The Future of COVID-19: What's Next in the Drug Pipeline
Presented at ISPOR 2023, May 7-10, 2023
Allison Carey
Introduction
COVID-19 has created a major health pandemic worldwide. Scientists, drugmakers and governments have moved with unprecedented speed to develop and manufacture vaccines and therapeutics for the prevention and treatment of the novel coronavirus. Many new and repurposed medicines such as antivirals and monoclonal antibodies have been studied for the treatment of COVID-19. This poster provides a snapshot of the global COVID-19 pipeline and a breakdown of the various vaccines and treatments undergoing clinical evaluation, as well as a look at what the future may hold for this pandemic, including themes predicted for 2023.
Approach
Using GlobalData's drug product database, medicines indicated for COVID-19 undergoing clinical trials were extracted based on a development stage of Phase I, II, III, and pre-registration. All such medicines were assessed for this analysis, both new and existing (repurposed medicines). New medicines were identified as those that have not yet been marketed for any indication, while existing medicines include previously marketed therapies that have been repurposed for new indications related to the treatment of COVID-19.
The World Health Organization data tracker was used to compile the COVID-19 future predictions and vaccine development on a global scale. Other sources include Our World in Data and the Organisation for Economic Co-Operation and Development (OECD).
1. What types of drugs are in the COVID-19 pipeline?
The global pipeline for COVID-19 medicines is growing rapidly, with clinical trials examining novel and existing drugs for the treatment and prevention of COVID-19.
- As of September 2022, the total COVID-19 drug pipeline contained 550 new and repurposed medicines and vaccines. Many of the treatments and vaccines are novel medicines undergoing clinical evaluation for the treatment or prevention of COVID-19. More than half of the drugs in the COVID-19 pipeline are new antivirals, anti-inflammatory drugs, and monoclonal antibodies undergoing clinical evaluation for the treatment of moderate to severe symptoms related to COVID-19.
Figure 1: Number of medicines indicated for the prevention and treatment of COVID-19 by stage of development, 2022
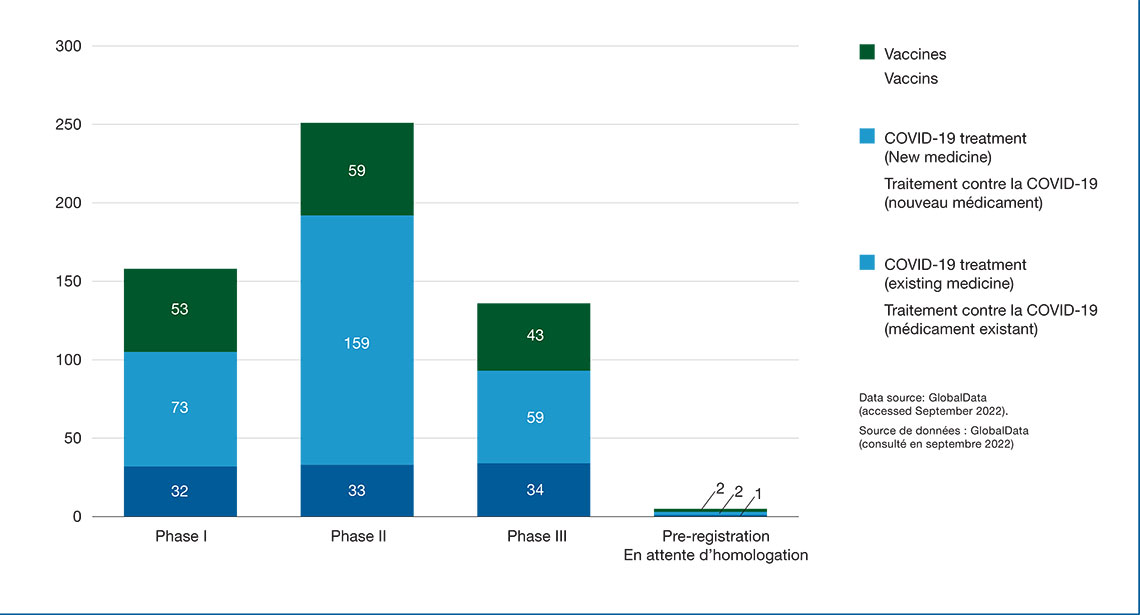
Figure 1 - Text version
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
| Vaccine | 53 | 59 | 43 | 2 |
| New treatment | 73 | 159 | 59 | 1 |
| Repurposed / Redirected treatment | 32 | 33 | 34 | 2 |
| Total | 158 | 251 | 136 | 5 |
Data source: GlobalData (accessed September 2022)
2. What types of vaccines are in the COVID-19 pipeline?
Figure 2 displays the various COVID-19 vaccines categorized by mechanism of action and highest development phase.Footnote 1 Vaccines are categorized into different types based on their mechanism of action; for example, while live attenuated vaccines target the whole virus, subunit and recombinant vaccines target specific parts of the virus.
Figure 2: Distribution of COVID-19 vaccines by mechanism of action and phase of clinical evaluation, 2022

Figure 2 - Text version
| Protein Subunit Vaccine | Inactivated Vaccine | mRNA Vaccine | Recombinant Vector Vaccine | DNA Vaccine | Live Attenuated Vaccine | Total | |
|---|---|---|---|---|---|---|---|
| Phase I | 20 | 2 | 7 | 10 | 12 | 2 | 53 |
| Phase II | 25 | 6 | 13 | 9 | 6 | 0 | 59 |
| Phase III | 16 | 13 | 4 | 5 | 3 | 2 | 43 |
| Pre-registration | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
Data source: GlobalData (accessed September 2022)
3. What’s happening on a global scale?
The global effort to develop and distribute effective vaccines against the COVID-19 coronavirus disease has produced various safe and effective options. The development of multiple vaccines within one year of the virus’s emergence is unprecedented; the process has typically taken eight to fifteen years.
More than thirty vaccines have been approved for general or emergency use in countries around the world. By the end of 2022, over 13 billion had been administered worldwide.
Who is involved in vaccine development?
Governments: Public health agencies have played critical roles in supplying funds to develop COVID-19 vaccines.
International institutions: The WHO and other multilateral institutions such as the World Bank are focused on financing and manufacturing COVID-19 vaccines for global use, in particular to ensure fair allocation among all countries.
Private sector: The pharmaceutical industry has driven much of the push. Companies ranging from biotech start-ups to giants such as Pfizer, Moderna, and Johnson & Johnson shifted their research and development efforts to focus on COVID-19.
Research institutions and non-profits: Many of the COVID-19 vaccine candidates have involved a university or college assisting in preclinical research or clinical trials.
The World Health Organization says COVID-19 remains a global health emergency as the world enters the fourth year of the pandemic. However, many sources including the WHO have forecasted that the world will transition out of the emergency phase of the pandemic in 2023.
Data source: World Health Organization (Coronavirus disease updates) accessed March, 2023, OECD.org (https://www.oecd.org/coronavirus) (accessed March, 2023)
Figure 3: Share of people who completed the initial COVID-19 vaccination protocol, as of March 2023

| Entity | People fully vaccinated per hundred |
|---|---|
| Asia | 72.83 |
| Bangladesh | 81.73 |
| Bulgaria | 30.63 |
| Czechia | 65.69 |
| Europe | 66.23 |
| European Union | 72.9 |
| Greece | 73.62 |
| India | 67.17 |
| Italy | 81.25 |
| Kyrgyzstan | 20.9 |
| Lithuania | 68.38 |
| Malaysia | 81.18 |
| Malta | 88.42 |
| North America | 65.68 |
| Russia | 55.08 |
| South America | 77.12 |
| South Korea | 85.64 |
| Uruguay | 84.8 |
| World | 64.29 |
4. What does the future hold for COVID-19?
As of March 2023, more than 5.5 billion people worldwide have received a dose of a COVID-19 vaccine, equal to about 72.3% of the world population. The following points describe upcoming themes for COVID-19 in 2023.
- New coronavirus variants
- If the coronavirus continues to circulate, new versions of the virus are expected to occur. What’s unknown, however, is how these future variants will impact the course of the pandemic.
- COVID-19 treatments
- The focus in 2023 will be less on development of new monoclonal antibodies and more on antiviral therapies and anti-inflammatory therapies.
- Making progress on long COVID
- Studies are underway to better understand long COVID and its causes, and how it can potentially be treated.
- A new generation of COVID vaccines
- There are several promising candidates in the pipeline, which may provide more durable protection against both severe illness and infection across a wide range of potential variants.
- Combatting COVID fatigue
- Many studies are suggesting that staying up to date on vaccines that target some of the more recently circulating coronavirus variants may prevent or reduce the effects of COVID fatigue.
Data source: World Health Organization (Coronavirus disease updates) accessed March, 2023
GlobalData Healthcare platform COVID-19 updates, accessed March, 2023
Page details
- Date modified:
