Regulatory modernization of foods for special dietary use and infant foods: Divisions 24 and 25 of the Food and Drug Regulations
Consultation closed
This document is part of the consultation on the Regulatory modernization of foods for special dietary use and infant foods. The consultation ran from November 28, 2023 to February 26, 2024.
Table of contents
- List of abbreviations
- Executive summary
- 1.0 Introduction
- 2.0 Background
- 3.0 Description of proposed modernized framework
- 4.0 A division for foods for a special dietary purpose
- 5.0 A division for foods that are not foods for a special dietary purpose
- 6.0 Conclusion
- Appendix 1: Lexicon
- Appendix 2: Consultation questions
List of abbreviations
- AI
- Adequate Intakes
- CDRR
- Chronic Disease Risk Reduction Intake
- Codex
- Codex Alimentarius Commission
- CFIA
- Canadian Food Inspection Agency
- DRI
- Dietary Reference Intakes
- DV
- Daily value
- EU
- European Union
- FLD
- Formulated liquid diets
- FDR
- Food and Drug Regulations
- FNF
- Formulated nutritional foods
- FOP
- Front-of-package
- FMR
- Formulated meal replacements
- FSANZ
- Food Standards Australia New Zealand
- FSMP
- Food for special medical purposes
- FSDU
- Foods for special dietary use
- FSDP
- Food for a special dietary purpose
- FVLED
- Foods for a very low energy diet
- HMF
- Human milk fortifiers
- MR
- Meal replacements
- NASEM
- National Academy of Science, Engineering and Medicine
- NFt
- Nutrition Facts table
- NIFI
- New infant formula ingredient
- NS
- Nutritional supplements
- PPHM
- Prepackaged human milk
- RDA
- Recommended Dietary Allowance
- TMAL
- Temporary Marketing Authorization Letter
- TDR
- Total diet replacement
- UL
- Tolerable Upper Intake Level
- WHO Code
- World Health Organization International Code of Marketing of Breast-milk Substitutes
Executive summary
This pre-consultation paper presents the modernization proposal for Divisions 24 and 25 of the Food and Drug Regulations (FDR), which govern foods for special dietary use (FSDU) and foods for infants in Canada. The current regulations, developed decades ago, are outdated and inflexible, hindering the integration of scientific advancements and updates to recommended intakes. This rigidity poses challenges for industry innovation, limits availability of products approved in other countries and leaves Canada more vulnerable to shortages.
Regulatory modernization would continue to support the safety and nutritional adequacy of these foods, while also fostering increased innovation and alignment with international jurisdictions, when possible and within the Canadian regulatory context. It would create a more competitive marketplace and improve access to critical nutrition products for Canadians. This modernization aligns with the 2019 amendment to the Food and Drugs Act, introducing the term "food for a special dietary purpose" (FSDP) and supports the ongoing development of a regulatory framework for human clinical trials on these products.
The modernization initiative proposes a comprehensive restructuring of the regulatory requirements. One crucial aspect is the introduction of a clear differentiation between products that meet the definition of FSDP and those that do not. FSDP would be subject to enhanced regulatory oversight, including premarket authorization for most infant FSDP, and stop-sale provisions to protect public health. Moreover, compositional requirements both for products that are FSDP and those that are not would be updated to reflect the latest Dietary Reference Intakes (DRI) and be incorporated by reference, when feasible. The proposal also aims to improve product labelling by requiring detailed information on FSDP labels for safe use and differentiation from products that are not FSDP.
In conclusion, the proposed regulatory modernization for Divisions 24 and 25 aims to address current limitations and challenges in the regulation of FSDU and infant foods. By promoting flexibility, aligning with scientific advancements, and encouraging innovation, regulatory modernization will continue to ensure product safety while enhancing accessibility and diversity of products for the Canadian market. This diversity will help mitigate the risk of shortages to better support the health and well-being of all Canadians, particularly vulnerable groups relying on specialized nutrition products. Health Canada's commitment to modernization underscores its dedication to public health, ensuring that Canadians have access to high-quality, safe, and nutritious foods for their unique dietary needs.
1.0 Introduction
Divisions 24 and 25 of the Food and Drug Regulations (FDR) prescribe the requirements for foods for special dietary useReference 1 (FSDU) and foods for infants (individuals less than one year old). Division 24 sets out the requirements for foods for individuals over 1 year through a prescribed list of product categories that are permitted to be represented asReference 2 FSDU, including formulated liquid diets (FLD), meal replacements (MR), and nutritional supplements (NS). Division 25 regulates foods for infants such as infant formula, human milk fortifiers (HMF), and conventional infant foods like cereals and strained fruit.
While the regulations for FSDU and infant foods aim to ensure safety and nutritional adequacy, they are prescriptive and outdated. To comply with the regulations, FSDU must adhere to compositional requirements that have remained unchanged for decades, despite scientific advancements and updates to recommended intakes that have occurredReference 3.
The limitations of the current framework pose challenges for industry in introducing innovative products to the Canadian market. As a result, many products available in other countries are not permitted for sale in Canada, leaving the country with a less diversified supply that may be more affected by a shortage. This vulnerability was evident in 2022 when Canada experienced the impact of global shortages, particularly with infant formula and HMF, and dietary products for individuals with inherited metabolic disorders (commonly known as metabolic products).
To help address these shortages, Health Canada published an interim policy recommending that the Canadian Food Inspection Agency (CFIA) exercise enforcement discretion on certain provisions of the FDR. This facilitated the importation of products manufactured to standards comparable to those in Canada. Whereas the interim policy helped provide short-term access to these products for vulnerable groups, lasting regulatory solutions are needed to reduce the likelihood of future shortages and mitigate the impact when they occur.
In 2019, the Food and Drugs Act was amended to introduce the term, "food for a special dietary purpose"Reference 4 (FSDP), supporting the development of a regulatory framework that would enable human clinical trials on these products. The term FSDP was specifically crafted to accurately scope comprehensive regulations for certain foods, such as infant formula, human milk fortifiers, and other medical foods, where stringent regulations are essential to prevent harm to health. Currently, FSDP that are non-compliant as well as FSDP with premarket requirements, such as new or majorly changed infant formula, that have not yet undergone premarket review by Health Canada are not permitted to be used in clinical trials. This modernization initiative aims to restructure the regulatory requirements in a manner that would complement the ongoing development of the FSDP clinical trial framework by differentiating FSDP from foods that do not meet the definition of an FSDP.
Since the current regulatory framework was developed, more flexible modern regulatory instruments have become available, including the incorporation by reference authority. This allows regulatory amendments, such as updates to compositional requirements, to be adopted administratively as soon as the scientific assessment and related consultations have been completed. Within the proposed modernized framework, compositional requirements, where prescribed, would reflect the latest Dietary Reference Intakes (DRI)Reference 5 and be published in an incorporated by reference document, when possible, to enable administrative amendments to the compositional requirements to align with new scientific evidence.
Recognizing the importance of clear and complete labelling to support the safe use of FSDP, revised labelling requirements are proposed for these products to provide more information on the product label, helping to ensure safe use and distinguishing them from lower-risk food products, like gluten-free foods. The labelling requirements for lower-risk food products, such as MR and NS would also be revised to better align with the general requirements for prepackaged foods, including the use of a Nutrition Facts table (NFt).
Overall, this regulatory modernization proposal aims to support increased innovation, improve alignment with international jurisdictions, and reduce barriers to importing these foods into Canada. The new regulatory framework would improve access to these critical nutrition products for Canadians as well as promote a diverse market to reduce the risk of shortages.
1.1 Purpose
The purpose of this pre-consultation document is to present Health Canada's proposal for modernizing the regulatory framework for FSDU and foods for infants in order to gather input from interested parties. Stakeholder comments in response to this proposal will inform the drafting of regulations, which will be pre-published in Canada Gazette Part I for public comment. Section 2.0 outlines the current Canadian regulatory landscape and its limitations, while also offering an international perspective on similar frameworks. Sections 3.0 to 5.0 provide details on the proposed modernized framework for stakeholder consideration, complemented by a set of guiding questions in Appendix 2: Consultation questions.
2.0 Background
2.1 Canadian context
2.1.1 A brief history of Division 24, Food and Drug Regulations
In 1974, Division 24 was added to the FDR, establishing the closed list of permitted FSDU products in B.24.003 that currently remains in effect. Initially, this list only included "dietetic" foods, such as carbohydrate-reduced, sugar-free, calorie-reduced, or low-sodium foods. However, in 1978, the closed list of FSDU categories was expanded to include FLD, foods represented for gluten-free diets, protein-restricted diets, and diets low in specific amino acids. This amendment also included the addition of the FSDU definition and the establishment of specific labelling and compositional requirements for each category of FSDU.
The latest major regulatory amendments to Division 24 occurred in 1995, when MR, NS, and foods for use in weight-reduction diets were added to the list of categories that can represent as FSDU. This amendment also repealed some of the older categories of FSDU that were introduced in 1974 and that no longer fit the scope of the FSDU framework (for example, carbohydrate-reduced foods, fat-modified foods, low fat foods, calorie-reduced foods, etc.).
Although the market has since evolved due to advances in science and innovation, the list of products that are permitted to be represented as FSDU and their requirements have remained unchanged since 1995. In 2005, regulatory amendments were proposed for MR, NS, and prepackaged meals for weight-reduction diets to amend the compositional requirements; however, this regulatory package did not proceed, and these regulatory amendments were never promulgated.
2.1.2 Issues with the current regulatory framework: Division 24, Food and Drug Regulations
Issues with the Canadian FSDU framework include its narrow scope, inflexible and outdated compositional and labelling requirements, and misalignment with regulatory frameworks internationally. These issues are outlined in more detail in this section.
a) Restricted access due to closed list of foods for special dietary use categories
Division 24 is characterized by a closed list of product categories that can be represented as FSDUReference 6. This list excludes several products that are available in other jurisdictions, including pre-and postoperative beverages to enhance recovery after surgery, metabolic products for inherited metabolic disorders unrelated to protein metabolism (for example, galactosemia), and other specially formulated foods that address the unique nutrient requirements of individuals with medical conditions. The narrow scope of the list hinders access to new and innovative products in Canada, making it difficult to meet consumer needs.
b) Outdated compositional requirements
The outdated and prescriptive compositional requirements mean that products with nutrient amounts that align with the latest recommendations of the National Academies of Sciences, Engineering, and Medicine (NASEM), as well as those specifically formulated for individuals with unique nutrient needs due to medical conditions, do not comply with the FDR and cannot be sold in Canada without a Temporary Marketing Authorization Letter (TMAL). These outdated requirements do not align with standards in other jurisdictions, forcing manufacturers to develop separate formulations exclusively for the Canadian market or apply for a TMAL. Industry stakeholders have expressed difficulties in manufacturing products that comply with the narrow nutrient ranges stipulated in the FDR. Meeting the minimum requirements at the end of shelf-life without exceeding the regulatory maximum poses burdensome technical challenges.
c) Insufficient labelling requirements
Existing labelling requirements for some FSDU do not meet the needs of consumers and do not support informed decisions. For example, the nutrition labelling requirements for products intended for use by the general population lack consistency not only among product categories but also with other prepackaged foods. While most conventional prepackaged foods are required to carry an NFt and, in some cases a front-of-package (FOP) nutrition symbol, MR and NS are prohibited from carrying this information. Not having access to information provided in an NFt makes it difficult for consumers to make healthier and more informed food choices, including for people that require certain information to manage their health conditions. For example, MR and NS are not required to declare sugars content on the label, and manufacturers often choose not to disclose this information. This makes it challenging for diabetic individuals to make appropriate decisions. Consequently, concerned consumers have contacted Health Canada, expressing their desire to know the sugars content of these foods.
Furthermore, labelling requirements for FSDU are very different from those in other jurisdictions. This adds unnecessary burden and may discourage companies from distributing their products in Canada, potentially limiting access to essential products for vulnerable Canadians.
d) Outdated advertising restrictions
Parts B and D of the FDR prescribe advertising restrictions for certain FSDU namely FLD, foods for a very low energy diet (FVLED), fortified foods for protein-restricted and low amino acid diets, and fortified foods for gluten-free diets. According to Section 2 of the Food and Drugs Act, any communication aimed at promoting the sale of a food is considered an advertisement. This means that any means of promotion falls under advertising and would thus be restricted for these categories of FSDU.
The purpose of these advertising restrictions was to help ensure that consumers consult their health care providers before using these products. However, these restrictions are no longer suitable in today's era, where many individuals actively participate in their health care decisions. Additionally, advertising restrictions have become impractical in the current context of online advertising and distribution. With these restrictions, manufacturers and distributors are unable to advertise these products using social media; including providing links to online marketplaces or information on how to purchase these products. As such, consumers searching online for products appropriate for their needs are not aware that these types of FSDU are available for purchase. This, in turn, makes it difficult to access a wide range of products, particularly for individuals living in remote communities with limited access to retail locations.
e) Outdated requirements for foods represented for use in weight reduction diets
Division 24 restricts the representation of foods intended for use in weight reduction diets to FVLED, MR, prepackaged meals, and foods sold by a weight reduction clinic. The intent of this restriction was to help ensure that only those products that control food intake while maintaining nutrient adequacy can be represented as suitable options for weight reduction diets. Consequently, these foods are subject to additional regulatory requirements, which are out of date.
FVLED are intended for use under medical supervision and have detailed compositional requirements that are based on outdated recommended intakes. Premarket notification is required prior to the sale of these foods, yet to date, Health Canada has not received any notifications, suggesting that the requirements for this category are not aligned with what is available on the market.
Similarly, MR for use in weight reduction diets, as well as prepackaged meals and food sold in a weight reduction clinic are required to include a sample seven-day menu that meets certain requirements based on the 1992 version of Canada's Food Guide to Healthy Eating. These foods and the accompanying sample menu must adhere to compositional requirements that are based on outdated recommended intakes.
2.1.3 A brief history of Division 25, Food and Drug Regulations
In 1976, Division 25 was added to the FDR, establishing requirements for infant foods, including infant formula. It introduced sodium restrictions to infant foods and set compositional requirements for infant formula, both of which have remained unchanged since their promulgation.
Significant amendments were made to Division 25 in 1983, including the addition of provisions enabling the Department to order the stop-sale of a potentially inadequate or unsafe infant formula. Additionally, the term human milk substitute was introduced to harmonize with international terminology, reserving the term infant formula for use as the common name for human milk substitutes. Division 25 was also expanded to include any product sold or represented as a substitute for human milk, any product represented for use as an ingredient of a human milk substitute and foods containing a human milk substitute.
The most recent major amendments to the infant formula regulations occurred in 1990 when premarket notification requirements were introduced for new and majorly changed human milk substitutes. Fast forward to 2021, Division 25 underwent further significant amendments to include the addition of provisions for HMF. Prior to this regulatory amendment, HMF were not permitted to be sold in Canada; however some HMF were temporarily made available following a thorough safety assessment by Health Canada.
2.1.4 Issues with the current regulatory framework: Division 25, Food and Drug Regulations
a) Infant food nomenclature and definition issues
The FDR broadly defines infant food as food that is labelled or advertised for consumption by infantsReference 7. The definition is inclusive of all infant foods, including infant formula, HMF, and conventional foods for infants aged 6 months or more such as infant cereals and strained fruit.
In the case of infant formula, the regulatory term prescribed is "human milk substitute". However, the regulations also require the use of the common name "infant formula" on product labels and in advertising. This dual naming is overly complicated and unnecessary. Additionally, the definition for "human milk substitute" includes foods represented for use as ingredients in human milk substitutes, but such ingredients do not seem to be marketed in Canada. The only ingredients permitted to be required for addition in the directions for use of infant formula are water and/or a source of carbohydrate.
Overall, the regulations concerning infant foods would benefit from clearer and more consistent terminology and definitions to avoid misunderstandings and ensure that the regulatory framework aligns with the intended purpose and categorization of infant foods.
b) Regulatory gap for certain infant foods
Due to the outdated nature of the existing regulatory framework for infant foods, there are a number of globally available infant foods that are not addressed in Division 25 of the FDR.
- Specialized infant formula
Infant formula must be formulated to meet specific compositional requirements. While some exemptions to these compositional requirements exist, they are limited to specific cases, such as infant formula represented for diets that are fat-modified, low amino acid, low mineral, low vitamin D, or any combination of these. These exemptions are narrow in scope and could pose barriers to accessing specialized infant formula that may be beneficial or even necessary to infants with specific medical needs. For example, the current regulations do not allow for modifications to preterm formulas needed in specific circumstances (for example, low birth weight, extremely low birth weight, etc.). Removing the unnecessary barriers would make approving new and innovative products less complicated and more streamlined.
- Prepackaged human milk
Prepackaged human milk (PPHM) from Canadian milk banks is carefully screened, processed, and packaged to ensure safety, and is currently used in hospital settings. It serves as a supplement when a mother's milk is insufficient or unavailable. Currently, most PPHM in Canada is sourced from four not-for-profit human milk banks operating in Calgary, Montreal, Toronto, and Vancouver. Health Canada is also aware of commercial human milk processing companies selling their products in other countries.
There are no specific provisions for PPHM in Division 25. Instead, PPHM is subject to the general requirements for conventional prepackaged foods outlined in the Food and Drugs Act and Regulations. The absence of specific regulatory requirements, including provisions related to safety and quality, as well as tailored labelling information for the safe use of these products, is concerning for this category of food intended for a vulnerable subpopulation.
- Other infant foods
The existing regulations do not account for some other types of infant foods, including infant medical foods that are not intended to replace infant formula and supplemental components that do not meet the definition of an HMF, such as single macronutrient 'modular' type products that may be mixed with other products before use (for example, protein, carbohydrate, or fat modulars). The lack of regulatory provisions for these products further limits the options available to infants with unique dietary requirements.
c) Misalignment with the World Health Organization International Code of Marketing of Breastmilk Substitutes
The World Health Organization established the International Code of Marketing of Breastmilk SubstitutesReference 8 (WHO Code) in response to the global decline in breastfeeding, influenced by sociocultural factors and the promotion of infant formula. Adopted by the World Health Assembly in 1981, and updated in 2017, the WHO Code aims to ensure the provision of safe and adequate nutrition for infants by protecting and promoting breastfeeding, while regulating the appropriate use of human milk substitutesReference 9, when necessary, through responsible marketing and distribution practices.
As a signatory of the WHO Code, Canada has an obligation to support and promote breastfeeding, facilitate breastfeeding by mothers through legislative and social action, and prevent inappropriate sales promotion of infant foods that can be used to replace human milk. However, the FDR does not reflect the recommendations outlined in the WHO Code, particularly regarding the restriction of advertising or other forms of promotion, as well as the requirement to label human milk substitutes to provide the necessary information on the appropriate use of the product to not discourage breastfeeding.
d) Outdated compositional requirements
The existing compositional requirements for infant formula have not been amended since their introduction into the regulations in 1976, despite advances in nutrition science and updates to recommended intakes. Similarly, the fortification provisionsReference 10 for infant cereal products, a conventional infant food, have not been amended since their introduction in the FDR in the 1960s. These provisions are not based on current nutrient recommendations for infants and do not include maximum levels for mineral nutrients despite the vulnerability of this subpopulation.
Additionally, the regulations for conventional infant foods establish sodium limits for a closed list of product categories and the amounts vary by product type. The rationale for differentiating sodium limits according to product type is unclear and the closed list fails to encompass all categories of conventional infant food available on the market.
e) Problematic premarket notification
Based on the current regulations, regulated parties must notify Health Canada at least 90 days before selling or advertising a new infant formula. However, the review of a premarket notification submission for a new infant formula or major changes to an existing infant formula generally takes longer than 90 days. This extended duration includes pauses in the review process to accommodate the submission of additional information by manufacturers. While regulated parties usually wait until Health Canada completes its review, there is no stipulation in the current regulations that prevents the sale of an infant formula before the review is completed, thus introducing a potential risk to the health and safety of infants. While the existing stop-sales provisions enable the Minister to halt the sale of products currently on the market, it is a reactionary measure.
f) Voluntary separate review of new infant formula ingredients
New ingredients that have not been previously used in an infant formula need to undergo a novelty determination. If the ingredient is determined to be novel, then information to support a novel food review is required. If the ingredient is determined not to be novel, a voluntary new infant formula ingredient (NIFI) assessment is conducted by Health Canada to determine its safe use for infants. However, the absence of regulatory requirements related to NIFI submissions means that there is no legal obligation to obtain a separate authorization for a NIFI before incorporating it into an infant formula premarket notification submission. Evaluating the NIFI during the assessment of an infant formula introduces complexity that could have been resolved before the infant formula submission and extends the review timeline. Therefore, clarifying the requirements and process for NIFI would help streamline the review. This would include clarifying questions related to data ownership and proprietary information between the infant formula manufacturer and the NIFI manufacturer.
2.1.5 Temporary measures to address an outdated regulatory framework
Throughout the years, Health Canada has implemented temporary measures to address challenges related to the outdated regulatory framework. One such measure was interim marketing authorizations, which were used as a mechanism to bridge the time between the completion of the scientific evaluation of certain enabling amendments and publication of the approved amendments in the Canada Gazette, Part II. Although this regulatory tool is no longer available under the FDR and interim marketing authorizations have expired, the changes that were enabled continue to be needed to address important public health needs. For instance, interim marketing authorizations were issued to increase the maximum levels of micronutrients in term and preterm infant formula as well as FLD, and to exempt FLD from the compositional requirements when formulated specifically for patients with renal failure.
TMALs are another regulatory tool under the FDR that have been used by Health Canada to authorize the sale of certain FSDU. A TMAL authorizes the temporary sale of a non-compliant product in Canada so that real world marketing research can be conducted in order to inform a regulatory amendment. Over the past decade, hundreds of TMALs for FSDU have been issued by the Department in order to inform this regulatory modernization initiative for FSDU. This includes TMALs issued for MR and NS to facilitate the safe transition of these products from the natural health products framework to the food regulatory framework. TMALs have also been issued for FSDU intended for children, whose revised nutrient needs are not reflected in the current compositional requirements.
Health Canada also periodically issues interim policy statements as a temporary measure to signal its intention that certain products that do not comply with the FDR remain available until regulatory amendments can be made. Health Canada has recently published two interim policy statements to address outdated regulatory requirements for prepackaged human milk (PPHM) and metabolic products. These policy statements signaled the Department's commitment to modernize the regulations for these types of products. Accordingly, proposals for PPHM and metabolic products have been included as part of this modernization initiative. Once final regulatory amendments are implemented, the interim policies for these types of products will no longer be in effect.
Temporary measures such as TMALs are inefficient, time-consuming and impose substantial administrative burden on both industry and Health Canada. Industry stakeholders have expressed that the often lengthy process required to obtain approvals can cause delays in getting products to market. Moreover, interim policy statements that rely on enforcement discretion lack regulatory certainty and transparency for industry and can create the appearance of an unequal playing field for industry.
2.1.6 Shortages of foods for a special dietary purpose
A shortage can arise from a range of circumstances, including manufacturing disruptions, difficulties in accessing raw materials, stockpiling, surges in demand, and unforeseen events affecting the global supply chain. In recent years, shortages of FSDP have become a significant concern due to global supply chain issues and product recalls.
Canada is particularly susceptible to shortages of FSDP due to its small market size and its reliance on a limited number of suppliers. Compounding this vulnerability is the outdated regulatory framework which poses unnecessary restrictions on the importation of FSDP available in other countries with similar safety standards.
Since early 2022, Canada has experienced shortages of FSDP, including infant formula, HMF, and metabolic products. These shortages were attributed to the recall of several powdered infant formula and the subsequent temporary closure of a major manufacturing facility in the United States (US), which globally supplies a significant portion of these products. This recall and production halt further exacerbated the strain on a supply chain challenged by the COVID-19 pandemic and the Russian invasion of Ukraine.
To help mitigate these shortages, Health Canada published an interim policy recommending that the CFIA temporarily exercise enforcement discretion for the importation of certain non-compliant products from other countries that adhere to high-quality manufacturing standards comparable to those upheld in Canada.
Shortages of FSDP can have serious consequences. These products serve as a critical source of energy and essential nutrients for vulnerable Canadians. Shortages can also lead to inflated prices for the limited supply available. Furthermore, these shortages place a heavy burden on the healthcare system, requiring health care providers to invest valuable resources and time in finding suitable alternative products. This can result in delayed or restricted access to FSDP, which can lead to adverse health outcomes.
The recent shortages of infant formulas and other FSDP have underscored the need for a more diverse and resilient supply for these products, and that updated regulations could contribute to this outcome. While interim measures were helpful in the short-term, lasting regulatory solutions are needed. Introducing regulatory amendments solely related to shortages would provide mechanisms to better address shortages when they occur, but these would not effectively minimize the risk of future occurrences. Modernizing the existing regulatory framework therefore requires a comprehensive approach that also addresses some of the underlying regulatory barriers that contribute to the risk of shortages. For example, this would include updating the outdated compositional requirements for certain FSDP.
2.2 Stakeholder engagement & market data
Over the years, consumers, health professionals, and industry stakeholders have consistently urged Health Canada to address the challenges associated with the outdated regulatory framework for FSDU and foods for infants. Feedback provided in response to multi-stakeholder consultations, a call for FSDU data as well as input obtained from external scientific articles and publications has been used to develop the proposed modernized regulatory framework described herein.
2.2.1 Foods for special dietary use
In 2017, the University of Toronto Program in Food Safety, Nutrition and Regulatory Affairs and the Canadian Nutrition Society co-hosted a multi-stakeholder workshop of experts, including health professionals, researchers, officials from Health Canada and industry stakeholders, to discuss issues with the Division 24 framework. This workshop, entitled "How to Optimize Foods for Special Dietary Use in Canada – Nutritional Requirements, Innovation, and Regulation"Reference 11, considered the context of global regulations, the importance of FSDU to nutrition and health, as well as next steps for addressing challenges and opportunities for regulating and using FSDU. There was consensus among workshop participants that the current Division 24 regulations do not best utilize current nutrition recommendations and food science/technology research, allow for innovation, meet the needs of users in clinical and nonclinical settings, nor support access to preferred products. The workshop resulted in the recommendation to develop a regulatory framework for FSDU in Canada that is modern, safe, flexible, innovative, and health driven.
In 2019, Health Canada requested data from manufacturers and distributors about FSDU that were sold in Canada, products that were planned for sale in Canada in the foreseeable future, as well as products that were sold in other jurisdictions but could not be sold in Canada under the current regulations. Information on about 100 products was provided by five respondents, including details on the intended use of the product, information on sales to date as well as projected, and product labels.
Respondents were also invited to communicate the challenges they encountered with the current FSDU regulations. A number of regulatory irritants were identified, including the closed list of product categories permitted to be represented as FSDU, outdated compositional requirements, issues with the current labelling requirements and lack of harmonization with respect to compositional requirements between Canada and main trading partners. These issues create barriers to the distribution of FSDU in Canada by preventing the use of global formulations and labels and therefore prevents access to innovative FSDU by Canadians.
In addition, an article published in 2019Reference 12 by the Regulatory Affairs Professionals Society highlights opportunities for the Canadian government to modernize its regulations for medical nutrition products and harmonize with international jurisdictions. The article underlines the challenges faced by patients in accessing these products due to the gaps in the current regulatory framework. The author suggests that, based on precedents from international regulatory systems for these products, the key to modernizing the Canadian framework will be to address the regulations' inherent limitations in flexibility, adaptability, and capacity to interface congruently with other regulatory systems.
2.2.2 Infant formula
In March 2017, an industry association submitted a discussion document to Health Canada regarding various aspects of the Department's guidance documents for infant formula. Their feedback included recommendations on topics such as the information required for establishing a new manufacturing facility, infant formula premarket notification, and the guide to industry on the scientific evidence needed to establish nutritional adequacy of infant formula. The association also expressed concerns about the current timeframes for premarket notification requirements, emphasizing the importance of sufficient scheduling and planning to effectively bring products to market.
In May 2018, Health Canada met with the industry association and committed to reviewing the feasibility of collaborating with international jurisdictions that share similar manufacturing standards to alleviate unnecessary burdens on the industry.
In June 2022, the industry association brought their ongoing concerns to the Treasury Board of Canada, as part of the Government of Canada's Let's Talk Federal Regulations initiative, specifically within the Breaking Down Inter-jurisdictional Barriers online consultation project. The association's primary concern revolved around Canada's regulatory misalignment with major international trading partners. They strongly urged the Department to implement risk-based processes for infant formula that undergo simple, low-risk changes. This would enable a faster review through an expedited process, ultimately facilitating smoother market entry for such products.
2.2.3 Shortages of foods for a special dietary purpose
In February 2023, Health Canada conducted a survey of key stakeholders involved in the Department's response to the shortages of certain FSDP for infants and children. The objective was to gather comprehensive feedback regarding the Department's management of the shortages and the effectiveness of the interim policy implemented to mitigate them. Stakeholders emphasized the importance of developing a comprehensive action plan to effectively manage potential future FSDP shortages. They highlighted the necessity of establishing regulations to ensure a consistent and reliable supply of infant formula and other FSDP in Canada. Furthermore, stakeholders proposed the development of a regulatory framework, similar to the one used for managing drug shortages, to help address and mitigate potential issues related to FSDP shortages in the future.
The survey and subsequent feedback provided valuable insights for Health Canada in assessing the effectiveness of their management of the FSDP shortages. The recommendations and concerns raised by stakeholders will inform ongoing efforts to enhance policies, regulations, and communication strategies to better address and mitigate such shortages in the future.
2.3 International context
Health Canada conducted a comprehensive review of comparable existing regulatory frameworks internationally. Similar to the Department's proposed approach, other frameworks make a clear distinction between "special dietary products" intended for more vulnerable populations (specifically FSDP) and those intended for self-selection by the general population. While the range of products recognized as "special dietary" varies, many jurisdictions have established separate and specific requirements for these products, in order to reflect their intended purpose and use by vulnerable populations.
Over the past decade, several jurisdictions, including the European Union (EU), and Australia and New Zealand, have embarked on regulatory modernization initiatives for FSDP. Health Canada has an opportunity to address deficiencies in the existing regulatory framework by leveraging similar updates and flexibilities already being implemented in those regions.
The proposed approach for modernization of Divisions 24 and 25 draws upon the principles and best practices established by these other frameworks while ensuring that it considers specific Canadian needs and regulatory context.
2.3.1 Codex Alimentarius Commission
The Codex Alimentarius Commission (Codex) is an international organization established by the Food and Agriculture Organization and the World Health Organization. Its purpose is to develop international food standards, guidelines, and codes of practice. These standards and guidelines are intended to help ensure that food meets specific nutrient composition, labelling and claims requirements, thereby facilitating international trade and maintaining consistent quality and safety across borders.
To ensure alignment, where possible, with international regulations and promote consistent quality and safety of special dietary foods, Health Canada considered various relevant Codex standards and guidelinesReference 13. These texts provide recommendations for the labelling and composition of food including infant formula, infant food, food for special medical purposes (FSMP) (which encompasses some categories of products considered FSDU in Canada as well as other types of medical foods not covered under Canada's current framework), and food for persons intolerant to gluten.
2.3.2 European Union
In 2013, the EU replaced their framework for foods for particular nutritional uses, with the introduction of foods for specific groups in order to strengthen provisions on foods for vulnerable populations that require protection and to ensure clear categorization, harmonization, and free movement of these foods across the EUReference 14. This change was driven by the need to enhance consumer protection concerning the content and marketing of these products, streamlining a complex and fragmented system that had evolved over three decades. This framework establishes requirements for the labelling and composition of infant formula, processed cereal-based food and other baby food, FSMP, and total diet replacement (TDR) for weight control.
Some foods are excluded from this framework as they were deemed sufficiently covered under horizontal legislation (for example, general food legislation on nutrition and health claimsReference 15 and the addition of vitamins and minerals and of certain other substances to foodsReference 16). Foods excluded from the framework include toddler milks and similar products intended for young children, foods intended for sportspeople, other fortified foods formulated for nutritionally vulnerable individuals such as those intended to replace a meal or to supplement the diet, and gluten-free/very low gluten foods and lactose-free foods.
2.3.3 Australia and New Zealand
Part 2.9 of the Food Standards CodeReference 17 of Australia and New Zealand establishes compositional and labelling requirements for special purpose foods, which are food products intended for physiologically vulnerable individuals and population subgroups. This includes infants, young children, pregnant and lactating women, and people with certain medical conditions or special dietary requirements. Special purpose foods include various products such as infant formula, food for infants, FSMP, formulated meal replacements, formulated supplementary foods, and formulated supplementary sports foods. While formulated meal replacements, supplementary foods and sports foods are intended for the general population, they are included in the framework because individuals who consume these products have unique dietary needs which may vary from dietary recommendations for the general population.
The regulatory modernization efforts in Australia and New Zealand are still ongoing, with a particular focus on infant formula products, including those intended for medical purposes. Food Standards Australia New Zealand (FSANZ) has been actively consulting on proposals to amend these regulations since 2011. The objective of these proposals is to bring clarity to the regulatory landscape, incorporate the latest scientific evidence, and align the regulations with international standards wherever possible.
2.3.4 United States
Unlike Canada and many other international jurisdictions, the United States does not have a distinct regulatory framework for special dietary foods. Although there is a framework for FSDUReference 18, it is rarely used, as most products shifted to being classified as medical foods in the 1990sReference 19. The definition of medical foods in the United States is limited to products intended to meet the distinct nutritional requirements of a specific disease or condition, used under medical supervision, and designed for the specific dietary management of that disease or condition.
There is a separate regulatory framework for infant formula in the United States, in which compositional and labelling requirements are prescribed. In early 2023, the United States Food and Drug Administration announced that a study will be conducted by NASEM to examine the challenges in regulating infant formula effectively. The study will contribute to the development of a long-term national strategy for better oversight and consumer protection.
3.0 Description of proposed modernized framework
3.1 Objectives
The objective of regulatory modernization is to establish a comprehensive framework encompassing products currently regulated under Divisions 24 and 25 of the FDR. To address the limitations of the current regulatory framework, Health Canada is proposing a modernized risk-based approach that is better aligned with international jurisdictions. The proposal stems from an extensive evaluation of Divisions 24 and 25 to identify outdated provisions and barriers to access critical nutrition products.
3.2 Restructuring Divisions 24 and 25 of the Food and Drug Regulations
The proposed modernization initiative would restructure Divisions 24 and 25 of the FDR, which currently differentiate products based on the age of intended consumers. The aim of restructuring is to redefine product categories and establish requirements based on the risk profile of the product and the vulnerability of the intended subpopulation. The proposed approach of one division for FSDP and another for products that are not FSDP would simplify the regulatory framework and establish clearer compositional and labelling requirements for all products commensurate with risk.
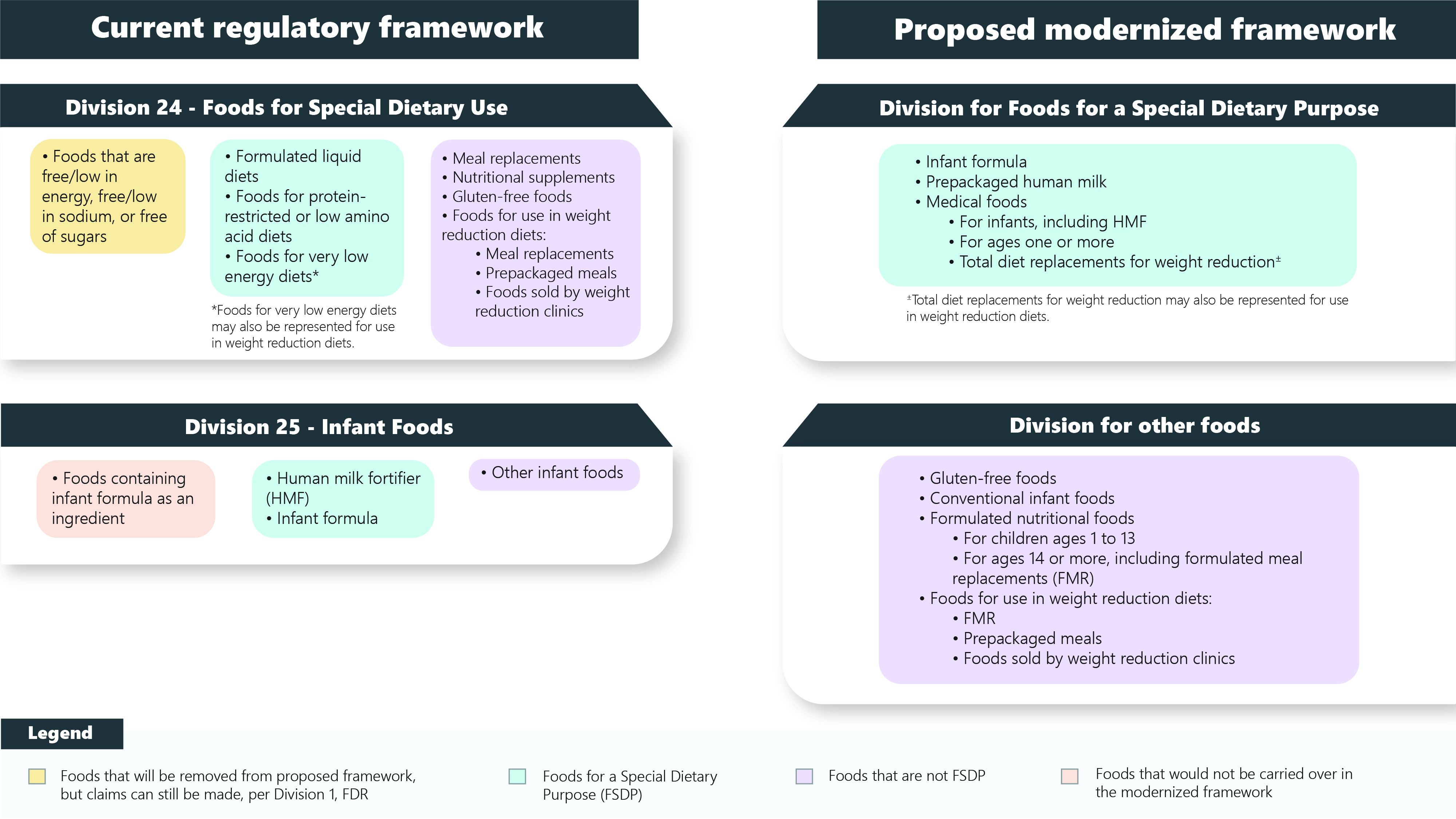
Figure 1 - Text description
Shown here is a figure that represents the current regulatory framework of Divisions 24 and 25 of the Food and Drug Regulations (FDR), and the proposed modernized framework.
The current regulatory framework for Division 24 of the FDR, foods for special dietary use (FSDU), includes three food categories. The first category includes foods that are free/low in energy, free/low in sodium, or free of sugars. These foods would be removed from the proposed framework, but claims would still be able to be made, per Division 1 of the FDR. The second category includes formulated liquid diets, foods for protein-restricted or low amino acid diets and foods for very low energy diets*. These foods would be captured in the proposed framework for foods for a special dietary purpose (FSDP). The third category includes meal replacements, nutritional supplements, gluten-free foods, and foods for use in weight reduction diets such as meal replacements, prepackaged meals and foods sold by weight reduction clinics. These foods would be moved to a division of the FDR that is separate from FSDP.
The current regulatory framework for Division 25 of the FDR, infant foods, includes three categories. The first category includes foods containing infant formula as an ingredient. and these foods would not be carried over into the modernized framework. The second category includes human milk fortifier and infant formula.
These foods would be captured in the proposed framework for FSDP. The third category includes all other infant foods. These foods would be moved to a division of the FDR that is separate from FSDP.
The proposed modernized framework includes a division for FSDP and a division for other foods. The food categories included in the proposed FSDP division are: infant formula, prepackaged human milk, medical foods for infants (including HMF), medical foods for ages one or more, and total diet replacements for weight reduction±.
The food categories in the proposed division for other foods are: gluten-free foods, conventional infant foods, formulated nutritional foods (for children ages 1 to 13 and for ages 14 or more, including formulated meal replacements), and foods for use in weight reduction diets (formulated meal replacements, prepackaged meals and foods sold by weight reduction clinics).
*Foods for very low energy diets may also be represented for use in weight reduction diets.
±Total diet replacements for weight reduction may also be represented for use in weight reduction diets.
To support access to safe products and provide a new pathway to manage future shortages, the regulations would provide a mechanism for exceptional importation in the case of shortages. As part of these provisions, companies would be required to report shortages of FSDP. The obsolete term ""FSDU" would be eliminated due to its redundancy and overlap with the term "FSDP". In addition, the term "medical foods" currently used in the United States would be adopted to refer to products intended for the dietary management of medical conditions and used under medical supervision.
The proposed division for FSDP would encompass higher-risk products intended for vulnerable subpopulations and require enhanced regulatory oversight. This includes infant formula, PPHM, and medical foods. This division would prescribe labelling requirements that differ from those of prepackaged foods for the general population. In most cases, premarket authorization would be required for FSDP intended for infants.
Products currently regulated under Divisions 24 and 25 that do not fall under the FSDP category would be placed in a separate division. This includes conventional infant foods, gluten-free foods, formulated nutritional foods, prepackaged meals and foods sold by a weight reduction clinic. These products are intended for self-selection by consumers and are not intended for use as a sole or primary source of nutrition under medical supervision. The regulatory requirements for these products would be updated to align with current nutrient recommendations and address consumer demand for nutrition information that is consistent with that on other prepackaged foods for the general population. Most of the nutrition labelling requirements unique to these foods would be removed, and these products would be required to carry an NFt and be subject to applicable FOP nutrition labelling requirements.
While the approach to modernizing the regulatory framework for products regulated under Divisions 24 and 25 is outlined in the following sections, some details, such as specific compositional requirements, will be available for stakeholder feedback during prepublication in Canada Gazette I.
To facilitate amendments in the future, the Department aims to incorporate by reference the compositional requirements for these foods, where appropriate. This approach would contribute to the creation of an agile regulatory framework, whereby compositional requirements can be amended administratively to align with the most up to date DRI and advancements in the field of nutrition science, ensuring that the framework remains responsive and in line with the evolving understanding of nutritional needs.
3.2.1 Product category exclusions from the revised framework
It is proposed to remove certain categories of foods from the regulatory framework. First, the framework would exclude foods on the sole basis of that they carry any of the following statements and claims set out in column 1 of the Table of Permitted Nutrient Content Statements and Claims: "free of energy", "low in energy", "free of sodium or salt", "low in sodium or salt" or "free of sugars". Although these foods are currently permitted to be represented as FSDUReference 20, Division 24 does not establish specific requirements for them.
Second, the framework would exclude foods containing infant formula. As these foods do not appear to be, nor are they expected to be on the market, they do not need to be retained in the modernized regulatory framework.
3.2.2 Transitioning from foods for special dietary use to foods for a special dietary purpose
The definitions for FSDU and FSDP both refer to foods that have been specially processed or formulated to meet the particular requirements of a person in whom a physical or physiological condition exists as a result of a disease or disorder (for example, medical foods such as FLDs, metabolic products, etc.). However, there are unique elements to both definitions as well, and are presented in Figure 2. FSDU includes foods for whom a particular effect, including but not limited to weight loss, is to be obtained by their controlled intake (such as MR for weight loss) while FSDP includes foods to be consumed as the sole or primary source of nutrition for an individual (such as infant formula).

Figure 2 - Text description
Shown here is a Venn diagram (two overlapping circles) that indicates the differences and similarities between the definitions of Foods for Special Dietary Use (FSDU) in B.24.001 for the Food and Drug Regulations and Foods for a Special Dietary Purpose (FSDP) in section 2 of the Food and Drugs Act. As indicated in the overlapping part of the circles, the definition of FSDU and the definition of FSDP both refer to foods that meet the requirements of a person in whom a physical or physiological condition exists due to an abnormal state for example, foods for protein-restricted diets).
There are unique elements to both the FSDU and FSDP definitions. FSDU include foods that impart a particular effect through controlled intake (for example, meal replacements for weight reduction), while FSDP includes foods to be consumed as the only or main source of nutrition (for example, infant formula for healthy term infants).
The modernized framework proposes removing the term FSDU and including a framework to regulate FSDP. This proposal would align with the addition, in 2019, of the term FSDP to the Food and Drugs Act and would eliminate confusion regarding these overlapping terms. By transitioning from FSDU to FSDP, the scope of products within this framework would be redefined, in particular, the framework would include products that are intended for use as a sole or primary source of nutrition for healthy individuals (for example, infant formula).
3.2.3 Clarifying current regulatory terms
Clarity on the term "infant foods" is required as the current nomenclature and definition create confusion. It is therefore proposed to revise the regulatory definition for infant foodReference 21 to address the issues with the current definition which has two uses in the regulations, by capturing all products intended for infants, including infant formula and other infant FSDP while also capturing conventional foods intended for infants. The proposed definition follows. Note that a new definition would be added to the regulations for conventional infant foods, which is proposed in subsection 5.1 Conventional infant foods.
a) Revised definition
The proposed revised definition for infant food would be:
infant food means a food that is labelled or advertised for consumption by infants including conventional infant foods and foods for a special dietary purpose
Note that certain regulatory requirements for infant foods would remain applicable to both conventional foods and infant FSDP. This includes the food additive provisionsReference 22 that are currently included in Division 25 of the FDR.
4.0 A division for foods for a special dietary purpose
The proposed modernization initiative includes establishing a division that would encompass products currently regulated under Divisions 24 and 25 of the FDR that meet the definition of an FSDP. Additionally, this division would cover FSDP that do not currently have specific provisions within the FDR.
This division for FSDP would include products that are currently regulated as FSDU, such as FLD, FVLED, and foods represented for protein-restricted and low amino acid diets (protein-related metabolic products). It would also encompass products that are not covered by the existing FSDU framework such as foods for dysphagia, fortified beverages for pre- and postoperative recovery, and metabolic products for disorders unrelated to protein metabolism, such as galactosemia and dyslipidemia.
With respect to foods for infants, the division for FSDP would include all infant formula, such as those intended for healthy infants and those for infants with diseases, disorders, or abnormal physical states (medical conditions), as well as PPHM, and medical foods for infants. Medical foods for infants are not intended to replace infant formula or human milk and are strictly intended for partial feeding. Examples include HMF, for which provisions were added to the FDR in 2021 and do not require any significant amendments to fit within the proposed framework. Additionally, single macronutrient type products that may be mixed with other products before use (such as protein, carbohydrate, or fat modulars) and other supplemental products (for example, feed thickeners) that can be added to infant formulas would be included within the division for FSDP.
The following categories of FSDP are proposed for this division, with details on each category outlined in the sections that follow:
- Infant formula
- Prepackaged human milk
- Medical food:
- Medical food for infants, including HMF
- Medical food for ages one or more
- Total diet replacement for weight reduction
4.1 Infant formula
This proposed category would include infant formula meeting the standard compositional requirements (such as infant formula intended for healthy term infants) as well as infant formula deviating from the standard compositional requirements (for example, infant formula intended for use under medical supervision such as metabolic infant formula for inherited metabolic disorders).
4.1.1 Definition
The following definition is proposed for infant formula:
infant formula means any food that is labelled or advertised for use as a partial or total replacement for human milk and is intended for consumption by infants
The proposed definition replaces the term "human milk substitute" in the existing definitionReference 23 with "infant formula" to match the common name. In addition, the proposed definition no longer refers to foods that are for use as an ingredient in a human milk substitute.
Water and/or a source of carbohydrate are the only substances that are currently permitted to be required for addition to infant formula in the directions for use. However, as it is no longer recommended to add a source of carbohydrate to infant formula other than under medical supervision, it is proposed to only allow the mention of water in the directions for use.
4.1.2 Compositional requirements
This proposed category of infant formula would include the following subcategories:
- Infant formula that meet the standard compositional requirements, such as infant formula for healthy term infants, lactose-free infant formula, and extensively and partially hydrolyzed infant formula
- Infant formula that deviate from the standard compositional requirements to meet the needs of infants with medical conditions. This could include preterm infant formula, metabolic infant formula for inherited metabolic disorders, and infant formula for catch-up growth
To ensure the nutritional requirements of infants with particular conditions are met, the proposed framework allows flexibility for the composition of infant formula to deviate from the standard requirements when medically justified. During the premarket authorization process, Health Canada would carefully evaluate the rationale and proposed deviations from the compositional requirements to assess their suitability for the target population.
The compositional requirements for infant formula will be updated to integrate the latest scientific evidence and align with international jurisdictions. To ensure transparency and gather valuable feedback, the draft compositional requirements for infant formula will be presented in a future consultation process.
a) New infant formula ingredients (NIFI)
To address the challenges associated with NIFI, a clear regulatory framework would be established, in which premarket authorization of a NIFI would be required prior to the submission of an infant formula containing the NIFI. By separating the NIFI review from the infant formula submission process, the timeline for reviewing an infant formula submission can be improved since the NIFI would have already received approval from Health Canada.
The premarket authorization requirements for NIFI would establish that the responsibility for preparing and submitting the necessary documentation lies with the NIFI manufacturer, rather than the infant formula manufacturer. If the ingredient in question was found to be safe for use in infant formula, then the infant formula manufacturer would be able to proceed with an infant formula submission for a product that contains the approved ingredient. This approach recognizes that the infant formula manufacturer may not have access to the proprietary information from the NIFI manufacturer, ensuring a more efficient and effective review process.
The following definition is proposed for NIFI:
new infant formula ingredient (NIFI) means a substance:
- that is intended for use as a food ingredient in infant formula, and
- that is not added to meet the compositional requirements for infant formula, and
- that is not a food additive or a novel food, and
- for which a history of safe use in infant formula has not been demonstrated in Canada
Furthermore, it is proposed to create a list of infant formula ingredients that have been authorized by Health Canada, which would be publicly accessible on the Government of Canada website. This list would also serve as a valuable resource to petitioners seeking to add an ingredient to an infant formula product, as they could easily identify which ingredients have already been accepted by Health Canada for addition to infant formula. Additionally, the guidance document would be updated to address current gaps, including providing a clear distinction between NIFIs and novel food for infants.
These improvements aim to enhance clarity and efficiency for adding NIFI to infant formula that are intended for sale in Canada. By establishing a robust regulatory framework, providing clear guidance, separating NIFI evaluations from infant formula submissions, and maintaining a public list of accepted new infant formula ingredients, Health Canada could effectively facilitate the introduction of safe NIFI while streamlining the regulatory process for manufacturers. These measures would help ensure transparency, support informed decision-making, and foster innovation in the development of infant formula.
4.1.3 Labelling requirements
The proposed labelling requirements for infant formula would aim to ensure that sufficient information is provided to caregivers to enable the safe use and preparation of these products while aligning with the Codex Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants (Codex Standard 72-1981)Reference 24 and the WHO Code, where appropriate, to reduce the burden on manufacturers. To streamline the review process and minimize ambiguity, it is essential to establish comprehensive and transparent labelling requirements for these products. This will help ensure a more efficient review process by reducing unnecessary back-and-forth communications to clarify label information.
a) Nutrition information
The current nutrition labelling requirements and prohibitions for infant formula would continue to apply, including the prohibition on the use of an NFt and FOP nutrition symbol on the label. However, minor changes are proposed related to outdated requirements. This would include removing the requirement to declare the content of ash in the productReference 25, which may be confusing to caregivers as ash is not considered a nutrient, and its declaration is not required in several other jurisdictions.
In addition, to enhance clarity and to facilitate compliance and enforcement activities, it is proposed to align the units of measure for vitamins and mineral nutrients in the nutrition information on the label with the units specified in the compositional requirements.
b) Mandatory label statements
- Principal display panel requirements
Mandatory statements on the principal display panel help caregivers make informed decisions about the most appropriate infant formula to select, such as differentiating between formula intended for infants 0-6 months of age (often called starter formula) and for infants 6-12 months of age (often called follow-up formula), standard or specialized formulas, liquid concentrated or ready-to-feed formulas, and more. As such, the following information would be required on the principal display panel of an infant formula product:
- Age range of the intended user of the product (for example 0 to 12 months, 6 months and more)
- For products that deviate from the standard compositional requirements: indication of how the composition deviates from the standard requirements and the purpose for which the product is intended (such as phenylalanine free for the dietary management of phenylketonuria in infants)
- Statement and/or symbol related to the addition of water:
- For liquid concentrated infant formula: "Add water", as well as a symbol demonstrating the addition of water
- For ready-to-feed infant formula: "Do not add water"
- Indication of the product format (for example powder, liquid concentrated, liquid ready-to-feed, etc.)
- Indication of the protein source (for example cow milk-based infant formula, goat milk-based infant formula, soy-based infant formula etc.)
Requiring on the label the source of protein, the age range of the intended user of the product and an indication of how the composition deviates from the standard compositional requirements aligns with recommendations in Codex Standard 72-1981 that states that the source of protein in the product shall be clearly shown on the label and the product should be labelled in such a way to avoid any risk of confusion between infant formula, follow-up formula, and formula for special medical purposes.
- Directions for use, including the preparation, storage and disposal
Mandatory statements related to the preparation, storage and disposal of the product would also be required on the product label. Prescribing these statements in regulation would provide consistency for the caregiver. Specifically, it is proposed that the label include the following directions for use or similar statements (in words and pictures):
- Clean countertops, top of infant formula container and hands with soap and warm water before preparing formula.
- Boil bottles, nipples, utensils and preparation equipment in safe water to sterilize before use. Each bottle must be prepared individually.
- To prepare powdered and liquid concentrated formula:
- For healthy, full-term infants: Boil safe water for two minutes. Cool to between body and room temperature (37 - 25 degrees Celsius).
- For premature and low birth weight infants under two months of age and infants with a weakened immune system: Boil safe water for two minutes. Cool to 70 degrees Celsius (30 minutes).
- The formula must be shaken well just prior to feeding, and served at body temperature (37 degrees Celsius).
- If a bottle of prepared formula is to be stored prior to use, it must be refrigerated and used within 24 hours of preparation.
- Throw away any formula that has not been consumed within two hours after a feed has begun.
- Do not store infant formula containers at excessive temperatures. Opened powdered formula containers must be used within one month. Opened liquid concentrated formula containers and ready to feed formula containers must be used within 24 hours.
Note: Item 3b in this list is not applicable to infant formula that carry a contraindication statement on the label against the use of these formulas in premature and low birth weight infants under two months of age and infants with a weakened immune system.
It is proposed that the following statement be required on the label immediately before the directions for use:
- For all infant formula: "Your baby's health depends on carefully following preparation instructions"
It is also proposed that the following statements be required on the label in close proximity to the directions for use:
- For powdered or liquid concentrated infant formula: "Do not change proportions of powder or concentrate or add other food except on medical advice"
- For ready-to-feed infant formula: "Do not dilute or add any other food except on medical advice"
These mandatory statements help reinforce to the caregiver that infant formula must be prepared according to the directions for use on the label.
This proposal aligns with recommendations in the WHO Code and Codex Standard 72-1981 which states that the label should include adequate directions for the appropriate preparations and use of the product, including its storage and disposal, as well as a warning about the health risks of inappropriate preparation.
- Lot numbers
It is proposed to require the lot number on the label of all infant formula. This information is important in case of a product recall, so that affected products can be easily identified by retailers and caregivers and removed from the market.
- Protecting and promoting breastfeeding
It is proposed that infant formula meeting the standard compositional requirements be required to carry a statement of the superiority of breastfeeding on the label under the heading "Important Notice". This statement would not be required for infant formula that deviate from the standard compositional requirement, because those products are intended for infants with distinct nutrient requirements that may impact their ability to safely consume human milk. This mandatory statement aligns with the WHO Code, as well as Codex Standard 72-1981.
Prohibiting certain images and text from the label would help Canada adhere to the WHO Code, as well as Codex Standard 72-1981. These recommend prohibiting the use of pictures or text that may idealize the use of infant formula (for example, pictures of infants or parents holding infants) as they can undermine the message that breastfeeding is best for babies. These also recommend prohibiting statements identifying an ingredient in infant formula as a key component of human milk which are considered misleading given that there are many components in human milk that are equally important. It is proposed to prohibit the following on the label of all infant formula:
- Pictures of infants, or other pictures or text, which may idealize the use of infant formula
- The terms "humanized", "maternalized", "human milk oligosaccharide", "human milk identical oligosaccharide" or similar terms, words, or abbreviations
- Statements comparing infant formula or its ingredient(s) to human milk, including comparison of the levels of a nutrient in infant formula to the level of the same nutrient in human milk
- Cautionary statements
It is proposed that cautionary statements currently recommended in Health Canada guidance, and those recommended during the premarket review, be added to the FDR to ensure consistent wording across all infant formula regarding their safe use. It is proposed that all cautionary statements be grouped on the product label under the heading "Caution".
Burn injuries related to heating infant formula can lead to emergency room visits and be life threateningReference 26. It is therefore proposed that the following be included on the label:
- A statement about the risks associated with heating and reheating infant formula in the microwave, such as "Never heat formula in a microwave oven. Serious burns can result"
- A statement about testing the temperature of prepared infant formula prior to feeding to prevent scalding the infant's mouth, such as "Place a drop of formula onto the inside of your wrist. If you do not feel a temperature difference, the formula temperature is just right"
It is proposed that infant formula meeting the standard compositional requirements be required to include a statement to the effect that the infant formula is to be used only on the advice of a health care professional. Similar statements already appear on infant formula on the Canadian market, and this aligns with recommendations in Codex Standard 72-1981 and the WHO Code.
It is proposed that infant formula deviating from the standard compositional requirements be required to include a statement on the label indicating that the infant formula is to be used only under medical supervision. These infant formulas are formulated to meet the needs of infants with medical conditions. As such, they may not be safe for consumption by healthy term infants.
It is proposed that all milk-based lactose-free infant formula be required to include a contraindication statement on the label against the use of these formulas in infants with galactosemia. Lactose-free, milk-based infant formula still contains a small amount of residual lactose. For this reason, these formulas are contraindicated for infants with galactosemia.
It is proposed that all powdered infant formula with heat sensitive ingredients (such as probiotics) include a contraindication statement on the label against the use of these formulas in premature and low birth weight infants under two months of age and infants with a weakened immune system. For these infants, the use of previously boiled water, cooled to 70 degrees Celsius is required for the preparation of the formula to kill bacteria that could be harmful to immunocompromised infants. However, infant formula with heat sensitive ingredients often have directions for use to prepare the product using previously boiled water, cooled to lower temperatures.
- Prohibition on cross branding
A study by Richter et al., showed that parents frequently mentioned the similarities between the labelling of infant formula and toddler milk (currently regulated as nutritional supplements for children)Reference 27. This may be due to cross branding (for example, when labels on nutritional supplements for children have a color scheme or design similar to labels on infant formula). This marketing strategy makes it challenging for caregivers to differentiate between infant formula and nutritional supplements for children, which may result in infants being fed nutritional supplements for children, which could pose a risk to health.
Nutritional supplements for children are proposed to be regulated as formulated nutritional foods (FNF) for children (refer to subsection 5.3.1 Formulated nutritional foods for children). As such, it is proposed that cross branding infant formula and FNF for children be prohibited.
- Labelling exemptions
Infant formulas that deviate from the standard compositional requirements are specially formulated to address the specific needs of infants with certain medical conditions. Considering the unique nature and purpose of these formula variations, some of the mandatory labelling requirements may not be applicable or necessary. Therefore, it is proposed that a provision be added to the regulations for infant formula deviating from the standard compositional requirements that would provide an exemption to certain labelling requirements specific to infant formula, where there is a valid rationale.
- Changes to label text or images
It is proposed that changes to the text or images on infant formula labels require advance notification to Health Canada, a minimum of 60 days prior to their implementation. This proactive measure would ensure that Health Canada possesses the most current version of product labels available on the market. Furthermore, this timeline allows Health Canada to identify any potential concerns related to claims and indications of use.
4.1.4 Additional regulatory requirements for infant formula
a) Claims
It is proposed that existing prohibitions for disease risk reduction claims and therapeutic claimsReference 28 as well as restrictions for nutrient content claimsReference 29 for foods intended solely for children under 4 years of age be maintained for all infant formula.
It is also proposed that health claimsReference 30 and/or indications of use made for all infant formula require substantiation with acceptable evidence from clinical research. However, certain indications of use already firmly established in scientific literature and widely accepted in medical practice would not necessitate additional substantiation. For instance, an infant formula specifically formulated to be phenylalanine-free for infants diagnosed with phenylketonuria would fall within this category. This measure is intended to help ensure that infant formula labels and advertisements do not contain misleading or unsubstantiated claims or indications of use. Furthermore, it is proposed that the regulations include font size restrictions for any claims made on the label to ensure that they do not overshadow the statement of the superiority of breastfeeding or mandatory cautionary statements.
b) Premarket authorization
It is proposed to require premarket authorization prior to the sale of a new infant formula product or an infant formula product that has undergone a major change. This would replace the existing premarket notification process and would require written authorization by Health Canada prior to the distribution of a product on the Canadian market. This approach would align with the current approach for HMF that was introduced in the regulations in 2021.
The information required for the premarket authorization submission would not change significantly from what is currently required in the FDR for the premarket notification. For example, it is proposed to require submission of the full label (including text and images) rather than the written text only. This would ensure that any images that are to be placed on the label would be reviewed by Health Canada.
c) Advertising restrictions
Advertisements that idealize the use of infant formula could create a belief that infant formula feeding is superior to breastfeeding and undermine the message that breastfeeding is best for babies. Presenting an ingredient in infant formula as a key component of human milk is misleading, as many components in human milk are equally important.
Prohibiting certain messaging in advertising would help Canada to adhere to the WHO Code and would not discourage breastfeeding. It is proposed to prohibit the following in the advertising of all infant formula:
- Messaging (statements, images, or videos) that implies or creates a belief that infant formula feeding is equivalent or superior to breastfeeding, including comparisons of ingredients in infant formula to components in human milk
- Any images, text or videos that may idealize the use of infant formula
- Messaging that highlights breastfeeding problems or emphasizes how formula feeding is easier than breastfeeding
- Terms such as "humanized", "maternalized", "human milk oligosaccharide", "human milk identical oligosaccharide" or similar terms, words, or abbreviations
- Any health claims or indications for use that have not been substantiated with acceptable evidence from clinical research as part of the infant formula premarket submission
Furthermore, messaging (statements or videos) in advertisements for infant formulas would be required to clearly indicate the superiority of breastfeeding.
These advertising restrictions would protect caregivers from advertisements that could negatively impact informed decision about breastfeeding. This approach is consistent with international jurisdictions like the EU, Australia and New Zealand, which restrict advertisements of infant formula in alignment with the WHO Code. While the advertising restrictions in the EU are through regulationReference 31, Australia and New Zealand has a voluntary agreement with industryReference 32.
d) Sales restrictions
No sales restrictions are proposed for infant formula.
4.2 Prepackaged human milk
PPHM refers to human milk that has been collected from individuals, processed, and packaged for safe consumption by infants. It is used in hospital settings for feeding infants who cannot obtain sufficient breast milk and, due to its limited supply, is generally prioritized for sick or preterm infants in neonatal intensive care units. While PPHM should not be a sole source of nutrition due to nutrient losses that occur during processing, its use has demonstrated health benefits for these infants (for example, less risk of developing necrotizing enterocolitis and contracting infections)Reference 33Reference 34.
PPHM would be a new category of FSDP not currently covered by Division 25. While PPHM is currently only available in Canada from a small number of human milk banks, the new category would include PPHM from both existing and new human milk banks as well as from commercial human milk processing companies that may in the future want to sell their PPHM products to hospitals or at retail in Canada. Human milk sold directly from one individual to another would be prohibited as it is not possible to implement the appropriate safeguards to prevent the sale of contaminated milk, which could cause severe harm to an infant.
Finally, prepackaged components of human milk (such as human milk cream) would not fall within the PPHM category but would be regulated under the medical foods for infants category (refer to subsection 4.3.1 Medical foods for infants). By implementing clear and comprehensive regulations for PPHM, Health Canada can further safeguard the well-being of infants who rely on these specialized products while providing clarity and guidance to stakeholders involved in their production, distribution, and use.
4.2.1 Definition
The following definition is proposed for PPHM:
prepackaged human milk means expressed human milk that is processed and prepackaged for consumption by an infant that is not the child of the individual supplying the expressed milk
It is proposed that PPHM must undergo a treatment process that eliminates harmful bacteria and viruses to ensure the milk is safe for consumption by infants. This language also allows for processing of human milk into formats with longer shelf lives (for example, powder and frozen formats).
4.2.2 Compositional requirements
The nutritional composition of human milk varies widely among individuals due to several factors, including stage of lactation, food and supplement intakeReference 35. PPHM is produced by pooling human milk from many individuals; thus, its nutrient composition can vary. Further, there are several steps within the PPHM production process that may impact nutrient composition (such as pasteurization, freezing, thawing, and storage)Reference 34. Given the natural variation in composition of human milk and the difficulty in meeting a standard set of compositional requirements, it is proposed that no compositional requirements be established for PPHM.
While PPHM may be supplemented with HMF in hospital according to medical practice and based on individual circumstances, it is proposed that PPHM contain human milk only; no other ingredients (such as vitamins and mineral nutrients, food additives) would be permitted to be added to these products.
4.2.3 Labelling requirements
a) Nutrition information
As an FSDP and recognizing the difficulty in generating accurate nutrient values when there can be significant variation in nutrient composition of human milk, it is proposed that PPHM be prohibited from carrying an NFt. For the same reason, no other form of nutrition information would be required for PPHM. However, producers could voluntarily declare the quantities of energy, protein, fat, carbohydrates, vitamins and mineral nutrients on the product label if they are able to generate accurate product-specific nutrient data.
PPHM would be prohibited from carrying an FOP nutrition symbol to align with the existing prohibition for foods intended for infants in B.01.350(15).
b) Mandatory label statements
Certain label statements are essential to provide health care professionals and caregivers with information about the safe use of PPHM. The following would be required to be displayed anywhere on the label:
- Directions for use, including the preparation, storage and disposal
- Expiration date
- Lot number
Mandatory label statements related to the preparation, storage and disposal of the product would be required on the product label. These are described below. Prescribing these statements in regulation would provide consistency for the caregiver. An expiration date would be required to indicate the date up to which the product maintains its microbiological stability. In addition, requiring the lot number (or batch ID) on the label is important in the case of a recall.
- Cautionary statements
To help caregivers make informed decisions for feeding their infant and to ensure the safe use of PPHM, it is proposed that PPHM carry the following cautionary statements on the principal display panel:
- "Not a complete source of nutrition"
- "Not a substitute for mother's milk or infant formula"
- "Not for infants under 6 months of age"
Due to strong public health messaging about mother's milk being the best food for infants, caregivers may think that PPHM is equivalent, or superior to, mother's milk or infant formula. Whereas mother's milk and infant formula are complete sources of nutrition, PPHM is not. Caregivers could mistakenly fully displace mother's milk or infant formula with PPHM if they do not have access to the important information conveyed by the "not a complete source of nutrition" and "not a substitute for mother's milk or infant formula" cautionary statements.
The cautionary statement "not for infants under 6 months of age" is intended to reflect Health Canada's recommendation for exclusive feeding of infants with mother's milk, or infant formula as a suitable alternative, from birth to 6 months.
These statements would not be required for PPHM used solely in hospitals because in this setting, PPHM is prescribed by health care professionals based on the nutrient requirements of the infant and supplemented with HMF as required. These products may be used by a health care professional in ways that do not align with the proposed cautionary statements, based on clinical judgement (for example, PPHM used for infants under 6 months of age).
It is proposed that the following cautionary statements be grouped anywhere on the label of PPHM under the heading "Caution":
- A statement about the risks associated with heating and reheating PPHM in the microwave, such as "Never heat product in a microwave oven. Serious burns can result"
- A statement about testing the temperature of prepared prepackaged human milk prior to feeding to prevent scalding the infant's mouth, such as "Place a drop of product onto the inside of your wrist. If you do not feel a temperature difference, the product temperature is just right"
- A statement indicating that the product is to be used only on the advice of a health care professional
The first two cautionary statements are intended to prevent burn injuries from consuming overheated human milkReference 36.
The benefits of using PPHM as a substitute for mother's milk depend on individual circumstances. As conveyed by the third cautionary statement, a health care professional should be consulted to determine whether the use of PPHM is appropriate for a given infant.
- Prohibition on statements and images that idealize prepackaged human milk
Pictures or text that may idealize the use of PPHM could undermine the message that breastfeeding is best for babies and give the impression that PPHM is equivalent or superior to mother's milk. As such, it is proposed that pictures of infants and any other pictures or text that may idealize the use of PPHM be prohibited on the label.
4.2.4 Additional regulatory requirements for prepackaged human milk
a) Claims
It is proposed that existing prohibitions for disease risk reduction claims and therapeutic claims as well as restrictions for nutrient content claims for foods solely for children under 4 years of age apply to PPHM. It is also proposed that health claims and/or indications for use for all PPHM require substantiation with acceptable evidence from clinical research.
In addition, it is proposed that the regulations include font size restrictions for any claim made on the label to ensure that they do not overshadow the mandatory cautionary statements on the principal display panel.
b) Premarket authorization
Most PPHM in Canada currently come from four existing Canadian milk banks that have well established risk mitigation strategies and quality control procedures in place (such as procedures for milk provider screening, milk provider education, milk processing, safety (microbial and chemical)). PPHM from these milk banks are regulated as conventional foods and they are not subject to premarket authorization.
Recognizing that commercial human milk processing is an emerging market, premarket authorization is proposed for new and majorly changed PPHM from commercial human milk processing establishments. Premarket authorization would also be required for products from new milk banks, noting that PPHM distributed by the four existing Canadian milk banks would be exempted from these premarket authorization requirements given their history of safe supply.
Premarket authorization would require a submission to Health Canada with the following information: procedures for milk provider screening, milk provider education, milk processing, safety (microbial and chemical), and quality controls for the determination of energy and nutrient content (where appropriate). This would allow Health Canada to determine whether sufficient risk mitigation strategies and quality control procedures are in place to ensure that safe products are produced, prior to their distribution in Canada.
c) Advertising restrictions
It is proposed to prohibit the following in the advertising of all PPHM:
- Messaging (statements, images, or videos) that implies or creates a belief that PPHM is a sole source of nutrition, or a substitute for mother's milk or infant formula
- Representation for infants under 6 months of age (exception: does not apply to PPHM used solely in hospitals)
- Any images, text, or videos that may idealize the use of PPHM
- Any health claims or indications for use that have not been substantiated with acceptable evidence from clinical research as part of the PPHM premarket submission
d) Sales restrictions
No sales restrictions are proposed for PPHM.
4.3 Medical foods
Medical foods encompass a diverse range of products that are specially processed or formulated to meet the distinct nutrient requirements of individuals with medical conditions. To enhance alignment with international standards and regulations and improve access to products available in other countries, the adoption of the term "medical foods" and the implementation of regulatory requirements for these foods are proposed.
The term "medical foods" refers to products intended for the dietary management of medical conditions and used under medical supervision.
Three categories are proposed: medical foods for infants, medical foods for ages 1 or more, and medical foods represented as a total diet replacement (TDR) for weight reduction. This categorization would allow for a more targeted approach in addressing the specific nutritional needs of the different age groups and for different intended uses of the products. In addition, it allows for greater regulatory oversight for medical foods for infants, who are a more vulnerable group.
4.3.1 Medical foods for infants
This proposed category is intended to capture FSDP for infants that do not meet the definition of an infant formula, specifically, the food is not intended for use as a partial or total replacement for human milk. Medical foods for infants are used to supplement the intake of primary nutrition sources (that is, human milk and infant formula) and are intended for the dietary management of a disease, disorder, or abnormal physical state. These products include but are not limited to HMF, modulars (for example, protein, carbohydrate, or fat modulars), and other products like food thickeners, metabolic foods in conventional food formats, and amino acid-based semi-solid medical foods that may be used to supplement a diet. This proposed FSDP category would capture a wide range of product types in order to support innovation in nutrition.
Medical foods for infants would exclude infant formula intended for use under medical supervision (for example, metabolic infant formula for inherited metabolic disorders) as these products are better captured under the infant formula category to effectively address the diverse needs of infants fed products intended to replace human milk.
The regulatory requirements for HMF introduced into the FDR in 2021 are proposed to be maintained. These regulations also helped inform the proposed regulatory requirements for other products captured in the new definition of medical foods for infants.
a) Definition
The following definition is proposed for medical foods for infants:
medical food for infants means a food that has been specially processed or formulated and is intended for the dietary management of infants, to be used under medical supervision. It is intended for the partial feeding of infants:
- with a limited or impaired capacity to take, digest, absorb, metabolize, or excrete ordinary food or certain nutrients contained therein, or metabolites, or
- with other medically determined nutrient requirements
whose dietary management cannot be reasonably achieved by modification of the normal diet alone
Note that the current definition of "human milk fortifier" in the regulations would be maintained.
b) Compositional requirements
Some medical foods for infants, such as modulars and feed thickeners, which are intended to be added to infant formula, may not require fortification for their intended purpose. However, other medical foods for infants, such as metabolic foods, may require fortification with specific nutrients to meet the nutritional requirements of the infants for whom they are intended. Therefore, medical foods for infants would be permitted to be fortified, however, given the wide variability of infant products that are intended to be captured under this category, it is proposed that compositional requirements would not be prescribed. Refer to the premarket authorization subsection for details.
c) Labelling requirements
- Nutrition information
As an FSDP, medical foods for infants would be prohibited from carrying an NFt as well as an FOP nutrition symbol. It is proposed that the nutrition information requirements for HMFReference 37 be applied to all medical foods for infants. This includes the following information:
- A statement of the quantity, expressed in grams, of protein, fat, available carbohydrate and, where present, fibre in the quantity of the food specified in the directions for use
- A statement of the energy value, expressed in Calories, in the quantity of the food specified in the directions for use
- A statement of the quantity, expressed in milligrams, micrograms, or International Units, of all vitamins, mineral nutrients and amino acids that are listed in item 6 of the table to section D.03.002 of the FDR that are present in the quantity of the food specified in the directions for use
- A statement of the quantity, expressed in grams, milligrams, micrograms, or International Units, of any other nutritive substance that is present in the quantity of the food specified in the directions for use
- Mandatory label statements
Label statements are essential to provide health care professionals and caregivers with information necessary to ensure the safe use and proper storage of food. It is proposed that mandatory label statements for HMF be applied to all medical foods for infants. This includes the following information:
- Directions for the storage of the food before and after the package has been opened
- Directions for use, including the preparation and storage of the food on its own or mixed if applicable
- A statement indicating that the food is to be used only under medical supervision
- The expiration date
- The lot number
The following additional mandatory statements are proposed for all medical foods for infants, including HMF as many of these statements are mandatory in other jurisdictions and are recommended under the Codex Standard for the Labelling of and Claims for Foods for Special Medical Purposes (Codex Standard 180-1991)Reference 38:
- A statement indicating that the product is not suitable for use as the sole or primary source of nourishment
- A statement on the intended purpose of the food
- Cautionary statements
The following cautionary statements are proposed for all medical foods for infants, including HMF, as these statements are recommended under Codex Standard 180-1991:
- Where appropriate, a statement concerning adequate precautions and contraindications
- Where appropriate, a warning that the product is not for parenteral use
d) Additional regulatory requirements
- Claims
It is proposed that existing prohibitions for disease risk reduction claims and therapeutic claims as well as restrictions for nutrient content claims for foods solely for children under 4 years of age apply to medical foods for infants.
It is also proposed that health claims and/or indications for use for all medical foods for infants would require substantiation with acceptable evidence from clinical research. However, certain indications of use already firmly established in scientific literature and widely accepted in medical practice would not necessitate additional substantiation. Furthermore, it is proposed that regulations include font size restrictions for any claims made on the label to ensure that they do not overshadow the mandatory cautionary statements.
- Premarket authorization
To help ensure nutrient adequacy and help prevent nutrient excess considering the wide variability in composition for these foods, premarket authorization would be required for all new and majorly changed medical foods for infants, except for non-fortified products like modulars and thickeners. By requiring premarket authorization, the goal is to establish appropriate safeguards and ensure the production of safe products that meet the specific dietary needs of vulnerable infants.
- Advertising restrictions
No advertising restrictions are proposed for medical foods for infants.
- Sales restrictions
It is proposed to apply the existing sales restriction for HMFReference 39 to all medical foods for infants that are subject to premarket authorization. For example, manufacturers and distributors would be allowed to sell these foods to individuals only if they have received a written order from a health care professional and the manufacturer or distributor has received a written request from a hospital.
4.3.2 Medical foods for ages one or more
The FSDU product categories in Division 24 that align with the proposed category of medical foods for ages 1 or more include FLD, FVLED, and foods represented for protein-restricted/low amino acid diets.
The proposed requirements for medical foods for ages 1 or more would be primarily based on the current regulatory requirements for FLD and would consider updates based on the latest DRI and alignment with international best practices and global standards, where appropriate.
It is also proposed that medical foods be permitted to represent as a TDR for weight reduction, which would replace the existing FSDU category of FVLED. These products are intended for use under medical supervision by individuals who are overweight or obese, and would be subject to additional regulatory provisions, outlined at the end of this section.
a) Definition
The following definition is proposed for medical foods for ages 1 or more:
medical food for ages one or more means a food that has been specially processed or formulated and is intended for the dietary management of individuals ages one or more, to be used under medical supervision. It is intended for the exclusive or partial feeding of those individuals:
- with a limited, or impaired capacity to take, digest, absorb, metabolize, or excrete ordinary food or certain nutrients contained therein, or metabolites, or
- with other medically determined nutrient requirements
whose dietary management cannot be reasonably achieved by modification of the normal diet alone
b) Compositional requirements
The proposed compositional requirements for medical foods for ages 1 or more aim to provide flexibility to accommodate rapidly evolving scientific evidence and diverse types of medical foods for various medical conditions/diseases. These requirements would be incorporated by reference, enabling amendments to be made administratively. This would lead to prompt updates to the composition of these products while ensuring alignment with evolving DRI and other scientific advancements in this field.
Medical foods are designed for the exclusive or partial feeding of individuals for an indefinite period, ranging from a few days to a lifetime. Individuals who consume medical foods as a sole or primary source of nutrition rely on these foods to meet all their nutrient requirements for the duration of use. Therefore, these foods must contain nutrients within an acceptable range to meet the minimum nutrient requirements of an individual without exceeding safe levels of intake. Minimum and maximum micronutrient requirements are relevant for products that provide a sole or primary source of nutrition.
Medical foods that are not intended for use as a sole source of nutrition are consumed alongside other foods in the diet, which reduces the risk of inadequate intake. However, there may still be a risk of excessive nutrient intake if nutritionally incomplete foods are fortified with high levels of nutrients. As such, maximum micronutrient requirements are relevant for these products.
It is proposed that compositional requirements be established for the following subcategories of medical foods for ages 1 or more:
- Nutritionally complete foods, which are represented for use as primary or sole source of nourishment
- Nutritionally incomplete foods, which are not represented for use as primary or sole source of nourishment
Medical foods that are represented as nutritionally complete would have to meet prescribed macronutrient requirements and provide all micronutrients determined to be mandatory, in amounts that meet prescribed minimum and maximum levels.
Medical foods that are represented as nutritionally incomplete would not have prescribed macronutrient requirements nor prescribed minimum micronutrient levels. However, they may be fortified with micronutrients if the amounts do not exceed prescribed maximum levels.
- Exemption when medically justified
To ensure the proposed medical food framework remains flexible and inclusive of the wide range of existing and future innovative products, as well as to accommodate scientific advancements, an exemption from meeting compositional requirements would be included when medically justified.
For example, medical foods intended for individuals with chronic kidney disease are specifically formulated to provide a lower level of protein and specific mineral nutrients. In such cases, there is a medical basis for deviating from the minimum compositional requirements for these specific nutrients. To mitigate potential risks, these products would be required to provide additional labelling information. This exemption would apply to both nutritionally complete and incomplete medical foods.
Moreover, this exemption would ensure that the formulation of these products can meet the specific dietary requirements of individuals with medical conditions. By allowing for scientifically justified exemptions, the regulatory framework acknowledges the need for personalized nutrition approaches in medical food formulation.
- Energy as the basis for compositional requirements
It is proposed that the compositional requirements for medical foods for ages 1 or more be prescribed per 100 kilocalories of the food, regardless of the recommended intake of the food. This approach is consistent with international jurisdictions. It also simplifies the current requirements for FLD, which differ based on total recommended daily energy intake, addressing stakeholder feedback received in the call for data from manufacturers and distributors of FSDU sold in Canada.
- Macronutrient requirements
It is proposed that macronutrient requirements for protein and fat similar to those for FLDReference 40 be applied only to medical foods represented as nutritionally complete. These requirements would be revised to reflect current DRI requirements. No carbohydrate requirements are proposed to allow for variability in composition.
- Fortification requirements
The current micronutrient requirements for FLD include a prescribed minimum for 18 micronutrients, with a maximum level established only for vitamins A and D. These requirements are based on outdated recommended intakes for adult males, and the approach used to establish these levels remains unclear, noting that the minimum requirements provided 25 to 150% of the respective recommended intake. Furthermore, the compositional requirements for FLD are intended for products that are nutritionally complete, leaving a significant gap for products that are not intended for use as a sole source of nutrition. Finally, the current requirements do not align with the requirements for products that fit this category in international jurisdictions, restricting access to products available in other countries.
Mandatory fortification with vitamins and mineral nutrients
The proposed micronutrient requirements for medical foods for ages 1 or more would be based on current DRIs. The proposed mandatory nutrients would be those for which a Recommended Dietary Allowance (RDA) or Adequate Intake (AI) is established, with possible exceptions. These nutrients include the current mandatory micronutrients for FLD, as well as additional micronutrients.
The proposed minimum levels would apply to all mandatory nutrients while the maximum levels would apply only to those nutrients for which a Tolerable Upper Intake Level (UL) or a Chronic Disease Risk Reduction Intake (CDRR) is established, indicating that long-term excessive intake has the potential to cause adverse effects.
Distinct nutrient requirements for different age groups may be established if deemed necessary (for example, children ages 1 to 13 years old).
If the medical food is nutritionally complete and represented as suitable as a sole or primary source of nourishment, it must contain all mandatory nutrients at levels within the prescribed minimum and maximum requirements.
If the food is nutritionally incomplete and not represented as suitable as a sole or primary source of nourishment, it may contain one or more specified micronutrients at any level that does not exceed the maximum prescribed level.
Voluntary fortification with vitamins, mineral nutrients and amino acids
In addition to the mandatory micronutrients prescribed for medical foods for ages 1 or more, it is proposed to allow voluntary fortification with certain nutrients, for which minimum levels would not be prescribed. This could include boron, which is permitted to be added to medical foods in other jurisdictions (EU, Australia and New Zealand). Similar to mandatory fortification for these foods, maximum levels would apply only to those nutrients for which a UL or CDRR is established.
The current provision that permits the addition of amino acids to FLD would be retained for all medical foods for ages 1 or more and expanded to enable the addition of other non-essential or non-proteinogenic amino acids, such as ornithine and citrulline, which have been shown to support the dietary management of critical illness and may provide benefits in the context of certain medical conditions. The addition of amino acids would be limited to improving the protein quality or achieving a medical purpose.
c) Labelling requirements
The proposed labelling requirements would ensure that sufficient information is provided to both consumers and health care providers to enable the safe use and preparation of these foods. The labelling requirements would align with other jurisdictions, as much as possible, to reduce barriers to the Canadian market. Together, these updated regulatory requirements would help Canadians access medical products that are available internationally.
- Nutrition information
The prohibitions on carrying an NFt and an FOP nutrition symbol that are currently applicable to FLD would be applied to medical foods.
It is proposed that the current requirements on nutrient content declaration and units of measure for FLD be applied to all medical foods for ages 1 or more. However, to enhance clarity and to facilitate compliance and enforcement activities, it is proposed to align the units of measure for vitamins and mineral nutrients in the nutrition information with the units specified in the compositional requirements. This harmonization of units between the label declaration and compositional requirements would enable regulatory authorities to easily verify whether the nutrient content stated on the label meets the specified requirements.
- Mandatory label statements
Label statements are essential to provide health care professionals and consumers with information necessary to ensure the safe use and proper storage of food. The following proposed mandatory label statements align with Codex Standard 180-1991 and many of these statements are mandatory in other jurisdictions.
- A statement that the product must be used under medical supervision
- A statement indicating whether the product is suitable for use as the sole or primary source of nourishment
- If a product deviates from the compositional requirements, a statement describing the deviation
- The statement "For the dietary management of …" where the blank shall be filled in with the disease, disorder, or abnormal physical state for which the product is intended
- Directions for use, including the preparation, storage and disposal
- The expiration date
- The lot number
Cautionary statements
The following cautionary statements are proposed, in alignment with Codex Standard 180-1991.
- Where appropriate, a statement concerning adequate precautions and contraindications
- A warning that the product is not for parenteral use
Labelling exemptions for metabolic products
Some medical foods are specially formulated for individuals with rare medical conditions, such as inherited metabolic disorders; these foods are known as metabolic products. Due to the low incidence of inherited metabolic disorders, the market for metabolic products is very small. Consequently, manufacturers often consolidate orders from multiple countries into a single production. This approach not only allows for the use of a standardized formulation, but also enables the packaging of products with uniform labels for global distribution. While these products comply with the regulatory requirements (composition and labelling) of the major market where the product is sold, they may not fully align with all the applicable regulations of the FDR.
To ensure the safe use and appropriate distribution of metabolic products, a system with robust safeguard has been put in place by distributors, including a requirement to provide a proof of diagnosis or written communication from a health care provider prior to purchase. This system aims to ensure that the products reach only those individuals who require them and for whom they are safe and beneficial. Additionally, it helps guarantee that individuals receive all the necessary information from their health care providers to ensure the proper use of these foods.
Health Canada has recently issued an interim policy statement recognizing that the risk of disrupted access to metabolic products for Canadians with inherited metabolic disorders outweighs the risks associated with non-compliance with current regulations. Restricted access to these products could pose a considerable risk to the health of medically vulnerable Canadians who rely on them to manage serious health conditions. As a result, a labelling exemption for metabolic foods is currently under consideration to ensure the continued availability of these products, even if they do not fully comply with all the applicable FDR labelling requirements. However, companies would still be required to provide information in a manner that permits safe preparation and use of these products. This may include a list of ingredients, nutrition information, and expiration date.
d) Additional regulatory requirements for medical foods for ages one or more
- Claims
It is proposed that existing prohibitions for disease risk reduction claims and therapeutic claims as well as restrictions for nutrient content claims for foods solely for children under 4 years of age apply to medical foods for ages 1 or more.
It is also proposed that health claims and/or indications for use for all medical foods for ages one or more require substantiation with acceptable evidence from clinical research. However, certain indications of use already firmly established in scientific literature and widely accepted in medical practice would not necessitate additional substantiation. Furthermore, it is proposed that regulations include font size restrictions for any claims made on the label to ensure that they do not overshadow mandatory cautionary statements.
- Premarket authorization
There are currently no premarket requirements for FLD in the FDR. It is proposed that there would be no premarket requirements for medical foods for ages 1 or more.
- Advertising restrictions
Health Canada has reassessed the current advertising restrictions for many FSDU and recognizes the need to update the approach in order to strike a balance between consumer protection and facilitating consumer access to medical foods, while also allowing manufacturers and distributors to effectively promote and distribute their products. Many other risk mitigation strategies would be used to protect consumer health, including the mandatory label statements; as such, it is proposed that the current advertising restrictions applicable to many FSDU would not be applied to medical foods for ages 1 or more.
- Sales restrictions
No sales restrictions are proposed for medical foods for ages 1 or more.
4.3.3 Medical foods represented as a total diet replacement for weight reduction
To address the specific needs of individuals seeking medically supervised weight reduction through TDR, it is proposed to establish a distinct subcategory of medical foods. This category would be designated as medical foods represented as a TDR for weight reduction, with unique requirements outlined below, in place of, or in addition to, the proposed regulatory requirements for medical foods for ages 1 or more. This proposal aligns with international jurisdictions and would replace the category for FVLED in Division 24.
a) Definition
The following definition is proposed for total diet replacement for weight reduction:
total diet replacement for weight reduction means a medical food specially formulated for use in energy restricted diets for weight reduction and which provides the sole source of nutrition when consumed as directed
b) Removal of existing definition
The regulatory definition for "very low energy diet" in Division 24, which refers to "a diet for weight reduction that provides less than 900 kilocalories per day when followed as directed", would be removed from the regulatory framework to accommodate the new proposed definition for TDR for weight reduction.
c) Compositional requirements
For medical foods represented as TDR for weight reduction, it is proposed that the energy restricted diet provide a minimum of 800 kcal per day and a maximum of 1200 kcal per day, in order to align with international jurisdictions. This requirement is intended to ensure that an individual consuming a medical food represented as a TDR for weight reduction would consume sufficient energy, while also reducing their energy intake in amounts necessary to achieve weight reduction.
This subcategory of medical foods would be subject to distinct compositional requirements, which would be prescribed per total daily intake. This approach is consistent with the current requirements in Division 24 for FVLED. For macronutrients, there would be minimum requirements for protein quantity and quality, carbohydrate quantity and linoleic and linolenic acid quantities. In addition, requirements for micronutrients would be set out, with no provision for deviation. Furthermore, international alignment and harmonization with the Codex Standard for Formula Foods for Use in Very Low Energy Diets for Weight Reduction (Codex Standard 203-1995)Reference 41 would be considered.
d) Labelling requirements
- Nutrition information
The nutrient content declaration requirements for this subcategory of medical foods would be consistent with the requirements for medical foods for ages 1 or more.
- Mandatory label statements
In addition to the mandatory label statements that would be required for medical foods for ages 1 or more, TDR for weight reduction would be required to include the following:
- The statement "for the dietary management of obesity" shall be declared on the label, near the common name of the food
- The recommended daily quantity to be consumed to provide the sole source of nutrition.
- A statement indicating that the food is suitable for use as a sole source of nutrition when consumed as directed
Cautionary statements
In addition to the cautionary statements that would be required for medical foods for ages 1 or more, TDR for weight reduction would be required to include the following:
- A statement to the effect that the food is not recommended for pregnant, nursing, or lactating women or individuals under 18 years of age
e) Additional regulatory requirements for total diet replacement for weight reduction
- Claims
It is proposed that existing prohibitions for disease risk reduction claims and therapeutic claims for FVLED apply to TDR for weight reduction. It is also proposed that health claims and/or indications for use for all TDR for weight reduction require substantiation with acceptable evidence from clinical research. In addition, it is proposed that the regulations include font size restrictions for any claim made on the label to ensure that they do not overshadow the mandatory cautionary statements on the principal display panel.
- Premarket authorization
Premarket authorization would not be required for these products, noting that compositional requirements would be established to ensure nutrient adequacy as a sole source of nutrition.
- Advertising restrictions
No advertising restrictions are proposed for this subcategory of medical foods, which would be consistent with the restrictions for medical foods for ages 1 or more.
- Sales restrictions
No sales restrictions are proposed for this subcategory of medical foods, which would be consistent with the restrictions for medical foods for ages 1 or more.
4.4 Proposed regulatory requirements applicable to all foods for a special dietary purpose
In addition to the proposed category-specific regulatory requirements, all FSDP would be subject to the following regulatory requirements.
4.4.1 Shortage provisions
The proposed regulatory framework for FSDP aims to reduce barriers to access for these products and improve alignment of Canadian regulations with those in international jurisdictions to help mitigate the risk of FSDP shortages. In addition to this effort, it is proposed to prescribe shortage provisions into the proposed regulatory framework for FSDP. This approach considers the FSDP provisions outlined in the Interim Orders Respecting Drugs, Medical Devices and Foods for a Special Dietary Purpose in Relation to COVID-19.
These provisions would align with the mechanisms already established for managing shortages of health products including medical devices and drugs, while also addressing regulatory gaps that hindered the advancement of FSDP shortage provisions in the September 2021 Regulations amending certain regulations concerning drugs and medical devices (shortages).
The proposed shortage provisions would provide Health Canada with enhanced capabilities to assess and address shortages of FSDP effectively. This would significantly strengthen support to healthcare systems nationwide. By helping to monitor, prevent, and alleviate shortages, these provisions would ultimately reduce the time health care professionals and patients spend searching for alternative products. Moreover, they would play a crucial role in preventing adverse outcomes resulting from treatment cancellations, deferrals, or the use of less effective substitute products.
a) Definition
The following definition is proposed for addition to the FDR:
shortage, in respect of a food for a special dietary purpose, means a situation in which the manufacturer of a food for a special dietary purpose is unable to meet the demand for the product in Canada
b) Reporting shortages
It is proposed that manufacturers of FSDP be required to report shortages or anticipated shortages to Health Canada. A shortage occurs when the existing supply no longer meets current demand, while an anticipated shortage refers to a future supply that is unable to meet projected demand. Criteria may be established to determine when mandatory reporting of an FSDP shortage is necessary; alternatively, it may be mandatory to report any shortage of an FSDP to Health Canada. Furthermore, the possibility of reporting product discontinuations is also being considered, particularly if the discontinuation has the potential to result in a shortage.
The reporting requirements would include notifying Health Canada when a shortage, or anticipated shortage is first known (within five business days), providing updated shortage information (within two business days of any modifications to the initially provided information) and reporting the end of the shortage (within two business days after the manufacturer's capability is restored). The Minister would have the authority to request specific information from regulated parties to assess or respond to an FSDP shortage. This information would be used to evaluate the level of risk and determine measures to prevent or alleviate the shortage.
Once a shortage, or anticipated shortage is reported to Health Canada, the proposed regulations would require that this information be promptly made publicly available on the Government of Canada website through an online list of FSDP that have been identified as being in a shortage situation. This transparent sharing of shortage information would enable manufacturers to effectively identify supply gaps and assist health care facilities in making well-informed decisions regarding patient care.
c) Exceptional importation of non-compliant foods for a special dietary purpose
The proposed shortage provisions would also introduce an exceptional importation framework to the FDR, helping to ensure access to FSDP in shortage situations. The framework would allow for the exceptional importation and sale of products that do not fully meet Canadian regulatory requirements, but that are manufactured to comparable quality standards. FSDP that are authorized for exceptional importation by Health Canada would be on a list of designated FSDP made available on the Department's webpage.
Manufacturers and importers of designated FSDP would be required to provide essential label information in both official languages, in a manner that permits the safe preparation and use of the food. This required information may include: the special dietary purpose for which the food is represented; a list of the food's ingredients; cautionary statements; directions for the preparation, use and storage of the food; the expiration date; and the lot number of the food, if applicable.
4.4.2 Stop-sale provisions
The proposed changes would include the extension of the existing stop-sale provisionsReference 42, currently applicable to FLD, MR, FVLED, HMF, and infant formula, to encompass all FSDP. These provisions empower the Minister to request written evidence from manufacturers regarding their products and to temporarily halt their sale until the requested evidence is provided. Moreover, the Minister holds the power to evaluate the adequacy of the submitted evidence, leading to the suspension of sales if deemed insufficient.
In 2021, additional requirements were added to the stop-sale provisions for HMF, specifying that the time limit for compliance should not be less than 24 hours following the request, unless the Minister has reasonable grounds to believe that there is a serious and imminent risk of harm to human health. These additional requirements would also be applicable to all FSDP.
Lastly, these provisions specify that the requested evidence primarily pertains to establishing the nutritional adequacy of the product when used as the sole source of nutrition. In the case of infant formula and HMF, evidence related to the product's expiration date is also included. Additionally, it is proposed that the scope of the requested evidence be expanded to include evidence related to other parameters regarding the safety of the product.
4.4.3 Clinical trial framework
As part of the Treasury Board of Canada Secretariat's Targeted Regulatory Review initiative and subsequent Health and Biosciences Sector Regulatory Review Roadmap, Health Canada committed to modernize clinical trial regulations across various product lines, including FSDP. Under this proposed risk-based framework, clinical trials for FSDP with premarket review requirements including premarket authorization and notification, could be pursued prior to the issuance of a Letter of Authorization or a Letter of No Further Questions, respectively. Additionally, non-compliant FSDP that do not require premarket review could also receive authorization for use in a clinical trial in Canada.
The clinical trial framework for FSDP is still under development. The proposed modernization of the regulatory framework for FSDP, as outlined in this consultation paper, would complement the proposed clinical trial framework by providing clarity on the classification of foods as FSDP and updating their regulatory requirements.
4.4.4 Premarket review requirements for infant foods for a special dietary purpose
As described in the previous sections, the vast majority of FSDP intended for infants would require premarket authorization prior to sale of a new or majorly changed product, with the exception being unfortified medical foods for infants. However, the premarket authorization submission requirements would vary depending on the category of infant FSDP. Additional details related to these requirements for PPHM and medical foods for infants, as applicable, will be proposed at a later date.
a) Major change
A major change to an existing HMF or infant formula currently does not require the submission of data on a range of significant topics including manufacturing, quality control, packaging, and stability testing for expiry dates. However, this information is often requested during the evaluation process to provide necessary context and complete the review.
The introduction of five additional requirements is proposed to support the premarket authorization of a major change to an infant formula or HMF. These new requirements would align with information requested for new infant formula and new HMF submissions, and consist of:
- the name and address of each establishment in which the infant formula or HMF is manufactured
- the nutrient specifications for the infant formula or HMF after incorporating the major change
- details of the changes to the infant formula or HMF manufacturing process and quality control procedures used throughout the process as a result of the major change
- the results of the tests carried out to determine the expiration date of the infant formula or HMF after incorporating the major change
- a description of the type of packaging to be used for the infant formula or HMF, if the major change affects the packaging
b) Revised definition
It is proposed to revise the current definition of a "major change" to address deficiencies in the current definition in the FDR. This includes replacing the term "human milk substitute" and "human milk fortifier" with "food for a special dietary purpose intended for infants", replacing the word "processing" with "manufacturing" and including the word "physical" to reflect the possibility of inadequate physical characteristics of the infant formula matrix (powder or liquid).
The following revised definition is proposed for major change:
major change means, in respect of a food for a special dietary purpose intended for infants, any change of an ingredient, the amount of an ingredient, or the manufacturing or packaging of the food for a special dietary purpose intended for infants where the manufacturer's experience or generally accepted theory would predict an adverse effect on the levels or availability of nutrients in, or the physical, microbiological or chemical safety of the food for a special dietary purpose intended for infants
4.4.5 Review of submission timelines
Noting that Health Canada would expand the premarket authorization requirements to apply to the vast majority of new and majorly changed FSDP intended for infants, the Department is exploring the possibility of introducing different streams of submissions for premarket authorization with specific timelines based on complexity. For example, submissions that are less complex could be placed in a stream with a shorter timeframe. Health Canada intends to propose and publish these timelines in guidance.
In certain instances, when Health Canada receives a new or major change submission for infant formula, further clarification may be needed, leading to additional communication with the manufacturer and potentially prolonging the review process. The number of deficiency letters sent by Health Canada to obtain all the necessary information for assessment can significantly impact the overall review time. To address this, Health Canada aims to strengthen the screening process to identify submissions that lack significant amounts of information, allowing for early identification and potential closure of poor-quality submissions if data gaps cannot be adequately addressed within a relatively short period. This approach would prioritize the evaluation of high-quality submissions, reducing correspondence with manufacturers and ultimately shortening the review time.
Health Canada is committed to updating the submission checklist and guidance documents for industry, ensuring that manufacturers have comprehensive information to prepare their submission packages. The Department will continue to support petitioners through pre-submission meetings, as is currently practiced.
5.0 A division for foods that are not foods for a special dietary purpose
The current regulatory framework set out in Divisions 24 and 25 includes several products that are not FSDP. This includes MR, NS, gluten-free foods, prepackaged meals, and foods sold by a weight reduction clinic (Division 24) as well as conventional infant foods (Division 25). The regulatory requirements for these products would be housed in a separate division within the FDR.
The following categories of products are proposed, with details on each category outlined in the sections that follow:
- Conventional infant food
- Gluten-free food
- Formulated nutritional food (FNF)
- FNF for children (ages 1 to 13)
- FNF for ages 14 or more, including products represented as formulated meal replacements
- Foods represented for use in weight reduction diets
- Formulated meal replacements
- Prepackaged meals
- Foods sold by a weight reduction clinic
5.1 Conventional infant foods
Health Canada is proposing to introduce the term "conventional infant food" to refer to a category of infant foods that are not FSDP, such as infant cereals and strained fruit. This proposed category would distinguish these products from the broad category of infant foods currently defined in the FDR, which includes all products intended for infants, including FSDP.
It is proposed that the category capture foods that are labelled or advertised for consumption by infants 6 months of age to less than 1 year of age, excluding FSDP. These foods are subject to the existing provision that prohibits any representation on the label respecting the consumption of the food by an infant who is less than 6 months of ageReference 43. Foods which are not labelled or advertised for consumption by infants, such as products solely targeted to young children aged 1 or more, would be excluded.
5.1.1 Definition
The following definition is proposed for conventional infant food:
conventional infant food means a food that is labelled or advertised for consumption by infants other than a food for a special dietary purpose
5.1.2 Compositional requirements
a) Fortification requirements
The FDR permits the addition of certain nutrients to infant cereal products. It is proposed that the fortification of infant cereal products remain voluntary. The current list of vitamins and mineral nutrients that may be added to infant cereal products would be reviewed. The current nutrient amounts rely on the general fortification provisions, which are not specific to infants and do not provide maximumReference 44 amounts for mineral nutrients. The proposed approach would set out minimum and maximum amounts for all vitamins and mineral nutrients that may be added, taking into consideration the latest nutrient intake recommendations for infants, evidence related to nutrient intakes and international approaches.
b) Sodium
Sodium is an essential mineral nutrient which is needed to maintain health, but excess consumption may lead to high blood pressure, a major risk factor for developing cardiovascular disease. Canadians, particularly children, teens and males, are consuming too much sodium. An important factor underlying excess sodium consumption is the preference for salted foods and the development of this preference may begin in infancyReference 45Reference 46. The FDR currently sets restrictions on sodium chloride addition and sodium levels in some infant foods.
It is proposed to maintain the prohibition of added sodium chloride to any infant food that contains strained fruit, fruit juice, fruit drink or cereal.
It is proposed to remove the exemption for strained desserts as set out in B.25.003(2) of the FDR. Instead, desserts would be added to the list of infant foods prohibited from containing added sodium chloride.
Regarding sodium limits, Table 1 demonstrates the proposed changes to the food categories and corresponding sodium limits set out in Table 1 of Division 25 that would be incorporated by reference into the FDR.
| Column I Food category |
Column II Total sodium in milligrams per 100 grams of food |
|---|---|
| Rusks, biscuits, dehydrated snacksFootnote * | 200 |
| All other ready to eat conventional infant foodsFootnote * | 100 |
|
|
This proposal would simplify the current regulatory approach and eliminate gaps resulting from the current closed list of foods.
The proposed sodium limit for rusks, biscuits and dehydrated snacks considers the typically small serving size (by weight) of these foods. Although the proposed limit is below the voluntary maximum targets set out for these types of foods in Health Canada's Voluntary sodium reduction targets for processed foods 2020-2025, those targets are inclusive of toddler foods.
The AI for sodium is more than two times higher for the toddler age group (1 to 3 years) than for infants (7 to 12 months), and foods represented for toddlers are subject to FOP labelling that identifies foods high in sodium, whereas prepackaged products intended solely for infants aged 6 months or more but less than 1 year are prohibited from carrying the FOP nutrition symbol. Given this, a lower sodium level in conventional infant foods than in toddler foods would be appropriate.
c) Sugars
Health Canada guidance recommends that complementary foods for infants be prepared and served with little or no added sugar. On food labels, sugars-based ingredients must be grouped in the list of ingredients to help caregivers understand their relative proportion in the food compared to other ingredients as well as identify unfamiliar sources of sugars in foods. Additionally, the FDR requires that prepackaged foods declare the total amount of sugar, in grams, in the NFt.
Although a scan of the Canadian marketplace indicates that most conventional infant foods contain little or no added sugar, certain food categories were more likely to contain added sugar (such as rusks or biscuits, infant snacks and desserts). For these food categories, Health Canada is considering introducing restrictions on sugars identified by the Word Health Organization as those to limitReference 47.
d) Saturated fats
Given that dietary fat restriction is not recommended for children younger than 2 years, saturated fat limits would not be proposed.
5.1.3 Labelling requirements
a) Nutrition information
It is proposed that the current nutrition labelling requirements for these foods be maintained as set out in the nutrition labelling requirements for prepackaged foods in Part B of the FDR. This includes the requirement to carry an NFt and the prohibition on the use of an FOP nutrition symbol on the label.
5.2 Gluten-free foods
Gluten is the main protein component of wheat and can also be found in other cereals like barley and rye, as well as their hybridized strains. Celiac disease is a chronic, immune-mediated condition that is triggered by gluten exposure. Exposure to gluten in individuals with Celiac disease can lead to damage to the lining of the small intestine, resulting in reduced nutrient absorption, including iron, folate, calcium, vitamin D, protein, fat, and other essential nutrientsReference 48. The only effective way to manage this disease and prevent health complications is by strictly avoiding gluten sources in the diet.
Division 24 sets out a prohibition on the representation of a food as gluten-free if it contains any form of gluten proteinReference 49. This prohibition helps to protect the health and safety of individuals who require the use of specially processed or formulated gluten-free food. Currently, only those foods that have been specially processed or formulated to meet the needs of individuals who are required to follow a gluten-free diet in order to protect their health, are considered FSDU and are allowed to carry a gluten-free claim. For example, bread made with substitute flours from grains that are not considered gluten sources can be labelled gluten-free if all other requirements are met.
It is challenging to differentiate foods that have been specially processed or formulated to be gluten-free from those that are inherently gluten-free (such as crackers made from rice flour). For this reason, many international jurisdictions have removed gluten-free foods from their special dietary food frameworks. Instead, these foods are regulated as conventional foods, subject to specific labelling requirements.
It is important to note that even foods without gluten-containing ingredients can still contain gluten due to cross-contamination during manufacturing or distribution. In 2012, regulatory amendments to the FDR came into effect to address this issue through enhanced labelling regulations for food allergens including gluten sources.
In the proposed modernized framework, gluten-free foods would not be considered FSDP which better aligns with international approaches. In addition, this would allow gluten-free foods to continue to apply the current labelling requirements for conventional prepackaged foods.
5.2.1 Compositional requirements
a) Fortification requirements
Following a gluten-free diet may increase the risk of nutrient deficiencies particularly for certain vitamins and minerals that must be added to gluten-containing staple foods, but not gluten-free substitute foods. For example, folic acid is mandatory in white flour to prevent neural tube defects like spina bifida, while calcium may be voluntarily added to white flour to support bone health.
There are no mandatory fortification requirements for gluten-free substitute foods (for example, gluten-free flour). An exemption exists in Part D of the FDRReference 50, which governs the addition of vitamins, minerals, and amino acids to foods. This exemption permits the fortification of specially processed or formulated gluten-free foods as long as no specific standard is prescribed in the FDR, and they are not advertised to the general public. This provision does not specify fortification amounts or the specific nutrients that can be added to fortified gluten-free foods.
- Voluntary fortification of gluten-free foods with vitamins and mineral nutrients
Industry stakeholders have expressed confusion regarding the regulations for the voluntary fortification of gluten-free foods, prompting the CFIA to publish guidance online. According to this guidance, the fortification of specially processed or formulated gluten-free foods should aim to achieve nutrient levels equivalent to those in white flour, corresponding to the amount of white flour replaced. The advertising restrictions aim to prevent the advertisement of fortified gluten-free foods as superior to their gluten-containing counterparts.
The exclusion of gluten-free foods from the FSDP framework would require amendments to the fortification provision for gluten-free foods. By removing the requirement for gluten-free foods to be specially processed or formulated, the criteria for fortification may apply to a broader range of foods, increasing the risk of over-fortification in the food supply. To address this, new restrictions would be considered. These could include limiting fortification to certain food commodities such as grains and bakery products, and/or specifying the nutrients and amounts that may be added to gluten-free foods to achieve nutritional equivalence.
5.2.2 Labelling requirements
a) Nutrition information
The proposed classification of gluten-free foods outside of the FSDP framework would mean nutrition labelling requirements for conventional foods would apply. Gluten-free foods would continue to be required to provide nutrient content information through an NFt and comply with FOP nutrition labelling requirements when applicable.
5.2.3 Additional regulatory requirements for gluten-free foods
a) Gluten-free label claim
The prohibition on the representation of a food as gluten-free if it contains any form of gluten proteinReference 51 would be maintained. However, by excluding gluten-free foods from the FSDP framework, the range of products permitted to carry a gluten-free claim would expand to include inherently gluten-free products, not just those specially processed or formulated to be gluten-free. This expansion could simplify shopping for individuals who must follow a gluten-free diet.
However, there may be concern that expanding the gluten-free claim to all foods, even when there is no risk of gluten contamination (for example, bottled water), could have a health halo effect and influence consumers' purchasing decisions. Therefore, it may be necessary to limit the use of this claim to certain food commodities where there is a possibility of inherent gluten content or cross-contamination.
b) Gluten-free threshold
Section B.24.018 of the FDR prohibits the use of gluten-free claims on foods that contain any gluten or modified gluten protein. While no specific threshold is mentioned in the regulations, Health Canada considers that gluten-free foods prepared under good manufacturing practices, with gluten levels not exceeding 20 parts per million (ppm) meet the health and safety intent of B.24.018 when a gluten-free claim is made. This threshold level is supported by the Codex Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten (Codex Standard 118-1979)Reference 52.
To ensure flexibility and the ability to adapt to the latest scientific evidence and gluten detection methodologies, Health Canada proposes to maintain this recommendation in guidance.
c) Advertising restrictions
Certain stakeholders have highlighted that advertising restrictions disincentivize manufacturers from fortifying gluten-free foods. To promote awareness of and accessibility to fortified gluten-free foods, Health Canada is proposing to remove the advertising restriction for these products.
5.3 Formulated nutritional foods
This proposed category of formulated nutritional foods (FNF) aims to capture the existing products currently regulated under Division 24 as MR and NS. MR are intended to replace a meal while NS are intended to supplement a diet that may be inadequate in energy or other essential nutrients. Given their similar objectives, it is proposed to merge these two categories into one overarching category named FNF, with the option to represent as formulated meal replacements (FMR) when certain conditions are met. To fulfill their intended purpose, FNF would provide well-balanced amounts of macronutrients and micronutrients relative to energy (kcal). In addition, insights gained from the TMALs issued for these products, including the common areas of regulatory non-compliance, have helped inform the following proposal.
Products targeted to individuals who generally maintain a nutritionally adequate diet, such as those intended for individuals working out (for example, sports food) would not fall within the scope of FNF. These products do not align with the intended purpose of this category, which is to supplement a diet that may be inadequate in energy or essential nutrients. Instead, such products may be more appropriately classified as supplemented foods, which are intended to be consumed in addition to a typical diet that already meets the nutritional needs of most Canadians. Accordingly, supplemented foods have defined maximum amounts for supplemental ingredients including vitamins and minerals that reflect this distinction.
Two categories are proposed: FNF for children and FNF for ages 14 or more. While many regulatory requirements would apply to both categories, the prescribed compositional requirements would be aligned with the DRI for the targeted age group.
FNF for ages 14 or more would be further subdivided to accommodate the varied use of these products, including permitting some FNF to represent as meal replacements, including those for use in weight reduction diets. Similarly, FNF for children would be subdivided into two subcategories, to reflect the target population of the products; however, FNF for children would not be allowed to be represented as FMR or for use in weight reduction diets. Table 2 provides information on the proposed categories and subcategories of FNF.
| Categories | Formulated nutritional foods (FNF) | |||
|---|---|---|---|---|
| FNF for children | FNF for ages 14 or more | |||
| Subcategories | FNF for ages 1 to 3 | FNF for ages 1 to 13 | FNF providing 150 – 199 kcal | FNF providing ≥200 kcal, which includes products represented as FMR, including those used in weight reduction diets |
5.3.1 Formulated nutritional foods for children
NS that are intended for children aged 1 year or more do not currently have age specific compositional requirements in Division 24. Instead, these products must comply with compositional requirements that encompass adult recommended intakes or apply for a TMAL to permit their sale despite regulatory non-compliance caused by aligning with DRI for children. Many international jurisdictions lack prescribed requirements for these products, as their fortification policies generally allow their manufacturing as conventional foods.
Some jurisdictions and health organizations are of the opinion that these products marketed as "growing-up milk", "toddler milk", and "young-child formula" are unnecessary for healthy children, emphasizing that they may displace healthy foods and are not essential to meet nutrient requirementsReference 53Reference 54. Health Canada recognizes that there may be instances where these products are used by non-medically supervised children as one of the means to supplement a diet when it may be inadequate in energy and essential nutrients. As such, this proposal includes the introduction of a subcategory for fortified and energy-balanced products specifically intended for children, with compositional requirements that align with the nutrient requirements of this particular age group.
To address the specific nutritional requirements of young children, two subcategories of FNF for children are proposed:
- FNF for ages 1 to 3
- FNF for ages 1 to 13
While most of the regulatory requirements for both subcategories of FNF for children would be the same, there would be variations in the micronutrient requirements between these two subcategories. This distinction aims to encompass the range of existing products available both in the Canadian and international markets, in which there exists products designed exclusively for children 1 to 3 years old or for a broader pediatric subpopulation, including children 1 to 13 years old.
By establishing micronutrient requirements for each subcategory, FNF can be tailored to meet the nutrient requirements of either young children (1 to 3 years old) or a wider age range of children (1 to 13 years old), aligning with current market demand. This approach helps to ensure that these products provide adequate nutrients while minimizing the risk of nutrient excess for this vulnerable population.
a) Definition
The following definition is proposed for FNF for children:
formulated nutritional food for children means a formulated food sold or represented for children 1 to 13 years old to be consumed in addition to their diet when it may be inadequate in energy and essential nutrients
b) Compositional requirements
Compositional requirements for FNF for children would be developed using the latest pediatric DRIs and would be incorporated by reference into the FDR.
- Energy requirements
It is proposed that all subcategories of FNF for children provide a minimum of 150 kcal per serving, aligning with the current minimum energy requirement for NS.
- Macronutrient requirements
It is proposed that quantitative requirements for macronutrients be prescribed to ensure that FNF provide balanced nutrition to help achieve the intended use of these products. The existing macronutrient requirements for MR and NS would be reviewed to reflect current DRI for the pediatric subpopulation and would be incorporated by reference into the FDR.
It is proposed that FNF for children be required to meet the current protein quality requirements for NS, in accordance with B.24.201(1)(c).
- Fortification requirements
Mandatory and voluntary addition of vitamins and mineral nutrients
FNF for children are intended to supplement a diet when it may be inadequate in energy or other essential nutrients. These products are generally not consumed in addition to a regular diet, instead they are used when the diet may be inadequate and are therefore replacing foods usually consumed in a regular diet. As such, it is important for these products to provide a broad spectrum of nutrients to adequately replace the portion of the regular diet being displaced.
To ensure consumers' needs are being met when using FNF instead of foods usually consumed in a regular diet, it is proposed that all micronutrients with an RDA set by NASEM be mandatory. If these micronutrients are not present in FNF there could be a risk of adverse effects since there is evidence of a causal relationship between intake of these micronutrients and an indicator of adequacy, as well as a demonstrated intake-response relationship. On the other hand, it is proposed that micronutrients with an AI set by NASEM be voluntary. If these micronutrients are not present in FNF the risk of adverse effects is low since deficiencies are either rarely seen in the general, healthy population or only seen when artificially induced (for example, individuals fed diets devoid of the micronutrient). Furthermore, FNF will not be used as a sole source of nutrition, so consumers will still be consuming these micronutrients with an AI from other foods or supplements.
This approach allows for some nutrients currently not permitted in MR or NS to be added to FNF for children, such as vitamin K and choline. Some nutrients that are currently voluntary would become mandatory, such as selenium and molybdenum; while others that are mandatory would become voluntary, such as pantothenic acid, biotin, manganese, and sodium.
Minimum amounts for vitamins and mineral nutrients
For those micronutrients which are voluntary, it is proposed that no minimum amount be prescribed. The prescribed minimum amounts for mandatory nutrients would apply to all sources of a nutrient but would not include overage.
The minimum amount for FNF for children would be set at 15% of the highest RDA (when applicable) for the respective target age groups, as consumed. This level corresponds to the amount necessary for making a "good source" claim for most nutrients.
Maximum amounts for vitamins and mineral nutrients
Maximum amounts would be applicable to all sources of the nutrient in the product, excluding any overages. It is proposed that the maximum amount for most micronutrients be set at 50% of the highest RDA or AI (when applicable) for the respective target age groups, as consumed. This approach aims to mitigate the risk of adverse events caused by excessive nutrient intake, considering that many nutrients have a narrow safe intake range and can lead to serious adverse effects when consumed in excess. For most nutrients, 50% of the highest RDA or AI is less than 25% of the corresponding UL or CDRR. Some adjustments to this general approach may be necessary after conducting additional safety analyses. In cases where nutrients have narrower safety margins (in other words, when the highest RDA or AI is closer to the corresponding UL or CDRR), exceptions to the general approach may be made to set lower maximum amounts.
Exception for potassium
Regarding the proposed distinction between micronutrients with an RDA as mandatory and those with an AI as voluntary, an exception is proposed for potassium. Although potassium does not have an established RDA, it is proposed that potassium remains a mandatory nutrient in these foods. This decision is supported by evidence highlighting the relationship between increased potassium intake and decreased blood pressure. While scientific literature on the relationship between potassium intake and blood pressure in children is limited, given the evidence related to potassium intake and blood pressure regulation in adultsReference 55, it is proposed to make potassium mandatory for all FNF for children.
However, instead of applying the same approach used for micronutrients with an established RDA, which proposes a minimum amount of 15% for FNF for children, lower minimum amounts are being contemplated specifically for potassium. The minimum amount currently prescribed in the FDR for NS is being considered for FNF for children, while the proposed maximum amount for potassium is 50% of the AI.
A summary of the proposed micronutrient requirements for FNF for children is presented in Table 3.
| Subcategories of FNF for children | ||
|---|---|---|
| FNF for ages 1 to 3 | FNF for ages 1 to 13 | |
| Minimum amounts for mandatory micronutrients | 15% of the RDA for children 1 to 3 years old | 15% of the highest RDA for children 1 to 13 years old |
| Minimum amounts for voluntary micronutrients | N/A | N/A |
| Maximum amounts for mandatory and voluntary micronutrients | 50% of the RDA or AI for children 1 to 3 years old | 50% of the highest RDA or AI for children 1 to 13 years old |
Voluntary addition of amino acids
The use of plant-based proteins in the food supply is on the rise. It is challenging for products that use plant-based proteins to meet the current protein quality requirements for MR and NS. To address this issue, it is proposed that essential amino acids may be added to FNF for children to improve protein quality and meet protein quality requirements. However, the amount of added amino acids cannot exceed the amount that would be needed to meet the protein quality requirements. Furthermore, the addition of amino acids would be limited to essential amino acids in the L-form, as the addition of non-essential amino acids does not improve protein quality.
c) Labelling requirements
- Nutrition information
It is proposed that FNF for children be required to carry an NFt. They would also be subject to the general labelling requirements for prepackaged foods, including the use of an FOP nutrition symbol when the product contains saturated fat, sugars, and/or sodium at or above the specified thresholds.
- Mandatory label statements
FNF for children would be required to carry the following label statements:
- Indication that the product is not intended for use as a sole source of nutrition
- Age range of the intended user of the product (for example, ages 1 to 3, ages 1 to 13, or age groups within this range, such as ages 1 to 10)
- Indication that the product is not intended for children under the age of 1 year
- Indication that the food is intended to be one of the means to supplement a diet when intakes of energy and nutrients may not be adequate to meet an individual's requirements
These label statements are intended to clarify the intended use of the food and to differentiate these products from infant formula and supplemented foods.
- Prohibited label statements
With respect to infant formula, in addition to the age range of the intended user of the product (for example, 0 to 6 months, 6 to 12 months), companies often voluntarily add "Stage 1" or "Stage 2" to help the caregiver differentiate between products intended for infants 0 to 6 months and 6 to 12 months. For FNF for children, it is proposed that product labelling as part of a staged infant formula product line (such as Stage 3 or 4) be prohibited. This marketing strategy makes it challenging for caregivers to differentiate between infant formula and FNF for childrenReference 56, which may result in infants being fed FNF for children.
Labelling of FNF for children as part of a staged infant formula product line may also imply that these products are required, or should be provided, once infant formula is weaned. However, there is no recommendation for children aged 12 months or more to consume these products.
d) Representation and advertising
FNF for children may not be represented (that is, labelled or advertised) as:
- A meal replacement: Children should eat a variety of foods recommended by Canada's Food Guide. Using FNF as meal replacements for children (intended to replace one or more daily meals) may displace a significant proportion of healthy foods in the diet, which goes against dietary guidance. FNF for children are intended to supplement a diet when it may be inadequate in energy and essential nutrients.
- A FNF represented for use in a weight reduction diet: When considering weight loss for children, it is important to consult a health care professional to ensure their diet provides adequate nutrition for growth and development.
- A fortified plant-based beverage or a substitute for cow's milk: Fortified plant-based beverages are subject to specific requirements outlined in an interim policy. This policy supports voluntary fortification to achieve nutrient levels comparable to cow's milk, thereby enabling these beverages to be positioned as a suitable substitute for cow's milk.
- A substitute for human milk: Products intended to substitute human milk fall under the regulatory category of infant formula and are subject to specific compositional requirements.
5.3.2 Formulated nutritional foods for ages 14 or more
FNF for ages 14 or more are intended to complement a diet that may be inadequate in energy and essential nutrients. This proposal introduces two distinct subcategories of FNF, determined by the products' energy content: FNF providing 150 – 199 kcal per serving and FNF providing ≥200 kcal per serving. This classification aims to ensure that consumers can access products that help meet their energy requirements while providing essential nutrients in appropriate proportions.
Moreover, FNF providing ≥200 kcal per serving would be permitted to be marketed and sold as formulated meal replacements (FMR), including those represented for use in weight reduction diets, when certain requirements are met. These additional requirements are intended to provide clarity and support their intended use as meal replacements. However, the compositional requirements for FMR would be congruent with FNF providing ≥200 kcal per serving, as detailed in the subsequent section.
a) Definition
The following definition is proposed for FNF for ages 14 or more:
formulated nutritional food for ages 14 or more means a formulated food sold or represented for individuals aged 14 or more to be consumed in addition to their diet when it may be inadequate in energy and essential nutrients or as a replacement for one or more daily meals, but not for all daily meals
b) Compositional requirements
Existing compositional requirements for MR and NS would be reviewed to reflect current DRI for FNF for ages 14 or more. The compositional requirements would be incorporated by reference into the FDR. The following proposed compositional requirements would apply to all FNF for ages 14 or more, including those represented as FMR, as well as those represented as FMR for use in weight-reduction diets.
- Energy requirements
It is proposed that all categories of FNF for ages 14 or more must provide a minimum of 150 kcal per serving, aligning with the current minimum energy requirement for NS. However, as previously noted, an additional subcategory of FNF for ages 14 or more is proposed, for products that provide ≥200 kcal per serving.
Only those FNF for ages 14 or more that provide ≥200 kcal per serving would be permitted to represent as FMR. This proposed energy requirement is 25 kcal lower than the current requirement for MR. This adjustment aims to harmonize with the minimum requirements for MR in other jurisdictions, fostering trade and enhancing access to imported products.
- Macronutrient requirements
It is proposed that quantitative requirements for macronutrients be maintained to ensure that FNF for ages 14 or more provide balanced nutrition to help achieve the intended use of these products. The existing macronutrient requirements for MR and NS would be reviewed to reflect current DRI.
It is proposed that FNF for ages 14 or more be required to meet the current protein quality requirements for MR, in accordance with B.24.200(1)(e).
Furthermore, maintaining macronutrient requirements may help consumers to distinguish FNF from supplemented foods, which may provide a variety of micronutrients but do not have specific macronutrient requirements.
- Fortification requirements
Mandatory and voluntary addition of vitamins and mineral nutrients
FNF for ages 14 or more are intended to supplement a diet when it may be inadequate in energy or other essential nutrients, or in some cases, to replace a meal. With both uses, these products are generally not consumed in addition to a regular diet, but they are used instead of foods usually consumed in a regular diet. As such, it is important for these products to provide a broad spectrum of nutrients to adequately replace the portion of the regular diet being displaced.
Similar to FNF for children, it is proposed that all micronutrients with a RDA set by NASEM be mandatory, while micronutrients with an AI be voluntary. This would mean that some micronutrients that are not currently permitted in these products would be permitted in the modernized regulations, such as vitamin K and choline. Additionally, some nutrients that are currently voluntary would become mandatory, such as selenium and molybdenum; while others that are mandatory would become voluntary, such as pantothenic acid, biotin, manganese, and sodium.
Minimum amounts for vitamins and mineral nutrients
For those micronutrients which are voluntary, it is proposed that no minimum amount be prescribed. The prescribed minimum amounts for mandatory nutrients would apply to all sources of a nutrient but would not include overage.
The minimum amounts for mandatory nutrients would be based on the caloric content of the food, ensuring that it provides a balanced source of nutrition (that is, FNF that provide more energy must provide more micronutrients). Specifically, the minimum amounts for FNF providing 150-199 kcal per serving would be set at 15% of the highest RDA for ages 14 or more, as consumed. This level corresponds to the amount necessary for making a "good source" claim for most nutrients. In contrast, the minimum amounts for FNF providing ≥200 kcal per serving, which are permitted to represent as an FMR, would provide at least 25% of the highest RDA for the applicable target age groups, as consumed. These amounts assume that a person typically has four eating occasions in a day, including three meals and one snack, and that a FNF representing as an FMR would replace one eating occasion. Therefore, it is appropriate for these products to provide 25% of the micronutrient requirements.
Maximum amounts for vitamins and mineral nutrients
These maximum amounts would be applicable to all sources of the nutrient in the product, excluding any overages. It is proposed that the maximum amount for most micronutrients be set at 50% of the highest RDA or AI (when applicable) for the respective target age groups, as consumed. However, in cases where nutrients have narrower safety margins (in other words, when the highest RDA or AI is closer to the corresponding UL or CDRR), exceptions to the general approach may be made to set lower maximum amounts.
Exception for potassium
As noted for FNF for children, although potassium does not have an established RDA, it is proposed that potassium remain a mandatory nutrient for FNF for ages 14 or more. However, instead of applying the same approach used for micronutrients with an established RDA, lower minimum amounts are being considered specifically for potassium. The minimum amount currently prescribed in the FDR for NS is being considered for FNF providing 150 – 199 kcal per serving, while the minimum amount currently prescribed for MR in the FDR is being considered for FNF providing ≥200 kcal per serving. The proposed maximum amount for potassium is 50% of the AI.
A summary of the proposed micronutrient requirements for FNF for ages 14 or more is presented in Table 4.
| Subcategories of FNF for ages 14 or more | ||
|---|---|---|
| FNF providing 150 – 199 kcal per serving | FNF providing ≥200 kcal per serving | |
| Minimum amounts for mandatory micronutrients | 15% of the highest RDA for individuals aged 14 or more | 25% of the highest RDA for individuals aged 14 or more |
| Minimum amounts for voluntary micronutrients | N/A | N/A |
| Maximum amounts for mandatory and voluntary micronutrients | 50% of the highest RDA or AI for individuals aged 14 or more | |
Voluntary addition of amino acids
It is proposed that essential amino acids may be added to FNF for ages 14 or more to improve protein quality and meet protein quality requirements. However, the amount of added amino acids cannot exceed the amount that would be needed to meet the protein quality requirements. Furthermore, the addition of amino acids would be limited to essential amino acids in the L-form, as the addition of non-essential amino acids does not improve protein quality.
c) Labelling requirements
- Nutrition information
It is proposed that FNF for ages 14 or more would be required to carry an NFt. As such, they would also be subject to the general labelling requirements for prepackaged foods, including the use of an FOP nutrition symbol. Further analysis will be done to determine how the FOP nutrition symbol will be applied to these products.
- Mandatory label statements
FNF for ages 14 or more, including those represented as FMR, would be required to carry a label statement that indicates that the product is not recommended for those under 14 years old. If an FNF is to be consumed by children under 14 years old, an FNF for children should be used because it has been formulated to meet pediatric DRI.
Formulated nutritional foods that are not represented as formulated meal replacements
For FNF for ages 14 or more that are not represented as FMR, the label would require an indication that the food is intended to be one of the means to supplement a diet where intakes of energy and nutrients may not be adequate to meet an individual's requirements.
The compositional requirements for FNF for ages 14 or more are based on the assumption that they are used to replace a meal or supplement an inadequate diet in terms of energy and essential nutrients. Consuming FNF alongside an already adequate or high-energy/nutrient diet could lead to excessive nutrient intake. This label statement ensures FNF are used appropriately to support healthy nutrition and meet the needs of individuals requiring additional energy and nutrients.
Formulated meal replacements
It is proposed that products represented as FMR be required to carry an indication that the product is not intended to replace all daily meals. This statement would be required because the proposed compositional requirements for FNF are not based on the use of the product as a sole source of nutrition. It is also worth noting that foods that are intended as the sole source of nutrition are regulated under the FSDP framework and subject to different regulatory requirements to ensure safe intake.
5.4 Foods represented for use in weight reduction diets
It is proposed that the current provision that permits certain foods to be represented for use in weight reduction dietsReference 57 would be retained in the modernized regulatory framework. These foods would include:
- Total diet replacement for weight reduction
- Formulated meal replacements
- Prepackaged meals
- Foods sold by a weight reduction clinic to clients of the clinic for use in a weight reduction program supervised by the staff of the clinic
While TDR for weight reduction is a proposed FSDP category, as it is intended for use under medical supervision and as a sole source of nutrition, the other three categories of food that would be permitted to be represented for use in weight reduction diets would not be FSDP, and would therefore not be permitted to be represented for use as a primary or sole source of nutrition.
Details on these three additional categories of foods that would be permitted to represent as suitable for use in a weight reduction diet are outlined in the following three sections, followed by a section that outlines the additional regulatory requirements applicable to all of these foods.
5.4.1 Formulated meal replacements represented for use in weight reduction diets
It is proposed that, consistent with the current approach, FMR would be permitted to be represented as suitable for use in weight reduction diets, provided they meet the additional regulatory requirements for foods represented for use in weight reduction diets.
FMR for use in weight reduction diets would be subject to the compositional requirements for FNF that provide ≥200 kcal per serving, as well as the labelling requirements for FNF that represent as FMR, as outlined in subsection 5.3.2 Formulated nutritional foods for ages 14 or more. In addition to these requirements, FMR represented for use in weight reduction diets must meet additional regulatory requirements which are outlined in subsection 5.4.4 Additional regulatory requirements for foods represented for use in weight reduction diets.
5.4.2 Prepackaged meals
a) Revised definition
The current definition of a prepackaged meal is housed in Division 1 of the FDR and is not specific to prepackaged meals that are represented for use in weight reduction diets. This definition is based on dietary guidelines from the 1992 version of Canada's Food Guide and includes the requirement to provide at least one serving of: a) meat, fish, poultry, legumes, nuts, seeds, eggs or milk or milk products other than butter, cream, sour cream, ice-cream, ice milk and sherbet; and b) vegetables, fruit or grain products. However, the 2019 version of Canada's Food Guide took a new approach to dietary guidance by moving away from traditional serving sizes and specific portion recommendations.
As part of regulatory amendments to the FDR for FOP nutrition symbols, a revised definition of a prepackaged meal was proposed in the Canada Gazette, Part I, Volume 152, Number 6. However, industry stakeholders commented that many prepackaged meals for use in weight reduction diets would no longer qualify as prepackaged meals under the proposed definition. Considering this feedback, Health Canada decided not to proceed with the proposed changes to the prepackaged meal definition and indicated the need to reevaluate the proposed revisions to the prepackaged meal definition within the context of the modernization of Division 24.
Since then, the Department has reconsidered the definition of a prepackaged meal and by following the direction of the 2019 version of Canada's Food Guide, is proposing that a prepackaged meal would not prescribe serving sizes or specific portions of food groups. In addition, feedback that was provided by stakeholders as part of the FOP consultation process indicated that the intent behind the requirement that a prepackaged meal "requires no preparation other than heating" is not clear. In response, and recognizing that some products, such as dehydrated meals, may require the addition of water, it is proposed to restrict the definition to foods that do not require the addition of ingredients (other than water) rather than foods that require no preparation other than heating. As well, some of the language in the definition has been updated for consistency with other newly added regulatory terms, such as "single-serving prepackaged product" and the description of food categories that align with those in the definition of "main dish".
The following revised definition is proposed for a prepackaged meal:
prepackaged meal means a single-serving prepackaged product that does not require the addition of ingredients, other than water, for its preparation, that is represented as a meal and that
- contains food from one of the following categories:
- dairy products and their alternatives, except butter, cream, sour cream, ice cream, ice milk, sherbet and alternatives for those foods; or
- meat products, poultry products, marine and fresh water animal products referred to in Division 21, and their alternatives, such as eggs, tofu, legumes, nuts, seeds, nut or seed butters and spreads made from legumes; and
- contains food from one of the following food categories:
- fruits and vegetables except pickles, relishes, olives and garnishes; or
- breads, breakfast cereals, rice and other grains, and alimentary pastes
b) Compositional requirements
It is proposed that prepackaged meals that are represented for use in weight reduction diets would be subject to certain compositional requirements in order to help ensure adequate energy and protein intake through a controlled intake of food.
- Energy requirements
Similar to FMR that are represented for use in weight reduction diets, it is proposed that prepackaged meals that are represented for use in weight reduction diets would be required to provide a minimum of 200 kcal per serving.
- Macronutrient requirements
It is proposed that carbohydrate and fat requirements are not prescribed, in order to support a diversity of foods that are represented as prepackaged meals for use in weight reduction diets. However, as adequate protein intake is an important component of a weight reduction diet, it is proposed that prepackaged meals for use in weight reduction diets be required to meet the current protein requirements for MR, in accordance with B.24.200(1)(e).
- Fortification requirements
In alignment with the current approach, it is proposed that prepackaged meals would not be permitted to be fortified.
c) Labelling requirements
- Nutrition information
It is proposed that these foods maintain their current nutrition labelling requirements for prepackaged foods outlined in Part B of the FDR (that is, carrying an NFt and including the use of an FOP symbol when the product contains saturated fat, sugars, and/or sodium at or above the specified thresholds).
5.4.3 Foods sold in weight reduction clinics
a) Definition
A regulatory definition does not exist for foods sold by a weight reduction clinic to clients of the clinic for use in a weight reduction program supervised by the staff of the clinic. However, given the level of details provided in the product category name, the introduction of a definition for this category of food is not required.
b) Compositional requirements
It is proposed not to prescribe compositional requirements for foods sold in weight reduction clinics, in order to support diversity in food options that may be used in a weight reduction program. Furthermore, in alignment with the current approach, it is proposed that foods sold in weight reduction clinics would not be permitted to be fortified.
c) Labelling requirements
- Nutrition information
It is proposed that these foods maintain their current nutrition labelling requirements for prepackaged foods outlined in Part B of the FDR (i.e., carrying an NFt and including the use of an FOP symbol when the product contains saturated fat, sugars, and/or sodium at or above the specified thresholds).
5.4.4 Additional regulatory requirements for foods represented for use in weight reduction diets
In addition to the product category specific regulatory requirements outlined above, the following additional regulatory requirements are proposed for all foods represented for use in weight reduction diets, with the exception of the proposed FSDP category, TDR for weight reduction, which would be formulated to provide a sole source of nutrition and are intended for use under medical supervision.
a) Labelling requirements
- Mandatory label statements
It is proposed to maintain the current requirements for products that make weight reduction claims, by requiring the statement, "Useful in weight reduction only as part of an energy reduced diet" on the product label.
It is also proposed that the product's label would be required to carry the following statements:
- A statement that indicates that the product is not intended to replace all daily meals
- A statement that indicates that the product is not recommended for those under 18 years old
These products are not intended to replace all daily meals. Products that are represented for use as primary or sole source of nutrition are regulated under the proposed framework for FSDP and are subject to different requirements to ensure safe and adequate nutrient intake. Additionally, a food that makes a weight reduction claim is not intended for use by children or adolescents. Individuals under 18 who are considering weight loss should consult a health care professional to ensure their diet provides adequate nutrients for proper growth and development.
- Prohibited label statements
Foods represented for use in weight reduction diets would be prohibited to include label statements that reference the rate or amount of weight loss to be expected. These effects are highly individualized and depend on factors such as the consumer's total daily calorie intake. Furthermore, a healthy rate or amount of weight loss should be determined in consultation with a health care professional. Restricting these types of label statements would help prevent misleading claims.
- Sample seven-day menu
It is proposed to retain the requirement to provide a sample seven-day menu for foods that are represented for use in weight reduction diets. The provision of a menu is essential for products that are represented for use in weight reduction, as weight loss is achieved through consistently reducing energy intake rather than solely consuming the product. Following a seven-day menu would assist consumers in achieving healthy weight loss by guiding them towards reduced energy consumption while ensuring the intake of essential nutrients and adequate energy levels. The minimum daily energy intake of 1200 Kcal per day would be retained; however, it would be the manufacturer's responsibility to ensure that the menu is based on principles of Canada's Food Guide and healthy weight loss, as well as ensuring that energy requirements, and macro- and micro-nutrient needs are met without exceeding upper limits (that is, daily intakes over the UL or CDRR) for the target groups.
Currently, the product label must include the required sample seven-day menu. However, manufacturers have expressed difficulties in adhering to this requirement, due to limited space on the package. This requirement was established several decades ago and does not reflect the current widespread use and accessibility of the internet. Therefore, the proposed amendments would allow the product label to include a link directing consumers to a website where the sample seven-day menu can be accessed.
6.0 Conclusion
The modernization proposal for Divisions 24 and 25 of the FDR is an important step towards ensuring the continued safety, nutritional adequacy, and accessibility of FSDP and other foods regulated under these Divisions in Canada. The existing regulations have proven to be outdated and inflexible, leading to limited availability of innovative products approved in other countries, and leaving Canada vulnerable to shortages.
To address these issues, the modernization initiative proposes a comprehensive restructuring of the regulatory requirements. A key aspect is the clear differentiation between FSDP and products that are not FSDP. FSDP would be subject to additional regulatory oversight throughout their lifecycle, including premarket authorization for most infant FSDP as well as stop-sale provisions for all FSDP to ensure public health protection. Moreover, the proposal includes shortage provisions requiring regulated parties to report shortages to Health Canada and facilitating access to safe products through an exceptional importation framework.
This proposal would update the compositional requirements for all product categories, where applicable, to reflect the latest DRI. To the extent possible, these requirements would be incorporated by reference, allowing a more flexible approach in which future amendments could be made administratively to reflect scientific advancements. Additionally, the compositional requirements for FSDP would allow for deviations when medically justified, subject to review during premarket authorization for most infant FSDP.
The proposal also aims to improve labelling requirements for FSDP, providing detailed product information for safe use and differentiation from products that are not FSDP. Labelling for products that are not FSDP would better align with conventional prepackaged food requirements, including the use of NFt and FOP nutrition symbols, where applicable. FOP nutrition symbols enable Canadians to more easily identify foods high in nutrients of public health concern (sodium, saturated fats and sugars) in order to make healthier and more informed decisions.
Moreover, the modernization effort aligns with the 2019 amendment of the Food and Drugs Act, introducing the term FSDP to enable the development of a regulatory framework for human clinical trials on these products. By modernizing the regulations, Canada can foster more research and development in this essential sector and improve access to products available in other countries. Furthermore, aligning the requirements with international jurisdictions, when possible, and within the Canadian regulatory context would improve access to critical nutrition products for Canadians.
In conclusion, the proposed regulatory modernization for Divisions 24 and 25 of the FDR demonstrates a comprehensive approach to address current limitations and challenges in the regulation of FSDU and infant foods. By promoting flexibility, aligning with scientific advancements, and encouraging innovation, the modernization initiative would maintain product safety while increasing their accessibility to support a more diverse market. This diversity would help mitigate the risk of shortages in the future, ensuring the health and well-being of all Canadians, particularly vulnerable groups relying on specialized nutrition products. Health Canada's commitment to regulatory modernization underscores its dedication to public health, ensuring that Canadians continue to have access to high-quality, safe, and nutritious foods for their unique dietary needs.
Appendix 1: Lexicon
Dietary Reference Intakes
The DRIs are a set of scientifically based nutrient reference values for healthy populations. They were established by Canadian and American scientists through a review process overseen by the NASEM which is an independent, non-governmental body in the Unites States (US). The US and Canadian governments jointly sponsor the development of the DRIs since 1994.
The DRI are an important part of the evidence underpinning government activities such as the development of regulatory standards, assessment of dietary intakes, food product safety assessment, and the development of dietary guidance for the general population and for specific life stage groups.
The main types of DRI reference values are the Estimated Average Requirement (EAR), the Recommended Dietary Allowance (RDA), the Adequate Intake (AI), the Tolerable Upper Intake Level (UL), and the Chronic Disease Risk Reduction Intake (CDRR).
- An EAR is the average daily nutrient intake that is estimated to meet the requirement of half the healthy individuals in a life-stage and gender group. A specific indicator of adequacy is used to determine the EAR. The EAR is used to calculate the RDA.
- An RDA is an estimate of the minimum daily average dietary intake level that is sufficient to meet the nutrient requirement of nearly all (97 to 98 percent) healthy individuals in a particular life-stage and gender group. The main use of the RDA is as a goal for usual intake of individuals. Since the RDA is calculated based on the EAR, an RDA can only be set for a particular nutrient if there is sufficient scientific evidence to establish an EAR for that nutrient.
- The AI is the recommended average daily nutrient intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people who are assumed to be maintaining an adequate nutritional state. If sufficient scientific evidence is not available to establish an EAR and to subsequently set an RDA, an AI is derived for the nutrient instead. An AI is based on much less data and incorporates substantially more judgment than is used in establishing an EAR and subsequently the RDA. The issuance of an AI indicates that more research is needed to determine, with some degree of confidence, the mean and distribution of requirements for that specific nutrient. The AI is expected to meet or exceed the needs of most individuals in a specific life-stage and gender group. The AI can be used as the goal for an individual's intake when an RDA is not available for a nutrient.
- A UL is the highest level of continuing daily nutrient intake that is likely to pose no risk of adverse health effects in almost all individuals in the life-stage group for which it has been designed. The term "tolerable" intake was chosen to avoid implying a possible beneficial effect. Instead, the term is intended to specify a level of intake with a high probability of being tolerated biologically. The UL is not intended to be a recommended level of intake. As intake increases above the UL, the potential risk of adverse effects increases.
- A CDRR is the lowest level of intake expected to reduce chronic disease risk. CDRR values were established for sodium.
Appendix 2: Consultation questions
Health Canada encourages respondents to provide their feedback by responding to the following questions. Please elaborate in your responses where appropriate. For example, if you do not agree with a proposal, please explain why.
Please provide your responses to the consultation questions and any other feedback to bns-bsn@hc-sc.gc.ca with the subject: "Regulatory Modernization of Foods for Special Dietary Use & Infant Foods". Acceptable formats include Microsoft Word and PDF.
Demographics
- From which point of view are you answering this questionnaire? Select one of the following options.
- Consumer
- Consumer association
- Industry
- Industry association
- Academics / research institutes
- Health care practitioners
- Health care organizations not including those listed as research institutes
- Provincial or territorial governments (please specify)
- Health Canada
- Other federal government department (please specify)
- Non-government organization
- International trading partner
- Indigenous Peoples
- Other (please specify)
- Prefer not to say
- While not required, please provide your contact information so that we may contact you if we have questions or need more details, including the following:
- Name
- Title (if applicable)
- Company or organization (if applicable)
- Telephone
Policy proposal
- Do you support the proposal to restructure Divisions 24 and 25 of the FDR into a division for FSDP and one for foods that are not FSDP (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for infant formula (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for prepackaged human milk (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for medical food for infants (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for medical food for ages 1 or more (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for medical food represented as a total diet replacement for weight reduction (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for shortage provisions applicable to all FSDP (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for stop-sale provisions applicable to all FSDP (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for premarket requirements for infant FSDP (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for conventional infant food (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for gluten-free food (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for formulated nutritional food for children (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for formulated nutritional food for ages 14 or older (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for food represented for use in weight reduction diets (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
- Do you support the proposal for prepackaged meals (for example, yes, no, partially)? Please explain your answer. Do you have any other comments? What additional factors should Health Canada consider, if any?
Footnotes
- Reference 1
-
FSDU are defined as "foods that has been specially processed or formulated to meet the particular requirements of a person, in whom a physical or physiological condition exists as a result of a disease, disorder or injury, or for whom a particular effect, including but not limited to weight loss, is to be obtained by a controlled intake of foods" (B.24.001, Food and Drug Regulations)
- Reference 2
-
In the context of the FDR, "represented as" refers to products that are labeled or advertised as such.
- Reference 3
-
Prior to 1994, the Recommended Nutrient Intakes (RNI) were used when establishing compositional requirements. Canada transitioned to using the Dietary Reference Intakes (DRI) starting in 1994, but Division 24 and 25 of the FDR have not been revised to reflect the DRIs.
- Reference 4
-
FSDP is defined as "food that has been specially processed or formulated to meet the particular requirements of an individual in whom a physical or physiological condition exists as a result of a disease, disorder or abnormal physical state, or to be the sole or primary source of nutrition for an individual" (Section 2, Food and Drugs Act)
- Reference 5
-
Refer to Appendix 1: Lexicon for more information on DRI.
- Reference 6
-
B.24.003, Food and Drug Regulations
- Reference 7
-
B.25.001, Food and Drug Regulations
- Reference 8
-
World Health Organization, 1981. International Code of Marketing of Breast-Milk Substitutes. January 27. https://www.who.int/publications/i/item/9241541601
- Reference 9
-
The WHO Code applies to human milk substitutes, which include infant formula and other milk products, foods and beverages represented for use as a partial or total replacement of breast milk.
- Reference 10
-
Section D.03.002(1), Food and Drug Regulations
- Reference 11
-
Wiggins AKA, Grantham, A and Anderson GH. 2019. "Optimizing foods for special dietary use in Canada: key outcomes and recommendations from a tripartite workshop." Applied physiology, nutrition, and metabolism 44 (11): 1258-1265. https://cdnsciencepub.com/doi/full/10.1139/apnm-2019-0013
- Reference 12
-
Oosterveld D. 2019. Modernizing Canada's Medical Nutrition Regulations for Food. June 24. https://www.raps.org/News-and-Articles/News-Articles/2019/6/Modernizing-Canadas-Medical-Nutrition-Regulations
- Reference 13
-
This includes the General Standard for the Labelling of and Claims for Prepackaged Foods for Special Dietary Uses (CXS-146-1985), as well as specific standards for different categories of FSDU such as IF and formulas for special medical purposes intended for infants (CXS 72-1981), follow-up formula (CXS 156-1987), processed cereal-based foods for infants and young children (CXS 74-1981), canned baby food (CXS 73-1981), foods for special medical purposes (CXS 180-1991), formula foods for use in very low energy diets for weight reduction (CXS 203-1995), formula foods for use in weight control diets (CXS 181-1991), FSDU for persons intolerant to gluten (CXS 118-1979), and FSDU with low-sodium content (CXS 53-1981).
- Reference 14
-
European Union. 2013. "REGULATION (EU) No 609/2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control." https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02013R0609-20170711&from=EN#:~:text=This%20Regulation%20establishes%20compositional%20and,diet%20replacement%20for%20weight%20control
- Reference 15
-
European Union. 2006a. "Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods." https://eur-lex.europa.eu/eli/reg/2006/1924/2014-12-13
- Reference 16
-
European Union. 2006b. "Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods." https://eur-lex.europa.eu/eli/reg/2006/1924/2014-12-13
- Reference 17
-
Food Standards Australia New Zealand. 2021. Food Standards Code. https://www.foodstandards.gov.au/code/Pages/default.aspx
- Reference 18
-
Food and Drug Administration, Department of Health and Human Services. 1977. 21 CFR Part 105. March 15. https://www.ecfr.gov/current/title-21/part-105
- Reference 19
-
Food and Drug Administration, Department of Health and Human Services. 2001. 21 CFR 101.9. https://www.ecfr.gov/current/title-21/part-101/section-101.9
- Reference 20
-
B.24.003(1.1), Food and Drug Regulations
- Reference 21
-
B.25.001, Food and Drug Regulations
- Reference 22
-
B.25.062, Food and Drug Regulations
- Reference 23
-
B.25.001, Food and Drug Regulations
- Reference 24
-
Food and Agriculture Organization (FAO) of the United Nations. 2007. "Standard for infant formula and formulas for special medical purposes intended for infants CXS 72-1981." https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B72-1981%252FCXS_072e.pdf
- Reference 25
-
Sections B.25.057(1)(a) and B.25.057(2)(c)(i), Food and Drug Regulations
- Reference 26
-
Chiang, KV, EH Anstey, and SA and Perrine, CG Abrams. 2023. "Infant burn injuries related to water heating for powdered infant formula preparation." Frontiers in pediatrics 11 (1125112). doi:10.3389/fped.2023.1125112. eCollection 2023.
- Reference 27
-
Richter APC, Duffy EW, Higgins ICA, Barrington C, Martin SL, Aquilina KH, Avendaño-Galdamez MI & Hall MG. 2023. "Toddler milk perceptions and responses to front-of-package claims and product warnings: A qualitative study of caregivers of toddlers." Journal of the Academy of Nutrition and Dietetics. doi: https://doi.org/10.1016/j.jand.2023.06.281
- Reference 28
-
B.01.601(1)(c)(i), Food and Drug Regulations
- Reference 29
-
B.01.503(2), Food and Drug Regulations
- Reference 30
-
A health claim is any representation in labelling or advertising those states, suggests, or implies that a relationship exists between consumption of a food or an ingredient in the food and a person's health.
- Reference 31
-
Government of the United Kingdom. 2022. Commission Delegated Regulation (EU) 2016/127 (supplementing Regulation (EU) No 609/2013): guidance. https://www.gov.uk/government/publications/infant-and-follow-on-formula-and-food-for-special-medical-purposes/commission-delegated-regulation-eu-2016127-supplementing-regulation-eu-no-6092013-guidance
- Reference 32
-
Australian Government Department of Health and Aged. 2023. Marketing in Australia of Infant Formulas: Manufacturers and Importers Agreement. https://www.health.gov.au/topics/pregnancy-birth-and-baby/breastfeeding-infant-nutrition/marketing-infant-formula
- Reference 33
-
Pound C, Unger S, Blair B. 2020. "Pasteurized and unpasteurized donor human milk." Paediatrics & Child Health 25 (8): 549-550. doi:https://doi.org/10.1093/pch/pxaa118.
- Reference 34
-
Sousa SG, Delgadillo I, Saraiva JA. 2016. "Human milk composition and preservation: Evaluation of high-pressure processing as a nonthermal pasteurization technology." Critical Reviews in Food Science and Nutrition 56 (6): 1043-1060. doi:10.1080/10408398.2012.753402.
- Reference 35
-
Ballard O, Morrow AL. 2013. "Human milk composition: nutrients and bioactive factors." Pediatric clinics of North America 60 (1): 49-74. doi:10.1016/j.pcl.2012.10.002.
- Reference 36
-
La Leche League International. 2023. Heating Human Milk in the Microwave. https://llli.org/breastfeeding-info/heating-human-milk/
- Reference 37
-
B.25.020(1)(a)-(d), Food and Drug Regulations
- Reference 38
-
Food and Agriculture Organization (FAO) of the United Nations. 1991. "Standard for the Labelling of and Claims for Foods for Special Medical Purposes: Codex Stan 180-1991." https://www.fao.org/fao-who-codexalimentarius/sh-proxy/it/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B180-1991%252FCXS_180e.pdf
- Reference 39
-
B.25.019, Food and Drug Regulations
- Reference 40
-
B.24.102(1)(a)-(b), Food and Drug Regulations
- Reference 41
-
Food and Agriculture Organization (FAO) of the United Nations. 1995. "Standard for formula foods for use in very low energy diets for weight reduction (CXS-203 1995)." https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B203-1995%252FCXS_203e.pdf
- Reference 42
-
Stop sale provisions currently exist within Division 24 for FLD, MR, FVLED in B.24.017, and in Division 25 for HMF (and infant formula in B.25.018), B.25.060, respectively.
- Reference 43
-
B.25.061(1), Food and Drug Regulations
- Reference 44
-
D.01.010, D.01.011 and D.02.009, Food and Drug Regulations
- Reference 45
-
Liem, DG. 2017. "Infants' and Children's Salt Taste Perception and Liking: A Review." Nutrients 9 (9): 1011. doi:10.3390/nu9091011.
- Reference 46
-
Stein LJ, Cowart BJ and Beauchamp GK. 2012. "The development of salty taste acceptance is related to dietary experience in human infants: a prospective study." The American Journal of Clinical Nutrition 95 (1): 123-129. doi:10.3945/ajcn.111.014282.
- Reference 47
-
World Health Organization. 2015. "Guideline: sugars intake for adults and children." https://www.who.int/publications/i/item/9789241549028
- Reference 48
-
Pulido O, Gillespie Z, Zarkadas M, et al. 2009. Introduction of oats in the diet of individuals with celiac disease: A systematic review. Advances in Food and Nutrition Research 57:235-85. https://www.researchgate.net/publication/26664127_Introduction_of_oats_in_the_diet_of_individuals_with_celiac_disease_a_systematic_review
- Reference 49
-
Gluten means (a) any gluten protein from the grain of any of the following cereals or from the grain of a hybridized strain that is created from at least one of the following cereals: (i) barley, (ii) oats, (iii) rye, (iv) triticale, (v) wheat; or (b) any modified gluten protein, including any gluten protein fraction, that is derived from the grain of any of the cereals referred to in paragraph (a) or from the grain of a hybridized strain referred to in that paragraph. (B.01.010.1(1) of the FDR)
- Reference 50
-
D.03.003, Food and Drug Regulations
- Reference 51
-
B.24.018, Food and Drug Regulations
- Reference 52
-
Food and Agriculture Organization (FAO) of the United Nations. 2008. "Standard for foods for special dietary use for persons intolerant to gluten (CXS 118-1979). https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B118-1979%252FCXS_118e_2015.pdf
- Reference 53
-
Alberta Health Services. 2022. "Nutrition Guideline: Healthy Infant and Young Children Milk." https://www.albertahealthservices.ca/assets/info/nutrition/if-nfs-ng-healthy-infants-other-milks-fluids-milk.pdf
- Reference 54
-
European Commission. 2016. "Report from the Commission to the European Parliament and the Council on Young Child Formulae." https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52016DC0169&from=LT
- Reference 55
-
National Academies of Sciences Engineering and Medicine (NASEM). 2019. "Dietary Reference Intakes for Sodium and Potassium." doi:https://doi.org/10.17226/25353.
- Reference 56
-
Berry NJ, Jones S and Iverson D. 2010. "It's all formula to me: women's understandings of toddler milk ads." Breastfeeding Review: Professional Publication of the Nursing Mothers' Association of Australia 21-30. https://pubmed.ncbi.nlm.nih.gov/20443436/
- Reference 57
-
B.24.003(3), Food and Drug Regulations
Page details
- Date modified: