2022–23 Departmental Results Report
ISSN: 2561-0732
Patented Medicine Prices Review Board
The Honourable Mark Holland
Minister of Health
Table of contents
From the Chairperson
It is my pleasure to present the 2022-23 Departmental Results Report for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB is an independent quasi-judicial body established by Parliament in 1987 under the Patent Act (the "Act"). The PMPRB has a dual regulatory and reporting mandate: our regulatory mandate is to ensure that the prices of patented medicines sold in Canada are not excessive, and our reporting mandate is to provide stakeholders with pharmaceutical trends information to help them make informed choices.
Long-awaited amendments to the Patented Medicine Regulations ("Regulations") were brought into force in July 2022, directing focus for PMPRB Staff on the redevelopment of new Guidelines to operationalize the regulatory changes. An initial proposal for new Guidelines in October 2022 was consulted on, but ultimately not implemented by the PMPRB. The Board will continue to refine its work into 2023-24 to develop new Guidelines that respond to the comments heard from its many stakeholders across the healthcare system. A complementary Guidelines Monitoring and Evaluation Plan (GMEP) is likewise under review, with the aim of gauging the impact of the amended regulatory framework on key indicators.
In the fall of 2022, the Board's Hearing Panel issued a decision that found that the price of Procysbi (cysteamine bitartrate) was excessive under section 83 and 85 of the Act, directing Horizon Therapeutics Canada to pay just over $22 million to the Receiver General of Canada. A Board Hearing Panel also issued a final Order in the matter of Soliris (eculizumab) in August 2022, discontinuing the redetermination proceedings following a settlement.
As a member of the Health Portfolio, the PMPRB continues to work closely with our federal, provincial, and territorial health partners to align and optimize our respective processes. Likewise, under our National Prescription Drug Utilization Information System (NPDUIS) reporting mandate established by the Minister under s. 90 of the Act, we provided analytical support and expertise to our health partners, as appropriate, in our collective efforts to advance policy work on pan-Canadian initiatives related to pharmaceuticals.
Internally, the PMPRB worked to support the growth of a sustainable, accessible, and equitable public service in 2022-23. The PMPRB's Accessibility Plan was developed in consultation with employees with disabilities and published in December 2022, laying a roadmap for the organization to assess and take action on employment, environmental, technological, communications, programs and services, and cultural barriers. The PMPRB is also committed to advancing its work in response to the Clerk of the Privy Council's Call to Action on Anti-Racism, Equity, and Inclusion in the Federal Public Service, and has implemented Sponsorship and Mentorship programs to support career advancement for equity-deserving employees.
The end of 2022-23 marked a point of change in the PMPRB's leadership, with announcements of the departure of long-term Executive Director, Douglas Clark, as well as my own appointment as Board Chairperson. I would like to take this opportunity to thank Doug for his dedication, commitment, and leadership over the past nine years, and look forward to working with PMPRB Staff in continuing to serve the Canadian public and our stakeholders.
Thomas J. Digby
Chairperson
Results at a glance
Priority 1 – Implement new pricing framework and begin evaluating its impact
The PMPRB responded to the implementation of the Health Canada-sponsored amendments to the Patented Medicines Regulations ("Regulations") in July 2022 with revisions to its draft Guidelines. Following a 60-day consultation process, the Guideline amendments were ultimately put on hold. Consultations will be revisited in 2023-24 under the PMPRB's new leadership.
The PMPRB also further developed its Guidelines Monitoring and Evaluation Plan (GMEP) to monitor and analyze trends in the pharmaceutical market before and after the implementation of the PMPRB's new regulations and proposed Guidelines to assess whether they are working as intended and to inform the need for any future adjustments.
Priority 2 – Support the Government's high-level priorities for the future of pharmaceutical management in Canada
The PMPRB continued to work with federal/provincial/territorial (F/P/T) health partners to align and optimize our respective processes in the context of the new framework and other recent or ongoing reforms that impact pricing and reimbursement.
In the past year, the PMPRB also continued to provide analytical support and expertise to health partners, as appropriate, in efforts to advance policy work in priority areas such as drugs for rare diseases, common formularies, the development of a Canadian drug agency, and other pan-Canadian initiatives to improve the pricing and reimbursement of pharmaceuticals in Canada.
Furthermore, the PMPRB has developed considerable policy and analytical capacity and is frequently used as a resource to support broader efforts by the federal Health Portfolio and pan-Canadian partners to foster a modern and sustainable health system. In 2022-23, the PMPRB focused reporting efforts on key areas for achieving greater savings for the Canadian health care systems in an era where very high-cost medicines pose an increasing threat to the sustainability of public and private drug plans.
For more information on the PMPRB's plans, priorities, and results achieved, see the "Results: what we achieved" section of this report.
Results: what we achieved
Core responsibilities
Regulate Patented Medicine Prices
Description
The Patented Medicine Prices Review Board (PMPRB) regulates the prices of patented medicines by setting non-excessive price ceilings and taking enforcement action before the Board in the event of non-complianceFootnote 1.
Results
The amended Patented Medicines Regulations ("Regulations") came into force on July 1, 2022. Final amendments revised the basket of schedule countries used as comparators in the price review process to the PMPRB11 but opted out from previously proposed amendments related to the submission of transaction-level pricing information from rights holders as well as provisions for the use of pharmacoeconomic factors in reviews.
Owing to these changes, the draft Guidelines proposed by the PMPRB to operationalize the amended Regulations were redeveloped. An additional 60-day consultation period on the proposed Guidelines opened October 6, 2022, but was ultimately put on hold, postponing the implementation of the Guidelines. Consultations are anticipated to resume in 2023-24.
The PMPRB saw a decrease in the rate of medicines with prices within the thresholds set by the 2010 Guidelines this fiscal year, from 84.6% in 2021-22 to 80.9% in 2022-23, although this comparison is not directly comparable since the latter number captures a smaller, and older set of medicines under the Interim Guidance issued in August 2022, which replaces the 2010 Guidelines while new Guidelines are being developed. For the same reason, there was a greater-than-usual percentage of medicines still under review at the point of measurement for this year’s result.
Two significant decisions were issued by the Board in 2022-23. On June 21, 2022, a Board Order granted the discontinuation of a redetermination on the matter of Soliris (eculizumab) and a related settlement agreement. A separate Board decision issued September 1, 2022, found the price of Procysbi (cysteamine bitartrate) to be excessive under sections 83 and 85 of the Act. The Board ordered a payment of excess revenues totalling $22,028,977.26 as well as the reduction of the ceiling price of Procysbi to a non-excessive level. In addition, the PMPRB Chair accepted five Voluntary Compliance Undertakings (VCUs) in 2022-23, three of which resulted in a price reduction. These VCUs, along with settlements and Board Orders over the fiscal year, resulted in excess revenues and potential excess revenues of more than $31 million that were offset by payments to the Government of Canada.
Under its Pharmaceutical Trends Program, the PMPRB published three analytical reports on Canada’s pharmaceutical market in 2022-23, exploring public drug plan expenditures, new medicines entering Canadian and international markets, and new medicines currently in clinical trials. The PMPRB also developed eight poster presentations on a variety of topics for presentation at academic and scientific conferences. These studies have served to support informed decision making on drug pricing in Canadian and international markets and have provided evidence-based context for the regulatory reform.
The introduction of new exceptionally high-priced patented medicines has been a major driver of sales growth in recent years. High-cost medicines now account for close to 60% of all patented medicine sales in Canada, despite being used by less than one percent of Canadians.
Gender-based analysis plus
The PMPRB recognizes that sex and gender differences, race, ethnicity, age and mental or physical disability are factors to consider in the accessibility, affordability and appropriate use of prescription medicines and medical devices. Differences in sex and gender+ roles, income and utilization of health care services can affect access to medicines and health insurance, as well as prescribing patterns and medicine use, and may have important repercussions for health and well-being.
Since the price of a patented medicine does not vary for the sex or gender+ of the user, the PMPRB's price review process does not take explicit account of the diversity of user groups or their economic situation. Lower patented medicine prices, and associated savings for all payers, will benefit all populations directly through lower out-of-pocket costs and indirectly through health system reinvestments and improved access to better care.
Work began in 2022-23 to scope out opportunities through the Pharmaceutical Trends program to report on research topics informed by GBA Plus. Data collected by the PMPRB under regulatory requirements of the Patent Act does not provide disaggregated demographic information. However, innovative use of supplementary data sources used for analysis conducted under the National Prescription Drug Utilization Information System (NPDUIS) reporting mandate established by the Minister under s. 90 of the Act has allowed the PMPRB to start to explore options for the application of GBA Plus in its analytic reporting. Data under NPDUIS includes age and sex but does not include other indicators such as race, ethnicity, or other socio-economic indicators. Also, analysis can be limited by the absence of diagnosis information which would provide more information on the health concern for which a drug is dispensed. The work that began this fiscal year is ongoing, and includes factors such as gender-based analysis of the cost of heart failure on healthcare systems, pharmaceutical spending for seniors, and demographic-driven analyses of drug plan beneficiaries.
United Nations 2030 Agenda for Sustainable Development and the Sustainable Development Goals
The Sustainable Development Goals (SDGs) set out in the UN 2030 Agenda reflect the interconnectedness of the social, economic, and environmental facets of sustainability.
While the majority of the PMPRB's contribution to the UN 2030 Agenda is conducted at an Internal Services level, the PMPRB's Patented Medicine Price Regulation program benefits those most affected by wealth inequality, contributing to a more equitable access to pharmaceuticals for all Canadians. As described in the GBA Plus section, non-excessive prices for patented medicines benefit uninsured Canadians who pay for their prescriptions out of pocket and allow for savings in the healthcare system to be reinvested. With the tools provided by amended Patented Medicine Regulations in July 2022, the strengthened price review process for patented medicines will support the work towards SDG 10: Reduce Inequality Within and Among Countries.
Similarly, the PMPRB is part of the response to health impacts and inequalities resulting from the effects of climate change in coming years under SDG 13: Take Urgent Action to Combat Climate Change and its Impacts. As health system payers look to find flexibility in their budgets and policy options to respond to the changing needs of Canadians, the PMPRB's analytic reporting can support informed decision making towards the development of a sustainable healthcare system, particularly as it provides public and private federal, provincial, territorial, and program-level information on pharmaceutical pricing, spending, and beneficiaries.
Innovation
The PMPRB did not engage in any high-impact innovations in 2022-23. The primary focus of the organization continues to be the reform and modernization of its regulatory framework. To that end, the PMPRB has conducted extensive and far-reaching public consultations. As the PMPRB switches gears from policy development of the new framework to its implementation, it will be monitoring and assessing the impacts of the Guidelines and the effects of the new legal regime both on the external operating context and on internal PMPRB operations. The PMPRB lacks the human or financial resources at this time to take on any additional innovative or experimental initiatives.
Key risks
The PMPRB has taken action to mitigate the potential risks to the achievement of results for its Core Responsibility identified in the 2022-23 Departmental Plan.
Although the amended Regulations were brought into force in 2022, delays to the implementation of corresponding Guidelines required mitigation efforts to prepare for the eventual use of the new regulatory framework in price reviews, to respond to the new terms of the amended Regulations, and to keep rights holders informed about what data to file and how this data will be used for ceiling price calculation and compliance purposes. Mitigation efforts included interim guidance documents published in August 2022, the collection of pricing data for the new basket of schedule countries ("PMPRB11"), the adaptation of technical infrastructure to correspond with the final Regulations, and the implementation of an outreach strategy with rights holders to share tools and information to support their compliance, as well as guidance on prices, as provided for under subsection 98(4) of the Act.
The PMPRB is also continuing its work on a comprehensive Guidelines Monitoring and Evaluation Plan (GMEP) for monitoring and evaluating the effect of the new regulatory framework on price, access, research, and development in Canada. This will allow the PMPRB to keep track of any unintended consequences on patient access to innovative new medicines or clinical trial activity in Canada and move forward with corrective action if warranted. However, the expectation and intent are that the cost savings achieved by public and private drug plans through these reforms will enable public and private drug plans to pay for new, innovative medicines that might otherwise not be affordable given current budget constraints.
The PMPRB is making use of additional funding received through Budget 2017 to prepare for the possible need to contend with contested pricing matters, as voluntary compliance with the PMPRB's non-binding Guidelines may initially decline after implementation. The PMPRB's own dedicated hearing facilities will ensure that it has the capacity to accommodate multiple parallel hearings, if necessary.
Results achieved
The following table shows, for Regulate Patented Medicine Prices, the results achieved, the performance indicators, the targets and the target dates for 2022–23, and the actual results for the three most recent fiscal years for which actual results are available.
| Departmental results | Performance indicators | Target | Date to achieve target | 2020–21 actual results | 2021–22 actual results | 2022–23 actual results |
|---|---|---|---|---|---|---|
| Affordable patented drug prices | % of patented drug prices in Canada below the median of the PMPRB's comparator countriesFootnote * | 50%Footnote † | March 31, 2023 | 58.2% | 59.5% | 43.9% |
| % of patented drug prices in Canada within the thresholds set out in the PMPRB's Guidelines | 95%Footnote ‡ | March 31, 2023 | 86.3% | 84.6% | 80.6%Footnote § |
Note: Results and targets identified in this section are not a factor in the Board's discretion in the exercise of its quasi-judicial powers.
The results for 2022-23 are influenced by several factors related to the PMPRB's pending regulatory reform.
As of July 1, 2022, the amended Regulations stipulate the use of a group of 11 schedule countries as comparators ("PMPRB11"): Australia, Belgium, France, Germany, Italy, Japan, the Netherlands, Norway, Spain, Sweden, and the United Kingdom. Prior to this change, a group of seven comparator countries was used ("PMPRB7"), including the United States and Switzerland, which have some of the highest prices for patented medicines in the Organisation for Economic Co-operation and Development (OECD). Because of this change, the result for the percentage of patented drug prices below the median of the PMPRB comparator countries in 2022-23 is not directly comparable to those calculated for previous years.
The target for this result was established at the introduction of the indicator in 2015-16, under the premise that the PMPRB would continue to conduct its price reviews without significant changes in its regulatory framework. Analysis in the PMPRB's 2015 Annual Report indicated that the percentage of patented medicines priced below the median price of the PMPRB's comparator countries was 51.8%, a decline from the previous two years. Based on these factors, it was determined that 50.0% would be a reasonable target, primarily as a result of voluntary compliance with the non-binding Guidelines by patentees. However, as the regulatory framework has since been modified and the schedule countries revised, these targets and indicators will need to be reassessed in the coming reporting cycles.
Similarly, as the terms of the price review process have changed to accommodate the amended Regulations, and in the process of development of a corresponding set of Guidelines, the number of medicines without a completed review was much greater as of March 31, 2023, than in previous years. This means that the result presented for this indicator in 2022-23 may not be directly comparable to previous years.
Finally, it should be noted that the results stated above do not have a direct cause-and-effect relationship with the price factors measured under the Guidelines. The PMPRB Guidelines are non-binding and the PMPRB cannot dictate price ceilings outside of the hearing context, which is a process that cannot be fettered by the Guidelines or the PMPRB's Departmental Results Framework. For this reason, the departmental targets and actual results should be considered as a comparative indicator of where pricing stands relative to a set metric, and not as a statement of cause-and-effect or a statement of what the definition of an "excessive price" may be for any particular medicine.
Financial, human resources and performance information for the PMPRB's program inventory is available in GC InfoBaseFootnote i.
Budgetary financial resources (dollars)
The following table shows, for Regulate Patented Medicine Prices, budgetary spending for 2022–23, as well as actual spending for that year.
| 2022–23 Main Estimates | 2022–23 planned spending | 2022–23 total authorities available for use | 2022–23 actual spending (authorities used) | 2022–23 difference (actual spending minus planned spending) |
|---|---|---|---|---|
| 13,870,473 | 13,870,473 | 14,229,198 | 8,687,581 | (5,541,617)Footnote * |
Financial, human resources and performance information for the PMPRB’s program inventory is available in GC InfoBaseFootnote ii.
Human resources (full-time equivalents)
The following table shows, in full-time equivalents, the human resources the department needed to fulfill this core responsibility for 2022–23.
| 2022–23 planned full-time equivalents | 2022–23 actual full-time equivalents | 2022–23 difference (actual full-time equivalents minus planned full-time equivalents) |
|---|---|---|
| 60 | 55 | (5)Footnote * |
Financial, human resources and performance information for the PMPRB's program inventory is available in GC InfoBaseFootnote iii.
Internal services
Description
Internal services are those groups of related activities and resources that the federal government considers to be services in support of programs and/or required to meet corporate obligations of an organization. Internal services refers to the activities and resources of the 10 distinct service categories that support program delivery in the organization, regardless of the internal services delivery model in a department. The 10 service categories are:
- acquisition management services
- communication services
- financial management services
- human resources management services
- information management services
- information technology services
- legal services
- material management services
- management and oversight services
- real property management services
Results
In 2022-23, the PMPRB engaged with employment equity and wellness initiatives to strengthen its internal operations and support staff.
As part of the Clerk of the Privy Council's Call to Action on Anti-Racism, Equity and Inclusion in the Federal Public Service,Footnote iv the PMPRB is on the Mentorship Plus Interdepartmental Working group for Small Departments and Agencies and has taken significant steps to implement this initiative over the past fiscal year. Mentorship Plus is a new initiative co‑developed by members of employment equity (EE) and equity‑seeking groups to better support leadership development, with specific emphasis on supporting members of underrepresented groups who aspire to leadership and executive positions. The PMPRB launched the Sponsorship component of this initiative in 2021-22, and the Mentorship component in May 2022. Work is ongoing on the remaining action items from the Chairperson's letter to the Clerk of the Privy CouncilFootnote v.
In response to Part 7(1)(a) of The Accessible Canada Act,Footnote vi the PMPRB released its first Accessibility PlanFootnote vii in December 2022. The Plan was developed in consultation with employees with disabilities to incorporate best accessibility practices and to create a work environment that is welcoming and conducive to success, with leadership that models and reinforces accessibility-positive attitudes and practices. Work is underway to implement the requirements set out in the Government of Canada's Accessibility StrategyFootnote viii and to build on this foundation to cultivate a pro-accessibility culture and operational standard at the PMPRB.
Work began in 2022-23 on the PMPRB's 2023 to 2027 Departmental Sustainable Development Strategy. The Strategy will lay out the PMPRB's sustainability vision and the actions the PMPRB has undertaken and will undertake in support of the targets set out in Canada's Federal Sustainable Development StrategyFootnote xi. It will be tabled and made publicly available by November 2, 2023.
As reforms to the PMPRB's Guidelines are ongoing, a review of the Departmental Results Framework (DRF) has not been initiated. Performance indicators will be reassessed following the full implementation of the amended regulatory framework.
Contracts awarded to Indigenous businesses
The PMPRB is a Phase 1 department and as such must ensure that a minimum 5.0% of the total value of the contracts it awards to Indigenous businesses by the end of 2022-23. In its 2023–24 Departmental Plan, the PMPRB forecasted that, by the end of 2022–23, it would award 5.7% of the total value of its contracts to Indigenous businesses.
As shown in the following table, the PMPRB far exceeded this estimate, awarding 18.4% of the total value of its contracts to Indigenous businesses in 2022–23.
| Contracting performance indicators | 2022-23 Results |
|---|---|
| Total value of contractsnote de bas de page * awarded to Indigenous businessesnote de bas de page † (A) | $245,219 |
| Total value of contracts awarded to Indigenous and non Indigenous businessesnote de bas de page ‡ (B) | $1,329,643 |
| Value of exceptions approved by deputy head (C) | $0 |
| Proportion of contracts awarded to Indigenous businesses [A / (B−C) × 100] | 18.4% |
In compliance with Indigenous Services Canada requirements, the PMPRB implemented a strategy to increase procurement from Indigenous businesses and suppliers to a minimum of 5.0% of the value of all contracts from 2022-23 onward. To meet this target, Indigenous suppliers were sourced for information technology (IT) equipment such as tablets and monitors, IT services, graphic design, and contracted web development services in 2022-23.
Procurement from Indigenous suppliers has been incorporated into the budget planning and monitoring cycle, as well as in the Operational Plan cycle, to ensure that the 5.0% target continues to be met or exceeded in coming years. Half of procurement staff at the PMPRB have completed Indigenous Considerations in Procurement (COR409) from the Canada School of Public Service, while the other half are in the process of completing the course.
Budgetary financial resources (dollars)
The following table shows, for internal services, budgetary spending for 2022–23, as well as spending for that year.
| 2022–23 Main Estimates | 2022–23 planned spending | 2022–23 total authorities available for use | 2022–23 actual spending (authorities used) | 2022–23 difference (actual spending minus planned spending) |
|---|---|---|---|---|
| 3,132,740 | 3,132,740 | 3,473,105 | 3,333,947 | (139,158) |
Human resources (full-time equivalents)
The following table shows, in full-time equivalents, the human resources the department needed to carry out its internal services for 2022–23.
| 2022–23 planned full-time equivalents | 2022–23 actual full-time equivalents | 2022–23 difference (actual full-time equivalents minus planned full-time equivalents) |
|---|---|---|
| 24 | 22 | (2) |
Spending and human resources
Spending
Spending 2020–21 to 2025–26
The following graph presents planned (voted and statutory spending) over time.
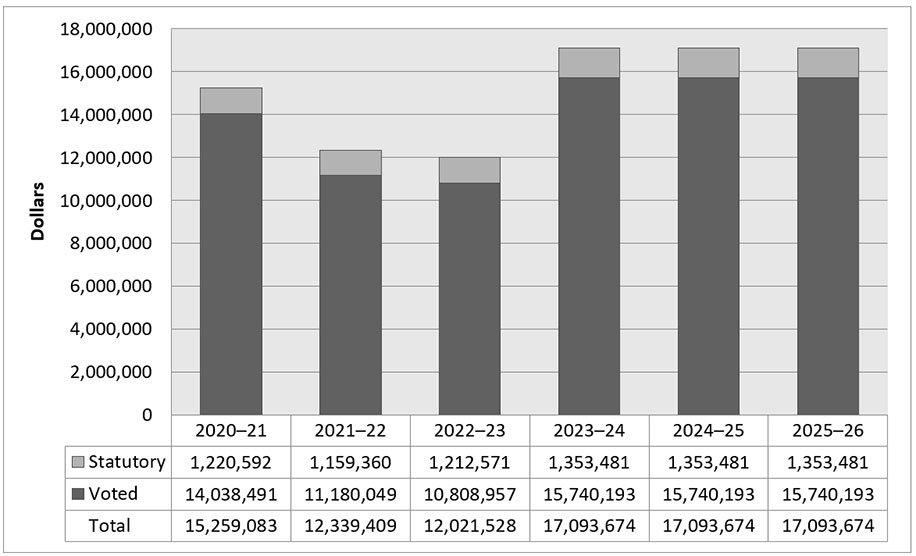
Figure 1 - Version texte
| 2020–21 | 2021–22 | 2022–23 | 2023–24 | 2024–25 | |
|---|---|---|---|---|---|
| Statutory | 1,220,592 | 1,159,360 | 1,212,571 | 1,353,481 | 1,353,481 |
| Voted | 14,038,491 | 11,180,049 | 10,808,957 | 15,740,193 | 15,740,193 |
| Total | 15,259,083 | 12,339,409 | 12,021,528 | 17,093,674 | 17,093,674 |
As announced in Budget 2017, the PMPRB received additional funding for future years; $3,849,215 in 2018-19, $5,694,677 in 2019-20, $6,671,853 in 2020-21, $7,668,725 in 2021-22 and $5,680,633 in 2022-23 and ongoing, including Employee Benefits Payments (EBP) and increased funding for the Special Purpose Allotment (SPA).
Voted spending in 2020-21 was higher than following years due in large part to hearing costs for the matter of the price of "Procysbi" by Horizon Therapeutics Canada, as well as costs associated with the construction of the PMPRB’s dedicated hearing facilities.
For purposes of forecasting Planned Spending for 2023-24 and future years, it is necessary to assume that the entire SPA funding for hearings will be spent because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict. The amount of the SPA for 2023-24 and beyond is $4,463,361.
Budgetary performance summary for core responsibilities and internal services (dollars)
The "Budgetary performance summary for core responsibilities and internal services" table presents the budgetary financial resources allocated for the PMPRB's core responsibilities and for internal services.
| Core respon-sibilities and internal services | 2022–23 Main Estimates | 2022–23 planned spending | 2023–24 planned spending | 2024–25 planned spending | 2022–23 total authorities available for use | 2020–21 actual spending (authorities used) | 2021–22 actual spending (authorities used) | 2022–23 actual spending (authorities used) |
|---|---|---|---|---|---|---|---|---|
| Regulate Patented Medicine Prices | 13,870,473 | 13,870,473 | 13,927,400 | 13,927,400 | 14,229,198 | 10,858,873 | 8,999,721 | 8,687,581 |
| Subtotal | 13,870,473 | 13,870,473 | 13,927,400 | 13,927,400 | 14,229,198 | 10,858,873 | 8,999,721 | 8,687,581 |
| Internal services | 3,132,740 | 3,132,740 | 3,166,274 | 3,166,274 | 3,473,105 | 4,400,210 | 3,339,688 | 3,333,947 |
| Total | 17,003,213 | 17,003,213 | 17,093,674 | 17,093,674 | 17,702,303 | 15,259,083 | 12,339,409 | 12,021,528 |
Planned spending in 2022-23 was higher than Actual spending largely due to a lapse of funding for the Special Purpose Allotment (SPA) to conduct Public Hearings. The SPA can only be used to cover the costs of public hearings, such as external legal counsel and expert witnesses, etc. For purposes of forecasting Planned Spending, it is necessary to assume that the entire SPA funding will be spent because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict. In 2022-23, the SPA was $4,463,360 and the PMPRB only spent $396,067, a difference of $4,067,293. Any unspent amount is returned to the Consolidated Revenue Fund.
Human resources
The "Human resources summary for core responsibilities and internal services" table presents the full-time equivalents (FTEs) allocated to each of the PMPRB's core responsibilities and to internal services.
Human resources summary for core responsibilities and internal services
| Core responsibilities and internal services | 2020–21 actual full-time equivalents | 2021–22 actual full-time equivalents | 2022–23 planned full-time equivalents | 2022–23 actual full-time equivalents | 2023–24 planned full-time equivalents | 2024–25 planned full-time equivalents |
|---|---|---|---|---|---|---|
| Regulate Patented Medicine Prices | 57 | 55 | 60 | 55Footnote * | 58 | 58 |
| Subtotal | 57 | 55 | 60 | 55 | 58 | 58 |
| Internal services | 23 | 23 | 24 | 22 | 23 | 23 |
| Total | 80 | 78 | 84 | 77 | 81 | 81 |
Expenditures by vote
For information on the PMPRB's organizational voted and statutory expenditures, consult the Public Accounts of CanadaFootnote xii.
Government of Canada spending and activities
Information on the alignment of the PMPRB's spending with Government of Canada's spending and activities is available in GC InfoBaseFootnote xiii.
Financial statements and financial statements highlights
Financial statements
The PMPRB's financial statements (unaudited) for the year ended March 31, 2023, are available on the PMPRB's websiteFootnote xiv.
Financial statement highlights
| Financial information | 2022–23 planned results | 2022–23 actual results | 2021–22 actual results | Difference (2022–23 actual results minus 2022–23 planned results) | Difference (2022–23 actual results minus 2021–22 actual results) |
|---|---|---|---|---|---|
| Total expenses | 18,668,777 | 13,404,304 | 13,884,528 | (5,264,473) | (480,224) |
| Total revenues | – | 3,384 | 527 | 3,384 | 2,857 |
| Net cost of operations before government funding and transfers | 18,668,777 | 13,400,920 | 13,884,001 | (5,267,857) | (483,081) |
The 2022–23 planned results information is provided in the PMPRB's Future-Oriented Statement of Operations and Notes 2022–23Footnote xv.
| Financial information | 2022–23 | 2021–22 | Difference (2022–23 minus 2021–22) |
|---|---|---|---|
| Total net liabilities | 1,542,151 | 1,867,625 | (325,474) |
| Total net financial assets | 802,701 | 952,913 | (150,212) |
| Departmental net debt | 739,450 | 914,712 | (175,262) |
| Total non-financial assets | 49,529 | 45,968 | 3,561 |
| Departmental net financial position | (689,921) | (868,744) | 178,823 |
The 2022–23 planned results information is provided in the PMPRB's Future-Oriented Statement of Operations and Notes 2022–23Footnote xvi.
Corporate information
Organizational profile
Appropriate minister[s]:
The Honourable Mark Holland
Institutional head:
Thomas J. Digby, Chairperson
Ministerial portfolio:
Health
Enabling instrument[s]:
Year of incorporation / commencement:
1987
Other:
The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act set out in sections 79 to 103. Although the PMPRB is part of the Health Portfolio, because of its quasi-judicial responsibilities the PMPRB carries out its mandate at arm's length from the Minister. It also operates independently of Health Canada, which approves drugs for safety, efficacy and quality; other Health Portfolio members, such as the Public Health Agency of Canada, the Canadian Institutes of Health Research, and the Canadian Food Inspection Agency; federal, provincial, and territorial (F/P/T) public drug plans, which approve the listing of drugs for their respective formularies for reimbursement purposes; and the Common Drug Review, administered by the Canadian Agency for Drugs and Technologies in Health (CADTH), which recommends drugs that should qualify for reimbursement purposes by participating public drug plans.
Raison d'être, mandate and role: who we are and what we do
"Raison d'être, mandate and role: who we are and what we do" is available on the PMPRB's websiteFootnote xix.
For more information on the department's organizational mandate letter commitments, see the Minister's mandate letterFootnote xx.
Operating context
Information on the operating context is available on the PMPRB's websiteFootnote xxi.
Reporting framework
The PMPRB's departmental results framework and program inventory of record for 2022–23 are shown below.
Departmental Results Framework |
Core Responsibility: Regulate Patented Medicine Prices |
Internal Services |
|
Departmental Results: Affordable patented drug prices |
Indicator 1: % of patented drug prices in Canada below the median price of the PMPRB’s compara-tor countries |
||
Indicator 2: % of patented drug prices in Canada within the threshold set out in the PMPRB’s Guidelines |
|||
Program Inventory |
Patented Medicine Price Regulation Program |
||
Pharmaceutical Trends Program |
|||
Supporting information on the program inventory
Financial, human resources and performance information for the PMPRB's program inventory is available in GC InfoBaseFootnote xxii.
Supplementary information tables
The following supplementary information tables are available on the PMPRB's website:
Federal tax expenditures
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals, and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Report on Federal Tax ExpendituresFootnote xxvi. This report also provides detailed background information on tax expenditures, including descriptions, objectives, historical information and references to related federal spending programs as well as evaluations and GBA Plus of tax expenditures.
Organizational contact information
Mailing address
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario
K1P 1C1
Telephone: 1-877-861-2350
TTY: 613-288-9654
Fax: 613-288-9643
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Website(s): https://www.canada.ca/en/patented-medicine-prices-review.htmlFootnote xxvii
Appendix: definitions
- appropriation
- Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
- budgetary expenditures
- Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
- core responsibility
- An enduring function or role performed by a department. The intentions of the department with respect to a core responsibility are reflected in one or more related departmental results that the department seeks to contribute to or influence.
- Departmental Plan
- A report on the plans and expected performance of an appropriated department over a 3-year period. Departmental Plans are usually tabled in Parliament each spring.
- departmental priority
- A plan or project that a department has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired departmental results.
- departmental result
- A consequence or outcome that a department seeks to achieve. A departmental result is often outside departments’ immediate control, but it should be influenced by program-level outcomes.
- departmental result indicator
- A quantitative measure of progress on a departmental result.
- departmental results framework
- A framework that connects the department’s core responsibilities to its departmental results and departmental result indicators.
- Departmental Results Report
- A report on a department’s actual accomplishments against the plans, priorities and expected results set out in the corresponding Departmental Plan.
- full-time equivalent
- A measure of the extent to which an employee represents a full person-year charge against a departmental budget. For a particular position, the full-time equivalent figure is the ratio of number of hours the person actually works divided by the standard number of hours set out in the person’s collective agreement.
- gender-based analysis plus (GBA Plus)
- An analytical tool used to support the development of responsive and inclusive policies, programs and other initiatives; and understand how factors such as sex, race, national and ethnic origin, Indigenous origin or identity, age, sexual orientation, socio-economic conditions, geography, culture and disability, impact experiences and outcomes, and can affect access to and experience of government programs.
- government-wide priorities
- For the purpose of the 2022–23 Departmental Results Report, government-wide priorities are the high-level themes outlining the government’s agenda in the November 23, 2021, Speech from the Throne: building a healthier today and tomorrow; growing a more resilient economy; bolder climate action; fighter harder for safer communities; standing up for diversity and inclusion; moving faster on the path to reconciliation; and fighting for a secure, just and equitable world.
- horizontal initiative
- An initiative where two or more federal organizations are given funding to pursue a shared outcome, often linked to a government priority.
- non-budgetary expenditures
- Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
- performance
- What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
- performance indicator
- A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
- performance reporting
- The process of communicating evidence-based performance information. Performance reporting supports decision making, accountability and transparency.
- plan
- The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally, a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead to the expected result.
- planned spending
- For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts presented in Main Estimates. A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
- program
- Individual or groups of services, activities or combinations thereof that are managed together within the department and focus on a specific set of outputs, outcomes or service levels.
- program inventory
- Identifies all the department’s programs and describes how resources are organized to contribute to the department’s core responsibilities and results.
- result
- A consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization’s influence.
- statutory expenditures
- Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
- target
- A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
- voted expenditures
- Expenditures that Parliament approves annually through an appropriation act. The vote wording becomes the governing conditions under which these expenditures may be made.
Page details
- Date modified: