals 50 years of age and older, a narrative review of the literature describing incidences prior to and following the implementation of childhood PNEU-C programs was conducted by the PWG.
IPD
Changes in adult (18 years of age and older) IPD incidence following infant PNEU-C program introduction in unvaccinated populations were recently reported in a systematic review and meta-analysis of 142 studies published between January 1994 and Jan 2016.(66) Data were available from 27 high-income and seven middle-income countries, including studies that reported on the impact of infant PNEU-C immunization programs in Canada. Using a random-effects model to estimate vaccine effectiveness over time, the authors predicted a 90% reduction in 8.5 years (95% CI 5.7- 19.7) for the additional 6 serotypes following a switch from PNEU-C-7 to PNEU-C-13 vaccine programs. The model showed similar decreases in IPD due to PNEU-C vaccine serotypes in adults aged 19-64 years and adults aged 65 years and older.
The PWG also conducted a review of 10 Canadian studies that reported on serotype specific IPD incidence in immunocompetent adults after PNEU-C infant program introduction in Alberta, Ontario, British Columbia and Quebec, including data from the CIRN SOS Network, which included 45 hospitals (18,000 beds) in 7 provinces. Based on empirical evidence (period 2000-2014) and theoretical considerations, Zhou et al.(67) predicted future trends in IPD rates in Quebec adults 65-74 years of age in the context of childhood PNEU-C vaccine programs. According to the multivariate Poisson regression model, proportion of PNEU-C-13 serotypes (including ST3) in IPD cases was expected to reach 20% (95% CI: 15% to 28%) in 2024. The study authors also conducted a sensitivity analysis in which, depending on the impact of PNEU-C-13 vaccine on ST3 IPD, the proportion of PNEU-C-13 types in overall IPD was expected to range from 23% [8% to 52%] to 18% [6% to 49%].
Kellner et al. analyzed over 1,150 IPD samples from adults 65 years of age and older in Calgary following the initiation of the provincial infant PNEU-C-7 program in 2002 (68). From the1998/2001 to 2003/2007 period, there was an observed 78% reduction in PNEU-C-7 serotypes in the 65-85 year-old age group (22.1 to 4.8/100,000 P-Y). The trend of decreasing IPD incidence among Calgary adults in the same age group was further documented by Leal et al. based on over 1,400 IPD cases reported from 1998 through 2010. (69) The authors observed a near eradication (2.2/100,000 P-Y) of PNEU-C-7 serotype IPD cases in the 65 years of age and older age group, with no reported PNEU-C-7 serotype cases in individuals 85 years and over in 2010. Sahni et al. reported similar trends following the introduction of PNEU-C-7 in British Columbia. (70) The proportion of PNEU-C-7 serotypes in samples submitted to the BC Centre for Disease Control from 2002 through 2010 significantly decreased by 94% (2.7 to 0.1 per 100,000 P-Y, p<0.01) in the 17-64 age group and by 91% (9.6 to 0.7 per 100,000 P-Y, p<0.01) in persons over 64 years of age. The most recent regional data analysis by Cabaj et al. (71) showed marked declines in adult IPD rates 10 years following the childhood PNEU-C program introduction. Compared to the pre-introduction rates (2000-2002 vs. 2010-2013), there was a 36% reduction in IPD in adults 65-84 years of age, and a 42% reduction in adults 85 years of age and older. The study authors noted a near-elimination of PCV7-serotype IPD in adults 65 years of age and older, particularly in immunocompetent individuals.
Two studies using TIBDN data from 1995 to 2011 reported consistent decreases in adult IPD after childhood PNEU-C program implementation.(72, 73) Rudnick et al., and Lim et al. reported an 88% reduction (95% CI, 93-78%) in IPD due to PNEU-C-7 serotypes among adults 15-64 years of age and an 89% reduction (95% CI, 94-80%) in adults over 65 years of age. During the same period, the incidence of IPD due to serotypes included in PNEU-P-23 but not in PNEU-C-13 remained stable. Desai et al.(74) also reported on IPD surveillance data from Ontario between 2007 and 2014. Based on data from over 3,800 adults aged 65 years and over, there was a 20% annual decrease in the incidence of PNEU-C-7 serotypes (from 3.0 to 0.7 cases per 100,000). A significant decrease (p < 0.001) in incidence was also observed for PNEU-C-13 serotypes 4 years after its introduction into the routine childhood program (9.8 to 5.3 per 100,000). For serotypes unique to PNEU-P-23, the study authors observed a significant increase (p < 0.001) in incidence over the study period, from 2.3 cases per 100 000 in 2007 to 5.8 cases per 100,000 in 2014.
Decreases in the proportion of IPD cases attributable to PNEU-C VT serotypes in Quebec were reported by LSPQ.(75) Based on the data from 21 sentinel hospitals which report approximately a third of all IPD cases among children < 5 years of age in Quebec, only one case (1.2% of total cases) of PNEU-C-7 and 10 cases (12.3% of all cases) of PNEU-C-13-unique serotype were reported in 2014, compared to 20 cases (26.3%) of PNEU-C-7 and 23 cases (30.3%) of PNEU-C-13-unique serotype reported in 2006.
National data from IPD samples submitted to the NML three years after the introduction of the PNEU-C-13 vaccine programs in Canada were reported by Demczuk et al. In this time period, the study authors observed an overall PNEU-C-13 serotype decrease from 50% to 39% among individuals 65 years of age and older (p<0.001).(76)
CAP
The literature search identified fourteen studies that reported on CAP incidence rates among adults following the implementation of childhood PNEU-C programs. A decrease in CAP incidence was observed in all studies beginning three years after program initiation.
A study by Nelson et al.(77) from the Group Health Cooperative in Washington State evaluated the impact of infant PNEU-C-7 immunization on pneumonia in approximately 800,000 members in the first 2 years post-childhood PNEU-C program introduction in year 2000. Pneumonia episodes were identified in 17,513 outpatient and 6,318 hospitalized events using diagnostic codes and confirmed chest radiograph reports or hospitalization records.
For the 65-74 year age group, study authors observed an increase in incidence rate ratios of confirmed hospitalized pneumonia (IRR 1.3; 95%CI: 1.12-1.5) and decrease in confirmed outpatient pneumonia (IRR 0.97; 95%CI: 0.88-1.08). Using the clinical discharge diagnosis from approximately 20% of all US hospital admissions, Grijalva et al.(78) compared the impact of PNEU-C-7 program during the 4 years after its introduction in the US. For the 65 years of age and older individuals, the study authors estimated a 20% hospital admission rate reduction for PP (from 73.9 to 59.3/100,00; 14.6/100,000 [95% CI: 2.0-27.6]) and a 15% hospital admission rate reduction for all-cause pneumonia (from 2,559 to 2,162/100,000; 396.5/100,000 [95% CI: 60.9-774.1]).
Simonson et al.(79) used Healthcare Cost and Utilization Project State Inpatient Databases from 10 states to evaluate the impact of PNEU-C-7 infant program introduction on PP hospitalizations. Compared to a pre-program baseline incidence, the authors observed a 54% (95% CI: 53-56) reduction in non-bacteremic PP in adults ≥ 65 years of age, six years after program implementation.
Griffin et al.(80) also analyzed the impact of PNEU-C-7 introduction on hospitalization for all cause pneumonia in the Nationwide Inpatient Sample database. The authors reported a 6.6% (95% CI: 0.5-12.7) reduction in all cause pneumonia in the 65-74-year-old age group 7 to 9 years after the infant PNEU-C-7 program introduction. Simonsen et al.(81) also used the IMS Charge Data Master hospital database that collects information from approximately 500 non-federal, short-stay hospitals (20% of all US hospital admissions) to analyze the impact of the infant PNEU-C-13 program introduction on non-invasive pneumococcal or lobar pneumonia two years following the PNEU-C-13 infant program introduction. For adults 65 years of age and older, the study authors estimated a 34% (95% CI: 27-41) decline in hospital admissions.
Decreasing incidence was also observed among individuals over 65 years of age in two studies in the UK and one study from Australia. Rodrigo et al.(82) reported on differences in VT PP 3 to 5 years following PNEU-C-13 childhood program introduction. An observed decrease in incidence of PNEU-C-13 VT CAP was reported in individuals aged 65-74 years of age (23.2 to 12.5 per 100,000) 3 years following PNEU-C-13 adoption. In this age group, the study authors estimated the annual change in rate ratio for pneumococcal CAP, CAP due to serotypes contained in PNEU-C-7 and CAP due to additional serotypes contained in PNEU-C-13 to be 0.84 (0.80-0.89), 0.52 (0.43-0.62) and 0.87 (0.80-0.95), respectively. Nair et al.(83) analyzed hospital records and death certification datasets from Scotland for the three-year period following PNEU-C-13 introduction in 2010. Compared to the incidence pre PNEU-C-7 program introduction in 2006, study authors found a 21.4% (95%CI: 42.9-7.1) decrease in hospital admissions for PP in the 65-74-year age group. Menzies et al.(84) evaluated the impact of infant PNEU-C-7 program on adult pneumonia hospitalization up to 6.5 years following program introduction, analyzing the Institute of Health and Welfare National Hospital Morbidity Database. For pneumococcal and lobar pneumonia in individuals 65-74 years of age, the study authors reported a post (2005-2011) vs. pre (1998-2004) program implementation incidence rate ratio of 0.86 (95% CI: 0.74-0.99).
Decrease in adult CAP following infant PNEU-C program introduction was also reported in studies from Japan, Nicaragua, Taiwan, Germany and Poland(85). In a single hospital study in Taiwan, among adults over 65 years of age, Lin et al.(86) observed a 64.1% reduction (95% CI: 13.3-115%) in non-bacteremic PP hospitalizations within three years of childhood program implementation (from 51 to 18 cases per 100,000 hospitalizations, p=0.009).
In a study from Poland, Patrzalek et al.(87)reported on the changes of all-cause pneumonia incidence among individuals 65 years of age and older following the implementation of a national childhood PNEU-C-7 program. The study authors reported a 56% reduction in incidence four years after the program (from 1,939 to 1,095 cases per 100,000 population). Katoh et al.(88) reported on the changes in the incidence of PP in Japan using data polled through a systematic review. In comparison to pre-program period, one to three years following the childhood PNEU-C-7 program implementation, study authors found an 18.1% decrease (95% CI: 24.6, 11.5%) in the total proportion of PNEU-C-7 serotypes.
Akata et al. also reported changes in PP rates from 2011 to 2015. During this period, there was a significant decrease in vaccine serotypes from 46.4% to 8.3% (p < 0.05) for PNEU-C-7 serotypes and from 71.4% to 33.3% (p < 0.05) for PNEU-C-13 serotypes. Pletz et al..(85), using the data from the CAPNETZ study in Germany, studied the impact of PNEU-C-7 program introduction in 2007 on the incidence of adult CAP (mean age, 58 years). The proportion of patients with PNEU-C-7 serotype non-bacteremic PP decreased from 31.3% to 14.8% in the four years following PNEU-C-7 program implementation. Becker-Dreps(89) reported changes (pre/post introduction) due to pneumonia two years after PNEU-C-13 program introduction in Nicaragua as IRR of 0.81 (95% CI: 0.61-1.06) for ambulatory visits and 2.07 (95% CI: 1.84-2.33) for hospitalizations.
In the Netherlands, van Werkoven et al.(90) reported incidence trends for non-IPD pneumococcal CAP caused by PNEU-C-13 types and non-PNEU-C-13 serotypes among patients 65 years of age and older who were recruited into the CAP-pilot and CAPITA studies. In 270 samples that were available for analysis, the proportion of PNEU-C-7 serotypes in non-IPD PP decreased linearly from 28% in 2008/2009 to 7% in 2012/2013 (p<0.001), 7 years after PNEU-C-7 childhood program introduction.
No statistically significant changes in the proportion of additional strains contained in PNEU-C-10 or PNEU-C-13 were observed over this time period.
Information on the impact of infant PNEU-C program introduction was also available from Shigayeva et al.(91)who analyzed the Ontario TIBDN data for non-bacteremic PP six years following the implementation of the provincial childhood PNEU-C-7 immunization program. The median age of adults included in the study was 64 years, with 46.6% being 65 years of age and older. The study authors reported a 24.6% (95% CI: 15-35.2%) reduction in non-bacteremic PP between 2003 and 2011.
IV.3 Immunogenicity
Information on PNEU-P-23 and PNEU-C-13 vaccine immunogenicity has previously been detailed in the NACI statement on PNEU-C-13 for individuals(41).
IV.4 Vaccine administration and schedule
Detailed information on PNEU-P-23 and PNEU-C-13 vaccine administration and recommended schedules is available in the Pneumococcal vaccine chapter of the Canadian Immunization Guide.
IV.5 Serological testing
Routine pre- or post-immunization serology for pneumococcal vaccines is not indicated.
IV.6 Storage requirements
Please refer to the Pneumococcal vaccine chapter of the Canadian Immunization Guide for information regarding storage of pneumococcal vaccines.
IV. 7 Simultaneous administration with other vaccines
Please refer to the Pneumococcal vaccine chapter of the Canadian Immunization Guide for information regarding simultaneous administration of pneumococcal vaccines with other vaccines.
IV.8 Adverse events
Adverse events (AE) following administration of PNEU-P-23 and PNEU-C-13 have been reported in previous NACI statements on PNEU-C-13(1, 40, 41) and in the Pneumococcal vaccine chapter of the Canadian Immunization Guide.
A recently published study by Miller et al.(92) evaluated the post-licensure safety of PNEU-P-23 (Pneumovax®23 only) using data from the US Vaccine Adverse Event Reporting System from 1990-2013. The authors identified injection site erythema, pain, and swelling as the most commonly reported adverse events among adults 18 years of age and older following administration of PNEU-P-23 and concluded that the evaluation revealed no novel or surprising results.
IV.9 Contraindications and precautions
Please refer to the previous statement and the Canadian Immunization Guide for more information on contraindications and precautions for PNEU-P-23 and PNEU-C-13.
IV.10 Economic analysis
Under the Erickson-De Wals framework, PWG evaluated a static model from previous work that was developed to simulate IPD and non-invasive pneumococcal community-acquired pneumonia (NIpCAP) epidemiology in the age-group 65 to 74 years of age.(93) PWG compared two different strategies:(i) one dose of PNEU-P-23 at age 65 years, and (ii) PNEU-C-13 at age 65 years followed by PNEU-P-23 one year later. Program costs included vaccine and administration costs, with the vaccine price differential in the base model set at $55 per dose, in favor of PNEU-P-23. Demographic and epidemiological parameters used in the model were extracted from published studies, surveillance and administrative databases originating from Quebec and Ontario.
Future trends in IPD and NIpCAP incidence and serotype distribution (2015-2024) were modelled using surveillance data from Quebec and considering the assumed serotype replacement and the indirect effects of PNEU-C-13 vaccine use in children. Benefits of vaccination included reduction in outpatient and emergency room visits, hospitalizations, long-term sequelae from meningitis and mortality, as well as improvement in quality of life. Direct disease costs to the healthcare system, including costs to individuals for treatment of disease were considered, but not indirect costs resulting from work absenteeism and productivity losses. All benefits and costs were discounted at a 3% annual rate with a lifetime horizon and incremental cost-effective ratios (ICER) expressed as $CAD/QALY.
The reference population was 100,000 persons 65 to 74 years of age, followed over a lifetime, with mutually exclusive outcomes associated with pneumococcal infections including IPD (meningitis, bacteremia-septicemia, bacteremic pneumonia, and other clinical presentations) and NIpCAP. PNEU-C-13 effectiveness values against VT-IPD and VT-NIpCAP in the 65-74 years age group were derived from results of the CAPITA trial in the Netherlands and PNEU-P-23 effectiveness values against VT-IPD were derived from the Cochrane review of randomized clinical trials and from a case-control study in the US. PNEU-P-23 effectiveness against NIpCAP was assumed to be zero. Forecasted changes in serotype distribution were based on Quebec LSPQ (Laboratoire de Sante Publique du Quebec) data (using 2000-2014 data to predict serotype distribution until 2024), which predicted the continued shifting of IPD burden from PNEU-C vaccine towards non-vaccine serotypes. Information on input parameters used in the base-case and sensitivity analyses is provided in Table 4 and Table 5.
| Model parameters | Base-case | Sensitivity analyses | References |
|---|---|---|---|
| Epidemiology | |||
| IPD incidence | 22.2/100,000 p-y | x 0.5 to x 2 | MED-ECHO Quebec(1-5) |
| Hospitalized CAP incidence | 333.4/ 100,000 p-y | x 0.5 to x 2 | MED-ECHO Quebec(1-5) |
| Proportion of CAP non- hospitalized | 40% | 20% to 70% | |
| Vaccine | |||
| PNEU-C-13 effectiveness | Table 5 | x 0.8 to x 1.2 (limit=100%) | (6) |
| PNEU-P-23 effectiveness against IPD | Table 5 | x 0.8 to x 1.2 (limit=100%) | (8, 7) |
| PNEU-P-23 effectiveness against NIpCAP | Table 5 | Table 5 | (8, 7, 9) |
| Economics | |||
| Vaccine price difference PNEU-C-13 (minus) PNEU-P-23 | $55 | $0 to $55 | Quebec Ministry of Health and Social Services, written communication, 2016 |
| Administration cost | $16 | $0 to $30 | (10) |
| Discounting rate annual | 3% | 0 to 6% | (11) |
- M. Jackson, O. Yu, C.G. Whitney, L. Bounds, R. Bittner, et coll. « Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults », Vaccine, 2008, 26(38) : 4947-54.
- Morrow, A., De Wals P., Petit G., Guay M. et L.J. Erickson. « The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine », Canadian Journal of Infectious Diseases and Medical Microbiology, 2007, 18(2) : 121-7.
- JOKINEN, C., L. HEISKANEN, H. JUVONEN, S. KALLINEN, K. KARKOLA, M. KORPPI, et coll. « Incidence of community-acquired pneumonia in the population of four municipalities in Eastern Finland », American Journal of Epidemiology, 1993, 137(9) : 977-88.
- JOKINEN, C., L. HEISKANEN, H. JUVONEN, S. KALLINEN, M. KLEEMOLA, M. KOSKELA, et coll. « Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland », Clinical Infectious Diseases, 2001, 32(8) : 1141-54.
- MARRIE, T.J., D.E. LOW, E. DE CAROLIS, R. DUPERVAL, S. FIELD, T. LOUIE, et coll. « A comparison of bacteremic pneumococcal pneumonia with nonbacteremic community-acquired pneumonia of any etiology - Results from a Canadian multicentre study », Canadian Respiratory Journal, 2003, 10(7) : 368-74.
- CCNI. Une déclaration du comité consultatif (DCC). Déclaration sur l'utilisation du vaccin conjugué contre le pneumocoque 13-valent chez l'adulte (Pneu-C-13). RMTC, 2013, 39 (DCC -5) : 1-52.
- Honkanen, P.O., T. Keistinen, L. Miettinen, E. Herva, U. Sankilampi, E. Läärä, et coll. « Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia and pneumococcal pneumonia among persons aged 65 years or older », Vaccine, 1999, 17(20-21) : 2493-500.
- Mise à jour sur l'utilisation des vaccins conjugués contre le pneumocoque chez les enfants [Internet], Canada, ASPC, 2010 [mis à jour en nov. 2010; recensé le 2 nov. 2017]. Disponible à l'adresse https://www.canada.ca/fr/sante-publique/services/rapports-publications/releve-maladies-transmissibles-canada-rmtc/numero-mensuel/2010-36/releve-maladies-transmissibles-canada-3.html.
- Menzies, R.I., S.H. Jayasinghe, V.L. Krause, C.K. Chiu et P.B. McIntyre. « Impact of pneumococcal polysaccharide vaccine in people aged 65 years or older », Medical Journal of Australia, 2014, 200(2) : 112-5.
- Link-Gelles, R., T. Taylor et M.R. Moore. « Forecasting invasive pneumococcal disease trends after the introduction of 13-valent pneumococcal conjugate vaccine in the United States, 2010-2020 », Vaccine, 2013, 31(22) : 2572-7.
- Pneumococcal Polysaccharide (PNEUMO-P) Vaccine [Internet], Alberta, Canada, Alberta Health Services, 2016 [mis à jour le 6 juillet 2017; recensé le 6 déc. 2017]. Disponible à l'adresse https://myhealth.alberta.ca/Alberta/Pages/immunization-pneumococcal-polysaccharide.aspx
| Years since vaccination | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| VT-IPD | ||||||||||
| PNEU-C-13 | 76% | 76% | 76% | 76% | 76% | 63% | 51% | 38% | 25% | 13% |
| PNEU-P-23 | 72% | 72% | 72% | 64% | 64% | 64% | 51% | 38% | 26% | 13% |
| PNEU-P-23 (sensitivity analysis) | 72% | 72% | 72% | 64% | 64% | 0% | 0% | 0% | 0% | 0% |
| VT-NIpCAP | ||||||||||
| PNEU-C-13 | 46% | 46% | 46% | 46% | 46% | 38% | 31% | 23% | 15% | 8% |
| PNEU-P-23 (base-case model) | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| PNEU-P-23 (sensitivity analysis) | 30% | 24% | 18% | 12% | 6% | 0% | 0% | 0% | 0% | 0% |

Figure 6.
| Variable/Uncertainty | Low | Up |
|---|---|---|
| % S. pneumonia among NIpCAP [0.5; 0] | $39,005.00 | $231,535.00 |
| CAP incidence [x2 to x0.5] | $32,599.00 | $98,836.00 |
| Discount rate [0%; 6%] | $45,571.00 | $77,288.00 |
| Ratio of PCV13-types in NIpCAP/IPD [1.0 to 0.6] | $54,931.00 | $81,017.00 |
| IPD incidence [x2 to 0.5] | $46,645.00 | $71,720.00 |
| Administration cost [$0 to $30] | $48,107.00 | $72,873.00 |
| PCV13 effectiveness against VT-NIpCAP [x1.2 to x0.8] | $52,462.00 | $72,332.00 |
| PPV23 effectiveness against VT-IPD [x0.8 to x1.2] | $54,919.00 | $70,048.00 |
| PPV23 effectiveness against VT-NIpCAP [not; effective] | $60,977.00 | $68,597.00 |
| Case-fatality outcomes [x1.2 to x0.8] | $57,765.00 | $64,568.00 |
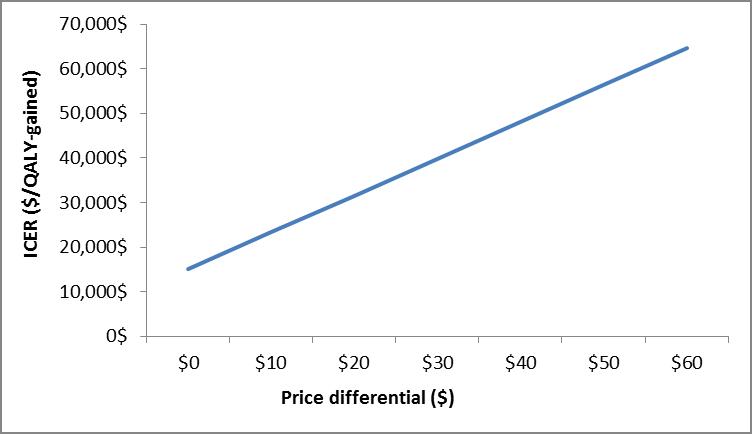
Figure 7.
| ICER ($/QALY-gained) | Price Differential ($) |
|---|---|
| $15,020.00 | $0.00 |
| $23,275.00 | $10.00 |
| $31,531.00 | $20.00 |
| $39,786.00 | $30.00 |
| $48,041.00 | $40.00 |
| $56,296.00 | $50.00 |
| $64,552.00 | $60.00 |
IV.11 Summary considerations
In adults 65 years of age and older, following the introduction of routine pediatric PNEU-C programs, the proportion of CAP and IPD caused by serotypes that are contained in PNEU-C-7 and PNEU-C-13 vaccines have been decreasing. The PNEU-C-7 vaccine strains in IPD have almost disappeared in the elderly population, with the similar trend being observed for PNEU-C-13; a 30% reduction has been observed in the first five years following pediatric PNEU-C-13 program implementation, with a more than 90% reduction in PNEU-C-13 vaccine containing strains expected to occur by 2019(66).
The only exception is ST3, persisting with approximately 180 cases per year in adults over 65 years of age. This is likely a result of greater circulation in this age group, in part due to decreased effectiveness of PNEU-C-13 in children against carriage and the low effectiveness of PNEU-P-23 vaccine against ST3. Among adults 65 years of age and older, serotypes not contained in the currently available vaccines as well as those unique to PNEU-P-23 vaccine continue to be the most important contributors to the IPD burden of illness in Canada.
NACI has therefore continued to recommend one dose of PNEU-P-23 vaccine for all adults 65 years of age and older. Comprehensive recommendations for the use of PNEU-P-23 are provided in the CIG.
According to 2015 data, in adults 65 years of age and older, approximately 30% of IPD cases and 10% of all-cause CAP requiring hospitalization are caused by PNEU-C-13 serotypes.
In immunocompetent adults aged 65 years and older, both PNEU-C-13 and PNEU-P-23 vaccines have been shown to be safe, immunogenic and effective against IPD. Comparative immunogenicity studies between PNEU-C-13 and PNEU-P-23 vaccine have indicated that antibody levels are higher in elderly subjects vaccinated with PNEU-C-13 for 8 serotypes that are common to both vaccines, but the clinical and population-level implications associated with this improved immunogenicity remain unclear.(102, 103) PNEU-C-13 vaccine has also been shown to be moderately efficacious against NIpCAP caused by the serotypes included in the vaccine.
Based on the model that was developed by De Wals et al. and with an assumed 50% vaccine coverage, introducing the PNEU-C-13 vaccine at 65 years of age could potentially annually prevent up to 35 cases of IPD and up to 250 cases of community-acquired pneumonia, of which 150 are hospitalized (Table 6). At the current price, adding PNEU-C-13 vaccine to routine immunization programs for adults 65 years and older was not found to be cost effective, despite the likely overestimates in the vaccine-preventable pneumococcal disease burden that was used in the De Wals et al. model (Table 6). At a stable vaccine cost, given the declining incidence of PNEU-C-13 serotypes in IPD and CAP as a result of pediatric PNEU-C-13 vaccine programs, the cost-effectiveness of PNEU-C-13 program in adults will likely decline over time. Despite the reduction in the proportion of PNEU-C-13 vaccine types, the IPD burden is likely to remain stable or increase over time as a result of replacement. The effect on overall NIpCAP remains unknown due to the lack of nationally representative data on outpatient CAP.
| Program options | Considerations (N=100,000) |
|---|---|
| PNEU-P-23 only at 65 years of age versus no vaccination |
Number (percentage) of IPD cases averted= 85 (33.8%) Number (percentage) of IPD deaths averted= 18 (33.8%) Number (percentage) of CAP cases averted= 0 (0%) Number (percentage) of CAP hospitalizations averted= 0 (0%) Number (percentage) of CAP deaths averted= 0 (0%) (0%) Program cost = $2.7 M Estimated cost savings to health system (medical cost only)= $688,391 Incremental Cost Effectiveness Ratio= $10,148/QALY saved |
| PNEU-C-13 and PNEU-P-23 (1 year apart) at 65 years of age versus PNEU-P-23 at 65 years of age |
Number (percentage) of IPD cases averted= 17 (9.9%) Number (percentage) of IPD deaths averted= 3 (9.9%) Number (percentage) of CAP cases averted= 125 (8.1%) Number (percentage) of CAP hospitalizations averted= 76 (8.1%) Number (percentage) of CAP deaths averted= 8 (8.1%) Difference in program cost = $8.1 M Estimated cost savings to health system (medical cost only)= $710,717 Incremental Cost Effectiveness Ratio= $63,318/QALY saved |
| Options PNEU-P-23 vs. PNEU-C-13 and PNEU-P-23 |
Considerations | Decision Points |
|---|---|---|
| PNEU-P-23 alone | Epidemiology:
|
Epidemiology |
| PNEU-C-13 and PNEU-P-23 |
Epidemiology:
ICER: ~$49,000/QALY to $63 000/QALY, compared to PNEU-P-23 only; using the epidemiology from 2015 - increasing ICER with decrease in circulating PNEU-C- 13 serotypes. |
V. Recommendations
In summary, based on the review of the literature and Canadian epidemiological data, PNEU-P-23 vaccine is a safe and effective tool in preventing IPD in immunocompetent adults over 65 years of age, while evidence on the effectiveness in preventing CAP remains inconclusive. The available Canadian epidemiological data indicate that the burden of IPD among individuals 65 years of age due to PNEU-C-13 serotypes is decreasing, but the burden of IPD caused by unique PNEU-P-23 serotypes and those not included in any currently available vaccine remains substantial.
Further studies on PNEU-P-23 and PNEU-C-13 vaccine effectiveness, the Canadian burden of S. pneumoniae disease, the impacts of childhood PNEU-C programs, and the burden of CAP in Canada will be needed to guide future recommendations for adults over 50 years of age.
Recommendation 1:
NACI recommends that PNEU-P-23 vaccine should be offered in routine immunization programs for all adults age 65 years and older for the prevention of IPD (Strong NACI recommendation).
NACI concludes that there is good evidence to recommend immunization (Grade B Evidence)
This recommendation is based on the results of the systematic review on PNEU-P-23 vaccine efficacy and effectiveness in preventing IPD, incidence of circulating IPD serotypes in Canada, and the evidence of changing incidence of pneumococcal disease following the implementation of childhood PNEU-C vaccination programs. The current IPD epidemiology and the results from systematic reviews and meta-analyses suggest that PNEU-P-23 may be as effective as PNEU-C-13 in preventing IPD in the general population of adults 65 years of age and older.
The evidence for the effectiveness of PNEU-P-23 in the prevention of CAP is however conflicting. Findings from systematic reviews and meta-analyses indicate that PNEU-P-23 may or may not be effective in preventing CAP in the general population of adults over 65 years of age.
Recommendation 2:
NACI recommends that PNEU-C-13 vaccine should not be publicly funded in routine immunization programs for adults 65 years of age and older without other risk factors increasing their risk of IPD (Strong NACI recommendation), unless PNEU-C-13 price decreases. NACI considered disease burden, herd immunity effects and the results of economic evaluation.
NACI concludes that there is fair evidence to recommend against immunization (Grade D Evidence)
This recommendation for adults aged 65 years and older, without other risk factors increasing their risk of IPD is based on the epidemiology of circulating serotypes causing IPD and CAP in Canada and the evidence of changing incidence of pneumococcal disease following the implementation of childhood PNEU C vaccination programs. Although there is clinical trial evidence for PNEU-C-13 vaccine efficacy in older adults for preventing CAP, within the Canadian context, such a publicly funded program would not significantly decrease the disease burden in a cost- effective manner.
IV. Research priorities
- Direct comparison of vaccine efficacy of PNEU-P-23 and PNEU-C-13 via randomized controlled trial among the general population of adults 65 years of age and older looking at the outcomes of IPD, VT IPD, CAP, and VT CAP
- Assessment of the herd effects of PNEU-C childhood programs over time on the incidence of IPD, VT IPD, CAP, and VT CAP and on carriage within the Canadian population of individuals 65 years of age and older
- Estimates of the vaccine effectiveness of PNEU-C-13 in the general population of individuals 65 years of age and older
- Assessment of program PNEU-C-13 vaccine effectiveness in additional specific population groups (e.g. Indigenous populations)
IIV. Surveillance issues
- Nationally representative data is not currently available on the burden of CAP and VT CAP in Canada
- National surveillance data on vaccination status are not available for identified cases of IPD and VT IPD in Canada, which limits extension of findings
- Additional risk factors (e.g. comorbidities) are not available for identified cases of IPD and VT-IPD, which limits extensions of findings to high-risk groups due to underlying health conditions
Missing data was present within both the CNDSS and NML datasets.
Tables
| Level | Description |
|---|---|
| I | Evidence from randomized controlled trial(s). |
| II-1 | Evidence from controlled trial(s) without randomization. |
| II-2 | Evidence from cohort or case-control analytic studies, preferably from more than one center or research group using clinical outcome measures of vaccine efficacy. |
| II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments (such as the results of the introduction of penicillin treatment in the 1940s) could also be regarded as this type of evidence. |
| III | Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
| Quality Rating |
Description |
|---|---|
| Good | A study (including meta-analyses or systematic reviews) that meets all design- specific criteria* well. |
| Fair | A study (including meta-analyses or systematic reviews) that does not meet (or it is not clear that it meets) at least one design-specific criterion* but has no known "fatal flaw". |
| Poor | A study (including meta-analyses or systematic reviews) that has at least one design-specific* "fatal flaw", or an accumulation of lesser flaws to the extent that the results of the study are not deemed able to inform recommendations. |
-
General design specific criteria are outlined in Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001;20:21-35.
| STRENGTH OF NACI RECOMMENDATION |
STRENGTH OF EVIDENCE |
|---|---|
| Based on factors not isolated to strength of evidence (e.g. public health need) |
Based on assessment of the body of evidence |
| Strong "should/should not be offered"
|
A - good evidence to recommend |
| B - fair evidence to recommend | |
| C - conflicting evidence, however other factors may influence decision-making | |
| D - fair evidence to recommend against | |
| E - good evidence to recommend against | |
| I - insufficient evidence (in quality or quantity), however other factors may influence decision-making | |
| Discretionary "may be considered"
|
A - good evidence to recommend |
| B - fair evidence to recommend | |
| C - conflicting evidence, however other factors may influence decision-making | |
| D - fair evidence to recommend against | |
| E - good evidence to recommend against | |
| I - insufficient evidence (in quality or quantity), however other factors may influence decision-making |
| Evidence for Safety for PNEU-P-23 | ||||||
|---|---|---|---|---|---|---|
| STUDY DETAILS | SUMMARY | |||||
| Study | Vaccine | Study Design |
Number of Participants |
Summary of Key Findings Using Text or Data |
Level of Evidence |
Quality |
| Miller et al., 20161 |
PPVS23 (Pneumovax®23)n | Case-series: surveillance system of AEs | Vaccine AE Reporting System (VAERS) in the USA received 25168 reports of AEs among individuals of all ages, between 1990-2013. | Among adults ≥19 years of age (21586 reports), injection site erythema (n=6119 [31%]), injection site pain (n=5161 [26%]), and erythema (n=4498 [23%]) were the most commonly reported non-serious AEs. The most commonly reported serious AEs among individuals who were ≥19 were pyrexia (770 [44%]), injection site erythema (520 [30%]), and cellulitis (515 [29%]). | Level III | N/A - data from a surveillance system |
-
Miller ER, Moro PL, Cano M, Lewis P, Bryant-Genevier M, Shimabukuro TT. Post-licensure safety surveillance of 23-valent pneumococcal polysaccharide vaccine in the Vaccine Adverse Event Reporting System (VAERS), 1990-2013. Vaccine. 2016 May 27;34(25):2841-6
| Evidence for efficacy for PNEU-P-23 in preventing IPD | ||||||
|---|---|---|---|---|---|---|
| STUDY DETAILS | SUMMARY | |||||
| Study | Vaccine | Study Details |
Participants | Summary of Key Findings Using Text or Data |
Level of Evidence |
Quality |
| Honkanen et al., 19991 | Pneu-P-23 Influenza | RCT Finland, initiated in 1992 Study open to all persons living in 35 northern districts Participant follow-up (vaccinated) 38,037 person years Individuals with immunocompromising conditions not excluded IPD reported as bacteraemia, with cases identified from the national register | Group 1: 13,980 individuals ≥65 years of age immunized with Pneu-P-23 and influenza vaccine; mean age 73.3 years Group 2: 12,945 individuals ≥65 years of age immunized with influenza vaccine; mean age 73.7 years | Efficacy: 63% (95% CI: -91 to 93); based on 2 cases in Group 1 and 5 cases in Group 2 | Level I | Poor - no allocation concealment |
| Maruyama2 | Pneu-P-23 | RCT, placebo controlled, double blind Japan, initiated in 2006 Participants recruitment in hospital affiliated nursing homes Participant follow-up (vaccinated) 1,140 person years IPD reported as bacteremic pneumonia | Group 1: 502 immunocompetent individuals ≥55 years of age immunized with Pneu-P-23 vaccine; mean age 84.7 years Group 2: 504 immunocompetent individuals ≥55 years of age not immunized with Pneu-P-23 vaccine; mean age 84.8 years | Efficacy: 86% (95% CI: -277 to 99); based on 0 cases in Group 1 and 3 cases in Group 2 | Level I | Fair |
| Ortqvist et al., 19983 | Pneu-P-23 | RCT, placebo controlled, double blind Sweden, initiated in 1991 Participants former CAP inpatients; recruitment done in infectious disease departments of 6 university hospitals Participant follow-up (vaccinated) 793 person years IPD reported as bacteremic pneumonia | Group 1: 339 immunocompetent individuals ≥50 years of age immunized with Pneu-P-23 vaccine; mean age 69.4 years Group 2: 352 immunocompetent individuals ≥50 years of age not immunized with Pneu-P-23 vaccine; mean age 69.1 years | Efficacy: 79% (95% CI: -77 to 98); based on 1 case in Group 1 and 5 cases in Group 2 | Level I | Good |
| Alfageme4 | Pneu-P-23 | RCT Spain, initiated in 1999 Participant recruitment done in one university hospital; all participants with confirmed COPD, primarily male (>93%) Participant follow-up (vaccinated): 2.7 years | Group 1: 298 immunocompetent individuals ≥60 years of age immunized with Pneu-P-23 vaccine; mean age 69 years Group 2: 298 immunocompetent individuals ≥60 years of age not immunized with Pneu-P-23 vaccine; mean age 69.1 years | No cases of bacteremic pneumococcal infection were observed during the study period | Level I | Fair |
| Evidence for efficacy for PNEU-P-23 in preventing CAP | ||||||
| STUDY DETAILS | SUMMARY | |||||
| Study | Vaccine | Study Details |
Participants | Summary of Key Findings Using Text or Data |
Level of Evidence |
Quality |
| Ortqvist et al., 1998 3 | Pneu-P-23 | RCT, placebo controlled, double blind Sweden, initiated in in 1991 Participants former CAP inpatients; recruitment done in infectious disease departments of 6 university hospitals Participant follow-up (vaccinated) 793 person years Diagnosis: clinical and radiological for CAP; positive culture from pleural fluid or sputum or a positive pneumococcal (pneumolysin) antibody test for PP | Group 1: 339 immunocompetent individuals ≥50 years of age immunized with Pneu-P-23 vaccine; mean age 69.4 years Group 2: 352 immunocompetent individuals ≥50 years of age not immunized with Pneu-P-23 vaccine; mean age 69.1 years | Efficacy CAP: -18% (95% CI: -75 to 20), Efficacy PP: -25% (95% CI: -147 to 36) Group 1: 63 (19%) individuals diagnosed with CAP, of which 19 were pneumococcal pneumonia (PP) Group 2: 57 (5.6%) individuals diagnosed with CAP, of which 16 were with PP CAP was diagnosed in 120 (17%) study participants on 177 occasions; 84 individuals had one episode of pneumonia, 24 had two, and 12 had three or more. | Level I | Good |
| Honkanen et al., 19991 | Pneu-P-23 Influenza | RCT Finland, initiated in 1992 Study open to all persons living in 35 northern districts Participant follow-up (vaccinated) 38,037 person years Individuals with immunocompromising conditions not excluded Diagnosis: clinical and radiological for CAP; positive pneumococcal (pneumolysin) antibody test for PP | Group 1: 13,980 individuals ≥65 years of age immunized with Pneu-P-23 and influenza vaccine; mean age 73.3 years Group 2: 12,945 individuals ≥65 years of age immunized with influenza vaccine; mean age 73.7 years | Efficacy CAP: -20% (95% CI: -50 to 10) Efficacy PP: -20% (95% CI: -90 to 20) During the influenza season, the relative risk of CAP in Group 1 was 1.2 (95% CI 0.7±1.9) and for PP 2.1 (95% CI 0.8±5.4); in non-influenza seasons relative risks were 1.2 (95% CI 0.9±1.6) for CAP and 1.2 (95% CI 0.8±1.9) for PP. | Level I | Poor - no adjustment for confounders and allocation |
| Alfageme4 | Pneu-P-23 | RCT Spain, initiated in 1999 Participant recruitment done in one university hospital; all participants with confirmed COPD Participant follow-up (vaccinated): 2.7 years Diagnosis: clinical and radiological for CAP; positive culture from pleural fluid, bronchial aspirate or sputum for PP | Group 1: 207 immunocompetent individuals ≥65 years of age immunized with Pneu-P-23 vaccine Group 2: 182 immunocompetent individuals ≥65 years of age not immunized with Pneu-P-23 vaccine | Efficacy CAP: -14% (95% CI: -107 to 38) Group 1: 22 individuals diagnosed with CAP Group 2: 17 individuals diagnosed with CAP | Level I | Fair |
| Maruyama5 | Pneu-P-23 | RCT, placebo controlled, double blind Japan, initiated in 2006 Participants recruitment in hospital affiliated nursing homes Participant follow-up (vaccinated) 1,140 person years Diagnosis: clinical and radiological for CAP; positive culture from pleural fluid or sputum, or a positive urine test for PP | Group 1: 502 immunocompetent individuals ≥55 years of age immunized with Pneu-P-23 vaccine; mean age 84.7 years Group 2: 504 immunocompetent individuals ≥55 years of age not immunized with Pneu-P-23 vaccine; mean age 84.8 years | Efficacy CAP: 45% (95% CI: 22 to 61) Efficacy PP: 64% (95% CI: 32 to 81) Group 1: 63 individuals diagnosed with CAP, of which 14 with PP Group 2: 104 individuals diagnosed with CAP, of which 37 with PP | Level I | Fair |
| Kawakami et al., 20105 | Pneu-P-23 Influenza | RCT Japan, initiated in 2005 Participants recruitment in hospitals and private clinics Participants followed-up for 2 years Diagnosis: clinical and radiological for CAP | Group 1: 391 immunocompetent individuals ≥65 years of age immunized with Pneu-P-23 and influenza vaccine; mean age 78.5 yearsGroup 2: 387immunocompetent individuals ≥65 years of age immunized with influenza vaccine; mean age 77.7 years | HR: 0.82 95% CI (0.55-1.23) Efficacy CAP, ≥65: 22% (95% CI: -12 to 45) Efficacy CAP, 65-75: -54% (95% CI: -239 to 30) Group 1: 67 individuals ≥65 with CAP; 16 in 127 individuals 65-75 years of age Group 2: 81 individuals ≥65 with CAP; 12 in 140 individuals 65-75 years of age | Level I | Fair - adjusted for some confounders and concealment allocation |
-
Honkanen PO, Keistinen T, Miettinen L, Herva E, Sankilampi U, Läärä E, et al. Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia and pneumococcal pneumonia among persons aged 65 years or older. Vaccine. 1999;17(20-21):2493-500
-
Maruyama T., O. Taguchi, M.S. Niederman, J. Morser, H. Kobayashi, T. Kobayashi, et coll. « Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: Double blind, randomised and placebo controlled trial », BMJ (en ligne), 2010, 340(7746) : 579.
-
Ortqvist A, Hedlund J, Burman L-, Elbel E, Hofer M, Leinonen M, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351(9100):399-403
-
Alfageme I, Vazquez R, Reyes N, Muñoz J, Fernández A, Hernandez M, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006;61(3):189-95
-
Kawakami K, Ohkusa Y, Kuroki R, Tanaka T, Koyama K, Harada Y, et al. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine. 2010;28(43):7063-9
| Evidence for Effectiveness for PNEU-P-23 in Preventing IPD | |||||
|---|---|---|---|---|---|
| STUDY DETAILS | SUMMARY | ||||
| Study | Study Details | Number of Participants |
Summary of Key Findings Using Text or Data |
Level of Evidence |
Quality |
| Andrews et al., 2012 1 | Indirect cohort UK Serotyped IPD cases (diagnosed between November 2003 and December 2010) with data on vaccination history, underlying risk factors and outcome of infection | Group 1: 1,272 Pneu-P-23 vaccine type IPD cases ≥65 years of age matched for age and time period Group 2: 1,270 non-Pneu-P-23 IPD cases ≥65 years of age | Effectiveness, non-immunocompromised individuals 65 to 74 years of age::
|
II-2 | Fair - adjusted but with potential exposure bias |
| Wright et al. 20132 | Indirect cohort UK HPA database of IPD cases in the North East of England (diagnosed between April 2006 and July 2012) | Group 1: 555 Pneu-P-23 vaccine type IPD cases ≥65 years of age matched for age and time period Group 2: 106 non-Pneu-P-23 IPD cases ≥65 years of age | Effectiveness, individuals 65-74 years of age vaccinated less than 5 years prior to diagnosis: 55.2% (95% CI: -18.6 to 82.8) Effectiveness, immunocompetent individuals 65-74 years of age: 70.3% (95% CI: -6.2 to 91.7) | II-2 | Good |
| Leventer-Roberts et al., 20153 | Case-Control Israel IPD cases extracted from health records of the Clalit Health Services database (diagnosed between January 2007 through December 2010) with over 470,000 adults 65 years of age and older | Group 1: 212 IPD cases ≥65 Group 2: 848 matched controls ≥65 years of age | Effectiveness, all individuals, all serotypes, immunization at less than 5 years since vaccination: 42% (95% CI: 19 to 59) | II-2 | Good |
| Rudnick et al, 20134 | Indirect cohort Canada IPD cases from Toronto Invasive Bacterial Diseases Network (TIBDN) database (diagnosed between 1995 to 2011) | Group 1: 1,461 Pneu-P-23 vaccine type IPD cases ≥65 years of age matched for age and time period Group 2: 234 non-Pneu-P-23 IPD cases ≥65 years of age | Effectiveness, all individuals, immunization <5years: 42.6% (95% CI; 26 to 55.5). Effectiveness, individuals ≥65, immunization <5years: 36.9% (95% CI: 12.7 to 54.4) Effectiveness, individuals ≥65 with no underlying conditions, immunization <5years: 51.1% (95% CI: -6.4 to 77.6) Effectiveness, all individuals, serotypes in Pneu-P-23 and not in Pneu-C-13: 45.2% (95% CI: 27.5 to 58.6) | II-2 | Good |
| Dominguez et al., 20055 | Case-Control Spain IPD cases in 12 hospitals (diagnosed between January 2001 through March 2002) matched with 2 hospital and 1 outpatient control subject on the basis of age and underlying medical conditions | Group 1: 149 IPD cases ≥65 Group 2: 447 matched controls ≥65 years of age | Effectiveness, non-immunocompromised individuals, all serotypes: 76% (95% CI: 51 to 88) Effectiveness, non-immunocompromised, vaccine serotypes: 78% (95% CI, 50 to 90) 131 IPD cases caused by vaccine or vaccine-related serotypes | II-2 | Good |
| Gutierez et al. 20146 | Indirect cohort Spain IPD cases registered in the Surveillance System of the Region of Madrid (diagnosed between 2008 and 2011) 18.6% of cases with immunodeficiency and/or cancer ion | Group 1: 588 Pneu-P-23 vaccine type IPD cases ≥6o years of age matched for age and time period Group 2: 211 non-Pneu-P-23 IPD cases ≥60 years of age | Effectiveness, all individuals, immunization at less than 5 years since vaccination: 44.5% (95% CI: 19.4 to 61.8) Effectiveness, individuals 60-69 years of age: 54.2% (95% CI: 15.3 to 75.2) Effectiveness, individuals 70-79 years of age: 54.1% (95% CI: 19.2 to 73.9) Effectiveness, all individuals, serotypes in Pneu-P-23 and not in Pneu-C-13: 46.8% (95% CI: 45.2 to 76.8) | II-2 | Fair |
| Vila-Corcoles et al., 20107 | Case-Control Spain IPD cases identified using an active surveillance system of 3 reference hospitals (diagnosis made between January 2002 and April 2007) | Group 1: 88 IPD cases ≥60 years of age Group 2: 176 matched controls ≥65 years of age | Effectiveness, all serotypes: 72% (95% CI: 46 to 85) Effectiveness, all serotypes, adults 60-79 years of age: 68% (95% CI: 26 to 86) Effectiveness, Pneu-P-23 serotypes: 77% (95% CI: 40 to 92) | II-2 | Good |
| Vila-Corcoles et al., 20068 | Cohort Spain Study conducted from January 2002 through April 2005; hospital records of reference used to identify IPD cases | 11,241 individuals aged ≥65 years assigned to one primary care center; immunized individuals follow-up for 17,401 person-years | Effectiveness, all serotypes, <5.5 years since immunization: 40% (95% CI: -65 to 78) Effectiveness, vaccine serotypes, <5.5 years since immunization: 39% (95% CI: -176 to 87) | II-2 | Good |
| Ochoa-Gondar et al. 20149 | Cohort Spain Hospital records of the 2 reference hospitals in the study area used to identify IPD cases (December 2008 to November 2011) | 27,204 individuals aged ≥60 years assigned to 9 primary care centers; immunized individuals follow-up for 29,065 person-years | Effectiveness, all serotypes, <5 years since immunization: 62% (95% CI: -68 to 91) | II-2 | Good |
| Hechter et al., 201210 | Cohort USA Participants of the longitudinal California Men's Health Study; mean follow-up period 6.4 years | 3,962 individuals immunized at age ≥65 years; mean follow-up period 7.3 years | Effectiveness, all serotypes, vaccination at age ≥65 years: 65% (95% CI: -91 to 94); only 3 cases of pneumococcal bacteremia during study period | II-2 | Poor - no noted adjustment |
| Jackson et al., 2003 11 | Cohort USA Members of the Group Health Cooperative; study period 2.7 years | 47,365 adults ≥65 years of age were followed for 84,203 person-years for pneumococcal vaccination. Of 26,313 individuals who were vaccinated before the beginning of the study, 91% received the vaccine ≥ 65 years of age and 81% were vaccinated <5 years before the beginning of the study. Of the 21,052 persons who had not received pneumococcal vaccine before study entry, 52% were vaccinated during the study period. There were 38,207 non-immunocompromised individuals. | Effectiveness, all serotypes, immunocompetent adults ≥65 years of age: 54% (95% CI: 13 to 76); estimate based on 39 cases of pneumococcal bacteremia | II-2 | Good |
| Tsai et al., 2015 12 | Cohort Taiwan Data extracted from the IPD notification database of Taiwan's Ministry of Health and Welfare | Cohort of 458,362 propensity score matched individuals ≥75 years of age with and without Pneu-P-23 immunization, (229,181in each group, mean age 81.7 years) | Effectiveness, all serotypes, <1 year since immunization: 76% (95% CI: 54 to 88) | II-2 | Good |
| Evidence for Effectiveness for PNEU-P-23 in Preventing CAP | |||||
| STUDY DETAILS | SUMMARY | ||||
| Study | Study Details | Number of Participants | Summary of Key Findings Using Text or Data | Level of Evidence | Quality |
| Dominguez et al., 201013 | Case-Control Spain Hospitalized patients with CAP admitted through the emergency department; diagnosis based on clinical and radiological findings; mean age (cases) 77.2 years Study conducted from May 2005 through January 2007. Similar proportion of individuals in both groups immunized with influenza vaccine | Group 1: 489 cases ≥65 years of age Group 2: 1,467 hospital patients matched for sex, age, date of hospitalisation and underlying disease | Effectiveness, all CAP, immunocompetent adults ≥65 years of age, <5 years since immunization: 23.6 % (-7.2 to 45.6) | II-2 | Good |
| Dominguez et al., 201714 | Case-Control Spain Hospitalized patients with CAP; diagnosis based on clinical and radiological findings Study conducted from September 2013 to June 2015. Similar proportion of individuals in both groups immunized with influenza vaccine | Group 1: 1,895 cases ≥65 years of age Group 2: 1,895 hospital patients matched for sex, age and date of hospitalisation | Effectiveness, all CAP, adults ≥65-74 years of age, <5 years since immunization: 23.6% (-3 to 43.3) | II-2 | Good |
| Jackson et al., 200915 | Case-Control USA Members of the Group Health Cooperative; study period 3 years CAP identified according to diagnosis codes assigned to outpatient and inpatient encounters, and validated by review of chest radiograph reports or medical records; data on CAP collected over three seasons between the date influenza vaccine first became available and the end of influenza season 38% of cases <75 years of age | Group 1: 1,173 immunocompetent adults ≥65 years of age with CAP Group 2: 2,346 age- and sex-matched controls | Effectiveness, all CAP, immunocompetent adults ≥65 years of age: 0% (95% CI: -30 to 20) | II-2 | Fair - adjusted for some potential confound-ers |
| Leventer-Roberts et al., 201516 | Case-Control Israel Data on hospital treated CAP cases extracted from health records of the Clalit Health Services database (diagnosed between January 2007 through December 2010) with over 470,000 adults 65 years of age and older | Group 1: 23, 441 CAP cases ≥65 Group 2: 46,882 matched controls ≥65 years of age | Effectiveness, all CAP, individuals 65-74 years of age, immunization at less than 5 years since vaccination: -12% (95% CI: -21 to -3) | II-2 | Good |
| Loeb et al., 200917 | Case-Control Canada CAP patients presenting to emergency departments of two hospitals; diagnosis based on clinical and radiological findings; mean age of cases 79.1 years (controls 74.4 years) Cases had a significantly higher proportion of comorbidities, including those associated with immunosuppression (cancer 27.1% vs. 18.1% and transplant 1.6% vs. 0.8%) Recruitment from September 2002 to April 2005 | Group 1: 717 CAP cases ≥65 Group 2: 867 controls ≥65 years of age from the same community | Effectiveness, all CAP: -45% (95% CI: -78 to -19) | II-2 | Poor - no adjustment for confound-ers |
| Skull et al., 200718 | Case-Control Australia Hospitalized CAP patients; diagnosis based on clinical and radiological findings; mean age of cases 78.4 years (controls 76.1 years) Approximately ¼ of individuals with immunosuppression Recruitment from April 2000 to March 2002 | Group 1: 1,952 CAP cases ≥65 Group 2: 2,927 controls ≥65 years of age. | Effectiveness, all CAP: -1% (95% CI: -16 to 13) | II-2 | Good |
| Vila-Corcoles et al., 2009 19 | Case-Control Spain Data collected from 19 primary health care centres and 3 reference hospitals PP confirmed on the basis of clinical and radiological findings, and culture or urine antigen testing Study conducted from January 2002 to April 2007. | Group 1: 210 patients ≥ 50 years of age with non-bacteremic pneumococcal pneumonia Group 2: 420 outpatient controls matched by age,, sex and chronic medical condition | Effectiveness, all non-bacteremic PP, all individuals ≥50 years of age: 42% (14-61) Effectiveness, all PP with or without bacteremia, individuals 65-79 years of age: 48% (19-66) Effectiveness, all PP with or without bacteremia, all non-immunocompromised individuals ≥50 years of age: 45% (95% CI: 20 to 62) | II-2 | Good |
| Vila-Corcoles et al., 201220 | Case-Control Spain Data collected from 19 primary health care centres and 2 reference hospitals PPa confirmed on the basis of clinical and radiological findings, and culture or urine antigen testing Study conducted from January 2002 to April 2007. | Group 1: 77 patients with chronic pulmonary disease (chronic bronchitis, emphysema and/or asthma) ≥ 60 years of age with non-bacteremic pneumococcal pneumonia Group 2: 98 outpatient controls matched by age,, sex and chronic medical condition Average age of study participants was 73 years | Effectiveness, all non-bacteremic PP, all individuals ≥60 years of age: 34% (-34-67) | II-2 | Fair |
| Wiemken et al., 201421 | Case-Control International Data extracted from the Community-Acquired Pneumonia Organization (CAPO) cohort study of adult hospitalized patients with CAP; PP diagnosis based on positive culture or urinary antigen test Mean age of cases, 79 years | Group 1: 279 individuals ≥65 years of age with pneumococcal. pneumonia; 35% with bacteremia Group 2: individuals with CAP of any other or unknown etiology | Effectiveness, PP: 37% (95% CI 16 to 60) Effectiveness,PP, males: 34% (95% CI: −1% to 57) Effectiveness, PP, females: 68% (95% CI 40-83). | II-2 | Good |
| Ansaldi et al., 200522 | Cohort Italy | RR: 0.72 95% CI (0.57 - 0.93) | II-2 | Good | |
| Suzuki et al, 201723 | Cohort Japan Inpatients and outpatients of 4 community hospitals screened for CAP from September 2011 through Aug 2014. CAP diagnosed based on clinical and radiological findings; PP confirmed with culture and urine antigen testing. | Group 1: 419 individuals ≥ 65 years of age with PP with or without bacteremia Group 2: 1,617 individuals with NIpCAP with or without bacteremia | Effectiveness, all PP, individuals 65-74 years of age, immunized <5 years prior to diagnosis: 32.2% (-20.7 to 61.9) Effectiveness, Pneu-P-23 type PP, individuals 65-74 years of age, immunized <5 years prior to diagnosis: 39.8% (-15.5 to 68.6) Effectiveness, Pneu-P-23 and non-Pneu-C-13 type PP, all adults ≥65 years of age, immunized <5 years prior to diagnosis: 12.0% (-62.8 to 52.4) | II-2 | Fair |
| Hechter et al., 201224 | Cohort USA Participants of the longitudinal California Men's Health Study; mean follow-up period 6.4 years Cases identified on the basis of ICD codes for pneumonia in hospital electronic medical records. | 3,962 individuals immunized at age ≥65 years | Effectiveness (hospitalization), all-cause CAP, immunization at or after 65 years of age: 5% (95% CI: -17 to 22) | II-2 | Poor - no adjustment for confound-ers |
| Jackson et al., 200325 | Cohort USA Members of the Group Health Cooperative; study period 3 years Outpatient and hospital records for CAP | 47,365 adults ≥65 years of age were followed for 84,203 person-years for pneumococcal vaccination. Of 26,313 individuals who were vaccinated before the beginning of the study, 91% received the vaccine ≥ 65 years of age and 81% were vaccinated <5 years before the beginning of the study. Of the 21,052 persons who had not received pneumococcal vaccine before study entry, 52% were vaccinated during the study period. | Effectiveness (hospitalization), immunocompetent individuals, all-cause CAP: -14% (95% CI: -31 to 1) Effectiveness (hospitalization), immunocompetent individuals, all-cause CAP, individuals immunized with influenza vaccine: -5% (-29 to 14) Effectiveness, all-cause CAP: -7% (95% CI: -14 to 1) No differences in risk found in time since vaccination | II-2 | Good |
| Ochoa-Gondar et al., 200826 | Cohort Spain Data extracted from electronic medical records from 8 primary care centres and regional reference hospitals PP diagnosis based on sputum culture or urinary antigen testing | 1,298 individuals with a diagnosis of chronic respiratory disease (chronic bronchitis, emphysema and asthma) aged ≥65 701 immunized individuals followed-up for 2,278 person-years 601 individuals immunized in prior 2 years and 98 in prior 3-5 years | Effectiveness, all CAP, outpatient: -15% (95% CI: 52 to -1.72) Effectiveness, all CAP, hospitalization: 30% (95% CI: 52 to 0) | II-2 | Good |
| Ochoa-Gondar et al., 2014 27 | Cohort Spain Hospital records of the 2 reference hospitals in the study Area; PP diagnosis based on sputum culture or urinary antigen testing | 27,204 individuals aged ≥60 years assigned to 9 primary care centers; immunized individuals follow-up for 29,065 person-years | Effectiveness, immunocompetent, PP: 7% (95% CI: -42 to 40) Effectiveness, immunocompetent, all CAP: 11% (95% CI: -9 to 18) Effectiveness, non-bacteremic PP, immunized <5 years prior to diagnosis: 48% (95% CI: 8 to 71) Effectiveness, all CAP, immunized <5 years prior to diagnosis: 25% (95% CI: 2 to 42) | II-2 | Good |
| Tsai et al., 2015 29 | Cohort Taiwan Data extracted from the Taiwan National Health Insurance Research Database (NHIRD) Proportion of participants who received the seasonal influenza vaccine similar in the Pneu-P-23 vaccinated and non-vaccinated group | Cohort of 458,362 propensity score matched individuals ≥75 years of age with and without Pneu-P-23 immunization, (229,181in each group, mean age 81.7 years) | Effectiveness (hospitalization), immunization <1 year prior to diagnosis: 60% (95% CI: 58 to 60) | II-2 | Good |
| Vila-Corcoles et al., 2006 30 | Cohort Spain Study conducted from January 2002 through April 2005 Over 85% of individuals received Pneu-P-23 vaccine within 2 years prior to study recruitment; PP diagnosed with urinary antigen testing | 11,241 individuals aged ≥65 years assigned to one primary care center; immunized individuals follow-up for 17,401 person-years | Effectiveness, non-bacteremic PP, immunized <5 years from diagnosis: 39% (95% CI: -6 to 65) Effectiveness (hospitalization), all CAP, immunized <5 years from diagnosis: 21% (95% CI: 2 to 36) | II-2 | Good |
| Rodriguez-Barradas et al. 200831 | Cohort USA Members of the Veterans Aging Cohort 5-Site Study; study period up to 3 years Outpatient and hospital records of Veterans Affairs (VA) medical centers | 692 individuals without HIV average age 55.5 years | Effectiveness, all cause CAP, <3 years since immunization: 15% (95% CI: -1.95 to 75) | II-2 | Good |
| Hung et al. 201032 | Cohort China (Hong Kong) | 1,875 immunocompetent individuals aged ≥65 years with chronic illness who attended the outpatient clinics in the Hong KongWest Cluster (HKWC) Study participants followed for 64 weeks | Effectiveness, hospitalization from all cause CAP: 23% (95% CI: 7 to 37%) Effectiveness, hospitalization from PP: 38% (95% CI: -5 to 70%) | II-2 | Fair |
-
Andrews NJ, Waight PA, George RC, Slack MPE, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802-8
-
Wright LB, Hughes GJ, Chapman KE, Gorton R, Wilson D. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in people aged 65 years and over in the North East of England, April 2006-July 2012. Trails Vaccinology. 2013;2(1):45-8
-
Leventer-Roberts M, Feldman BS, Brufman I, Cohen-Stavi CJ, Hoshen M, Balicer RD. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive disease and hospital-treated pneumonia among people aged ≤ 65 years: A retrospective case-control study. Clin Infect Dis. 2015;60(10):1472-80
-
Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995-2011. Vaccine. 2013;31(49):5863-71
-
Domínguez À, Salleras L, Fedson DS, Izquierdo C, Ruíz L, Ciruela P, et al. Effectiveness of pneumococcal vaccination for elderly people in Catalonia, Spain: A case-control study. Clin Infect Dis. 2005;40(9):1250-7
-
Gutiérrez Rodríguez MA, Ordobás Gavín MA, García-Comas L, Sanz Moreno JC, CóRdoba Deorador E, Lasheras Carbajo MD, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the region of Madrid, Spain, 2008–2011. Eurosurveillance. 2014;19(40)
-
Vila-Corcoles A., O. Ochoa-Gondar, J.A. Guzmán, T. Rodriguez-Blanco, E. Salsench et C.M. Fuentes. « Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older », BMC Infect Dis, 2010, 10.
-
8. Vila-Córcoles, A., O. Ochoa-Gondar, I. Hospital, X. Ansa, A. Vilanova, T. Rodríguez, et coll. « Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: The EVAN-65 study », Clinical Infectious Diseases, 2006, 43(7) : 860-8.
-
Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged =60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis. 2014;58(7):909-17
-
Hechter RC, Chao C, Jacobsen SJ, Slezak JM, Quinn VP, Van Den Eeden SK, et al. Clinical effectiveness of pneumococcal polysaccharide vaccine in men: California Men's Health Study. Vaccine. 2012;30(38):5625-30
-
Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. New Engl J Med. 2003;348(18):1747-55
-
Tsai Y-, Hsieh M-, Chang C-, Wen Y-, Hu H-, Chao Y-, et al. The 23-valent pneumococcal polysaccharide vaccine is effective in elderly adults over 75 years old-Taiwan's PPV vaccination program. Vaccine. 2015;33(25):2897-902
-
Domínguez, A., C. Izquierdo, L. Salleras, L. Ruiz, D. Sousa, J- Bayas, et coll. « Effectiveness of the pneumococcal polysaccharide vaccine in preventing pneumonia in the elderly », European Respiratory Journal, 2010, 36(3) : 608-14.
-
Domínguez, À., N. Soldevila, D. Toledo, N. Torner, L. Force, M.J. Pérez, et coll. « Effectiveness of 23-valent pneumococcal polysaccharide vaccination in preventing community-acquired pneumonia hospitalization and severe outcomes in the elderly in Spain », PLoS ONE, 2017, 12(2).
-
Jackson ML, Nelson JC, Jackson LA. Risk factors for community-acquired pneumonia in immunocompetent seniors. J Am Geriatr Soc. 2009;57(5):882-8
-
Leventer-Roberts, M., B.S. Feldman, I. Brufman, C.J. Cohen-Stavi, Hoshen M et R.D. Balicer. « Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive disease and hospital-treated pneumonia among people aged ≤ 65 years: A retrospective case-control study », Clinical Infectious Diseases, 2015, 60(10) : 1472-80.
-
Loeb M, Neupane B, Walter SD, Hanning R, Carusone SC, Lewis D, et al. Environmental risk factors for community-acquired pneumonia hospitalization in older adults. J Am Geriatr Soc. 2009;57(6):1036-40
-
Skull SA, Andrews RM, Byrnes GB, Kelly HA, Nolan TM, Brown GV, et al. Prevention of community-acquired pneumonia among a cohort of hospitalized elderly: Benefit due to influenza and pneumococcal vaccination not demonstrated. Vaccine. 2007;25(23):4631-40
-
Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Gutierrez-Perez A, Vila-Rovira A, Group ES. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in patients with chronic pulmonary diseases. Human Vaccines & Immunotherapeutics. 2012 05/01;8(5):639-44
-
Vila-Corcoles, A., O. Ochoa-Gondar, T. Rodriguez-Blanco, A. Gutierrez-Perez, A. Vila-Rovira et E.S. Group. « Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in patients with chronic pulmonary diseases », Human Vaccines & Immunotherapeutics, 2012 05/01; 8(5) : 639-44.
-
Wiemken TL, Carrico RM, Klein SL, Jonsson CB, Peyrani P, Kelley RR, et al. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumoniae community-acquired pneumonia in the elderly differs between the sexes: RESULTS from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine. 2014;32(19):2198-203
-
Ansaldi F, Turello V, Lai P, Batone G, De Luca S, Rosselli R, et al. Effectiveness of a 23-valent polysaccharide vaccine in preventing pneumonia and non-invasive pneumococcal infection in elderly people: A large-scale retrospective cohort study. J Int Med Res. 2005;33(5):490-500.
-
Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313-21
-
Hechter RC, Chao C, Jacobsen SJ, Slezak JM, Quinn VP, Van Den Eeden SK, et al. Clinical effectiveness of pneumococcal polysaccharide vaccine in men: California Men's Health Study. Vaccine. 2012;30(38):5625-30
-
Jackson, L.A., K.M. Neuzil, O. Yu, P. Benson, W.E. Barlow, A.L. Adams, et coll. « Effectiveness of pneumococcal polysaccharide vaccine in older adults », New England Journal of Medicine, 2003, 348(18) : 1747-55.
-
Ochoa-Gondar O, Vila-Corcoles A, Ansa X, Rodriguez-Blanco T, Salsench E, de Diego C, et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine. 2008 Apr 7;26(16):1955-62
-
Ochoa-Gondar, O., A. Vila-Corcoles, T. Rodriguez-Blanco, F. Gomez-Bertomeu, E. Figuerola-Massana, X. Raga-Luria, et coll. « Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged =60 years: 3 years of follow-up in the CAPAMIS study », Clinical Infectious Diseases, 2014, 58(7) : 909-17.
-
Rodriguez-Barradas MC, Goulet J, Brown S, Goetz MB, Rimland D, Simberkoff MS, et al. Impact of Pneumococcal Vaccination on the Incidence of Pneumonia by HIV Infection Status among Patients Enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis. 2008 Apr 1;46(7):1093-100
-
TSAI, Y.H., M.J. HSIEH, C.J. CHANG, Y.W. WEN, H.C. HU, Y.N. CHAO, et coll. « The 23-valent pneumococcal polysaccharide vaccine is effective in elderly adults over 75 years old-Taiwan's PPV vaccination program », Vaccine, 2015, 33(25) : 2897-902.
-
Vila-Córcoles, A., O. Ochoa-Gondar, I. Hospital, X. Ansa, A. Vilanova, T. Rodríguez, et coll. « Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: The EVAN-65 study », Clinical Infectious Diseases, 2006, 43(7) : 860-8.
-
Rodriguez-Barradas, M.C., J. Goulet, S. Brown, M.B. Goetz, D. Rimland, M.S. Simberkoff, et coll. « Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the veterans aging cohort 5-site study », Clinical Infectious Diseases, 1er avril 2008, 46(7) : 1093-100.
-
Hung IF, Leung AY, Chu DW, Leung D, Cheung T, Chan CK, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis. 2010 Nov 1;51(9):1007-16
| Changes in Pneumococcal Disease correlated to childhood PNEU-C programs in Canada* | ||||||
|---|---|---|---|---|---|---|
| Study | Surveillance systems used | Incidence of Overall IPD pre-program | Incidence of Overall IPD post-program | Incidence of Vaccine-Type IPD pre-program | Incidence of Vaccine-Type IPD post-program | STUDY DETAILS |
| Desai et al.1 | Data from 3,825 laboratory confirmed IPD cases reported by hospital and private laboratories to public health units from the integrated Public Health Information System were obtained for the period between Jan. 1, 2007, and Dec. 31, 2014. | N/A | N/A | Between 2007 and 2014 the incidence of Pneu-C-7 included serotypes decreased from 3.0 to 0.7 cases per 100, 000. Between 2010 and 2014, there was also a significant decrease in Pneu-C-13 cases from 12 to 5.3 per 100 000. Decrease was observed in Pneu-C-13 unique serotypes (i.e., serotypes 1, 3, 5, 6A, 7F and 19A) showed a similar trend, with decrease in rates from 9.8 cases per 100 000 in 2010 to 4.6 cases per 100 000 in 2014. Further serotype specific analysis showed that within those serotypes included in PCV13, from 2011 to 2014 there was a significant decrease in the incidence per year caused by serotypes 7F (27.9% decrease), 19A (23.0% decrease) and 3 (12.7% decrease). Pneu-P-23 incidence increased from 2.3 cases per 100 000 in 2007 to 5.8 cases per 100 000 in 2014. | All studies NACI Evidence Level: III) | |
| Lefebvre et al, 2016 2 | 21 sentinel hospitals that provided 318/891 (35.7%) of laboratory-confirmed IPD case samples in the province of Quebec in 2014 | N/A | N/A | 2006 (n = 76) : Pneu-C-7 serotypes 20 (26.3%) Additional Pneu-C-10 serotypes 10 (13.2%) Additional Pneu-C-013 serotypes 23 (30.3%) Non-Pneu-C serotypes 23 (30.3%) 2014 (n = 81) : Pneu-C-7 serotypes 1 (1.2%) Additional Pneu-C-10 serotypes 1(1.2%) Additional Pneu-C-013 serotypes 10 (12.3%) Non-Pneu-C serotypes 69 (85.2%) | ||
| Kellner et al., 20093 | Active, population-based laboratory surveillance from Calgary, Alberta and surrounding area from 1998 - 2007 were reported and analysed. | 1998-2001: 65-84: 36.2 per 100,000 P-Y 85+: 55.0 per 100,000 P-Y | 2003-2007: 65-84: 23.9 per 100,000 P-Y 85+: 60.1 per 100,000 P-Y | PCV7: 1998-2001: 65-84: 22.1 per 100,000 P-Y 85+: 29.1 per 100,000 P-Y | PCV7: 2003-2007: 65-84: 4.8 per 100,000 P-Y 85+: 22.5 per 100,000 P-Y | |
| Leal et al, 20124 | Active, population-based laboratory surveillance from Calgary, Alberta and surrounding area from 1998 - 2007 were reported and analysed. | 1998-2001: 65-84: 36.2 per 100,000 P-Y 85+: 55.0 per 100,000 P-Y | 2007-2010: 65-84: 20.8 per 100,000 P-Y 85+: 25.3 per 100,000 P-Y | PCV7: 1998-2001: 65-84: 22.1 per 100,000 P-Y 85+: 29.1 per 100,000 P-Y | PCV7: 2007-2010: 65-84: 2.2 per 100,000 P-Y 85+: 0 per 100,000 P-Y | |
| Rudnick et al., 20135 | Active, population-based surveillance of laboratory data from Toronto, Ontario and surrounding area from 1995 - 2011 were reported and analysed. | 1995/1996: 57.1 per 100,000 2001: 30.6 per 100,000 | 2005: 22.2 per 100,000 | Between 2001 and 2011, an 88% reduction (95% CI, 93-78%) in IPD due to Pneu-C-7 serotypes occurred among adults 15-64 years old and an 89% reduction (95% CI, 94-80%) occurred among adults over 65 years of age | ||
| Lim et al. 20136 | Active, population-based surveillance of laboratory data from Toronto, Ontario and surrounding area from 1995 - 2011 were reported and analysed. | 2001: 30.6 per 100,000 | 2008/2010: 23.6 per 100,000 | N/A | 2008/2010: 13.3 per 100,000 | |
| Demczuk et al, 20137 | Data on 8,047 serotyped isolates submitted to the National Microbiology Laboratory (Public Health Agency of Canada) from 2010-2012; 4,040 cultures submitted from all Canadian provincial and territorial public health and regional hospital laboratories except for the provinces of Alberta and Quebec and greater metropolitan Toronto region; 1,366 isolates were typed by Laboratoire de santé publique du Québec; 1260 isolates typed by the Toronto Invasive Bacterial Diseases Network (TIBDN); and 1381 isolates typed by the Provincial Laboratory for Public Health, Edmonton, Alberta. 2,818 (35% of total number of samples) from adults ≥65 years of age | Overall Pneu-C-13 serotypes decreased significantly (p<0.001) over the 3-year period in the ≥65-year-old age groups, from 50% (487/967) to 39% (369/937), respectively. | ||||
| Sahni et al., 20128 | Data were collected from all confirmed cases of IPD that were reported to the BC Centre for Disease Control between 2002 and 2010. | N/A | N/A | PCV7: 2002: 9.6 per 100,000 | PCV7: 2010: 0.7 per 100,000 | |
-
Desai S, Policarpio ME, Wong K, Gubbay J, Fediurek J, Deeks S. The epidemiology of invasive pneumococcal disease in older adults from 2007 to 2014 in Ontario, Canada: a population-based study. CMAJ Open. 2016 Sep 29;4(3):E545-50
-
Programme de surveillance du pneumocoque: rapport 2014 [Internet]. Québec, Canada: Laoratoire de santé publique du Québec; 2016 [updated Feb 4, 2016; cited Dec 6, 2017]. Available from: https://www.inspq.qc.ca/en/node/4680
-
Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998-2007: Update from the calgary-area Streptococcus pneumoniae research (Casper) study. Clin Infect Dis. 2009;49(2):205-12.
-
Leal J, Vanderkooi OG, Church DL, MacDonald J, Tyrrell GJ, Kellner JD. Eradication of invasive pneumococcal disease due to the seven-valent pneumococcal conjugate vaccine serotypes in Calgary, Alberta. Pediatr Infect Dis J. 2012;31(9).
-
Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995-2011. Vaccine. 2013;31(49):5863-71.
-
Lim GH, Wormsbecker AE, McGeer A, Pillai DR, Gubbay JB, Rudnick W, et al. Have changing pneumococcal vaccination programmes impacted disease in Ontario? Vaccine. 2013;31(24):2680-5.
-
Demczuk W.H.B., Martin I, Griffith A., Lefebvre B., McGeer A., Lovgren M., et al. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010-2012. Can J Microbiol. 2013;59:778-88.
-
Sahni V, Naus M, Hoang L, Tyrrell GJ, Martin I, Patrick DM. The epidemiology of invasive pneumococcal disease in British Columbia following implementation of an infant immunization program: Increases in herd immunity and replacement disease. Can J Public Health. 2012;103(1):29-33.
Notes: All data is for individuals 65+ unless otherwise noted
P-Y = Person-Years
Data collected from Rudnick et al., 2013 compares pre-licensing to post-licensing due to data availability
* PCV7 programs were implemented variably across Canada. Three doses of PCV 7 were recommended in Quebec in 2004 followed by a switch to PCV10 in 2010 and four doses were recommended in Alberta in 2002, in Ontario in 2001, with a publicly funded program beginning in 2005, and in British Columbia in 2003, which changed to a 3-dose schedule in 2007 and PCV-13 in 2010. Vaccination coverage was approximately 70-95%.
| Changes in Pneumococcal Disease correlated to childhood PNEU-C programs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STUDY DETAILS (All studies NACI Evidence Level: III) | |||||||||||||
| Details of childhood PNEU-C programsa | Adult PNEU-P-23 vaccination programsa | Study | Surveillance systems used | Incide nceb of Vaccine-Type CAP pre-program |
Incide |
Incidence of Overall CAP pre-program | Incidence of Overall CAP post-program | ||||||
| Australia (n=1) | |||||||||||||
| In 2005, three doses of PCV7 were recommended for infants in Australia reaching approximately 91% coverage. | In 2005, Pneu-P-23 was introduced for all adults 65 years of age and older. | Menzies et al., 20151 | Data were collected from a national database (Australian Institute of Health and Welfare National Hospital Morbidity Database) of electronic records and ICD-10-AM codes in Australia between 1998 and 2011; contains data from >99% of public and private hospitals In Australia | Incidence was not provided. Effect estimates (IRRs) were reported comparing 2005-2011 to 1998-2004. Pneumococcal and lobar pneumonia: 65-74: 0.86 (95% CI: 0.74 - 0.99) 75-84: 0.86 (95% CI: 0.76 - 0.98) 85+: 0.91 (95% CI: 0.77 - 1.09) Unspecified cause of pneumonia: 65-74: 0.95 (95% CI: 0.88 - 1.01) 75-84: 0.96 (95% CI: 0.90 - 1.03) 85+: 0.96 (95% CI: 0.89 - 1.02) All cause pneumonia: 65-74: 0.96 (95% CI: 0.90 - 1.04) 75-84: 0.98 (95% CI: 0.91 - 1.04) 85+: 0.97 (95% CI: 0.90 - 1.03) | |||||||||
| Canada (n=1) | |||||||||||||
| PCV7 programs were implemented variably across Canada. Three doses of PCV 7 were recommended in Quebec in 2004 followed by a switch to PCV10 in 2010 and four doses were recommended in Alberta in 2002, in Ontario in 2001, with a publicly funded program beginning in 2005, and in British Columbia in 2003, which changed to a 3-dose schedule in 2007 and PCV-13 in 2010. Vaccination coverage was approximately 70-95%. | PPV23 was suggested for all individuals 65 years of age and older throughout all study periods. | Shigayeva et al. 20162 | Data from the Toronto Invasive Bacterial Dis-eases Network (TIBDN) IPD surveillance database (2003 to 2011); median age of adults 64 years(46.6% >65). | 24.6% (95% CI: 15-35.2%) reduction in non-bacteremic PP between 2003 and 2011 | |||||||||
| United Kingdom (n=2) | |||||||||||||
| In 2006, childhood vaccination with 3 doses of PCV7 was introduced. Coverage reached approximately 90%d. PCV13 replaced PCV7 by 2010. | By 2005, PPV23 vaccination was recommended for all individuals 65 years of age and older. | Nair et al. 20163 | Data collected from hospital records for the entire Scottish population for the period 2000 to 2012 | N/A | N/A | Annual hospitalization rate for all-cause pneumonia 2000-2005 (per 100,000 persons): 65-74 years: 373 (365-380 75-84 years: 875 (861-889) ≥85 years: 1909 (1872-1946) Annual hospitalization rate for PP 2000-2006 65-74 age group: 205.2 | Annual hospitalization rate for all-cause pneumonia 2010-2012 (per 100,000 pop.): 65-74 years: 634 (621-647 75-84 years: 1426 (1402-1451 ≥85 years: 2785 (2728-2842) Annual hospitalization rate for PP 2010-2012 65-74 age group: 155 -21.4% (-42.9, -7.1) hospital admissions for PP in the 65-74 age group | ||||||
| Rodrigo et al., 20154 | Data collected from hospital records of all individuals admitted to a hospital with CAP between 2008 and 2013; from 383 adults in the study diagnosed with pneumococcal pneumonia by urine antigen testing, 56% had received Pneu-P-23 | Additional PCV13 serotypes 2008-2009: VT CAP: 65-74: 23.2 (95% CI: 12.3 - 39.6) per 100,000 75-84: 44.7 (95% CI: 25.6 - 72.6) per 100,000 85+: 104.2 (95% CI: 50.0 - 191.6) per 100,000 | Additional PCV13 serotypes 2010-2011: VT CAP: 65-74: 17.8 (95% CI: 8.6 - 32.8) per 100,000 75-84: 11.2 (95% CI: 3.0 - 28.6) per 100,000 85+: 52.1 (95% CI: 16.9 - 121.6) per 100,000 2011-2012: 65-74: 23.2 (95% CI: 12.3 - 39.6) per 100,000 75-84: 19.6 (95% CI: 7.9 - 40.3) per 100,000 85+: 52.1 (95% CI: 16.9 - 121.6) per 100,000 2012-2013: 65-74: 12.5 (95% CI: 5.0 - 25.7) per 100,000 75-84: 22.3 (95% CI: 9.6 - 44.0) per 100,000 85+: 20.8 (95% CI: 2.5 - 75.3) per 100,000 | 2008-2009: Overall CAP: 65-74: 201.4 (95% CI: 166.0 - 242.2) per 100,000 75-84: 360.3 (95% CI: 300.8 - 428.1) per 100,000 85+: 1052.1 (95% CI: 856.9 - 1278.4) per 100,000 Pneumo-coccal CAP: 65-74: 69.5 (95% CI: 49.4 - 95.0) per 100,000 75-84: 136.9 (95% CI: 101.3 - 181.0) per 100,000 85+: 416.7 (95% CI: 297.7 - 567.4) per 100,000 | 2010-2011: Overall CAP: 65-74: 137.2 (95% CI: 108.3 - 171.6) per 100,000 75-84: 254.2 (95% CI: 204.7 - 312.1) per 100,000 85+: 489.6 (95% CI: 359.7 - 651.0) per 100,000 Pneumo-coccal CAP: 65-74: 39.2 (95% CI: 24.6 - 59.4) per 100,000 75-84: 50.3 (95% CI: 29.8 - 79.5) per 100,000 85+: 114.6 (95% CI: 57.2 - 205.0) per 100,000 2011-2012: Overall CAP: 65-74: 190.7 (95% CI: 156.3 - 230.5) per 100,000 75-84: 413.4 (95% CI: 349.5 - 485.6) per 100,000 85+: 937.5 (95% CI: 753.9 - 1152.3) per 100,000 Pneumo-coccal CAP: 65-74: 35.6 (95% CI: 21.8 - 55.1) per 100,000 75-84: 67.0 (95% CI: 43.0 - 99.8) per 100,000 85+: 156.2 (95% CI: 87.4 - 257.7) per 100,000 2012-2013: Overall CAP: 65-74: 137.2 (95% CI: 108.3 - 171.6) per 100,000 75-84: 245.8 (95% CI: 197.2 - 302.8) per 100,000 85+: 427.1 (95% CI: 306.5 - 579.4) per 100,000 Pneumococcal CAP: 65-74: 41.0 (95% CI: 26.0 - 61.5) per 100,000 75-84: 69.8 (95% CI: 45.2 - 103.1) per 100,000 85+: 166.7 (95% CI: 95.3 - 270.7) per 100,000 Change in rate ratios for pneumococcal CAP = 0.84 (0.80-0.89), CAP due to serotypes contained in Pneu-C-7 = 0.52 (0.43-0.62) and CAP due to additional serotypes contained in Pneu-C-13 = 0.87 (0.80-0.95) | ||||||||
| Poland (n=1) | |||||||||||||
| In 2006, 3 doses of PCV7 were introduced in Kielce, Poland. Coverage reached approximately 99%. | There was no publicly funded PPV23 program for 65+ in Poland at the time of publication. | Patrzalek et al., 20125 | Data were collected in the form of ICD-10 codes from the provincial division of the National Health Fund in Kielce between 2005 and 2010. | N/A | N/A | All cases of pneumonia 2006: 1939 per 100,000 | All cases of pneumonia 2007: 2049 per 100,000 2008: 1692 per 100,000 2009: 1061 per 100,000 2010: 1095 per 100,000 | ||||||
| Nicaragua (n=1) | |||||||||||||
| In 2010 the pediatric pneumococcal immunization program with Pneu-C-13 was added to the g National Immunization Schedule; coverage between 60 and 65% | Pneu-P-23 was provided to adults aged 50 years and older since 2010; coverage approximately 25% | Becker-Dreps et al., 20156 | Hospital data from a single public referral hospital in one region of Nicaragua were analyzed from 2008 to 2012. Adjusted incidence rate ratios (IRR) based on the total number of ambulatory visits for pneumonia and pneumonia hospitalizations were provided for pre and post vaccine periods | IRR in ambulatory visits, individuals ≥65 years of age: 0.81 (95% CI: 0.61 to 1.06) IRR in pneumonia hospitalizations, individuals ≥65 years of age 2.07 (95% CI: 1.84, 2.33 | |||||||||
| Germany (n=1) | |||||||||||||
| Routine immunization of children was initiated in 2007 with Pneu-C-7 vaccine, which was replaced by Pneu-C-13 in 2010 | Pletz et al, 20167 | Data on the distribution of the vaccine-serotypes covered by Pneu-C vaccines in adult patients with CAP was obtained from a CAPNETZ, a German multicenter prospective cohort study for periods 2002-2006 and 2007-2011 using a serotype-specific multiplex urinary antigen detection assay; mean age, pre Pneu-C introduction 63 and post Pneu-C introduction 57.7 | Pneumococcal serotypes in non-bacteremic pneumococcal pneumonia 2002-2006, proportion Pneu-C-7 serotypes: 57/182 Pneumococcal serotypes in non-bacteremic pneumococcal pneumonia 2007-2011, proportion Pneu-C-7 serotypes: 26/176 | ||||||||||
| Japan (n=1) | |||||||||||||
| PCV7 became commercially available in February 2010 and was widely employed through a subsidiary from the local government, which increased the estimated vaccination rate among infants to over 80% in 2012; PCV13 was incorporated into the routine immunization schedule in November 2013 | Routine PPV23 for adults aged 65 or older in troduced in October 2014; prior to that coverage approximately 25% | Katoh et al. 20178 | Systematic literature search conducted for studies reporting CAP incidence in Japan; pooled analysis of studies provided estimates on incidence pre and post Pneu-C-7 introduction | Proportion of vaccine-covered serotypes 2001-2006: Pneu-C-7: 44.7% Pneu-P-23 but not Pneu-C-7: 32.6% Non-VT: 11.1% | Proportion of vaccine-covered serotypes 2011-2013 Pneu-C-7: 26.6% Pneu-P-23 but not Pneu-C-7: 42% Non-VT: 15.7% | ||||||||
| Taiwan (n=1) | |||||||||||||
| In 2005, PCV7 became available in Taiwan. Less than 20% of children were vaccinated by 2007.f | PPV23 is recommended for all individuals aged 65 years of age and older. | Lin et al., 20109 | Data collected from one hospital's records between 2000 and 2008 were analyzed. | N/A | N/A | 2004-2005: Non-bacteremicPP: 51 per 100,000 hospital-izations | 2006-2008: Non-bacteremic PP: 18 per 100,000 hospital-izations 64.1% reduction, (95% CI 13.3-115.0%) - adults 65 years of age and older | ||||||
| The Netherlands (n=1) | |||||||||||||
| In 2006, 4 doses of PCV7 were added to the national infant immunization program. The program attained approximately 95% coverage. PCV7 was replaced by PCV10 in 2011 | No national recommendation for PPV23 for the general population 65+. | Van Werkhoven et al, 201510 | Post-hoc analysis of two studies : the CAP-pilot study (prospective study of 1095 patients hospitalized with CAP between January2008 and April 2009) and the CAPiTA trial (double-blind randomized placebo-controlled trial evaluating the efficacy of Pneu-C-13 in 84,496 community-dwelling immunocompetent adults ≥65 years and older.) A total of 288 unimmunized individuals were diagnosed with non-bacteremic pneumococcal CAP between 2009 and 2013. | Proportion of Pneu-P-7 isolates in total reported NIpCAPsamples: 2008: 34/118 2009: 11/37 2010: 3/23 2011: 5/32 2012: 1/37 2013: 3/23 Proportion of Pneu-P-10 - Pneu-C-7 isolates in total reported NIpCAP samples: 2008: 22/118 2009: 4/37 2010: 5/23 2011: 6/32 2012: 10/37 2013: 5/23 | |||||||||
| United States of America (n=5) | |||||||||||||
| In 2000, 3 doses of PCV7 were recommended for use in all children younger than 2 years of age in the U.S.A. PCV13 replaced PCV7 in 2010. Coverage has remained at approximately 90% since 2009.g | PPV23 was recommended for all adults 65 years of age and older until 2014. In 2014, PCV13 was recommended for this age group. | Griffin et al., 201311 | Data were collected between 1994 and 2012 from the National Inpatient Sample, which is comprised of 20% of all discharge diagnoses in U.S. hospitals across 44 states. | N/A | N/A | All cause pneumonia hospitaliza-tion: 1997-1999: 65-74:1293 per 100,000 75-84:2758 per 100,000 85+: 5697 per 100,000 | All cause pneumonia hospitaliza-tion: 2001-2006: 65-74: 1268 per 100,000 75-84:2615 per 100,000 85+: 5209 per 100,000 2007-2009: 65-74: 1208 per 100,000 75-84:2398 per 100,000 85+: 4396 per 100,000 6.6% (95% CI: 0.5-12.7) reduction in all cause pneumonia in the 65-74 year age group | ||||||
| Simonsen et al., 201412 | N/A | N/A | 2007-2009: All cause pneumonia: 1438.4 per 100,000 Non-invasive pneumococcal or lobar pneumonia: 48.4 per 100,000 | 2011-2012: All cause pneumonia: 1375.2 per 100,000 Non-invasive pneumococcal or lobar pneumonia: 32.7 per 100,000 34% (95% CI: 27-41) decline in hospital admissions,non-invasive pneumococcal or lobar pneumonia | |||||||||
| Nelson et al., 200813 | Data were collected from the ICD-9 diagnostic codes from the Group Health study population in Washington state between 1998 and 2004. | N/A | N/A | 1998-2000: Hospitalized pneumonia: 65-74: 490 per 100,000 P-Y 75+: 1530 per 100,000 P-Y Confirmed outpatient pneumonia: 65-74: 1230 per 100,000 P-Y 75+: 2170 per 100,000 P-Y | 2003-2004: Hospitalized pneumonia: 65-74: 640 per 100,000 P-Y 75+: 2250 per 100,000 P-Y Confirmed outpatient pneumonia: 65-74: 1200 per 100,000 P-Y 75+: 2190 per 100,000 P-Y IRR for 65-74 pre/post program implementation: 1.3 [95%CI: 1.12-1.5]) for hospitalization and 0.97 [95%CI: 0.88-1.08] for outpatient pneumonia | ||||||||
| Simonsen et al., 201114 | Data was collected from the Health Case Utilization Project State Inpatient Databases between 1996 and 2006. The databases include all ICD-9 coded discharge diagnoses from 10 states; diagnosis by X-ray in the majority of cases | N/A | N/A | 1996-1999: Lobar PP: 144.9 per 100,000 Non-bacteremic PP: 126.1 per 100,000 All cause pneumonia: 1875.2 per 100,000 | 2005-2006: Lobar PP: 64.7 per 100,000 Non-bacteremic PP: 55.9 per 100,000 All cause pneumonia: 1672.6 per 100,000 54% (95% CI: 53-56) reduction in non-bacteremic PP in adults ≥65 years of age | ||||||||
| Grijalva et al. 200715 | Data from the Nationwide Inpatient Sample, the largest inpatient database available in the USA, were analyzed with an interrupted time-series analysis that used pneumonia (all-cause and pneumococcal) admission rates | PP admissions 1997-1999, persons ≥65 years of age: 73.9/100,000 | PP admissions 2001-2004, persons ≥65 years of age: 59.3/100,000 | All cause pneumonia admissions 1997-1999, persons ≥65 years of age: 2,559.2/100,000 | All cause pneumonia admissions 2001-2004, persons ≥65 years of age: 2,162.7/100,000 | ||||||||
-
Menzies RI, Jardine A, McIntyre PB. Pneumonia in Elderly Australians: Reduction in Presumptive Pneumococcal Hospitalizations but No Change in All-Cause Pneumonia Hospitalizations Following 7-Valent Pneumococcal Conjugate Vaccination. Clin Infect Dis. 2015;61(6):927-33.
-
Shigayeva A, Rudnick W, Green K, Tyrrell G, Demczuk WH, Gold WL, et al. Association of serotype with respiratory presentations of pneumococcal infection, Ontario, Canada, 2003-2011. Vaccine. 2016 Feb 3;34(6):846-53.
-
Nair H, Watts AT, Williams LJ, Omer SB, Simpson CR, Willocks LJ, et al. Pneumonia hospitalisations in Scotland following the introduction of pneumococcal conjugate vaccination in young children. BMC Infect Dis. 2016;16(1).
-
Rodrigo, C., T. Bewick, C. Sheppard, S. Greenwood, T.M. Mckeever, C.L. Trotter, et coll. « Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia », European Respiratory Journal, 2015, 1er juin 2015, 45(6) : 1632-41.
-
Patrzalek M, Gorynski P, Albrecht P. Indirect population impact of universal PCV7 vaccination of children in a 2 + 1 schedule on the incidence of pneumonia morbidity in Kielce, Poland. Eur J Clin Microbiol Infect Dis. 2012;31(11):3023-8.
-
Becker-Dreps S, Amaya E, Liu L, Rocha J, Briceño R, Moreno G, et al. Impact of a combined pediatric and adult pneumococcal immunization program on adult pneumonia incidence and mortality in Nicaragua. Vaccine. 2015;33(1):222-7.
-
Pletz MW, Ewig S, Rohde G, Schuette H, Rupp J, Welte T, et al. Impact of pneumococcal vaccination in children on serotype distribution in adult community-acquired pneumonia using the serotype-specific multiplex urinary antigen detection assay. Vaccine. 2016;34(20):2342-8.
-
Katoh S, Suzuki M, Ariyoshi K, Morimoto K. Serotype replacement in adult pneumococcal pneumonia after the introduction of seven-valent pneumococcal conjugate vaccines for children in Japan: a systematic literature review and pooled data analysis. Jpn J Infect Dis. 2017 Mar 28.
-
Lin SH, Tan CK, Lai CC, Wang CY, Hsueh PR. Declining incidence of nonbacteremic pneumococcal ppneumonia in hospitalized elderly patients at a tertiary care hospital after the introduction of pneumococcal vaccines in Taiwan, 2004 to 2008. J Am Geriatr Soc. 2010 Jan;58(1):195,196 Erratum in J.Am.Geriatr.Soc 2010, 58, 4, 810.
-
van Werkhoven CH, Hollingsworth RC, Huijts SM, Bolkenbaas M, Webber C, Patterson S, et al. Pneumococcal conjugate vaccine herd effects on non-invasive pneumococcal pneumonia in elderly. Vaccine. 2016;34(28):3275-82.
-
Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. New Engl J Med. 2013;369(2):155-63.
- Footnote 12
-
Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: A time series analysis. Lancet Respir Med. 2014;2(5):387-94.
-
Nelson JC, Jackson M, Yu O, Whitney CG, Bounds L, Bittner R, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26(38):4947-54.
-
Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio. 2011;2(1).
-
Nelson JC, Jackson M, Yu O, Whitney CG, Bounds L, Bittner R, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26(38):4947-54.
List of abbreviations
| Abbreviation | Term |
|---|---|
| AE | Adverse event |
| AMR | Antimicrobial resistance |
| CAP | Community-acquired pneumonia |
| CI | Confidence Interval |
| CIRN | Canadian Immunization Research Network |
| CNDSS | Canadian Notifiable Disease Surveillance System |
| HIV | Human Immunodeficiency Virus |
| HSCT | Hematopoetic Stem Cell Transplant |
| ICD | International Classification of Diseases |
| ICER | Incremental cost-effective ratio |
| IM | Intramuscularly |
| IPD | Invasive pneumococcal disease |
| IRR | Incidence rate ratio |
| LSPQ | Laboratoire de santé publique du Québec |
| MED-ECHO | Maintenance et exploitation des données pour l'étude de la clientèle hospitalière |
NACI |
National Advisory Committee on Immunization |
NIpCAP |
Non-invasive pneumococcal community-acquired pneumonia |
| NML | National Microbiology Laboratory |
| NOC | Notice of compliance |
| NVT | Non-vaccine type |
| NWT | Northwest Territories |
| OR | Odds ratio |
| Pop, | Population |
| PP | Pneumococcal pneumonia |
| P-Y | Population per year |
| PHAC | Public Health Agency of Canada |
| PNEU-C | Pneumococcal conjugate vaccine |
| PNEU-C-7 | 7-valent pneumococcal conjugate vaccine |
| PNEU-C-10 | 10-valent pneumococcal conjugate vaccine |
| PNEU-C-13 | 13-valent pneumococcal conjugate vaccine |
| PNEU-P | Pneumococcal polysaccharide vaccine |
| PNEU-P-23 | 23-valent pneumococcal polysaccharide vaccine |
| PWG | Pneumococcal Working Group |
| QALY | Quality adjusted life-years |
| RCT | Randomized controlled trial |
| RR | Risk ratio |
| SAE | Serious adverse events |
| SC | Subcutaneously |
| SOS | Serious Outcome Surveillance |
| ST3 | Serotype 3 |
| TIBDN | Toronto Invasive Pneumococcal Burden of Disease Network |
| UAD | Urine antigen detection |
| US | United States of America |
| UK | United Kingdom |
| VT | Vaccine type |
References
- Update on the use of 13-valent pneumococcal conjugate vaccine (PNEU-C-13) in addition to 23-valent pneumococcal polysaccharide vaccine (PNEU-P-23) in immunocompetent adults 65 years of age and older - Interim Recommendation [Internet].: National Advisory Committee on Immunization (NACI); October 2016 []. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/update-use-of-13-valent-pneumococcal-conjugate-vaccine-pneu-c-13-in-addition-to-23-valent-pneumococcal-polysaccharide-vaccine-pneu-p-23-immunocompetent-adults-65-years-and-older-interim-recommendation.html
- Vaccination Coverage Goals and Vaccine Preventable Disease Reduction Targets by 2025 [Internet].: Public Health Agency of Canada; 2017 []. Available from: https://www.canada.ca/en/public-health/services/immunization-vaccine-priorities/national-immunization-strategy/vaccination-coverage-goals-vaccine-preventable-diseases-reduction-targets-2025.html?wbdisable=true
- Kraicer-Melamed H, O'Donnell S, Quach C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: A systematic review and meta-analysis. Vaccine. 2016;34(13):1540-50.
- Kraicer-Melamed H, O'Donnell S, Quach C. Corrigendum to "The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: A systematic review and meta-analysis" [ Vaccine 34 (2016) 1540-1550]. Vaccine. 2016;34(34):4083-4.
- Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (ppv23) against pneumococcal disease in the elderly: Systematic review and meta-analysis. PLoS ONE. 2017;12(1).
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology. 2007;7(10).
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1.
- Schiffner-Rohe J, Witt A, Hemmerling J, Von Eiff C, Leverkus F-. Efficacy of PPV23 in preventing pneumococcal pneumonia in adults at increased risk - A systematic review and meta-analysis. PLoS ONE. 2016;11(1).
- Erickson LJ, De Wals P, Farand L. An analytical framework for immunization programs in Canada. Vaccine. 2005;23(19):2468-74.
- Streptococcus Pneumoniae. Pathogen Safety Data Sheet - Infectious Substances [Internet].: Public Health Agency of Canada (PHAC); April 2012 []. Available from: http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/streptococcus-pneumoniae-eng.php
- Invasive Pneumococcal Disease - For Health Professionals [Internet]. Canada: Public Health Agency of Canada; 2016 [updated March 10, 2016; cited October 31, 2017]. Available from: http://www.phac-aspc.gc.ca/im/vpd-mev/pneumococcal-pneumococcie/professionals-professionnels-eng.php
- National Laboratory Surveillance of Invasive Streptococcal Disease in Canada - Annual Summary 2014. Executive Summary [Internet]. Canada: Public Health Agency of Canada; 2016 [updated March 10, 2016; cited October 31, 2017]. Available from: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/national-laboratory-surveillance-invasive-streptococcal-disease-canada-annual-summary-2014.html#a2
- Pneumococcal Polysaccharide (PNEUMO-P) Vaccine [Internet]. Alberta, Canada: Alberta Health Services; 2016 [updated July 6, 2017; cited Dec 6, 2017]. Available from: https://myhealth.alberta.ca/Alberta/Pages/immunization-pneumococcal-polysaccharide.aspx
- Routine Immunization Schedules [Internet]. Canada: Government of Manitoba; 2016 [updated September 15, 2016; cited Oct 31, 2017]. Available from: http://www.gov.mb.ca/health/publichealth/cdc/div/schedules.html
- 15. What you need to know about Immunization [Internet]. Canada: Office of the Chief Medical Officer of Health, New Brunswick; 2017 [updated 2017; cited Oct 31, 2017]. Available from: http://www2.gnb.ca/content/gnb/en/departments/ocmoh/cdc/content/immunization.html
- Protect yourself and your child from Pneumococcal Disease [Internet]. Canada: Health and Community Services, Government of Newfoundland and Labrador; 2012 [updated January, 2012; cited October 31, 2017]. Available from: http://www.health.gov.nl.ca/health/publichealth/cdc/Protect_yourself_and_your_child_from_Pneumonococcal_Disease.pdf
- Immunization Schedules [Internet]. British Columbia, Canada: Provincial Health Services Authority, British Columbia Centre for Disease Control; 2017 [updated 2017; cited November 1, 2017]. Available from: http://www.bccdc.ca/health-info/immunization-vaccines/immunization-schedules
- NWT Immunization Schedule [Internet]. Northwest Territories, Canada: Health and Social Services, Government of Northwest Territories; 2017 [updated June, 2017; cited November 1, 2017]. Available from: http://www.hss.gov.nt.ca/sites/www.hss.gov.nt.ca/files/immunization-schedule-general-public.pdf
- Immunization Schedule for Adults [Internet]. Nova Scotia, Canada: Communicable Disease Prevention and Control, Province of Nova Scotia; 2013 [updated November, 2013; cited November 1, 2017]. Available from: http://novascotia.ca/dhw/CDPC/documents/13155_AdultImmunizationSchedule_En.pdf
- Routine Immunization Schedule 2010 - Adults Previously Immunized [Internet]. Nunavut, Canada: Department of Health, Government of Nunavut; 2010 [updated March 22, 2010; cited November 1, 2017]. Available from: http://gov.nu.ca/sites/default/files/files/Approved%20Immunization%20Schedules(adult)(1).pdf
- Publicly Funded Immunization Schedules for Ontario - December 2016 [Internet]. Ontario, Canada: Ontario Ministry of Health and Long-Term Care; 2016 [updated December, 2016; cited November 1, 2017]. Available from: http://www.health.gov.on.ca/en/pro/programs/immunization/docs/immunization_schedule.pdf
- Childhood Immunization in PEI [Internet]. Prince Edward Island, Canada: Government of Prince Edward Island; 2017 [updated Apri l 12, 2017; cited November 1, 2017]. Available from: https://www.princeedwardisland.ca/en/information/health-and-wellness/childhood-immunization-pei
- The Québec Immunisation Program [Internet]. Québec, Canada: Ministre de la Santé et des Services sociaux du Québec; April 2017 [updated April 3, 2017; cited November 1, 2017]. Available from: http://sante.gouv.qc.ca/en/programmes-et-mesures-daide/programme-quebecois-d-immunisation/
- Immunization Services [Internet]. Saskatchewan, Canada: Saskatchewan Ministry of Health; 2017 [updated September, 2017; cited November 1, 2017]. Available from: http://www.saskatchewan.ca/residents/health/accessing-health-care-services/immunization-services
- Pneumococcal Polysaccharide Vaccine [Internet]. Yukon, Canada: Yukon Health and Social Services; 2015 [updated June, 2009; cited Dec 6, 2017]. Available from: http://www.hss.gov.yk.ca/pdf/immunization_pneumococca_en.pdf
- Routine Immunization Schedule [Internet]. Alberta, Canada: Alberta Health; 2017 [updated July 1, 2017; cited November 1, 2017]. Available from: http://www.health.alberta.ca/health-info/imm-routine-schedule.html
- Routine Childhood Immunization Schedule [Internet]. Nova Scotia, Canada: Nova Scotia Department of Health and Wellness; 2015 [updated April, 2017; cited Dec 6, 2017]. Available from: http://novascotia.ca/dhw/CDPC/documents/13151_ChildhoodImmunizationSchedule_En.pdf
- Invasive Pneumococcal Disease - For Health Professionals [Internet]. Canada: Public Health Agency of Canada, Government of Canada; 2016 [updated March 10, 2016; cited November 1, 2017]. Available from: https://www.canada.ca/en/public-health/services/immunization/vaccine-preventable-diseases/invasive-pneumococcal-disease/health-professionals.html
- Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839-46.
- Slotved H-, Dalby T, Harboe ZB, Valentiner-Branth P, de Casadevante VF, Espenhain L, et al. The incidence of invasive pneumococcal serotype 3 disease in the Danish population is not reduced by PCV-13 vaccination. Heliyon. 2016;2(11).
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir Med. 2016;4(5):399-406.
- Azzari C, Cortimiglia M, Nieddu F, Moriondo M, Indolfi G, Mattei R, et al. Pneumococcal serotype distribution in adults with invasive disease and in carrier children in Italy: Should we expect herd protection of adults through infants' vaccination? Hum Vaccines Immunother. 2016;12(2):344-50.
- Savulescu C, Pastore Celentano L, Hanquet G. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease. Results of SpIDnet2 - A European multicentric study (2012-2014). (Poster). 10th International Symposium on Pneumococci and Pneumococcal Diseases. In press 2016.
- Andrews NJ, Waight PA, George RC, Slack MPE, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802-8.
- Slotved H. Other age groups than children need to be considered as carriers of Streptococcal pneumoniae serotypes. Hum Vaccines Immunother. 2016;12(10):2670-4.
- Canadian Antimicrobial Resistance Surveillance System - Report 2016 [Internet]. Canada: Public Health Agency of Canada; 2016 [updated September, 2016; cited November 1, 2017]. Available from: http://healthycanadians.gc.ca/publications/drugs-products-medicaments-produits/antibiotic-resistance-antibiotique/alt/pub-eng.pdf
- LeBlanc JJ, ElSherif M, Ye L, MacKinnon-Cameron D, Li L, Ambrose A, et al. Burden of vaccine-preventable pneumococcal disease in hospitalized adults: A Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) network study. Vaccine. 2017 Jun 22;35(29):3647-54.
- Vaccine uptake in Canadian adults: Results from the 2014 adult National Immunization Coverage Survey (aNICS) [Internet]. Canada: Government of Canada; 2016 [updated February 24, 2016; cited November 1, 2017]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-uptake-canadian-adults-results-2014-adult-national-immunization-coverage-survey.html
- Childhood National Immunization Coverage Survey, 2015 [Internet]. Canada: Statistics Canada; 2016 [updated 28 June, 2016; cited November 1, 2017]. Available from: http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5185
- National Advisory Committee on Immunization. An Advisory Committee Statement (ACS). Re-Immunization with Polysaccharide 23-Valent Pneumococcal Vaccine (Pneu-P-23). CCDR. 2015;41(ACS-3):1-28.
- National Advisory Committee on Immunization. An Advisory committee Statement (ACS). Statement on the Use of Conjugate Pneumococcal Vaccine-- 13 Valent in Adults (Pneu-C-13). CCDR. 2013;39(ACS-5):1-52.
- Update on the Use of Conjugate Pneumoccoccal Vaccines in Childhood [Internet]. Canada: Public Health Agency of Canada; 2010 [updated November, 2010; cited November 2, 2017]. Available from: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2010-36/canada-communicable-disease-report-3.html
- Canadian Immunization Guide: Part 4 - Active Vaccines. Pneumoccocal Vaccine [Internet]. Canada: Public Health Agency of Canada; 2016 [updated December 22, 2016; cited November 2, 2017]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-16-pneumococcal-vaccine.html
- Kraicer-Melamed H, O'Donnell S, Quach C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: A systematic review and meta-analysis. Vaccine. 2016;34(13):1540-50.
- Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. New Engl J Med. 2015;372(12):1114-25.
- Statement on the Use of Conjugate Pneumococcal Vaccine - 13 Valent in Adults (Pneu-C-13) [Internet].: Government of Canada; 2013 []. Available from: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2013-39/statement-on-use-conjugate-pneumococcal-vaccine-13-valent-adults-pneu-13.html
- Domínguez À, Salleras L, Fedson DS, Izquierdo C, Ruíz L, Ciruela P, et al. Effectiveness of pneumococcal vaccination for elderly people in Catalonia, Spain: A case-control study. Clin Infect Dis. 2005;40(9):1250-7.
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. New Engl J Med. 2003;348(18):1747-55.
- Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: The EVAN-65 study. Clin Infect Dis. 2006;43(7):860-8.
- Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med. 1988;108(5):653-7.
- Shapiro ED, Clemens JD. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Ann Intern Med. 1984;101(3):325-30.
- Ansaldi F, Turello V, Lai P, Batone G, De Luca S, Rosselli R, et al. Effectiveness of a 23-valent polysaccharide vaccine in preventing pneumonia and non-invasive pneumococcal infection in elderly people: A large-scale retrospective cohort study. J Int Med Res. 2005;33(5):490-500.
- Christenson B, Hedlund J, Lundbergh P, Örtqvist Å. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J. 2004;23(3):363-8.
- Christenson B, Pauksen K, Sylvan SPE. Effect of influenza and pneumococcal vaccines in elderly persons in years of low influenza activity. Virol J. 2008;5.
- Hechter RC, Chao C, Jacobsen SJ, Slezak JM, Quinn VP, Van Den Eeden SK, et al. Clinical effectiveness of pneumococcal polysaccharide vaccine in men: California Men's Health Study. Vaccine. 2012;30(38):5625-30.
- Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged =60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis. 2014;58(7):909-17.
- Song JY, Lee JS, Wie S-, Kim HY, Lee J, Seo YB, et al. Prospective cohort study on the effectiveness of influenza and pneumococcal vaccines in preventing pneumonia development and hospitalization. Clin Vaccine Immunol. 2015;22(2):229-34.
- Tsai Y-, Hsieh M-, Chang C-, Wen Y-, Hu H-, Chao Y-, et al. The 23-valent pneumococcal polysaccharide vaccine is effective in elderly adults over 75 years old-Taiwan's PPV vaccination program. Vaccine. 2015;33(25):2897-902.
- Jackson ML, Nelson JC, Jackson LA. Risk factors for community-acquired pneumonia in immunocompetent seniors. J Am Geriatr Soc. 2009;57(5):882-8.
- Leventer-Roberts M, Feldman BS, Brufman I, Cohen-Stavi CJ, Hoshen M, Balicer RD. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive disease and hospital-treated pneumonia among people aged ≤ 65 years: A retrospective case-control study. Clin Infect Dis. 2015;60(10):1472-80.
- Loeb M, Neupane B, Walter SD, Hanning R, Carusone SC, Lewis D, et al. Environmental risk factors for community-acquired pneumonia hospitalization in older adults. J Am Geriatr Soc. 2009;57(6):1036-40.
- Skull SA, Andrews RM, Byrnes GB, Kelly HA, Nolan TM, Brown GV, et al. Prevention of community-acquired pneumonia among a cohort of hospitalized elderly: Benefit due to influenza and pneumococcal vaccination not demonstrated. Vaccine. 2007;25(23):4631-40.
- Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, et al. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: A matched case-control study. Vaccine. 2009;27(10):1504-10.
- Wiemken TL, Carrico RM, Klein SL, Jonsson CB, Peyrani P, Kelley RR, et al. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumoniae community-acquired pneumonia in the elderly differs between the sexes: RESULTS from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine. 2014;32(19):2198-203.
- Htar MTT, Stuurman AL, Ferreira G, Alicino C, Bollaerts K, Paganino C, et al. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. PLoS ONE. 2017;12(5).
- Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Global Health. 2017;5(1):e51-9.
- Zhou Z, Deceuninck G, Lefebvre B, De Wals P. Forecasting Trends in Invasive Pneumococcal Disease among Elderly Adults in Quebec. Can J Infect Dis Med Microbiol. 2017;2017.
- Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998-2007: Update from the calgary-area Streptococcus pneumoniae research (Casper) study. Clin Infect Dis. 2009;49(2):205-12.
- Leal J, Vanderkooi OG, Church DL, MacDonald J, Tyrrell GJ, Kellner JD. Eradication of invasive pneumococcal disease due to the seven-valent pneumococcal conjugate vaccine serotypes in Calgary, Alberta. Pediatr Infect Dis J. 2012;31(9).
- Sahni V, Naus M, Hoang L, Tyrrell GJ, Martin I, Patrick DM. The epidemiology of invasive pneumococcal disease in British Columbia following implementation of an infant immunization program: Increases in herd immunity and replacement disease. Can J Public Health. 2012;103(1):29-33.
- Cabaj JL, Nettel-Aguirre A, MacDonald J, Vanderkooi OG, Kellner JD. Influence of Childhood Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Adults with Underlying Comorbidities in Calgary, Alberta (2000-2013). Clin Infect Dis. 2016;62(12):1521-6.
- Lim GH, Wormsbecker AE, McGeer A, Pillai DR, Gubbay JB, Rudnick W, et al. Have changing pneumococcal vaccination programmes impacted disease in Ontario? Vaccine. 2013;31(24):2680-5.
- Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995-2011. Vaccine. 2013;31(49):5863-71.
- Desai S, Policarpio ME, Wong K, Gubbay J, Fediurek J, Deeks S. The epidemiology of invasive pneumococcal disease in older adults from 2007 to 2014 in Ontario, Canada: a population-based study. CMAJ Open. 2016 Sep 29;4(3):E545-50.
- Programme de surveillance du pneumocoque. Rapport 2014 [Internet]. Quebec, Canada: Institut national de santé publique Québec, Laboratoire de santé publique du Québec; 2015 [updated May, 2015; cited November 2, 2017]. Available from: https://www.inspq.qc.ca/sites/default/files/publications/2081_surveillance_pneumocoque.pdf
- Demczuk W.H.B., Martin I, Griffith A., Lefebvre B., McGeer A., Lovgren M., et al. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010-2012. Can J Microbiol. 2013;59:778-88.
- Nelson JC, Jackson M, Yu O, Whitney CG, Bounds L, Bittner R, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26(38):4947-54.
- Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369(9568):1179-86.
- Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio. 2011;2(1).
- Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. New Engl J Med. 2013;369(2):155-63.
- Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: A time series analysis. Lancet Respir Med. 2014;2(5):387-94.
- Rodrigo C., Bewick T., Sheppard C., Greenwood S., Mckeever T.M., Trotter C.L., et al. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. European Respiratory Journal. 2015 01 Jun 2015;45(6):1632-41.
- Nair H, Watts AT, Williams LJ, Omer SB, Simpson CR, Willocks LJ, et al. Pneumonia hospitalisations in Scotland following the introduction of pneumococcal conjugate vaccination in young children. BMC Infect Dis. 2016;16(1).
- Menzies RI, Jardine A, McIntyre PB. Pneumonia in Elderly Australians: Reduction in Presumptive Pneumococcal Hospitalizations but No Change in All-Cause Pneumonia Hospitalizations Following 7-Valent Pneumococcal Conjugate Vaccination. Clin Infect Dis. 2015;61(6):927-33.
- Pletz MW, Ewig S, Rohde G, Schuette H, Rupp J, Welte T, et al. Impact of pneumococcal vaccination in children on serotype distribution in adult community-acquired pneumonia using the serotype-specific multiplex urinary antigen detection assay. Vaccine. 2016;34(20):2342-8.
- Lin SH, Tan CK, Lai CC, Wang CY, Hsueh PR. Declining incidence of nonbacteremic pneumococcal ppneumonia in hospitalized elderly patients at a tertiary care hospital after the introduction of pneumococcal vaccines in Taiwan, 2004 to 2008. J Am Geriatr Soc. 2010 Jan;58(1):195,196 Erratum in J.Am.Geriatr.Soc 2010, 58, 4, 810.
- Patrzalek M, Gorynski P, Albrecht P. Indirect population impact of universal PCV7 vaccination of children in a 2 + 1 schedule on the incidence of pneumonia morbidity in Kielce, Poland. Eur J Clin Microbiol Infect Dis. 2012;31(11):3023-8.
- Katoh S, Suzuki M, Ariyoshi K, Morimoto K. Serotype replacement in adult pneumococcal pneumonia after the introduction of seven-valent pneumococcal conjugate vaccines for children in Japan: a systematic literature review and pooled data analysis. Jpn J Infect Dis. 2017 Mar 28.
- Becker-Dreps S, Amaya E, Liu L, Rocha J, Briceño R, Moreno G, et al. Impact of a combined pediatric and adult pneumococcal immunization program on adult pneumonia incidence and mortality in Nicaragua. Vaccine. 2015;33(1):222-7.
- van Werkhoven CH, Hollingsworth RC, Huijts SM, Bolkenbaas M, Webber C, Patterson S, et al. Pneumococcal conjugate vaccine herd effects on non-invasive pneumococcal pneumonia in elderly. Vaccine. 2016;34(28):3275-82.
- Shigayeva A, Rudnick W, Green K, Tyrrell G, Demczuk WH, Gold WL, et al. Association of serotype with respiratory presentations of pneumococcal infection, Ontario, Canada, 2003-2011. Vaccine. 2016 Feb 3;34(6):846-53.
- Miller ER, Moro PL, Cano M, Lewis P, Bryant-Genevier M, Shimabukuro TT. Post-licensure safety surveillance of 23-valent pneumococcal polysaccharide vaccine in the Vaccine Adverse Event Reporting System (VAERS), 1990-2013. Vaccine. 2016 May 27;34(25):2841-6.
- De Wals P, Petit G, Erickson LJ, Guay M, Tam T, Law B, et al. Benefits and costs of immunization of children with pneumococcal conjugate vaccine in Canada. Vaccine. 2003;21(25-26):3757-64.
- Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol. 2007;18(2):121-7.
- Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Karkola K, Korppi M, et al. Incidence of community-acquired pneumonia in the population of four municipalities in Eastern Finland. Am J Epidemiol. 1993;137(9):977-88.
- Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Kleemola M, Koskela M, et al. Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin Infect Dis. 2001;32(8):1141-54.
- Marrie TJ, Low DE, de Carolis E, Duperval R, Field S, Louie T, et al. A comparison of bacteremic pneumococcal pneumonia with nonbacteremic community-acquired pneumonia of any etiology - Results from a Canadian multicentre study. Can Respir J. 2003;10(7):368-74.
- Honkanen PO, Keistinen T, Miettinen L, Herva E, Sankilampi U, Läärä E, et al. Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia and pneumococcal pneumonia among persons aged 65 years or older. Vaccine. 1999;17(20-21):2493-500.
- Menzies RI, Jayasinghe SH, Krause VL, Chiu CK, McIntyre PB. Impact of pneumococcal polysaccharide vaccine in people aged 65 years or older. Med J Aust. 2014;200(2):112-5.
- Link-Gelles R, Taylor T, Moore MR. Forecasting invasive pneumococcal disease trends after the introduction of 13-valent pneumococcal conjugate vaccine in the United States, 2010-2020. Vaccine. 2013;31(22):2572-7.
- O'Reilly R, Kowng J, McGeer A, To T, Sander B. The Cost-Effectiveness of a Pneumococcal Conjugate Vaccine (PCV13) Program for Older Adults (65+) in Ontario, Canada in the Context of Infant Immunization and Changing Serotype Distributions. Society for Medical Decision Making 39th Annual North American Meeting (Poster Session 1); October 22, 2017; Pittsburgh, PA.; 2017.
- Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2013;31(35):3577-84.
- Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31(35):3585-93.
- Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: Double blind, randomised and placebo controlled trial. BMJ (Online). 2010;340(7746):579.
- Ortqvist A, Hedlund J, Burman L-, Elbel E, Hofer M, Leinonen M, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351(9100):399-403.
- Alfageme I, Vazquez R, Reyes N, Muñoz J, Fernández A, Hernandez M, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006;61(3):189-95.
- Kawakami K, Ohkusa Y, Kuroki R, Tanaka T, Koyama K, Harada Y, et al. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine. 2010;28(43):7063-9.
- Wright LB, Hughes GJ, Chapman KE, Gorton R, Wilson D. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in people aged 65 years and over in the North East of England, April 2006-July 2012. Trails Vaccinology. 2013;2(1):45-8.
- Gutiérrez Rodríguez MA, Ordobás Gavín MA, García-Comas L, Sanz Moreno JC, CóRdoba Deorador E, Lasheras Carbajo MD, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the region of Madrid, Spain, 2008-2011. Eurosurveillance. 2014;19(40).
- Vila-Corcoles A, Ochoa-Gondar O, Guzmán JA, Rodriguez-Blanco T, Salsench E, Fuentes CM. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infect Dis. 2010;10.
- Domínguez A, Izquierdo C, Salleras L, Ruiz L, Sousa D, Bayas J-, et al. Effectiveness of the pneumococcal polysaccharide vaccine in preventing pneumonia in the elderly. Eur Respir J. 2010;36(3):608-14.
- Domínguez À, Soldevila N, Toledo D, Torner N, Force L, Pérez MJ, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccination in preventing community-acquired pneumonia hospitalization and severe outcomes in the elderly in Spain. PLoS ONE. 2017;12(2).
- Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Gutierrez-Perez A, Vila-Rovira A, Group ES. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in patients with chronic pulmonary diseases. Human Vaccines & Immunotherapeutics. 2012 05/01;8(5):639-44.
- Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313-21.
- Ochoa-Gondar O, Vila-Corcoles A, Ansa X, Rodriguez-Blanco T, Salsench E, de Diego C, et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine. 2008 Apr 7;26(16):1955-62.
- Rodriguez-Barradas MC, Goulet J, Brown S, Goetz MB, Rimland D, Simberkoff MS, et al. Impact of Pneumococcal Vaccination on the Incidence of Pneumonia by HIV Infection Status among Patients Enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis. 2008 Apr 1;46(7):1093-100.
- Hung IF, Leung AY, Chu DW, Leung D, Cheung T, Chan CK, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis. 2010 Nov 1;51(9):1007-16.
- Programme de surveillance du pneumocoque: rapport 2014 [Internet]. Québec, Canada: Laoratoire de santé publique du Québec; 2016 [updated Feb 4, 2016; cited Dec 6, 2017]. Available from: https://www.inspq.qc.ca/en/node/4680
Page details
- Date modified: