Guidelines for Canadian Drinking Water Quality: Dicamba Guideline Technical Document

Download the alternative format
(PDF format, 645 KB, 45 pages)
Organization: Health Canada
Type: Guidelines
Date published: 2022-01-14
Related Topics
Table of Contents
- Guideline
- Executive summary
- 1.0 Exposure considerations
- 2.0 Health considerations
- 3.0 Derivation of the health-based value
- 4.0 Analytical and treatment considerations
- 5.0 Management strategies
- 6.0 International considerations
- 7.0 Rationale
- Appendix A: List of abbreviations
- Appendix B: Canadian water quality data
- 8.0 References
Guideline value
The maximum acceptable concentration (MAC) for dicamba in drinking water is 0.11 mg/L (110 μg/L).
Executive summary
This guideline technical document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water and is based on the assessment of dicamba completed by Health Canada's Pest Management Regulatory Agency.
Exposure
Dicamba is a selective systemic herbicide registered for use on lawn and turf, as well as on industrial and agricultural sites. In 2018, the most recent year for which data are available, more than 100,000 kg of dicamba (as active ingredient) was sold in Canada. Dicamba is released into the environment through surface runoff, spray drift, and leaching from soils. It has the potential to leach into groundwater or move into surface water.
Data provided by provinces and territories that monitor for dicamba indicate that dicamba is not commonly found in source or drinking water in Canada. However, low levels of dicamba have been found in source and treated drinking water in a few Canadian provinces during targeted monitoring programs in agricultural areas where dicamba is applied. Although dicamba is used on food crops, it is rarely detected in foods.
Health effects
In general, dicamba has a low acute toxicity, and repeated dose studies in animals tend to show mostly mild effects, such as decreased body weight, decreased food consumption and behavioural effects. The MAC of 0.11 mg/L (110 µg/L) is based on alterations in clinical chemistry and inflammation of the prostate seen in a 1-year dog study.
Analytical and treatment considerations
The development of drinking water guidelines takes into consideration the ability to both measure the contaminant and remove it from drinking water supplies. Several analytical methods are available for measuring dicamba in water at concentrations well below the MAC.
At the municipal level, treatment technologies are available to decrease dicamba concentrations in drinking water. Advanced oxidation processes achieved the highest removal, with lower removals achieved through oxidation. When using these degradation processes, utilities should be aware of the potential formation of degradation byproducts. Few studies were available on activated carbon adsorption and membrane processes. However, these technologies may be effective. Pilot- and/or bench-scale testing are recommended prior to full-scale implementation.
In cases where dicamba removal is desired at a small system or household level—for example when the drinking water supply is from a private well—a residential drinking water treatment unit may be an option. Adsorption (activated carbon) represents the best potential technology for dicamba removal, while reverse osmosis might also be effective. When using a residential drinking water treatment unit, it is important to take samples of water entering and leaving the treatment unit and to send them to an accredited laboratory for analysis to ensure that adequate dicamba removal is occurring.
Application of the guidelines
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority.
The guidelines are protective against health effects from exposure to dicamba in drinking water over a lifetime. Any exceedance of the MAC should be investigated and followed by the appropriate corrective actions if required. For exceedances in source water where there is no treatment in place, additional monitoring to confirm the exceedance should be conducted. If it is confirmed that dicamba concentrations in the water source are above the MAC, then an investigation to determine the most appropriate way to reduce exposure to dicamba should be conducted. This may include use of an alternate water supply or installation of treatment. Where treatment is already in place and an exceedance occurs, an investigation should be conducted to verify treatment and to determine whether adjustments are needed to lower the treated water concentration below the MAC.
1.0 Exposure considerations
1.1 Sources and uses
Dicamba, or 3,6-dichloro-2-methoxybenzoic acid (C8H6Cl2O3), is a selective systemic herbicide registered for use on lawn and turf, as well as on industrial and agricultural sites (Health Canada, 2007a, 2007b, 2008). It is used for the control of annual and perennial broadleaf weeds and brush (Health Canada, 2008). According to Health Canada's Pest Management Regulatory Agency (PMRA), more than 100 000 kg of dicamba active ingredient was sold in 2018 (Health Canada, 2018). In Alberta, dicamba was listed as one of the top 15 commercial or industrial active ingredients sold in 1998, 2003, 2008 and 2013 (Alberta Environment and Parks, 2015). In Ontario, it was one of the top 10 active ingredients sold or used for agricultural purposes in 2003 (Environment Canada, 2011).
Contamination of water may occur through runoff, spray drift, entry into groundwater, or leaching from soils (CCME, 1999; Health Canada, 2008; NHMRC and NRMMC, 2011). Dicamba has high mobility in soil (Koc = 3.5–21) and may enter surface and groundwater as a result (Health Canada, 2007a, 2007b; EFSA, 2011; US EPA, 2016). Dicamba is highly soluble and does not adsorb onto sediment or other organic particles in water, allowing residues to be easily moved by water (Health Canada, 2007a, 2007b, 2008). Dicamba is moderately persistent in water (half-life = up to 55.9 days) (Health Canada, 2007a, 2007b, 2008; EFSA, 2011). It is more persistent if found in anaerobic groundwater sources (half-life = 141 days), compared to sources with aerobic conditions (half-life = 39.8–45.5 days) (Health Canada 2007a, 2007b; US EPA, 2016). Dicamba may dissipate into the atmosphere, and there is a potential for long-range transport (Health Canada, 2007a, 2007b; EFSA, 2011).
Aerobic biotransformation is the main degradation pathway for dicamba in soil and in aquatic systems. Anaerobic transformation and photodegradation do not contribute substantially to the removal of dicamba from aquatic systems. The major biotransformation product of dicamba, 3,6-dichlorosalicylic acid (3,6-DCSA), is very soluble in water. However, 3,6-DCSA has a low mobility (KKoc = 242–2930), preferentially partitioning to organic matter, and therefore is unlikely to enter groundwater sources (Health Canada, 2007a, 2007b, 2008). 3,6-DCSA is considered to be non-persistent in aerobic conditions (DTK50 = 8.5 days) (Health Canada, 2008). 3,6-DCSA is not expected to dissipate into the atmosphere due to its low volatility (Health Canada, 2007a, 2007b). Other salt forms of dicamba, including the diglycolamine salt, dimethylamine salt, isopropylamine salt, sodium salt, and potassium salt, are expected to dissociate into the dicamba anion and the cation when found in the environment (Health Canada, 2007b). The use of the diethanolamine salt of dicamba has been phased out (Health Canada, 2008).
1.2 Substance identity
Dicamba belongs to the benzoic acid chemical family (Health Canada, 2007a). Properties of dicamba relevant to its presence in drinking water are provided in Table 1.
| Property | Dicamba | Interpretation | |
|---|---|---|---|
| CAS RNFootnote a | 1918-00-9 | Not applicable | |
| Molecular formula | C8H6Cl2O3 | Not applicable | |
| Molecular weight (g/mol) | 221.0 | Not applicable | |
| Water solubility | 6.1 g/L (25°C) | Very soluble | |
| Vapour pressure (volatility) | 3.4 × 10-5 mm Hg at 25°C | Slight potential for volatilization | |
| Octanol-water partition coefficient (Kow) | pH 5.0 6.8 8.9 |
log Kow -0.55 -1.88 -1.9 |
Unlikely to bioaccumulate |
| Henry's law constant | 6.1 × 10-5 Pa m3 mol-1 | Low potential to volatilize from water or moist sediment | |
|
|||
1.3 Exposure
The main sources for Canadians' exposure to dicamba are through food and water, as well as contact with treated plants and sites (Health Canada, 2008).
Water monitoring data from the provinces and territories (municipal and non-municipal supplies), PMRA and Environment Canada (Environment Canada, 2011)(Appendix B) were available for dicamba.
The information provided by the provinces and territories includes fairly small datasets that did not specifically target dicamba for sampling. Where monitoring occurred, the data indicate that dicamba levels are below the method reporting limit (MRL) or method detection limit (MDL) in most samples collected from a variety of water supplies, including surface water, groundwater and treated and distributed water (British Columbia Ministry of Health, 2019; Government of Ontario, 2019; Indigenous Services Canada, 2019; Manitoba Sustainable Development 2019; Ministère de l'Environnement et de la Lutte contre les changements climatiques, 2019; Nova Scotia Environment, 2019; Prince Edward Island Department of Communities, Land and Environment, 2019; Saskatchewan Water Security Agency, 2019). Table 2 summarizes the monitoring data for jurisdictions in which all samples were reported below the MDL. Table 3 summarizes the data for jurisdictions in which dicamba detections were reported. The maximum dicamba concentrations reported are well below the MAC. There were no monitoring data available in New Brunswick, Newfoundland and Labrador or Yukon (New Brunswick Department of Environment and Local Government, 2019; Newfoundland and Labrador Municipal Affairs and Environment, 2019; Yukon Environmental Health Services, 2019).
| Jurisdiction (MDL µg/L) |
Monitoring Period | Type of Water System | Water Type (Municipal: ground/surface - raw, treated, distributed) |
# Detects/Samples |
|---|---|---|---|---|
| British Columbia (0.005–1) |
2013–2018 | Municipal | Surface – raw | 0/18 |
| FNIHBFootnote a Ontario Region (0.2–1) |
2014–2018 | Public water systems | Ground – raw | 0/13 |
| Ground – treated | 0/190 | |||
| Ground – distribution | 0/16 | |||
| Surface – raw | 0/33 | |||
| Surface – treated | 0/308 | |||
| Surface – distribution | 0/23 | |||
| Semi-public water systems | Ground – raw | 0/3 | ||
| Ground – treated | 0/16 | |||
| Ground – distribution | 0/68 | |||
| Surface – raw | 0/1 | |||
| Surface – treated | 0/9 | |||
| Surface – distribution | 0/2 | |||
| Private water systems | Ground – treated | 0/3 | ||
| Ground – distribution | 0/50 | |||
| Surface – treated | 0/5 | |||
| FNIHBFootnote a Atlantic Region (0.50–1) | 2014–2018 | Public water systems | Ground – treated | 0/4 |
| Ground – distribution | 0/4 | |||
| Surface – treated | 0/1 | |||
| FNIHBFootnote a Québec Region (0.03) | 2014–2018 | Drinking water system | Not available | 0/4 |
| Nova Scotia (0.05–2) |
2007–2018 | Municipal | Ground – raw | 0/71 |
| Ground – treated | 0/34 | |||
| Surface – raw | 0/35 | |||
| Surface – treated | 0/40 | |||
| Distributed | 0/1 | |||
| Prince Edward Island (0.001) |
2004–2017 | Municipal | Ground – raw | 0/54 |
| Non-municipal | Ground – raw | 0/52 | ||
| Saskatchewan (0.0001–1) |
2014–2019 | Municipal | Ground and surface – distribution | 0/31 |
| Ground and surface – treated | 0/4 | |||
| Ground – raw | 0/17 | |||
|
||||
| Jurisdiction (MDL µg/L) |
Monitoring Period | Water Type (Municipal: ground/surface – raw, treated, distribution and Non-Municipal: ground) |
# Detects/Samples | Maximum Value (µg/L) |
|---|---|---|---|---|
| Manitoba (0.006–0.075) |
2012–2018 | Surface – ambient | 107/393 | 1.08 |
| Ontario (0.2–10) |
2011–2020 | Surface – treated (municipal) | 4/3807 | 0.42 |
| Ground – treated (municipal) | 2/3957 | 2.49 | ||
| Distribution (municipal) | 0/60 | Not available | ||
| Quebec (0.03) |
2012–2018 | Ground – distribution (municipal) | 0/291 | Not available |
| Surface – distribution (municipal) | 2/1040 | 0.5 | ||
| Ground – rawFootnote a (municipal) | 1/46 | 0.03 | ||
| Ground – treatedFootnote a (municipal) | 0/17 | Not available | ||
| Ground – distributionFootnote a (municipal) | 1/5 | 0.03 | ||
| Ground – rawFootnote b (municipal) | 7/83 | 0.08 | ||
| Ground – rawFootnote b (non-municipal) | 0/19 | Not available | ||
|
||||
As part of its assessment, PMRA collected water quality monitoring data on dicamba from several sources, including scientific studies and provincial reporting. The data included ambient surface water, groundwater and treated municipal drinking water and were supplemented by relevant monitoring information from the United States. These data differ from the provincial and territorial data presented previously as they address water monitoring related to agricultural activity and show that dicamba is ubiquitous in Canadian waters as evidenced by frequent detection. A common detection value (one most often observed) of 0.5 μg/L was determined for municipal drinking water and ambient water sources, and a value of 5 μg/L was determined for farm dugouts that could possibly be used for drinking water. The maximum values estimated from the monitoring data ranged from 5 μg/L in municipal drinking water and ambient water sources to 15 μg/L in farm dugouts (Health Canada, 2007b).
Additional Canadian water monitoring data were available from the literature. In a study of 19 sites in urban rivers and streams across Canada, dicamba was frequently detected in all geographic areas, with concentrations being the highest in Ontario. Median concentrations across all sites ranged from approximately 10 to 40 ng/L, while the maximum concentration was 176 ng/L. Concentrations of dicamba were lower in the spring than in the summer and fall across all geographic areas (Glozier et al., 2012). Another study of 10 urban streams in Ontario examined concentrations of dicamba before (2003–2008) and after (2009–2012) a ban on the sale and use of pesticides for cosmetic purposes. Dicamba was frequently detected (371 out of 386 samples), although concentrations in a majority of the streams decreased significantly after the implementation of the ban. Median concentrations ranged from 2 ng/L to 62 ng/L before the ban, and from 0.1 ng/L to 12 ng/L after the ban. The maximum concentration was 601 ng/L (Todd and Struger, 2014). In a study that looked at the distribution and concentrations of a range of pesticides in watersheds that drain into the lower Great Lakes in Ontario, dicamba was detected at all 25 monitoring sites (Metcalfe et al., 2019). Mean time-weighted average concentrations ranged from 1.2 ng/L to 539 ng/L, while the maximum concentration was 602 ng/L.
Information on dicamba residues in Canadian food was unavailable. The United States Department of Agriculture's (USDA) Pesticide Data Program examined dicamba residues on various food items for the years 1994, 1996–1998 and 2003–2016; no residues were detected in any of the products sampled (fruit, vegetable, or milk) (USDA, 2019). The United States Food and Drug Administration (FDA)'s Total Diet Study from 1991 to 2003 detected dicamba in 3 of 44 samples of white enriched bread, in 23 of 44 samples of oat ring cereal, and in 1 of 44 cracked wheat bread samples, with mean concentrations of 0.00105, 0.00454, and 0.00085 ppm, respectively (FDA, 2019). From 2003 to 2005, dicamba was detected in 4 of 8 samples of oat ring cereal and not in any other products (FDA, 2019).
2.0 Health considerations
All pesticides, including dicamba, are regulated by PMRA. PMRA conducts extensive evaluations and cyclical reviews of pesticides, including unpublished and proprietary information, as well as foreign reviews by other regulatory agencies such as the United States Environmental Protection Agency (US EPA). This health assessment is based primarily on PMRA's evaluations and supporting documentation (Health Canada, 2007a, 2007b, 2008). Additionally, any reviews and relevant literature available since PMRA's evaluations were completed were also considered.
2.1 Kinetics
The available data do not appear to show any species or sex differences in the toxicokinetics of dicamba (FAO/WHO, 2011).
Absorption: Dicamba is readily and rapidly absorbed following oral exposure. Animal studies show estimated absorptions to be above 80% (EFSA, 2011) and peak levels to occur within the first couple of hours after dosing (FAO/WHO, 2011; US EPA, 2016). In rats, absorption was not saturated at doses tested up to 1000 mg/kg (USDA, 2004). Dermal absorption of dicamba is expected to be minimal, although data are limited as compared to oral absorption (USDA, 2004).
Distribution: Dicamba is widely distributed throughout the body, but there is no evidence of accumulation (EFSA, 2011). In animal studies, only 3% of the test dose was found in tissues 4 hours after dosing, with the highest residues found in the kidneys, plasma and uterus (FAO/WHO, 2011).
Metabolism: Dicamba is poorly metabolized and is generally excreted largely unchanged. Observed metabolism pathways include demethylation, hydroxylation and glucuronidation (FAO/WHO, 2011). In a single-dose study with radiolabelled dicamba in rats, mice, rabbits and dogs, 67% to 83% of the radioactivity was eliminated in the urine as the parent compound within 48 hours. Approximately 1% of the administered dose was metabolized to 3,6-DCSA and another 1% to an unidentified metabolite (USDA, 2004). In other studies, very low levels of glucuronidated dicamba, 3,6-DCSA, 5-hydroxy-dicamba, and a DCSA phenolic glucuronide metabolite were found in the urine (FAO/WHO, 2011).
Elimination: Dicamba is rapidly eliminated, with studies showing the half-life to be less than 4 hours and virtually all dicamba to be eliminated in 48 hours (US EPA, 2016). At doses greater than 125 mg/kg bw in rats, the elimination half-life of dicamba equivalents increased, indicating saturation of renal excretion at higher doses (USDA, 2004). Upwards of 95% of dicamba is eliminated in the urine, and less than 5% is eliminated in the feces. Excretion via exhaled air is considered negligible (FAO/WHO, 2011).
2.2 Health effects
The database for the toxicity of dicamba is comprehensive, covering several endpoints and various types of exposure (see USDA (2004) and FAO/WHO (2011) for a more thorough review). In general, dicamba has a low acute toxicity, and repeated dose studies in animals tend to show mostly mild effects.
2.3 Effects in humans
In terms of acute exposures, patients treated for intentional ingestion of dicamba presented with an altered mental state and with elevated levels of lactate, creatine kinase, metabolic acidosis and lipase (Moon and Chun, 2014). Workers exposed in spray operation incidents developed muscle cramps, dyspnea, nausea, vomiting, skin rashes, loss of voice, or swelling of cervical glands (US EPA, 1988). Regarding longer-term exposures, epidemiological studies have investigated various outcomes following dicamba exposure.
Agricultural Health Study: The Agricultural Health Study (AHS) is a large, ongoing questionnaire-based prospective cohort study (over 89,000 participants) investigating cancer and non-cancer endpoints in a cohort of licensed pesticide applicators and their spouses in Iowa and North Carolina. It began in 1993 with the collection of baseline information on farming practices (including pesticide use), lifestyle and health. Follow-up interviews/questionnaires (including dietary information) and DNA collection were done periodically. Cancer registries were used to assess cancer incidence. Overall, strengths of the AHS include its large size, the inclusion of a large number of women, the collection of genetic factors, baseline, health and lifestyle information, the use of cancer registries and the many different pesticides and diseases assessed. Its limitations include the indirect assessment of exposure (questionnaire-based), the lack of exposure refinement measurements (no induction time or latency discussion), and selection bias when controlling for multiple confounders due to the exclusion of many subjects with missing data (Sathiakumar et al., 2011).
Cancer: Several investigators have conducted analyses of the AHS data and found no association between exposure to dicamba and the incidence of bladder cancer (Samanic et al., 2006; Koutros et al., 2016), pancreatic cancer (Andreotti et al., 2009), melanoma (Samanic et al., 2006; Dennis et al., 2010), childhood cancer (Flower et al., 2004) or hematopoietic cancers (Samanic et al., 2006). A significant trend was found between exposure to dicamba and the incidence of lung cancer when the "low-exposure group" was used as a reference but not when the "no-exposure group" was the reference (Alavanja et al., 2004; Samanic et al., 2006). The authors suggest that this might be due to unidentified factors in the non-exposed group confounding results. A significant trend was also found between exposure to dicamba and the incidence of colorectal cancer in a study by Samanic et al. (2006) but not in a study by Lee et al. (2007). The difference in these findings may have been due to differences in exposure classification (intensity-weighted exposure days versus never-ever). No association was found between dicamba and non-Hodgkin lymphoma (NHL) in an analysis of the AHS cohort (Samanic et al., 2006) or in two other studies (De Roos et al., 2003; Hartge et al., 2005). However, a cross-Canada case-control study did find an association between NHL and exposure to dicamba-containing herbicides among men in a diversity of occupations (McDuffie et al., 2001). While no association was found between exposure to dicamba and prostate cancer in the AHS (Samanic et al., 2006), a case-control study of British Columbian farmers did find a significant association (Band et al., 2011).
Non-cancer: In terms of non-cancer endpoints, the risk of hypothyroidism was significantly increased with ever- versus never-use of dicamba in the AHS (Goldner et al., 2013; Shrestha et al., 2018), and an analysis of the Ontario Farm Family Health Study data showed some indication that pre-conception exposure to dicamba could be associated with an increased risk of birth defects in male offspring (Weselak et al., 2008).
Overall, the epidemiological database provides only uncertain indications of associations between dicamba exposure and various health outcomes. In addition to the absence of a clear endpoint and point of departure for dose–response analysis, limitations in the epidemiological studies include small numbers of cases, inconsistency in exposure classification and failure to control for confounders. These limitations mean that the results cannot be used in a quantitative risk assessment.
2.4 Effects in animals
Dicamba has a low acute oral toxicity, with LD50 values in rats ranging from approximately 750 to 3000 mg/kg (USDA, 2004). Short-term oral repeated dose studies in rats and dogs revealed mostly mild effects, including a decrease in body weight gain and food consumption, alterations to hematology and clinical chemistry, and effects in the liver (rat only) (Edson and Sanderson, 1965; Laveglia et al., 1981; Minnema, 1994; FAO/WHO, 2011). In long-term studies with mice and rats, the only adverse effect noted was a slightly reduced body weight gain in mice at 364 mg/kg bw per day, the highest dose tested (Goldenthal, 1985; Crome, 1987). In a 1-year dog study, animals fed dicamba experienced a transient reduction in body weight and food consumption (Blair, 1986). At the highest dose level (65 mg/kg bw per day), males experienced anemia (statistically significant decreases in red blood cell count, hematocrit and hemoglobin levels) at the 6-month mark and small decreases in these parameters at the 12-month mark. A moderate inflammation of the prostate was also observed in two of the four high-dose males. In a 2-year dog study, decreased body weight was observed in males at 0.625 and 1.25 mg/kg bw per day (Davis et al., 1962). However, due to deficient reporting, a lack of statistical analysis, and a lack corroboration in either a 3-month dog toxicity study (Wazeter,1966) or the 1-year dog toxicity study with higher treatment doses (Blair, 1986), the significance of the body weight effect was dismissed.
Behavioural effects were observed in several studies of rabbits and rats at doses over 150 mg/kg bw per day. However, a subchronic neurotoxicity study revealed few signs of neurotoxicity (e.g., impaired gait, increased rigidity, abnormal righting reflex) and only at very high dose levels (males: 767.9 mg/kg bw per day; females: 1028.9 mg/kg bw per day). No evidence of histological effects in nervous system tissues was observed (Minnema, 1994).
Based on developmental studies, dicamba did not cause fetal effects in rats or rabbits, and there was no evidence of the young being more sensitive than adult animals (Smith, 1981; Hoberman, 1992). In a two-generation study in rats, no effects on fertility or reproductive performance were observed (Masters, 1993). However, offspring appeared to be more sensitive than parental animals as demonstrated by a decrease in birth weight in all litters in the absence of any maternal toxicity. Delayed sexual maturation was also seen in the F1 males at the highest concentration. These effects were likely associated with decreased initial growth rates, although other causes (e.g., changes in endocrine function) remain a possibility. The sensitivity of the young to dicamba was thought to be associated with intermediate to long-term exposure of the maternal animal because no similar sensitivity of the young was observed under the short-term exposure scenario of the developmental studies. Furthermore, sensitivity of the young was considered to result from indirect (i.e., in utero) exposure because effects were noted at birth. Parental effects were almost exclusively limited to the first filial generation, suggesting that it developed a higher sensitivity to dicamba, which may be due to in utero exposure (Health Canada, 2007a).
2.5 Genotoxicity and carcinogenicity
The data on the genotoxicity of dicamba are mixed. Both positive results (Waters et al., 1980; Plewa et al., 1984) and negative results (Anderson et al., 1972; Poole et al., 1977; Waters et al., 1980; Eisenbeis et al., 1981; Moriya et al., 1983) have been observed in microbial test systems. In mammalian cells (Chinese hamster ovary (CHO) cells), exposure to dicamba has resulted in a significant increase in micronuclei, nucleoplasmic bridges and nuclear buds (Gonzalez et al., 2011), as well as an increase in the frequency of sister chromatid exchanges (SCE) (Gonzalez et al., 2007). Exposure to dicamba has also resulted in DNA damage (as measured by the single cell gel electrophoresis assay) in one study (Gonzalez et al., 2007) but not in another (Sorensen et al., 2005), possibly due to higher cytotoxic concentrations in the latter study. In human cells, SCEs were observed in whole blood lymphocyte cultures (Gonzalez et al., 2006), and unscheduled DNA synthesis, as well as a very slight but significant increase in SCE frequency, was observed in cultured human peripheral blood lymphocytes (Perocco et al., 1990). In in vivo studies, exposure to dicamba increased DNA unwinding in the rat (Perocco et al., 1990) but was negative for chromosome aberrations in rat bone marrow (Hrelia et al., 1994).
In 2-year dietary carcinogenicity studies in mice and rats, there was no evidence of dicamba being carcinogenic (Goldenthal, 1985; Crome, 1987). However, the rat study was deemed inadequate because the highest dose tested (107 mg/kg bw per day) was below the maximum tolerated dose (MTD) and did not elicit significant effects. It is noted that in a shorter-term study (Minnema, 1994) where rats received approximately fourfold the high dose of rats in the carcinogenicity study, only minor effects were exhibited (Health Canada, 2007a).
The US EPA designated dicamba as not likely to be carcinogenic to humans (US EPA, 2018a), while the International Agency for Research on Cancer has not reviewed the carcinogenicity of dicamba. Given the existence of several positive genotoxicity results and the rat carcinogenicity study that did not reach the MTD, the conclusion that dicamba is non-carcinogenic cannot be considered definitive (Health Canada, 2007a).
2.6 Mode of action
Little information exists on the mechanisms of toxicity of dicamba in humans or animals. Evidence that dicamba can produce DNA and cellular damage in the absence of exogenous metabolic activation (e.g., S9) indicates that damage is likely due to dicamba itself and not to any metabolite. In rats, dicamba has been shown to induce hepatic peroxisomal enzymes and to transcriptionally activate the peroxisomal proliferator activator receptor (Espandiari et al., 1995, 1998). However, in a two-stage hepatocarcinogenesis model, dicamba alone did not increase the number of altered hepatic foci and was inactive as a tumour promoter (Espandiari et al., 1999). Peixoto et al. (2003) investigated the effects of dicamba on rat liver mitochondrial bioenergetic activities. They found that exposure to dicamba resulted in the uncoupling of oxidative phosphorylation through a combination of inhibition of redox complexes and stimulation of proton leakage through the mitochondrial inner membrane. The results indicate that the reduced energy efficiency of the mitochondria following exposure to dicamba may account for some of the observed cytotoxic effects. In another study, Gonzalez et al. (2009) found that the frequency of SCEs and alterations to the cell cycle induced in CHO cells through exposure to dicamba could be attenuated following the addition of vitamin E, a known antioxidant. The results of this study suggest that dicamba may cause genotoxicity through oxidative damage.
2.7 Selected key study
No major data gaps have been identified in the toxicological database for dicamba. In its re-evaluation for the continuing registration of dicamba (PACR2007-02), PMRA identified the study by Blair (1986) as the key study (Health Canada, 2007a, 2019). Of the studies reviewed in the current risk assessment of dicamba, the study by Blair (1986) had the lowest point of departure. In this "good laboratory practice" study, 39-week-old male and female beagle dogs (4/sex/group) were administered technical dicamba (purity 86.8%) in the diet for 52 consecutive weeks. Dogs were exposed to concentrations of 0, 100, 500 or 2500 ppm, equivalent to 0/0, 2.0/2.2, 11.2/11.7 and 58.5/52.2 mg/kg bw per day in males and females, respectively.
Animals were checked twice a day for toxicity and mortality, and body weight was determined weekly. Neurological/behavioural effects in the control and high-dose animals were assessed on three occasions during the study. Clinical chemistry and hematology were evaluated prior to study initiation, at 6 months and prior to study termination. Histology was performed and organ weights were determined at study termination.
During the first week of exposure to the test article, hypophagia was observed in two males at 500 ppm and in two males and one female at 2500 ppm. During the first week, the mean body weights in the control and treated male groups decreased compared with the pretest period values. The weight losses were recovered during week 2, but in the 2500-ppm group, male mean body weight did not increase until week 5. One male did not eat for 3 weeks. Two dogs—one male at 500 ppm and another at 2500 ppm—lost about 11% of their body weight during the first week of the study. Mean food consumption values (g/dog/day) were decreased at 100 ppm. At 2500 ppm, the mean food consumption values were generally increased in males but decreased in females. Statistically significant differences from the control were noted at week 3 for the 100-ppm male group and at weeks 5, 6, 7 and 9 for the 2500-ppm male group.
Erythrocyte counts, hemoglobin and hematocrit were slightly, but statistically significantly, reduced in high-dose males at 6 months. Small but non-significant decreases were also observed at 12 months. In females, serum calcium, total protein and globulin values were slightly decreased and aspartate aminotransferase increased at 2500 ppm at 6 months.
The congestion of the spleen was seen macroscopically and microscopically. Microscopically, congestion was noted in all males at 2500 ppm and in one male at 500 ppm. In females, splenic congestion was observed in two animals at 500 ppm. This finding was considered to be related to the method of sacrifice. Spleen absolute and relative weights were increased in males. Moderate inflammation of the prostate was observed in two males at 2500 ppm.
The no-observed-effect level (NOEL) was approximately 11.2 mg/kg bw per day in this study based on toxicologically significant alterations at the next dose level of 58.5 mg/kg bw per day in males.
3.0 Derivation of the health-based value
As noted above, the study by Blair (1986) was selected as the basis for the current risk assessment. The NOEL of 11.2 mg/kg bw per day is based on alterations in clinical chemistry and inflammation of the prostate at the next dose of 58.5 mg/kg bw per day. Also considered in the derivation of the health-based value is the two-generation rat reproduction study (Masters, 1993), which demonstrated sensitivity in the young following indirect (in utero) exposure. Lack of an acceptable rat carcinogenicity study was also taken into account in the uncertainty factor in the risk assessment.
Using the NOEL of 11.2 mg/kg bw per day, the acceptable daily intake (ADI) (Health Canada, 2019) for dicamba is calculated as follows:
Equation 1
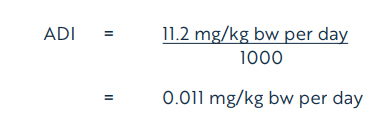
where:
- 11.2 mg/kg bw per day is the NOEL, based on alterations in clinical chemistry and inflammation of the prostate; and
- 1000 is the uncertainty factor, selected to account for interspecies variation (×10), intraspecies variation (×10), and potential sensitivity to the young and the lack of an acceptable carcinogenicity study in the rat (×10)
The ADI of 0.011 mg/kg bw per day is protective of potential concerns identified in the toxicology database for dicamba, including data gaps and potential endocrine effects and potential sensitivity of the young. Based on the ADI of 0.011 mg/kg bw per day, a health-based value (HBV) for dicamba in drinking water is derived as follows:
Equation 2

where:
- 0.011 mg/kg bw per day is the ADI derived above;
- 74 kg is the adult body weight (Health Canada, 2021);
- 1.53 L per day is the daily volume of tap water consumed by an adult (Health Canada, 2021);
- 0.20 is the allocation factor for drinking water.
Since drinking water is not a major source of exposure to dicamba and there is evidence of dicamba in other exposure sources (i.e., food), a floor value of 0.20 (20%) was applied implying that drinking water might contribute anywhere from 0% to 20% of the daily dose (Krishnan and Carrier, 2013).
4.0 Analytical and treatment considerations
4.1 Analytical methods to detect dicamba
Standardized methods available for the analysis of dicamba in source and drinking water and their respective MDLs are summarized in Table 4. MDLs are dependent on the sample matrix, instrumentation, and selected operating conditions and will vary between individual laboratories. These methods are subject to a variety of interferences, which are outlined in the respective references.
A number of accredited laboratories in Canada were contacted to determine MDLs and MRLs for dicamba analysis. The MDLs were in the same order of magnitude as the lower range of those reported in Table 4, and the MRLs were as follows: 0.1 μg/L using high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection; 1 μg/L using gas chromatography mass spectrometry (GC/MS); 0.11 to 0.2 μg/L using gas chromatography with electron capture detector (GC/ECD); and 0.006 to 0.2 μg/L using liquid chromatography with tandem mass spectrometry (LC/MS/MS) (AGAT Laboratories Ltd., 2019; ALS Environmental (Waterloo), 2019; Bureau Veritas Laboratories, 2019; CARO Analytical Services (Richmond Laboratory), 2019; Element Materials Technology Canada Inc., 2019; SGS Environmental Services, 2019).
The MDLs or MRLs from provincial and territorial data are in the range of 0.0001 to 10 μg/L (see section 1.3).
Additional analytical methods that are not currently standardized are available for the measurement of dicamba in water. These methods are based on high-performance liquid chromatography with tandem mass spectrometry (Mann et al., 2016). Similar MDLs to the standard methods listed below have been reported and these methods are suitable for use in commercial laboratories (Haist-Gulde and Sacher, 2019).
Drinking water utilities should discuss sampling requirements with the accredited laboratory conducting the analysis to ensure that quality control procedures are met and that MRLs are low enough to ensure accurate monitoring at concentrations below the MAC. Sample processing considerations and method interferences for the analysis of dicamba in drinking water (e.g., sample preservation, storage) can be found in the references listed in Table 4. It is important to note that quenching is critical if an oxidant is present in samples in order to prevent additional degradation of dicamba prior to analysis.
| Method (Reference) |
Methodology | MDL (µg/L) | Interferences/CommentsFootnote a |
|---|---|---|---|
| EPA 515.1 Rev. 4.1 (US EPA 1995a) |
Gas chromatography with electron capture detector (GC/ECD) | 0.085 | Sample carryover;Footnote b phthalate esters; samples and working standards should be contained in the same solvent |
| EPA 515.2 Rev. 1.1 (US EPA 1995b) |
Liquid-solid extraction and GC/ECD | 0.28 | Reagent contamination; sample carryover;Footnote b phthalate esters; samples and working standards should be contained in same solvent |
| EPA 515.3 Rev.1.0 (US EPA 1996a) |
Liquid-liquid extraction, derivatization, and GC/ECD | 0.30 | Solvent contamination; sample carryover;Footnote b phthalate esters; variable solvents |
| EPA 515.4 Rev. 1.0 (US EPA 2000) |
Liquid-liquid microextraction, derivatization, and fast GC/ECD | 0.032–0.042 | Sodium sulphate; phthalate esters |
| EPA 555 Rev. 1.0 (US EPA 1992) |
High-performance liquid chromatography (HPLC) with a photodiode array ultraviolet (UV) detector | 2.1 | Reagent contamination |
| EPA 8151A Rev. 1 (US EPA 1996b) |
Gas chromatography using methylation or pentafluorobenzylation derivatization | 0.081 | Reagent and solvent contamination |
| ASTM D5317 (ASTM 2011) |
GC/ECD | 0.081Footnote c | Reagent and solvent contamination; Sample carryover;Footnote b alkaline substances; organic acids and phenols; phthalate esters (e.g., flexible plastics) |
|
|||
4.2 Treatment considerations
Treatment technologies available to decrease dicamba concentrations in drinking water have varying effectiveness. These technologies include activated carbon, membrane filtration, oxidation and advanced oxidation.
4.2.1 Municipal-scale treatment
There are a few studies that cover dicamba removal. The information on the removal efficiencies and the operational conditions from these studies are reported in Tables 5 to 7 as they provide an indication of the effectiveness of specific treatment technologies. The selection of an appropriate treatment process will depend on many factors, including the raw water source and its characteristics, the operational conditions of the selected treatment method and the utility's treatment goals. Bench- or pilot-scale testing is recommended to ensure the source water can be successfully treated and optimal process design is established.
When using oxidation or advanced oxidation processes (AOPs) for pesticide removal in drinking water, it is important to be aware of the potential for formation of byproducts due to degradation of the target compound (Ikehata and Gamal El-Din, 2006; Beduk et al., 2012; Li et al., 2019). The primary objective should be removal of the pesticide, with the secondary objective being the minimization of byproduct formation if they are of health concern. In addition, water utilities should consider the potential for the formation of disinfection byproducts depending on the oxidant selected and the source water quality.
4.2.1.1 Conventional treatment
Conventional drinking water treatment processes (chemical coagulation, clarification, rapid sand filtration) and chlorine disinfection are reported to be ineffective in decreasing the concentration of a variety of classes of pesticides, including polar pesticides like phenoxyacetic acids (Robeck et al., 1965; Miltner et al., 1989; Croll et al., 1992; Haist-Gulde et al., 1993; Frick and Dalton, 2005; Chowdhury et al., 2010; Hughes and Younker, 2011). Studies specifically investigating dicamba removal using conventional drinking water treatment processes were not available and pilot-scale testing is recommended prior to full-scale implementation.
4.2.1.2 Activated carbon adsorption
Activated carbon adsorption is a widely used technology to reduce the concentration of micropollutants, including pesticides, in drinking water (Haist-Gulde and Happel, 2012; van der Aa et al., 2012). Activated carbon can be applied in two ways: slurry applications using powdered activated carbon (PAC) or fixed-bed reactors with granular activated carbon (GAC) (Chowdhury et al., 2013).
There is very limited published literature on the removal of dicamba using activated carbon, and no data are available on adsorption capacity or performance. Therefore, prior to full-scale implementation, it is essential to conduct appropriate pilot- or bench-scale testing. Dicamba removal from natural water using activated carbon can be negatively affected by competition from other contaminants or natural organic matter (NOM), biofilm development, temperature, influent concentration, carbon size and hydraulic loading rate (Speth and Miltner, 1998; Haist-Gulde and Happel, 2012).
Data generated through bench-scale testing to determine adsorption coefficients for pesticides are useful in predicting whether activated carbon adsorbs a particular pesticide (US EPA, 2011). In general, pesticides with an adsorption capacity constant (e.g., Freundlich coefficient) greater than 200 µg/g(L/µg)1/n are considered to be amenable to removal by carbon adsorption (Speth and Adams, 1993; Speth and Miltner, 1998, US EPA, 2011). The authors noted, however, that the adsorption capacity of activated carbon is affected by many factors, including the compound's ionic character and the solution pH. Speth and Miltner (1990) performed batch-scale experiments to generate adsorption isotherms for various synthetic organic compounds. For dicamba, the adsorption experiments were conducted using distilled-deionized water and GAC. The Freundlich coefficient from this study was 33,100 µg/g(L/µg)1/n. The high value of the Freundlich coefficient indicates that activated carbon could remove dicamba.
The use of PAC offers the advantage of providing virgin carbon when required (e.g., during the pesticide application season) (Miltner et al., 1989). The removal efficiency of PAC depends on the PAC type, particle size, dose, contact time, adsorbability of the contaminant and presence of NOM (Gustafson et al., 2003; Summers et al., 2010; Haist-Gulde and Happel, 2012; Chowdhury et al., 2013). The capacity of GAC to remove pesticides by adsorption depends on the filter velocity, empty bed contact time, GAC characteristics (type, particle size, reactivation method), adsorbability of the contaminant, and filter run time (Haist-Gulde and Happel, 2012). In addition, because GAC fixed-bed adsorbers are typically operated on a continuous basis, the GAC can become fouled (or preloaded) with NOM and may be completely or partially ineffective for pesticide removal (Knappe et al., 1999; Summers et al., 2010; Haist-Gulde and Happel, 2012; Chowdhury et al., 2013).
4.2.1.3 Membrane filtration
In general, nanofiltration (NF) and reverse osmosis (RO) are effective pressure-driven membrane processes for the removal of pesticides from drinking water (Van der Bruggen and Vandecasteele, 2003; US EPA, 2011). Their effectiveness is dependent on the membrane characteristics, pesticide properties, feed water composition, operating conditions and membrane fouling (Van der Bruggen and Vandecasteele, 2003; Plakas and Karabelas, 2012).
Since the main mechanism for pesticide removal using NF and RO membranes is size exclusion, the molecular weight cut-off (MWCO) of the membrane is an important characteristic. In choosing a membrane, the molecular weight of dicamba (221 Da) should be considered. As dicamba is hydrophilic, no additional removal through physicochemical interactions will be achieved.
Bellona et al. (2004) present a flow chart using the characteristics of the pesticide in water (e.g., molecular weight, log Kow, molecular diameter) and those of the membrane (e.g., MWCO, pore size) to determine the potential for removal of dicamba by membrane filtration. It is important to perform appropriate testing prior to full-scale implementation with membrane and source water under the proposed operating conditions to ensure that adequate dicamba removal is occurring.
4.2.1.4 Biological treatment
Biological treatment involves targeting the removal of the biodegradable organic material fraction. The effectiveness of biological treatment therefore depends on the initial concentration, microbial community and temperature (Drewes et al., 2009; Diem et al., 2013). The main biological treatment processes for drinking water include riverbank filtration, rapid granular media filtration without the maintenance of a disinfectant residual across the bed, and slow sand filtration. No studies investigating riverbank filtration or rapid biofilters were found in the literature.
One study investigating biological treatment included the investigation of biofilters using three different materials, including sand, through a bench-scale column study (Matamoros and Franco, 2018) (see Table 5). The hydraulic loading rate was fairly low, indicating that the results would be representative of a slow sand filter or riverbank filter. Overall, the study showed that average dicamba removal was low and declined with increasing hydraulic loading rate to the column.
| Influent (µg/L) | Average Removal (%) | Hydraulic Loading Rate (m/day) | Overall Process Description | Reference |
|---|---|---|---|---|
| 10 | 25 | 0.3 | Bench-scale: Agricultural runoff water (background concentration of dicamba < 0.1 μg/L) Acclimation period of 30 days Column: 100 cm sand; 15 cm diameter 10 pesticide mixture Test period of 20 days |
Matamoros and Franco, 2018 |
| 6 | 1.4 |
4.2.1.5 Oxidation and hydrolysis
Degradation of pesticides by chemical oxidation depends on the nature of the pesticides (i.e., molecular structures) as well as on the water matrix (Camel and Bermond, 1998; Wols and Hofman-Caris, 2012). The studies examining degradation of dicamba using various oxidants are presented in Table 6.
In a bench-scale study, typical drinking water oxidation/disinfection processes using free chlorine (Cl2), monochloramine (NH2Cl), chlorine dioxide (ClO2), permanganate (MnO4), hydrogen peroxide (H2O2), ozone (O3), and UV photolysis at 254 nm achieved less than 20% removal of dicamba. Hydrolysis tests conducted at pH 2, 7 and 12 reported similar results (Chamberlain et al., 2012). These results were consistent with the ozonation rate constant for dicamba reported by Hu et al. (2000). A bench-scale study evaluated oxidation rate constants of 24 pesticides using O3. The tests were carried out using synthetic raw water at a pH of 7.5, ionic strength of 10-3 M and 100 μM NaHCO3. Using an O3 dose of 1.3 mg/L, a rate constant of 183 M-1s-1 was obtained, which was the fifth lowest of all 24 pesticides examined.
A study examining ozone degradation of 23 pesticides found that dicamba was difficult to degrade with molecular O3 (Meijers et al., 1995). The authors reported a low reduction of dicamba using typical O3 dosages applied for disinfection (reported as the O3 to dissolved organic carbon (DOC) ratio). The results indicated that removal increased when the pH and O3 dose increased. No bromate formation was observed through these tests (Meijers et al., 1995). Kruithof et al. (2002) reported a similar result for dicamba degradation using UV photolysis with UV dose higher than needed for disinfection.
| Oxidant | Influent (µg/L) | Oxidant and O3/DOC Dose | Removal % | Test Conditions | Reference | |
|---|---|---|---|---|---|---|
| Cl2 | 25 | 2–5 mg/L | <20 | Bench scale: buffered water (sodium phosphate); 23 ± 1°C and pH of 6.6 and 8.6 | Chamberlain et al., 2012 | |
| NH2Cl | 9–14 mg/L | <20 | ||||
| MnO4- | 3–5 mg/L | <20 | ||||
| ClO2 | 2–3 mg/L | <20 | ||||
| H2O2 | 100 mg/L | <20 | ||||
| O3 | 1–2 mg/L | <20 | ||||
| UV254 | 77–97 mW·s/cm2 | <20 | ||||
| O3 | 0.9–6.4Footnote a | 0.53g/g | 26 | Footnote bC*T10 =2.0; pH=7.2; 5°C | Bench-scale: Pre-treated river water (coagulation and flotation); DOC = 2.2 mg C/L; Br- = 100 μg/L, HCO3- = 1.6 mM; 23 pesticides | Meijers et al., 1995 |
| 0.55 g/g | 26 | Footnote bCT =1.0; pH=7.2; 20°C | ||||
| 0.95 g/g | 53 | Footnote bCT =1.0; pH= 8.3; 20°C | ||||
| UV | 1 | Not available | 63 | Pilot-scale: Pre-treated surface water (breakpoint chlorination, coagulation, sedimentation, filtration and post disinfection); 3 UV reactors in series, each equipped with 2 medium pressure lamps; electric energy of 1.0 kWh/m3; 10 pesticides |
Kruithof et al., 2002; Kruithof and Martijn, 2013 | |
|
||||||
4.2.1.6 Advanced oxidation processes
Limited scientific literature has been published on the effectiveness of AOPs for dicamba removal from drinking water (Table 7). Meijers et al. (1995) found that for persistent pesticides, such as dicamba, the removal can increase when ozonation was preceded by H2O2 dosage. The authors reported a further increase of dicamba removal when the ozonation process was followed by an advanced oxidation process using H2O2/O3. However, bromate formation was observed through these tests. pH was shown to have a minor effect on pesticide degradation using H2O2/O3.
A pilot-scale study using a combined UV/H2O2 process reported that most pesticides studied were degraded by approximately 65% to 99%, with the exception of dicamba, which was degraded by approximately 55% to 57% under the experimental conditions described in Table 7. GAC filtration was required following UV/H2O2 oxidation to remove assimilable organic carbon and residual H2O2. The results were used to design and implement a full-scale UV/H2O2 system for drinking water treatment. No specific data on the formation of degradation byproducts of dicamba were reported (Kruithof and Martijn, 2013).
| Oxidant | Influent (µg/L) | Removal % | Process Description | Reference | |
|---|---|---|---|---|---|
| H2O2/O3 | 0.9–6.4Footnote a | 78 | 1.5 mg/L H2O2 and 3.0 mg/L O3 (H2O2/O3=0.5); pH range 7.2-8.3; 20°C; | Bench-scale: River water; DOC = 2.2 mg C/L; Br- = 100 μg/L, HCO3- = 1.6 mM; 23 pesticides. |
Meijers et al., 1995 |
| O3 followed by H2O2/O3 | 97 | 3 mg/L O3 followed by 1.5 mg/L H2O2/3.0 mg/L O3 (H2O2/O3=0.5); pH=8.3; 20°C; Bromate formation of 4 µg/L |
|||
| UV/H2O2 | 1 | 55–57Footnote b | Pilot-scale: Pre-treated surface water (breakpoint chlorination, coagulation, sedimentation, filtration, post disinfection); UV reactor equipped with 4 medium pressure lamps; electric energy of 0.56 kWh/m3 and H2O2 dose of 6 mg/L. |
Kruithof et al., 2002; Kruithof and Martijn, 2013 | |
|
|||||
4.2.2 Residential-scale treatment
In cases where dicamba removal is desired at the household level—for example, when a household obtains its drinking water from a private well—a residential drinking water treatment unit may be an option for decreasing dicamba concentrations in drinking water. Before a treatment unit is installed, the water should be tested to determine the general water chemistry and dicamba concentration in the source water.
To verify that a treatment unit is effective, water entering and leaving the treatment unit should be sampled periodically and submitted to an accredited laboratory for analysis. Units can lose removal capacity through use and time and will need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in the treatment unit according to the manufacturer's recommendations and service it when required. Systems classified as residential scale may have a rated capacity to treat volumes greater than that needed for a single residence, and thus, may also be used in small systems.
Health Canada does not recommend specific brands of drinking water treatment units, but it strongly recommends that consumers use units that have been certified by an accredited certification body as meeting the appropriate NSF International /American National Standards Institute (NSF/ANSI) standards for drinking water treatment units. The purpose of the standards is to establish minimum requirements for the materials, design and construction of drinking water treatment units that can be tested by a third party. This ensures that materials in the unit do not leach contaminants into the drinking water (i.e., material safety). In addition, the standards include performance requirements that specify the level of removal that must be achieved for specific contaminants (e.g., reduction claim) that may be present in the water supply. Third-party certification organizations provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). Accredited organizations in Canada include the following:
- CSA Group
- NSF International
- Water Quality Association
- UL LLC
- Bureau de Normalisation du Québec (available in French only)
- International Association of Plumbing and Mechanical Officials
- Truesdail Laboratories Inc.
An up-to-date list of accredited certification organizations can be obtained from the SCC.
The drinking water treatment technologies that are expected to be effective for dicamba removal at the residential scale include adsorption and RO. Currently, dicamba is not included in the performance requirements of the NSF/ANSI standards. However, consumers can use a treatment unit that is certified to the standards for adsorption or RO to ensure that the material safety has been tested.
Water that has been treated using reverse osmosis may be corrosive to internal plumbing components. Therefore, these units should be installed only at the point of use. Also, as large quantities of influent water are needed to obtain the required volume of treated water, these units are generally not practical for point-of-entry installation.
5.0 Management strategies
All water utilities should implement a risk management approach, such as the source-to-tap or water safety plan approach, to ensure water safety (CCME, 2004; WHO, 2011, 2012). These approaches require a system assessment to characterize the source water, to describe the treatment barriers that prevent or reduce contamination, to identify the conditions that can result in contamination, and to implement control measures. Operational monitoring is then established, and operational/management protocols are instituted (e.g., standard operating procedures, corrective actions and incident responses). Compliance monitoring is established and other protocols to validate the water safety plan are implemented (e.g., record keeping, consumer satisfaction). Operator training is also required to ensure the effectiveness of the water safety plan at all times (Smeets et al., 2009).
5.1 Monitoring
Dicamba can be present in groundwater and surface water in areas where it is being used depending on the type and extent of its application, environmental factors (e.g., amount of precipitation, soil type, hydrogeological setting) and environmental fate (e.g., mobility, leaching potential, degradation) in the surrounding area. Water utilities should consider the potential for dicamba to enter source water (e.g., raw water supply to the drinking water system) based on site-specific considerations.
When it is established that dicamba may be present and monitoring is necessary, surface and groundwater sources should be characterized to determine the concentration of dicamba. This should include monitoring of surface water sources during periods of peak use and rainfall events and/or monitoring of groundwater annually. Where baseline data indicate that dicamba is not present in source water, monitoring may be reduced.
Where treatment is required to remove dicamba, operational monitoring should be implemented to confirm whether the treatment process is functioning as required. The frequency of operational monitoring will depend on the water quality, fluctuations of the raw water concentrations and the treatment process. Responsible authorities should be aware of the impact of NOM on activated carbon systems, as it may affect water quality objectives for dicamba removal.
Where treatment is in place for dicamba removal, compliance monitoring (i.e., paired samples of source and treated water to confirm the efficacy of treatment) should be conducted at a minimum, on an annual basis. When routine operational monitoring indicates the potential for contaminant breakthrough, such as with GAC, monitoring should be conducted at least quarterly to plan for the regeneration or replacement of the media. When a degradation process like oxidation is utilized, byproduct formation should also be considered.
6.0 International considerations
This section presents drinking water guidelines, standards and/or guidance from other national and international organizations. Variations in these values can be attributed to the age of the assessments or to differing policies and approaches, including the choice of key study and the use of different consumption rates, body weights and source allocation factors.
Australia has set a guideline value of 0.1 mg/L for dicamba in drinking water (NHMRC and NRMMC, 2011) based on maternal toxicity (decreased body weights) in rabbits in a short-term developmental toxicity study. The US EPA and the World Health Organization do not have regulatory values for dicamba in drinking water.
The European Union (EU) does not have a specific chemical parametric value for individual pesticides. Instead, it has a value of 0.1 µg/L for any individual (single) pesticide and a value of 0.5 µg/L for total pesticides found in drinking water. In establishing these values, the EU did not consider the science related to each pesticide, such as health effects. The values are based on a policy decision to keep pesticides out of drinking water (European Union, 2020).
7.0 Rationale
Dicamba is registered in Canada as a selective systemic herbicide for use on lawn and turf, as well as on industrial and agricultural sites. Despite its common use in Canada, data provided by provinces and territories that monitor for dicamba in source and drinking water indicate that when detected, levels of dicamba are well below the MAC. In terms of health effects, no one critical endpoint has been identified in either animal or human studies. Repeated dose studies in animals tend to show mostly mild effects, such as decreased body weight, decreased food consumption and behavioural effects. Epidemiological studies showed no association between various cancers and exposure to dicamba.
Health Canada, in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water, has established a MAC of 0.11 mg/L (110 µg/L) based on the following considerations:
- An HBV of 0.11 mg/L (110 µg/L) based on alterations in clinical chemistry and inflammation of the prostate in beagle dogs.
- Dicamba can be accurately measured at concentrations well below the MAC.
- Drinking water treatment technologies are available to remove dicamba to below the MAC.
The MAC is protective of potential health effects from dicamba exposure. As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area, including the outcomes of PMRA's evaluations, and recommend any change to this guideline technical document that it deems necessary.
Appendix A: List of abbreviations
- 3,6-DCSA
- 3,6-dichlorosalicylic acid
- ADI
- Acceptable daily intake
- AHS
- Agricultural Health Study
- ANSI
- American National Standards Institute
- AOP
- Advanced oxidation process
- CAS RN
- Chemical Abstracts Service Registry Number
- CHO
- Chinese hamster ovary
- DOC
- Dissolved organic carbon
- DT50
- time required for 50% dissipation
- EFSA
- European Food Safety Authority
- EU
- European Union
- FAO
- Food and Agriculture Organization of the United Nations
- FDA
- Food and Drug Administration (US)
- FNIHB
- First Nations and Inuit Health Branch
- GAC
- Granulated activated carbon
- GC/ECD
- Gas chromatography with electron capture detector
- HBV
- Health-based value
- HPLC
- High-performance liquid chromatography
- Koc
- Soil adsorption coefficient
- Kow
- Octanol-water partition coefficient
- MAC
- Maximum acceptable concentration
- MDL
- Method detection limit
- MRL
- Method reporting limit
- MTD
- Maximum tolerated dose
- MWCO
- Molecular weight cut-off
- NF
- Nanofiltration
- NHL
- Non-Hodgkin lymphoma
- NHMRC
- National Health and Medical Research Council (Australia)
- NRMMC
- National Resource Management Ministerial Council (Australia)
- NOEL
- No-observed-effect level
- NOM
- Natural organic matter
- NSF
- NSF International
- PAC
- Powdered activated carbon
- PMRA
- Pest Management Regulatory Agency
- RO
- Reverse osmosis
- SCE
- Sister chromatid exchange
- USDA
- United States Department of Agriculture
- US EPA
- United States Environmental Protection Agency
- UV
- Ultraviolet
- WHO
- World Health Organization
Appendix B: Canadian water quality data
| Jurisdiction (Year Sampled) | No. Detects/Samples | MDL (ng/L) | Range (ng/L) | 25th Percentile (ng/L) | Median (ng/L) | 75th Percentile (ng/L) | |
|---|---|---|---|---|---|---|---|
| Min | Max | ||||||
| Tap water | |||||||
| AB, SK, MB – rural communities (2004–2005)Footnote a | 28 samples | Not available |
0.73 | 748.00 | Not available |
Not available |
Not available |
| Surface water | |||||||
| BC – Lower Fraser Valley and Okanagan Basin (2003–2005) | 64/92 | 0.05 | <0.05 | 179 | 0.044 | 0.452 | 3.298 |
| BC – Lower Fraser Valley (2003–2005) | Not available |
Not available |
0.08 | 179 | Not available |
Not available |
Not available |
| ON (2003) | 133/161 | 0.73 | 0.75 | 826 | 1.68 | 9.07 | 23.80 |
| ON (2004) | 188/228 | 0.73 | 0.73 | 105000 | 1.90 | 11.25 | 53.40 |
| ON (2005) | 138/183 | 0.73 | 0.75 | 5380 | 0.75 | 4.38 | 18.6 |
| QC (2003) | 18/51 | 30 | <30 | 1900 | Not available |
Not available |
Not available |
| QC (2004) | 31/70 | 10–30 | <10 | 430 | Not available |
Not available |
Not available |
| QC (2005) | 27/59 | 30 | <30 | 2600 | Not available |
Not available |
Not available |
| NB (2003–2005) | 0/33 | 600 | Not available |
Not available |
Not available |
Not available |
Not available |
| PEI (2003–2005) | 0/55 | 600 | Not available |
Not available |
Not available |
Not available |
Not available |
| NS (2003–2005) | 0/48 | 600 | Not available |
Not available |
Not available |
Not available |
Not available |
| Rivers | |||||||
| AB, SK, MB – 8 sites (2003) | 53/64 | 0.73 | <0.73 | 68.9 | 2.32 | 4.19 | 13.30 |
| Reservoir water | |||||||
| AB, SK, MB – 15 sites (2003-2004) | 177/206 | 0.73 | 0.4 | 1040 | 2.10 | 3.82 | 10.40 |
|
|||||||
8.0 References
AGAT Laboratories Ltd. (2019). Personal communication with N. Boulton, Mississauga, ON.
Alavanja, M.C., Dosemeci, M., Samanic, C., Lubin, J., Lynch, C.F., Knott, C., Barker, J., Hoppin, J.A., Sandler, D.P., Coble, J., Thomas, K. and Blair, A. (2004). Pesticides and lung cancer risk in the Agricultural Health Study cohort. Am. J. Epidemiol., 160(9): 876–885.
Alberta Environment and Parks. (2015). Overview of 2013 Pesticide Sales in Alberta. Land Policy Branch, Edmonton, Alberta. Available at https://Open.Alberta.Ca/Dataset/482d80ff-D4be-402a-b5b4-a0b641a3a019/Resource/31ea4dc9-01ea-4fe4-8f06-4107ca078626/Download/overview2013pesticidesales-Aug-2015.Pdf
ALS Environmental. (2019). Personal communication with A. Ganouri-Lumsden. Waterloo, ON.
Anderson, K.J., Leighty, E.G. and Takahashi, M.T. (1972). Evaluation of herbicides for possible mutagenic properties. J. Agric. Food Chem., 20(3): 649–656.
Andreotti, G., Freeman, L.E., Hou, L., Coble, J., Rusiecki, J., Hoppin, J.A., Silverman, D.T. and Alavanja, M.C. (2009). Agricultural pesticide use and pancreatic cancer risk in the agricultural health study cohort. Int. J. Cancer, 124(10): 2495–2500.
ASTM. (2011). D5317–98 Standard test method for determination of chlorinated organic acid compounds in water by gas chromatography with an electron capture detector. ASTM International, West Conshohocken, Pennsylvania.
Band, P.R., Abanto, Z., Bert, J., Lang, B., Fang, R., Gallagher, R.P. and Le, N.D. (2011). Prostate cancer risk and exposure to pesticides in British Columbia farmers. Prostate, 71(2): 168–183.
Beduk, F., Aydin, M.E. and Ozcan, S. (2012). Degradation of malathion and parathion by ozonation, photolytic ozonation, and heterogeneous catalytic ozonation processes. Clean-Soil, Air, Water. 40(2): 179–187.
Bellona, C., Drewes, J.E., Xu, P. and Amy, G. (2004). Factors affecting the rejection of organic solutes during NF/RO treatment – a literature review. Water Res. 38(12): 2795–2809.
Blair, M. (1986). Dicamba: One Year Dietary Toxicity Study in Dogs. Unpublished Report no. 1986/5183 from International Research and Development Corporation, Mattawan, MI, USA. Project no 158187. Submitted to WHO by BASF Corporation (as cited in Health Canada, 2019).
British Columbia Ministry of Health. (2019). Personal communication with D. Fishwick.
Bureau Veritas Laboratories. (2019). Personal communication with C. MacDermid. Mississauga, ON.
Camel, V. and Bermond, A. (1998). Review paper: The use of ozone and associated oxidation processes in drinking water treatment. Water Res. 32(11): 3208–3222.
CARO Analytical Services. (2019). Personal communication with K. Fyffe, Richmond, BC.
CCME. (1999). Canadian Water Quality Guidelines for the Protection of Agricultural Water Uses: Dicamba. In: Canadian Environmental Quality Guidelines, 1999, Canadian Council of Ministers of the Environment. Winnipeg, Manitoba.
CCME. (2004). From source to tap: Guidance on the multi-barrier approach to safe drinking water. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba. Available at www.ccme.ca/assets/pdf/mba_guidance_doc_e.pdf.
Chamberlain, E., Shi, H., Wang, T., Ma, Y., Fulmer, A. and Adams, C. (2012). Comprehensive screening study of pesticide degradation via oxidation and hydrolysis. J. Agric. Food Chem., 60(1): 354–363.
Chowdhury, Z., Traviglia, A., Carter, J., Brown, T., Summers, R.S., Corwin, C.J., Zearley, T., Thurman, M., Ferrara, I., Olson, J., Thacker, R. and Barron, P. (2010). Cost-effective regulatory compliance with GAC biofilters. Report No. 4155. Water Research Foundation, Denver, Colorado.
Chowdhury, Z.K., Summers, R.S., Westerhoff, G.P., Leto, B.J., Nowack, K.O. and Corwin, C.J. (2013). Activated carbon: Solutions for improving water quality. Passantino, L. B.(ed.). American Water Works Association. Denver, Colorado.
Croll, B.T., Chadwick, B. and Knight, B. (1992). The removal of atrazine and other herbicides from water using granular activated carbon. Water Sci. Technol. Water Supply 10(2): 111–120.
Crome, S. (1987). Dicamba: Potential Tumorigenic Effects in Prolonged Dietary Administration to Mice: Report no. VCL 72/871205. Unpublished study prepared by Huntingdon Research Centre Ltd. Huntingdon, Cambridgeshire, England. Submitted to WHO by BASF Corporation. (as cited in USDA, 2004; EFSA 2011; FAO/WHO, 2011).
Davis, R.K., Jolley, W.P. and Stemmer, K.L. (1962). The feeding for two years of the herbicide 2-methoxy-3,6-dichlorobenzoic acid to rats and dogs. Unpublished study. (as cited in US EPA, 1988).
De Roos, A.J., Zahm, S.H., Cantor, K.P., Weisenburger, D.D., Holmes, F.F., Burmeister, L.F. and Blair, A. (2003). Integrative assessment of multiple pesticides as risk factors for non-Hodgkin's lymphoma among men. Occup. Environ. Med., 60(9): E11.
Dennis, L.K., Lynch, C.F., Sandler, D.P. and Alavanja, M.C. (2010). Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural heath study. Environ. Health Perspect., 118(6): 812–817.
Diem, S., Rudolf Von Rohr, M., Hering, J.G., Kohler, H-E., Schirmer, M. and Von Gunten, U. (2013). NOM degradation during river infiltration: Effects of the climate variables temperature and discharge. Water Res., 47(17): 6585–6595.
Drewes, J.E., Hoppe, C., Oldham, G., McCray, J. and Thompson, K. (2009). Removal of bulk organic matter, organic micropollutants, and nutrients during riverbank filtration. Report number 3180. Water Research Foundation, Denver, Colorado.
Edson, E.F. and Sanderson, D.M. (1965). Toxicity of the herbicides, 2-methoxy-3,6-dichlorobenzoic acid (dicamba) and 2-methoxy-3,5,6-trichlorobenzoic acid (tricamba). Food Cosmet. Toxicol., 3(2): 299–304.
EFSA. (2011). Conclusion on the peer review of the pesticide risk assessment of the active substance dicamba. European Food Safety Authority. EFSA Journal, 9(1): 1965.
EFSA. (2013). Reasoned opinion on the modification of the MRL for dicamba in genetically modified soybean. European food safety authority. EFSA Journal, 11(10): 3440.
Eisenbeis, S.J., Lynch, D.L. and Hample, A.E. (1981). Ames mutation assay tested against herbicides and herbicide combinations. Soil Sci., 131: 44–47.
Element Materials Technology Canada Inc. (2019). Personal communication with H. Du, Calgary, AB.
Environment Canada. (2011). Presence and Levels of Priority Pesticides in Selected Canadian Aquatic Ecosystems. En14-40/2011E-PDF. Water Science and Technology Directorate, Environment Canada, Ottawa, Ontario.
Espandiari, P., Thomas, V.A., Glauert, H.P., O'Brien, M., Noonan, D. and Robertson, L.W. (1995). The herbicide dicamba (2-methoxy-3,6-dichlorobenzoic acid) is a peroxisome proliferator in rats. Fundam. Appl. Toxicol., 26(1): 85–90.
Espandiari, P., Ludewig, G., Glauert, H.P. and Robertson, L.W. (1998). Activation of hepatic NF-kappaB by the herbicide dicamba (2-methoxy-3,6-dichlorobenzoic acid) in female and male rats. J. Biochem. Mol. Toxicol., 12(6): 339–344.
Espandiari, P., Glauert, H.P., Lee, E.Y. and Robertson, L.W. (1999). Promoting activity of the herbicide dicamba (2-methoxy-3, 6-dichlorobenzoic acid) in two stage hepatocarcinogenesis. Int. J. Oncol., 14(1): 79–84.
European Union (2020). Council Directive 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast).
FAO/WHO. (2011). Pesticide Residues in Food 2010. Report of the Joint Meeting of the FAO Party of Experts on Pesticide Residues in Food and the Environment and WHO Core Assessment Group on Pesticide Residues. Food and Agriculture Organization of the United Nations. World Health Organization, FAO Plant Production and Protection Paper 200, Rome, Italy.
FDA. (2019). Analytical results of the Total Diet Study. Available at https://www.fda.gov/food/total-diet-study/analytical-results-total-diet-study.
Flower, K.B., Hoppin, J.A., Lynch, C.F., Blair, A., Knott, C., Shore, D.L. and Sandler, D.P. (2004). Cancer risk and parental pesticide application in children of Agricultural Health Study participants. Environ. Health Perspect., 112(5): 631–635.
Frick, E. A. and Dalton, M.S. (2005). Characterization of anthropogenic organic compounds in the source water and finished water for the City of Atlanta, October 2002–September 2004. In: Hatcher, K.S. (ed.), Proceedings of the 2005 Georgia Water Resources Conference, April 25–27, Institute of Ecology, University of Georgia, Athens, Georgia. Available at https://smartech.gatech.edu/bitstream/handle/1853/47123/FrickE%20paper%20March15%20rev.pdf
Glozier, N.E., Struger, J., Cessna, A.J., Gledhill, M., Rondeau, M., Ernst, W.R., Sekela, M.A., Cagampan, S.J., Sverko, E., Murphy, C., Murray, J.L. and Donald, D.B. (2012). Occurrence of glyphosate and acidic herbicides in select urban rivers and streams in Canada, 2007. Environ. Sci. Pollut. Res. Int., 19(3): 821–834.
Goldenthal, E. (1985). Lifetime Dietary Toxicity and Oncogenicity Study in Rats: Technical Dicamba: 163–694. Unpublished study prepared by International Research and Development Corp. Mattawan, MI, USA. Submitted to WHO by BASF Corporation. (as cited in USDA, 2004; EFSA 2011; FAO/WHO 2011).
Goldner, W.S., Sandler, D.P., Yu, F., Shostrom, V., Hoppin, J.A., Kamel, F. and LeVan, T.D. (2013). Hypothyroidism and pesticide use among male private pesticide applicators in the Agricultural Health Study. J. Occup. Environ. Med., 55(10): 1171–1178.
Gonzalez, N.V., Soloneski, S. and Larramendy, M.L. (2006). Genotoxicity analysis of the phenoxy herbicide dicamba in mammalian cells in vitro. Toxicol. in. Vitro., 20(8): 1481–1487.
Gonzalez, N.V., Soloneski, S. and Larramendy, M.L. (2007). The chlorophenoxy herbicide dicamba and its commercial formulation Banvel induce genotoxicity and cytotoxicity in Chinese hamster ovary (CHO) cells. Mutat. Res., 634(1–2): 60–68.
Gonzalez, N.V., Soloneski, S. and Larramendy, M.L. (2009). Dicamba-induced genotoxicity in Chinese hamster ovary (CHO) cells is prevented by vitamin E. J. Hazard. Mater., 163(1): 337–343.
Gonzalez, N.V., Nikoloff, N., Soloneski, S. and Larramendy, M.L. (2011). A combination of the cytokinesis-block micronucleus cytome assay and centromeric identification for evaluation of the genotoxicity of dicamba. Toxicol. Lett., 207(3): 204–212.
Government of Ontario. 2019. Drinking water surveillance program. Available at https://www.ontario.ca/data/drinking-water-surveillance-program.
Gustafson, D.K., Carr, K.H., Carson, D.B., Fuhrman, J.D., Hackett, A.G., Hoogheem, T.J., Snoeyink, V.L., Curry, M., Heijman, B., Chen, S., Herti, P. and van Wesenbeeck, I. (2003). Activated carbon adsorption of chloroacetanilide herbicides and their degradation products from surface water supplies. J. Water Supply Res. Technol. AQUA, 52(6): 443–454.
Haist-Gulde, B. and Happel, O. (2012). Removal of pesticides and their ionic degradates by adsorptive processes. Report no. 4022. Water Research Foundation, Denver, Colorado.
Haist-Gulde, B., Baldauf, G. and Brauch, H.–J. (1993). Removal of pesticides from raw waters. Water Sci. Technol. Water Supply, 11(1): 187–196.
Haist-Gulde, B. and Sacher, F. (2019). Personal communication, TZW German Water Centre, Karlsruhe, Germany.
Hartge, P., Colt, J.S., Severson, R.K., Cerhan, J.R., Cozen, W., Camann, D., Zahm, S.H. and Davis, S. (2005). Residential herbicide use and risk of non-Hodgkin lymphoma. Cancer Epidemiol. Biomarkers Prev., 14(4): 934–937.
Health Canada. (2007a). Proposed Acceptability for Continuing Registration. PARC2007–02. Re-Evaluation of Dicamba for Lawn and Turf Uses. Pest Management Regulatory Agency (PMRA), Health Canada, Ottawa, Ontario.
Health Canada. (2007b). Proposed Re-Evaluation Decision. PRVD2007–05. The use of Dicamba in Agricultural and Industrial Sites. Pest Management Regulatory Agency (PMRA), Health Canada, Ottawa, Ontario.
Health Canada. (2008). Re-Evaluation Decision. Dicamba. RVD2008–28. Pest Management Regulatory Agency (PMRA), Health Canada, Ottawa, Ontario.
Health Canada. (2018). Pest Control Products Sales Report for 2018. Pest Management Regulatory Agency (PMRA), Health Canada, Ottawa, Ontario.
Health Canada. (2019). Personal communication with the Health Evaluation Directorate, Pest Management Regulatory Agency (PMRA).
Health Canada. (2021). Canadian exposure factors used in human health risk assessments. Fact Sheet. Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/canadian-exposure-factors-human-health-risk-assessments.html
Hoberman, A. (1992). Developmental toxicity (embryo-fetal toxicity and teratogenic potential) study of technical dicamba administered orally via capsule to New Zealand White rabbits: Final report: Lab Project Number: 1819–004. Unpublished study prepared by Argus Research Lab. Horsham, PA, USA. Submitted to WHO by BASF Corporation. (as cited in USDA, 2004; EFSA, 2011; FAO/WHO, 2011).
Hrelia, P., Vigagni, F., Maffei, F., Morotti, M., Colacci, A., Perocco, P., Grilli, S. and Cantelli-Forti, G. (1994). Genetic safety evaluation of pesticides in different short-term tests. Mutat. Res., 321(4): 219–228.
Hu, J., Morita, T., Magara, Y. and Aizawa, T. (2000). Evaluation of reactivity of pesticides with ozone in water using the energies of frontier molecular orbitals. Water Res., 34(8): 2215–2222.
Hughes, W.B. and Younker, C.L. (2011). Organic compounds assessed in Chattahoochee River water used for public supply near Atlanta, Georgia, 2004–05. Fact Sheet 2011–3062. U.S. Geological Survey, Reston, Virginia. 6 p.
Ikehata, K. and Gamal El-Din, M. (2006). Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and Fenton-type advanced oxidation processes: A review. J. Environ. Eng. Sci., 5: 81–135.
Indigenous Services Canada. (2019). Personal communication with X. Redhead.
Knappe, D.R.U., Snoeyink, V.L., Roche, P., Prados, M.J. and Bourbigot, M-M. (1999). Atrazine removal by preloaded GAC. J. Am. Water Works Assoc., 91(10): 97–109.
Koutros, S., Silverman, D.T., Alavanja, M.C., Andreotti, G., Lerro, C.C., Heltshe, S., Lynch, C.F., Sandler, D.P., Blair, A. and Beane Freeman, L.E. (2016). Occupational exposure to pesticides and bladder cancer risk. Int. J. Epidemiol., 45(3): 792–805.
Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B, 16(1): 39–51.
Kruithof, J.C. and Martjin, B.J. (2013). UV/H2O2 treatment: an essential process in a multi barrier approach against trace chemical contaminants. Water Sci. Technol. Water Supply, 13(1): 130–138.
Kruithof, J.C., Kamp, P.C. and Belosevic, M. (2002). UV/H2O2–treatment: the ultimate solution for pesticide control and disinfection. J. Water Supply Res. Technol. AQUA, 2(1): 113–122.
Laveglia, J., Rajasekaran, D. and Brewer, L. (1981). Thirteen-week dietary toxicity study in rats with dicamba. IRDC no. 163–671. Unpublished study. (as cited in USDA, 2004; FAO/WHO, 2010).
Lee, W.J., Sandler, D.P., Blair, A., Samanic, C., Cross, A.J. and Alavanja, M.C. (2007). Pesticide use and colorectal cancer risk in the agricultural health study. Int. J. Cancer, 121(2): 339–346.
Li, W., Zhao, Y., Yan, X., Duan, J., Saint, C.P. and Beecham, S. (2019). Transformation pathway and toxicity assessment of malathion in aqueous solution during UV photolysis and photocatalysis. Chemosphere, 234: 204–214.
Manitoba Sustainable Development. (2019). Personal communication with D. Coulibaly, Water Quality Management Section.
Mann, O., E. Pock, K. Wruss, W. Wruss, and Krska. R. (2016). Development and validation of a fully automated online-;SPE-;ESI-LC-MS/MS multi-residue method for the determination of different classes of pesticides in drinking, ground and surface water. Int. J. Environ. Anal. Chem. 96(4): 353–372.
Masters, R. (1993). Technical dicamba: A study of the effect on reproductive function of two generations in the rat: Lab Project Number: SNC 140/921437. Unpublished study prepared by Huntingdon Research Centre Ltd. Huntingdon, Cambridgeshire, England. Submitted to WHO by BASF Corporation. (as cited in USDA, 2004; EFSA, 2011).
Matamoros, V. and Franco, J. (2018). Assessing the use of sand, peat soil, and pine bark for the attenuation of polar pesticides from agricultural run-off: A bench-scale column experiment. Environ. Sci. Pollut. Res., 25: 20640–20647.
McDuffie, H.H., Pahwa, P., McLaughlin, J.R., Spinelli, J.J., Fincham, S., Dosman, J.A., Robson, D., Skinnider, L.F. and Choi, N.W. (2001). Non-Hodgkin's lymphoma and specific pesticide exposures in men: Cross-Canada study of pesticides and health. Cancer Epidemiol. Biomarkers Prev., 10(11): 1155–1163.
Meijers, R.T., Oderwald-Muller, E.J., Nuhn, P.A.N.M. and Kruithof, J.C. (1995). Degradation of pesticides by ozonation and advanced oxidation. Ozone Sci. Eng., 17(6): 673–686.
Metcalfe, C.D., Helm, P., Paterson, G., Kaltenecker, G., Murray, C., Nowierski, M. and Sultana, T. (2019). Pesticides related to land use in watersheds of the Great Lakes basin. Sci. Total Environ., 648: 681–692.
Miltner, R.J., Baker, D.B., Speth, T.F., and Fronk, C.A. (1989). Treatment of seasonal pesticides in surface waters. J. Am. Water Works Assoc. 81: 43–52. https://doi.org/10.1002/j.1551–8833.1989.tb03321.x.
Ministère de l'Environnement et de la Lutte contre les changements climatiques. (2019). Personal communication with P. Cantin.
Minnema, D. (1994). Subchronic neurotoxicity study of dietary technical dicamba in rats: Final report. Lab Project no. HWA 686–178: 535: 0686178. Unpublished study prepared by Hazleton Washington, Inc. 426 p. (as cited in USDA, 2004).
Moon, J.M. and Chun, B.J. (2014). Clinical characteristics of patients after dicamba herbicide ingestion. Clin. Toxicol. (Phila)., 52(1): 48–53.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. and Shirasu, Y. (1983). Further mutagenicity studies on pesticides in bacterial reversion assay systems. Mutat. Res., 116(3–4): 185–216.
New Brunswick Department of Environment and Local Government. (2019). Personal communication with K. Gould.
Newfoundland and Labrador Municipal Affairs and Environment. (2019). Personal communication with H. Khan.
Nova Scotia Environment. (2019). Personal communication with A. Polegato.
NHMRC and NRMMC. (2011). Australian Drinking Water Guidelines 6. National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. Available at https://www.nhmrc.gov.au/about-us/publications/australian-drinking-water-guidelines.
Peixoto, F., Vicente, J.A. and Madeira, V.M. (2003). The herbicide dicamba (2-methoxy-3,6-dichlorobenzoic acid) interacts with mitochondrial bioenergetic functions. Arch. Toxicol., 77(7): 403–409.
Perocco, P., Ancora, G., Rani, P., Valenti, A.M., Mazzullo, M., Colacci, A. and Grilli, S. (1990). Evaluation of genotoxic effects of the herbicide dicamba using in vivo and in vitro test systems. Environ. Mol. Mutagen., 15(3): 131–135.
Plakas, K.V. and Karabelas, A.J. (2012). Removal of pesticides from water by NF and RO membranes – a review. Desalination, 287: 255–265.
Plewa, M.J., Wagner, E.D., Gentile, G.J. and Gentile, J.M. (1984). An evaluation of the genotoxic properties of herbicides following plant and animal activation. Mutat. Res., 136(3): 233–245.
Poole, D.C., Simmon, V. and Newell, G.W. (1977). In vitro mutagenic activity of fourteen pesticides. Toxicol. Appl. Pharmacol., 41(1): 196.
Prince Edward Island Department of Communities, Land and Environment. (2019). Personal communication with G. Somers.
Robeck, G., Dostal, K.A., Cohen, J.M. and Kreissl, J.F. (1965). Effectiveness of water treatment processes in pesticide removal. J. Am. Water Works Assoc., 57(2): 181–199.
Samanic, C., Rusiecki, J., Dosemeci, M., Hou, L., Hoppin, J.A., Sandler, D.P., Lubin, J., Blair, A. and Alavanja, M.C. (2006). Cancer incidence among pesticide applicators exposed to dicamba in the Agricultural Health Study. Environ. Health Perspect., 114(10): 1521–1526.
Saskatchewan Water Security Agency. (2019). Personal communication with S. Ferris.
Sathiakumar, N., MacLennan, P.A., Mandel, J. and Delzell, E. (2011). A review of epidemiologic studies of triazine herbicides and cancer. Crit. Rev. Toxicol., 41 Suppl 1: 1–34.
SGS Environmental Services. (2019). Personal communication with D. Griffin. Lakefield, ON.
Shrestha, S., Parks, C.G., Goldner, W.S., Kamel, F., Umbach, D.M., Ward, M.H., Lerro, C.C., Koutros, S., Hofmann, J.N., Beane Freeman, L.E. and Sandler, D.P. (2018). Pesticide use and incident hypothyroidism in pesticide applicators in the Agricultural Health Study. Environ. Health Perspect., 126(9): 97008.
Smeets, P.W.M.H., Medema, G.J., and van Dijk, J.C. (2009). The Dutch secret: How to provide safe drinking water without chlorine in the Netherlands. Drink. Water Eng. Sci., 2: 1–14.
Smith, S.H. (1981). Teratology study in albino rats with technical dicamba. Toxigenetics study no. 450–0460. Unpublished study. Toxigenics Inc., Decatur, IL, USA. Submitted to WHO by BASF Corporation. (as cited in USDA, 2004; EFSA, 2011; FAO/WHO, 2011).
Sorensen, K.C., Stucki, J.W., Warner, R.E., Wagner, E.D. and Plewa, M.J. (2005). Modulation of the genotoxicity of pesticides reacted with redox-modified smectite clay. Environ. Mol. Mutagen., 46(3): 174–181.
Speth, T.F. and Adams, J.Q. (1993). GAC and air stripping design support for the Safe Drinking Water Act. In: Clark, R. and S. Summers (eds.), Strategies and technologies for meeting SDWA requirements. Lewis Publishers, Ann Arbor, MI, pp. 47–89.
Speth, T.F. and Miltner, R.J. (1990). Technical note: Adsorption capacity of GAC for synthetic organics. J. Am. Water Works Assoc., 82(2): 72–75.
Speth, T.F. and Miltner, R.J. (1998). Technical note: Adsorption capacity of GAC for synthetic organics. J. Am. Water Works Assoc., 90(4): 171–174.
Summers, R.S., Knappe, D.R.U., and Snoeyink, V.L. (2010). Chapter 14: Adsorption of organic compounds by activated carbon. In: Edzwald, J.K. (ed.), Water quality and treatment: A handbook on drinking water. 6th edition. McGraw-Hill, New York, NY.
Todd, A. and Struger, J. (2014). Changes in acid herbicide concentrations in urban streams after a cosmetic pesticides ban. Challenges, 5(1): 138–151.
US EPA. (1988). Health Advisories for 50 Pesticides. United States Environmental Protection Agency, Office of Drinking Water, Washington, DC. NTIS/PB88–245931. (as cited in USDA, 2004).
US EPA. (1992). Method 555. Determination of Chlorinated Acids in Water by High Performance Liquid Chromatography with a Photodiode Array Ultraviolet Detector. Revision 1.0.
US EPA. (1995a). Method 515.1. Determination of Chlorinated Acids in Water by Gas Chromatography with an Electron Capture Detector. Revision 4.1.
US EPA. (1995b). Method 515.2. Determination of Chlorinated Acids in Water Using Liquid-Solid Extraction and Gas Chromatography with an Electron Capture Detector. Revision 1.1.
US EPA. (1996a). Method 515.3. Determination of Chlorinated Acids in Drinking Water by Liquid-Liquid Extraction, Derivatization and Gas Chromatography with Electron Capture Detection. Revision 1.0.
US EPA. (1996b). Method 8151A. Chlorinated Herbicides by GC using Methylation or Pentafluorobenzylation Derivatization. Revision 1.
US EPA. (2000). Determination of Chlorinated Acids in Drinking Water by Liquid-Liquid Microextraction, Derivatization, and Fast Gas Chromatography with Electron Capture Detection. Revision 1.0.
US EPA. (2011). Finalization of Guidance on Incorporation of Water Treatment Effects on Pesticide Removal and Transformation in Drinking Water Exposure Assessments.
US EPA. (2016). Dicamba and Dicamba BAPMA Salt: Human-Health Risk Assessment for Proposed Section 3 New Uses on Dicamba-Tolerant Cotton and Soybean. DP no. D378366, D404917. Office of Chemical Safety and Pollution Prevention, United States Environmental Protection Agency, Washington, D.C.
US EPA. (2018a). Chemicals Evaluated for Carcinogenic Potential. Annual Cancer Report 2018. United States Environmental Protection Agency, Office of Pesticide Programs.
US EPA. (2018b). 2018 Edition of the Drinking Water Standards and Health Advisories. United States Environmental Protection Agency, Office of Water, EPA 822-F-18-001, Washington, D.C.
USDA. (2004). Dicamba, Human Health and Ecological Risk Assessment, Final report. Prepared by Syracuse Environmental Research Associates, Inc. United States Department of Agriculture, Forestry Service, SERA TR 04-43-17-06d, Arlington, VA.
USDA. (2019). United States Department of Agriculture, Pesticides Data Program Databases and Annual Summaries. Available at https://www.ams.usda.gov/datasets/pdp/pdpdata
van der Aa, L.T.J., Kolpa, R.J., Rietveld, L.C. and van Dijk, J.C. (2012). Improved removal of pesticides in biological granular activated carbon filters by pre-oxidation of natural organic matter. J. Water Supply Res. Technol. AQUA, 61(3): 153–163.
Van der Bruggen, B. and Vandecasteele, C. (2003). Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in drinking water industry. Environ. Pollut., 122: 435–445.
Waters, M.D., Simmon, V.F., Mitchell, A.D., Jorgenson, T.A. and Valencia, R. (1980). An overview of short-term tests for the mutagenic and carcinogenic potential of pesticides. J. Environ. Sci. Health B, 15(6): 867–906.
Wazeter, F.X. (1966). International Research and Development Corporation (IRDC), 163–009, Unpublished study. (as cited in Health Canada, 2019).
Weselak, M., Arbuckle, T.E., Wigle, D.T., Walker, M.C. and Krewski, D. (2008). Pre- and post-conception pesticide exposure and the risk of birth defects in an Ontario farm population. Reprod. Toxicol., 25(4): 472–480.
WHO. (2011). Guidelines for drinking-water quality. Fourth edition. World Health Organization, Geneva, Switzerland. Available at http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/
WHO. (2012). Water safety planning for small community water supplies. World Health Organization, Geneva, Switzerland. Available at http://www.who.int/water_sanitation_health/publications/small-commwater_supplies/en/
Wols, B.A. and Hofman-Caris, C.H.M. (2012). Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res., 46: 2815–2827.
Yukon Environmental Health Services. (2019). Personal communication with P. Brooks.
Page details
- Date modified: