Proposed Guidance technical document for waterborne pathogens
Table of Contents
- Purpose of consultation
- Executive Summary
- Part A
- Part B. Technical information
- Part C. References
- Appendix A: List of Acronyms
- Appendix B: Table B1 - Summary of waterborne enteric pathogens
- Appendix C: Table C1 - Summary of waterborne naturally-occurring pathogens
- Appendix D: Figure D1 - Relative CT values for various waterborne pathogens, Free chlorine (2 log inactivation, 5-25°C, pH 6-9)
- Appendix E: Figure E1 - Relative UV dose requirements for various waterborne pathogens (4 log inactivation)

Download the alternative format
(PDF format, 1.518 MB, 85 pages)
Organization: Health Canada
Date Published: February 3, 2021
Purpose of consultation
This document has been developed with the intent to provide regulatory authorities and decision-makers with guidance on waterborne pathogens not covered in other Guideline Technical Documents.
This document is available for a 60-day public consultation period. Please send comments (with rationale, where required) to Health Canada via email:
All comments must be received before February 3, 2021. Comments received as part of this consultation will be shared with members of the Federal-Provincial-Territorial Committee on Drinking Water (CDW), along with the name and affiliation of their author. Authors who do not want their name and affiliation shared with CDW members should provide a statement to this effect along with their comments.
It should be noted that this guidance document may be revised following the evaluation of comments received and the final document will be published. Drinking water guidelines will be established, if required. This document should be considered as a draft for comment only.
Executive Summary
Many types of pathogenic microorganisms can spread through contaminated or inadequately treated drinking water to cause human illness. Some are present in human or animal feces and can cause gastrointestinal illness when fecally-contaminated water is consumed. Others are naturally found in aquatic environments and can cause opportunistic infections when the conditions in engineered water systems allow them to multiply and spread primarily to individuals who are susceptible to infection. The health effects caused by these opportunistic pathogens are diverse and range from respiratory illness to infections of the eye, skin, central nervous system or gastrointestinal tract.
A basic understanding of the different types of waterborne pathogens—their sources, the measures that are important for their control and the people that are most at risk for becoming sick—is necessary for effective drinking water management and for preventing outbreaks of waterborne disease. Health Canada completed its review of waterborne pathogens of potential human health concern. This guidance document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water (CDW) and describes these organisms, their health effects, how they are transmitted and best practices to ensure safe drinking water.
Assessment
Setting maximum acceptable concentrations for the pathogens described in this document remains impractical and is not necessary in order for drinking water utilities to adequately manage risks. Implementing a source-to-tap approach is a universally recommended strategy for reducing the concentration of waterborne pathogens in drinking water and controlling their potential risks. Important elements of this strategy include source water protection, treatment and disinfection requirements based on health-based treatment goals for enteric protozoa (Giardia and Cryptosporidium) and enteric viruses, and managing microorganism survival and growth in drinking water distribution systems. Maintaining microbiological control in water systems in buildings and residences is also a critical component of providing safe drinking water at the consumer’s tap. The intent of this document is to provide stakeholders, such as provincial and territorial regulatory authorities, decision makers, water system owners and operators and consultants with guidance on waterborne pathogens that are not addressed in the Guidelines for Canadian Drinking Water Quality, with the objective of minimizing public health risks in Canadian water systems.
International Considerations
Drinking water guidelines, standards and/or guidance from other national and international organizations may vary due to the date of the assessments as well as differing policies and approaches.
International organizations have not established numerical limits for these waterborne pathogens in drinking water. The United States Environmental Protection Agency (US EPA), the World Health Organization (WHO), the European Union (EU) and the Australian National Health and Medical Research Council all recommend a risk management strategy based on a multiple barrier approach to prevent the entry and transmission of these waterborne pathogens. The WHO and Australia have developed fact sheets providing information on waterborne pathogens that may contaminate the water supply.
Part A
A.1 Goal & Scope
The goal of this document is to provide provinces, territories, other government departments and stakeholders with guidance on waterborne pathogens of potential human health concern, which are not addressed in the Guidelines for Canadian Drinking Water Quality.
A wealth of important research has advanced understanding of the public health relevance of these waterborne pathogens in drinking water systems. Management strategy considerations focus on the treatment plant and the distribution system; however, some guidance is provided for plumbing systems in buildings and residences. The responsible drinking water authority in the affected jurisdiction should be consulted for specific information on guidance and requirements for plumbing systems.
A.2 Introduction
The microorganisms covered in this document are listed in Table 1. This document addresses the waterborne bacterial pathogens that are of enteric origin and are known to cause gastrointestinal illness when there is fecal contamination of inadequately treated drinking water. The document also describes naturally-occurring waterborne pathogens, as these organisms are often associated with infections in susceptible individuals (such as infants, the elderly and immunocompromised individuals), and are referred to as opportunistic pathogens. Engineered water systems are important habitats for naturally-occurring waterborne pathogens. Many possess features that present challenges for drinking water utilities, such as increased resistance to disinfection, growth under low nutrient and oxygen conditions and growth in biofilms. Effective management requires control of these organisms in drinking water distribution systems, and within building plumbing systems that generally fall outside of the utility's responsibility. More information is required on their ecology in biofilms and the effectiveness of treatment procedures to better understand best management practices.
Overall strategies for management are summarized in Part A.3. In Part B, brief technical information is presented on the individual pathogens (See Table 1), their effects on human health, as well as sources and exposure. Analytical and treatment considerations are also summarized.
Table 1. Microorganisms addressed in this guidance document
Waterborne enteric pathogens
- Campylobacter spp.
- Enteric pathogenic Escherichia coli (E. coli) and Shigella spp.
- Helicobacter pylori
- Salmonella spp.
- Yersinia spp.
Waterborne naturally-occurring pathogens
Bacteria:
- Aeromonas spp.
- Legionella spp.
- Mycobacterium spp.
- Pseudomonas spp.
Protozoa:
- Naegleria fowleri
- Acanthamoeba spp.
A.3 Management strategies
Setting maximum acceptable concentrations for these microorganisms remains impractical and is not required in order for drinking water utilities to adequately manage risks. Instead, a priority focus on drinking water process management, for example, through the implementation of a source-to-tap or water safety plan approach, is the recommended strategy for water utilities to manage potential risks. Important elements of this strategy include:
- source water protection (where feasible);
- optimized treatment performance for turbidity and natural organic matter removal;
- proper application of disinfection technologies;
- performance/verification testing using multiple operational parameters and water quality indicators;
- a well-designed and well-maintained distribution system; and
- maintenance of an effective disinfectant residual.
A.3.1 Water treatment plant
When properly designed and operated, physical removal and disinfection technologies commonly used in drinking water treatment are very effective in reducing or inactivating the waterborne pathogens described in this document. Current treatment requirements are based on health-based treatment goals for enteric protozoa (Giardia and Cryptosporidium), and enteric viruses. This is because of their importance as causes of waterborne disease, high infectivity, difficulty of removal through water treatment, and high disinfectant resistance. The physical removal and disinfection requirements for the waterborne pathogens discussed here are less than or equivalent to those for enteric protozoa and enteric viruses. As a result, surface water and groundwater under the direct influence of surface water systems that meet the guidelines for enteric protozoa and enteric viruses (minimum 3-log removal and/or inactivation and minimum 4 log removal and/or inactivation, respectively), are capable of controlling these pathogens. Groundwater systems that meet the guidelines for enteric viruses (minimum 4-log removal and/or inactivation) are capable of controlling of these pathogens. The Health Canada Guideline Technical Documents: Enteric Viruses and Enteric Protozoa: Giardia and Cryptosporidium include more information on the requirements for drinking water treatment and disinfection.
A.3.2 Drinking water distribution system
Even with treatment technologies in place, microorganisms can enter drinking water distribution systems as a result of inadequate treatment or through post-treatment contamination via intrusions, cross-connections or during construction or repairs. Biofilms and loose deposits in drinking water systems provide habitats that can support the survival, growth and dissemination of pathogenic microorganisms, particularly opportunistic pathogens (e.g., Legionella).
Information on managing microorganism survival and growth in drinking water distribution systems is found in Health Canada’s publications: Guidance on Monitoring the Biological Stability of Drinking Water in Distribution Systems and Guidance on Natural Organic Matter in Drinking Water. Key distribution system operational and maintenance practices include:
- use of proper construction materials;
- treatment optimization to minimize the amounts of nutrients, scaling and corrosion within the system;
- managing water age and controlling the effects of temperatures where possible;
- maintaining an effective disinfectant residual;
- preventing the entry of contamination (e.g., pressure maintenance, preventing cross-contamination/backflow, hygienic practises during mains constructions and repairs); and
- keeping the distribution system clean (e.g., use of appropriate flushing and cleaning techniques).
A.3.3 Premise plumbing
Maintaining microbiological control in premise plumbing systems, especially in large buildings, is a critical component of providing safe drinking water at the consumer's tap. Important elements of control strategies for plumbing systems include:
- limiting nutrient levels through an emphasis on system design and materials;
- minimizing areas of low flow/stagnation;
- keeping temperatures of hot and cold water systems outside of the ideal range for microorganism growth (e.g., cold water less than 20°C, hot water tank temperature greater than 60°C); and
- reducing the formation and transmission of contaminated aerosols from distal devices.
It is also important to highlight that in management strategies for complex water systems, many control measures are interrelated. Changes in the microbiological diversity of drinking water systems can occur with changes in materials or operational procedures. Understanding the effects of changes in water management operations on drinking water ecology is necessary to minimize unintended consequences such as creating conditions that favour the growth (i.e., enrichment) of specific microbiological groups.
Part B. Technical information
B.1 Enteric bacteria
B.1.1 Campylobacter spp.
B.1.1.1 Description
Campylobacter (Class: Epsilonproteobacteria) is a bacterial genus that contains over 30 recognized species, but only some have relevance for human health (Wagenaar et al., 2015; Backert et al., 2017; LPSN, 2019). Campylobacter jejuni (C. jejuni) and Escherichia coli (E. coli) are the primary and secondary species of most relevance as causes of human gastrointestinal illness, accounting for greater than 90% of cases of human campylobacteriosis worldwide (Huang et al., 2015; Wagenaar et al., 2015). Other species are known to cause gastrointestinal illness, but their occurrence is rare or is associated with specific risk groups (e.g., immunocompromised individuals) or geographical areas (Wagenaar et al., 2015). Some Campylobacter species (spp.) have been associated with prenatal and neonatal infections and human periodontal disease (Backert et al., 2017; Huang et al., 2015).
Campylobacter spp. are Gram-negative, motile, curved or spiral rod-shaped cells (Percival and Williams, 2014b). They are fastidious and microaerophilic (require lower oxygen levels) bacteria that have a growth temperature of 30 to 45°C (optimum: 40-42°C) (Percival and Williams, 2014b; Wagenaar et al., 2015; Zautner and Masanta, 2016).
B.1.1.2 Health effects
Gastroenteritis caused by Campylobacter spp. includes a watery, profuse and sometimes bloody diarrhea occasionally accompanied by fever and abdominal pain (Backert et al., 2017; Percival and Williams, 2014b). Some severe infections may lead to hospitalization and can be life threatening, although fatalities are usually associated with the very young, very old, or patients with underlying disease or immune system deficiencies (Kvalsvig et al., 2014). Symptoms generally occur within one to five days of infection and the illness lasts less than seven to ten days (Backert et al., 2017). Shedding of the organism in feces typically ceases within a few weeks of infection, but can persist for three months or more. Asymptomatic infections with Campylobacter spp. are also possible (Percival and Williams, 2014b). While Campylobacter spp. can cause illness in healthy individuals across all age groups, in developed countries, infections are more prevalent in young children, young adults and the elderly (Kaakoush et al., 2015; PHAC, 2018c). Estimates of the infectious dose for Campylobacter spp. vary considerably, however data suggests ingestion of a few hundred bacteria is sufficient to cause infection (Kothary and Babu, 2001; Percival and Williams, 2014b; Backert et al., 2017).
Post-infection complications associated with Campylobacter spp. illness include Guillain-Barré Syndrome and reactive arthritis, though these are relatively rare (Backert et al., 2017; Percival and Williams, 2014b). Campylobacter spp. infection may be associated with the development of inflammatory bowel diseases such as Crohn's Disease, ulcerative colitis and irritable bowel syndrome (Backert et al., 2017; Huang et al., 2015). A meta-analysis estimated that Campylobacter spp. cases develop long-term complications in the following proportions: Guillain-Barré Syndrome, 0.07% (95% confidence interval: 0.03-0.15%); reactive arthritis, 2.86% (95% CI: 1.40-5.61%); and, irritable bowel syndrome, 4.01% (95% CI: 1.41-10.88%) (Keithlin et al., 2014b). Campylobacter spp. is the leading cause of bacterial gastrointestinal illness in Canada and other developed countries worldwide (Backert et al., 2017; Huang et al., 2015). Cases of campylobacteriosis in Canada and internationally are predominantly sporadic, with most illness associated with consumption of contaminated food (Huang et al., 2015; Wagenaar et al., 2015). In Canada, reported annual incidence rates (all causes) over the period from 2013-2017 ranged from 25.4 to 29.2 (median: 28.4) cases per 100,000 population (PHAC, 2019). Infections (all sources) are more common in the summer months (Fleury et al., 2006; Lal et al., 2012; Kaakoush et al., 2015).
Illness caused by Campylobacter spp. is generally self-limiting. Antibiotics are prescribed only in severe cases (Wagenaar et al., 2015). No vaccines for Campylobacter spp. are available (Wagenaar et al., 2015). Campylobacter spp. are resistant to ciprofloxacin and azithromycin have been given a threat level of serious by the Centers for Disease Control and Prevention (CDC, 2013a). The WHO and the Public Health Agency of Canada (PHAC) consider these organisms a medium to high priority for surveillance, research and public health attention (Garner et al., 2015; WHO, 2017, PHAC, 2018a).
B.1.1.3 Sources and exposure
Campylobacter spp. are zoonotic pathogens (i.e., transmitted from animals to humans) that are naturally found in the intestinal tract of a wide range of wild and domestic birds and mammals (Wagenaar et al., 2015; Backert et al., 2017). Poultry are considered the major reservoir (Wagenaar et al., 2015; Backert et al., 2017). Cattle, sheep and domestic pets are also important sources of the organisms (Wagenaar et al., 2015; Backert et al., 2017). Transmission of Campylobacter spp. occurs via the fecal-oral route, with the main pathways for exposure being contaminated food or water and direct contact with animals (Percival and Williams, 2014b; Wagenaar et al., 2015). Person-to-person transmission is uncommon (Percival and Williams, 2014b; Wagenaar et al., 2015). Important sources of fecal contamination that can impact drinking water sources, including groundwater and surface water, are surface runoff contaminated with livestock waste and municipal sewage (e.g., from wastewater discharges, leaking sanitary sewers) (Whiley et al., 2013). Intrusion of animal feces following heavy rainfall or snowmelt is a particularly important cause of contamination of vulnerable groundwater wells (Moreira and Bondelind, 2017).
Although food and waterborne outbreaks are comparatively rare (Huang et al., 2015; Moreira and Bondelind, 2017), Campylobacter spp. has been recognized as the enteric waterborne bacterial pathogen most frequently involved in drinking water outbreaks in developed countries (Moreira and Bondelind, 2017). According to United States (US) data, Campylobacter spp. was identified as a causative or co-occurring agent in 11% of the drinking water outbreaks reported between 2001 and 2014 (the last year for which data is available). Outbreaks occurred in all months of the year, with the largest outbreaks occurring in the spring and summer months (CDC, 2004, 2006, 2008, 2011, 2013c, 2015, 2017d). Periods of higher risk for waterborne illness coincide with peak periods for precipitation-induced (e.g., rainfall, snowmelt) agricultural runoff (Sterk et al., 2013, Galanis et al., 2014).
Notable large drinking water outbreaks involving Campylobacter spp. worldwide include New Zealand (2016: greater than 1000 cases), Denmark (2010: 409 cases), Ohio, US (2004: 1450 cases), Finland (2001: 1000 cases), Walkerton, Ontario (2000: greater than 2300 cases) and France (2000: 781 cases, two deaths) (Hrudey and Hrudey, 2004, Government Inquiry into Havelock North Drinking Water, 2017; Moreira and Bondelind, 2017). Drinking water outbreaks have largely been associated with small drinking water supplies (i.e., private wells or small community supplies) with contamination from infiltration of animal feces or wastewater and inadequate disinfection reported as the most frequent causes (Moreira and Bondelind, 2017). Private and small community water systems are recognized as being more likely to contribute to cases of human enteric illness than municipally-operated systems (Hrudey and Hrudey, 2004; Murphy et al., 2016; Butler et al., 2016). Using a Quantitative Microbial Risk Assessment (QMRA) approach, Murphy et al. (2016) estimated that roughly 5% of the total number of Canadian cases of Campylobacter spp. acquired annually might be attributable to consumption of water from contaminated small drinking water systems. In municipal drinking water systems, inadequate disinfection and post-treatment contamination via intrusions or cross-connections are the most common causes of Campylobacter spp. outbreaks (Moreira and Bondelind, 2017).
B.1.2 Escherichia coli/Shigella spp. (pathogenic strains)
B.1.2.1 Description
Escherichia coli (Class Gammaproteobacteria, Family: Enterobacteriaceae) are Gram-negative bacteria that are a member of the natural intestinal microbial community of humans and animals. They are facultatively anaerobic, motile or non-motile rod-shaped bacteria that can grow over a broad temperature range (7-45°C) with an optimal growth temperature of 37°C (Ishii and Sadowsky, 2008, Percival and Williams, 2014c). Whereas most strains (i.e., variants) of E. coli are harmless and function as indicators of fecal contamination, some have acquired virulence traits through gains and losses of genetic material (Croxen et al., 2013). These pathogenic E. coli strains can cause numerous human diseases including serious gastrointestinal infections, urinary tract and bloodstream infections and neonatal meningitis (Croxen et al., 2013; Percival and Williams, 2014c). Non-pathogenic E. coli strains and their role in drinking water risk management are addressed in Health Canada's Guideline Technical Document on Escherichia coli (Health Canada, 2019e).
Pathogenic E. coli are broadly categorized into functional groups based on the mechanisms with which they interact with their target cells and cause symptoms. Different types can bind to, invade, or cause structural alterations of cells and produce specific types of toxins. There are six major groups of pathogenic E. coli that cause gastrointestinal infections: enterohaemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC) and diffuse adherent E. coli (DAEC) (Croxen et al., 2013; Percival and Williams, 2014c). Categorization of pathogenic E. coli strains has previously been determined by serotyping based on the classic Kauffmann-White classification scheme for surface O and H antigens (Croxen et al., 2013; Robins-Browne et al., 2016). Molecular methods have been developed that allow for more rapid detection and identification of the different pathogenic strains (Croxen et al., 2013; Robins-Browne et al., 2016). Serotyping information nevertheless remains helpful in outbreak investigations and disease surveillance (Robins-Browne et al., 2016). Additional pathogenic E. coli groups have been proposed, but these have not been completely defined. Comparative genomics studies have shown that these group designations are not always clear cut, and that there is considerable overlap in the virulence mechanisms possessed by different E. coli strains (Croxen et al., 2013). Shigella spp. are closely related to E. coli but have historically been considered separate species on the basis of biochemical characteristics and clinical features of disease. Advanced molecular typing and sequencing analyses have demonstrated that Shigella spp. clearly belongs within the species E. coli, forming a single group with the EIEC (Croxen et al., 2013, Robins-Browne et al., 2016). A re-evaluation of the Shigella spp. classification may be required to take into account its genetic relationship to the Escherichia genus. The genus name Shigella spp. and disease name shigellosis (i.e., disease caused by Shigella spp.) are still used for historical purposes (Croxen et al., 2013). Conventionally, Shigella spp. has four major species (S. dysenteriae, S. flexneri, S. boydii and S. sonnei); with Shigella sonnei and Shigella flexneri being the most important in developed countries (Percival and Williams, 2014h).
Among the pathogenic E. coli, the EHEC (synonyms: shiga toxin-producing Escherichia coli and verotoxin-producing Escherichia coli (VTEC)) are of most concern to the drinking water industry (Percival and Williams, 2014c; Saxena et al., 2015). EHEC are the subset of E. coli that can produce one or more of the potent Shiga toxins and are considered to be highly pathogenic to humans. E. coli O157:H7 is the most prevalent EHEC serotype; however, other serotypes, i.e., O26, O45, O103, O111, O121, and O145 are also important causes of human illness (Croxen et al., 2013, Saxena et al., 2015; PHAC, 2018c).
B.1.2.2 Health effects
In developed countries, most E. coli illness occurs as sporadic cases or outbreaks associated with contaminated food and water or travel (Croxen et al., 2013; Saxena et al., 2015). In developing countries, enteric pathogenic E. coli are a significant cause of illness and mortality, particularly among children.
Enteric pathogenic E. coli/ Shigella spp. cause diarrheal illness that can range in severity from mild and self-limiting to severe and life-threatening depending on the group and strain involved. The first symptom is a watery diarrhea. This can be followed by bloody diarrhea in EHEC infections and occasionally during EIEC/Shigella spp. and EAEC infections. (Croxen et al., 2013, Percival and Williams 2014c; 2014h). Additional symptoms can include nausea, vomiting, abdominal pain, fever, headache and muscle pain. Symptom onset generally occurs within one to three days of infection. The duration of diarrhea is usually one to two weeks, but can persist longer with some strains (Croxen et al., 2013; Percival and Williams, 2014c, 2014h). Infected individuals can become asymptomatic carriers capable of shedding the organisms in their feces for weeks to months after infection (Croxen et al., 2013; Percival and Williams, 2014c, 2014h). Doses required to cause infection are estimated to range from less than 100 to 1000 organisms for EHEC and EIEC/Shigella spp. to greater than one million to ten billion organisms for the other groups (Kothary and Babu, 2001; Croxen et al., 2013, Percival and Williams, 2014c; 2014h).
EHEC illness is particularly concerning as it can progress to the serious and potentially life-threatening hemolytic uremic syndrome (HUS), which results in decreased blood cell and platelet counts and acute kidney failure. A meta-analysis showed that HUS was the predominant long-term complication following cases of E. coli O157 illness, with an estimated rate of occurrence between 4-17% (Keithlin et al., 2014a). HUS can also lead to further long-term effects in the pancreas, gastrointestinal system and central nervous system (Spinale et al., 2013). Complications arising from non-EHEC infections are uncommon (Croxen et al., 2013). A link between infections with some pathogenic E. coli types (i.e., DAEC and some invasive E. coli) and chronic intestinal disorders such as irritable bowel syndrome and Crohn's disease has been suggested (Croxen et al., 2013). In developed countries, enteropathogenic. E. coli can cause gastrointestinal infections in healthy individuals in all age groups. Young children and the elderly are at higher risk of experiencing illness and complications as a result of infection (Percival and Williams, 2014c, 2014h; Gargano et al., 2017).
EHEC and Shigella spp. are among the leading causes of bacterial gastrointestinal illness in Canada, the US and Europe (Scallan et al., 2011; CDC, 2018; ECCDC, 2018a; PHAC, 2019). Case reports and outbreaks of E. coli-related diarrheal illness and shigellosis in North America have mostly been tied to food, and travel-related exposures, though waterborne exposure remains an important cause of infections (Croxen et al., 2013; PHAC, 2018c). Reported annual incidence rates in Canada (all causes) over the period from 2013 to 2017 were EHEC (as VTEC): 1.78-2.24 (median: 1.82) cases per 100,000 persons; and Shigella spp.: 1.94-2.53 (median: 2.28) per 100,000 persons (PHAC, 2019). Seasonal trends in EHEC and Shigella spp. infections (all sources) have generally been observed worldwide, with more cases occurring in summer to early fall (Fleury et al., 2006; PHAC, 2010; Lal, 2012).
In most cases, E. coli diarrheal infections are self-limiting. Treatment generally involves oral rehydration to maintain fluid and electrolyte balance. Antibiotics can be prescribed in severe cases with some strains of E. coli. Antibiotics are normally not recommended for EHEC infections, as they can stimulate Shiga toxin production, increasing the risk of HUS (Croxen et al., 2013).
The CDC, WHO and PHAC have identified carbapenem-resistant E. coli and extended spectrum β-lactamase (ESBL)-producing E. coli as public health threats of serious to critical importance (CDC 2013a; WHO, 2017, PHAC, 2018a). ESBL-producing E. coli are generally resistant to many antibacterial drugs; for persons with severe infections with these strains, carbapenems are one of the main treatment options. Resistance to carbapenems means resistance to one of the last available treatment options (CDC 2013a, WHO, 2017). Pathogenic E. coli strains resistant to ESBL antibiotics and carbapenems have been recovered from humans and animals (Mir and Kudva, 2018). Shigella spp. resistant to ciprofloxacin and azithromycin also have been designated as a serious threat level by the CDC, and have been designated as a low to medium priority for research and surveillance by PHAC and the WHO (CDC, 2013a, Garner et al., 2015; WHO, 2017). As Shigella spp. resistance to first-line drugs has increased, treatment has shifted to reliance on these two drugs for treating resistant infections (CDC, 2013a; WHO, 2017). A vaccine based on the cholera toxin (which is structurally similar to the ETEC heat-labile toxin) has been licensed for use to protect against ETEC-associated traveller's diarrhea (Croxen et al., 2013; O'Ryan et al. 2015). More data is required to determine the effectiveness of this and other candidate vaccines for ETEC (O'Ryan et al. 2015). No vaccines are available for other E. coli groups (Croxen et al., 2013).
B.1.2.3 Sources and exposure
Humans are the primary reservoir for the EPEC, ETEC and EAEC groups and the only known reservoir for EIEC/Shigella spp. (Croxen et al., 2013). EHEC are important zoonotic pathogens. Ruminants, particularly cattle, are the primary reservoir for EHEC; humans are a secondary reservoir (Croxen et al., 2013, Percival and Williams, 2014c). Animals (e.g., cattle, dogs, sheep, rabbits) are also a reservoir for certain EPEC strains (Croxen et al., 2013). Transmission of pathogenic E. coli occurs through the fecal-oral route and the main routes of infection are contaminated food or water, person-person spread and direct contact with animals. Important sources of fecal contamination for drinking water sources are much the same as those discussed for Campylobacter spp. (see B.1.1) (Hrudey and Hrudey, 2004; Moreira and Bondelind, 2017).
Warmer temperatures and extreme rainfall have been identified as contributing factors to waterborne disease outbreaks in Canada (Thomas et al., 2006). Heavy rainfall causing flooding contributed to the Walkerton, Ontario E. coli O157:H7 and Campylobacter spp. outbreak of 2000 (O'Connor, 2002). In the US, pathogenic E. coli (largely E. coli O157:H7) was identified as a causative or co-occurring agent in roughly 4% of the drinking water outbreaks reported over the period between 2001 and 2014 (the last year for which data is available) (CDC, 2004, 2006, 2008, 2011, 2013c, 2015, 2017d). Most E. coli drinking water outbreaks have been associated with small drinking water supplies (i.e., private wells or small community supplies) (Craun et al., 2010; CDC, 2011, 2013c, 2015, 2017d). QMRA estimates suggest that consumption of untreated or inadequately treated water from small drinking water supplies may be responsible for 4% of all cases of E. coli O157 illness in Canada (Murphy et al., 2016). Locations of large drinking water outbreaks involving pathogenic E. coli include Korea (2015: 188 cases, 0 deaths), Missouri, US (2010: 28 cases, 0 deaths) Walkerton, Ontario (2000: greater than 2300 cases, 7 deaths) and New York, US (1999: 781 cases, two deaths) (Hrudey and Hrudey, 2004; Missouri Department of Health and Senior Services, 2011; Park et al., 2018). Shigella spp. is rarely linked to drinking water outbreaks (Hrudey and Hrudey, 2004; Craun et al., 2010). Three reports of drinking water outbreaks involving Shigella spp. have been recorded in the US between 2001 and 2014; all associated with irregular sources of drinking water (pond, lake water, commercially bottled water system) (CDC, 2006, 2011, 2015).
B.1.3 Helicobacter pylori
B.1.3.1 Description
Helicobacter pylori (H. pylori, Class: Epsilonproteobacteria) is a pathogenic bacterium that can colonize the human stomach and is responsible for causing gastrointestinal diseases which can include gastritis, peptic ulcers and gastric cancer (Percival and Williams, 2014d; Posteraro et al., 2015). Helicobacter are closely related to the genus Campylobacter (Percival and Williams, 2014d). Over 20 different Helicobacter species have been determined by gene sequencing (Percival and Williams, 2014d; Posteraro et al., 2015). H. pylori is the predominant pathogenic species of the genus, accounting for the vast majority of human infections. Some other Helicobacter species have occasionally been associated with human gastrointestinal illness (Percival and Williams, 2014d).
H. pylori are Gram-negative, motile, fastidious and microaerophilic (require lower oxygen levels) bacteria that grow over the temperature range of 30-42°C (optimum: 37°C) (Mégraud and Lehours, 2007; Posteraro et al., 2015). They are not acidophilic (acid-loving) bacteria, but possess mechanisms that enable the bacteria to tolerate the acid conditions of the human stomach. H. pylori have two morphological forms: a spiral (S-shaped) rod form and a viable but non-culturable (VBNC) coccoid form that is adopted under conditions of environmental stress. The VBNC form is an important component of the organism's survival strategy; however, its role in pathogenesis is unknown (Percival and Williams, 2014d).
B.1.3.2 Health effects
The vast majority of H. pylori infections are asymptomatic (Percival and Williams, 2014d). H. pylori infection can cause a chronic and superficial gastritis, and some infections develop into peptic (i.e., duodenal or gastric) ulcers (Posteraro et al., 2015). Symptoms of gastritis and ulcers include nausea, abdominal pain, heartburn and bleeding (Percival and Williams, 2014d; Posteraro et al., 2015). In a very small fraction of the infected population, infections can develop into gastric cancer. H. pylori has been classified by the International Agency for Research on Cancer (IARC) as carcinogenic to humans (IARC, 2014), and the organism is considered to be the single most common cause of gastric cancer worldwide (Percival and Williams, 2014d; Posteraro et al., 2015). The infectious dose of H. pylori is not known. Challenge studies suggest it is less than 10,000 cells (Solnick et al., 2001; Graham et al., 2004); however, evidence from case reports of illness suggests the dose could be orders of magnitude lower (Langenberg et al., 1990; Matysiak-Budnik et al., 1995).
The varying health outcomes of H. pylori infection seem to be related to human genetic variability, environment and dietary factors and differences in strain virulence (Brown, 2000; Posteraro et al., 2015). Since the majority of persons do not develop clinical disease, it can be difficult to determine when infection occurs (Brown, 2000). People living in low socioeconomic status, poor hygiene or sanitary conditions and crowded or high-density living conditions are associated with higher prevalence of H. pylori infections (Brown, 2000). Rates of infection are higher in developing countries and in at-risk populations, with most infections being acquired during childhood in these locations (Brown, 2000, Posteraro et al., 2015). Childhood rates in developed countries are lower and may be decreasing with improvements to sanitary practices (Brown, 2000). H. pylori have been cited as the most prevalent bacterial pathogen of humans (Posteraro at al., 2015). Roughly one half of the world's population is infected with H. pylori (Percival and Williams, 2014d). Rates of asymptomatic H. pylori infections vary widely by geographical area but are broadly estimated to fall in the range from 20 to 50% in developed regions and from 50 to >70% in developing countries (Brown, 2000; Hooi et al., 2017; Zamani et al. 2018). The rates of H. pylori infections in Canada are not well understood as they are not reportable illnesses. Studies of H. pylori infections in Ontario adults 50 to 80 years of age and Canadian children with upper gastrointestinal symptoms have reported rates of 23.1% and 7.1% respectively (Naja et al., 2007; Segal et al., 2008). Higher rates (>40%) have been reported among First Nations populations in Canada (Bernstein et al., 1999; Sethi et al., 2013; Fagan-Garcia et al., 2019).
Once colonized by H. pylori, infections can be lifelong unless intensive antimicrobial therapy is provided (Percival and Williams, 2014d). Eradication of H. pylori has been shown to be the definitive cure for duodenal and most gastric ulcers (Percival and Williams, 2014d). Helicobacter resistant to clarithromycin and multi-drug-resistant Helicobacter have been identified as a medium to high priority for research and the development of new antibiotic strategies by PHAC and the WHO (Garner et el., 2015; WHO, 2017). No effective vaccines against H. pylori infection have been developed (Posteraro et al., 2015).
B.1.3.3 Sources and exposure
Hosts for H. pylori include humans, domestic cats and non-human primates (i.e., old world macaques) (Percival and Williams, 2014d). The human stomach is considered the major reservoir (Percival and Williams, 2014d). Domestic cats are also thought to be a source of the organism relevant for human infections (Percival and Williams, 2014d).
The principal means through which H. pylori infections are acquired is unclear. Person-to-person transfer is presumed to be the most likely route of transmission via fecal-oral, gastric-oral or oral-oral routes (Percival and Williams, 2014d; Posteraro et al., 2015). Direct contact with domestic cats is also thought to be a pathway for infection; however, there is no confirmed data on transmission from animals to humans (Brown, 2000). Consumption of contaminated drinking water is suspected as a potential source of infection. Infection occurring through multiple transmission pathways is likely (Percival and Williams, 2014d). Attempts to culture H. pylori from environmental water samples have mostly been unsuccessful, and this absence of cultured data has limited epidemiological and risk assessments (Percival and Williams, 2014d). Evidence for a waterborne transmission comes largely from epidemiological studies conducted in developing countries (Percival and Williams, 2014d). Further support for water as vehicle of transmission has been provided by culture of H. pylori in feces in infected individuals; detection of H. pylori by molecular methods in drinking water supplies; and the finding of an association between H. pylori in untreated groundwater supplies and clinical infection in individuals drinking the water (Baker and Hagerty, 2001). In countries with adequate drinking water treatment, drinking water is unlikely to be a significant source of infection (Percival and Williams, 2014d). Nevertheless, further research on the role of water in the spread of H. pylori infections is needed. Studies on the detection of H. pylori in municipal drinking water supplies have been limited. Surveys of public facilities and domestic taps detected H. pylori by polymerase chain reaction (PCR) in water and biofilm samples at 7-66% of the locations sampled (Watson et al., 2004; Santiago et al., 2015; Richards et al., 2018). H. pylori are not a recognized cause of waterborne outbreaks (Percival and Williams, 2014d).B.1.4 Salmonella spp.
B.1.4.1 Description
Salmonella (Class Gammaproteobacteria, Family: Enterobacteriaceae)is a large and diverse group of bacteria that can cause gastrointestinal infections in animals and humans. Molecular methods have shown that the genus consists of only two species, Salmonella enterica and Salmonella bongori (Percival and Williams, 2014g; Graziani et al., 2017). Salmonella enterica is further divided into six subspecies and contains the majority of the over 2500 serotypes that have been identified (Grimont and Weill, 2007; Percival and Williams, 2014g). Early in Salmonella identification, serotypes were treated as species and given names that reflected the host or disease it was associated with or, later, the geographic location where it was found (Grimont and Weill, 2007). When the current taxonomic structure of Salmonella was established, these names had become so familiar that they have been maintained, substituting for the O and H group naming structure that is more commonly used with other bacterial species (Grimont and Weill, 2007).
Salmonella of human importance are broadly categorized into two main groups according to the type of disease they cause. The typhoidal Salmonella (S. serotype Typhi and S. serotype Paratyphi) are the causative agents of enteric fever, a serious and life-threatening illness (Sanchez-Vargas et al., 2011). The non-typhoidal Salmonella are a large group containing the remainder of the serotypes which cause gastrointestinal illness of varying severity (Sanchez-Vargas et al., 2011). In developed countries, it is the non-typhoidal Salmonella that are the most important as food and waterborne pathogens (Sanchez-Vargas et al., 2011; Percival and Williams, 2014g). Salmonella serotype Enteritidis and S. serotype Typhimurium are the serotypes most commonly encountered as causes of human infections (Sanchez-Vargas et al., 2011).
Salmonella are Gram-negative, facultative anaerobic, predominantly motile rod-shaped bacteria that can grow over the temperature range of 5-47°C, and optimally at 35-37°C (Graziani et al., 2017).
B.1.4.2 Health effects
Salmonella infections follow different courses of disease depending on whether the serotype is typhoidal or non-typhoidal (Sanchez-Vargas et al., 2011). Non-typhoidal Salmonella cause a gastroenteritis characterized by diarrhea, fever and abdominal pain (Percival and Williams, 2014g, Graziani et al., 2017). Symptoms occur within 12-72 hours of infection and the illness may last for four to seven days. In severe cases, infection can spread to other parts of the body (e.g., blood, urine, joints, brain) and can be life-threatening (Percival and Williams, 2014g; Sanchez-Vargas et al., 2011). Children have the highest incidence of Salmonella infections (Christenson, 2013; PHAC, 2018c). Severe infections and fatal cases are rare and are more commonly reported among the very young, the very old and those with compromised immune systems or underlying illness (Sanchez-Vargas et al., 2011; Dekker and Frank, 2015). A meta-analysis of non-typhoidal Salmonella cases developing long-term complications estimated the proportion developing reactive arthritis at 5.8% (95% CI: 3.2-10.3%) and irritable bowel syndrome at 3.3 % (95% CI: 1.6-6.6%) (Keithlin et al., 2015). Estimates for other outcomes (e.g., HUS, Guillain-Barré Syndrome) were impeded by a lack of data (Keithlin et al., 2015). Typhoidal Salmonella cause enteric fever, an invasive and systemic disease which involves high fever, vomiting, headaches and numerous potentially fatal complications (Sanchez-Vargas et al., 2011). Enteric fever predominantly occurs in developing countries. In developed countries the incidence of enteric fever is infrequent and mostly related to travel (Sanchez-Vargas et al., 2011). The dose required to cause infection varies depending on the serotype involved and the susceptibility of the host. Data suggests that the infective dose (non-typhoidal Salmonella) can range from a low of less than 100 organisms to a high of 100,000 to 10 billion organisms (Kothary and Babu, 2001).
Salmonella is the second-leading cause of bacterial gastrointestinal illness in Canada, the US and Europe (Scallan et al., 2011; CDC, 2018; ECCDC, 2019; PHAC, 2019). In Canada, reported annual incidence rates (all sources) in 2013-2017 ranged from 17.6 to 21.7 (median: 21.38) cases per 100,000 population (PHAC, 2019). Cases of illness are predominantly sporadic, with most illness associated with consumption of contaminated food. Peak incidence of disease (all sources) occurs in the summer and fall (Fleury et al., 2006; Lal et al., 2012).
Infections with non-typhoidal Salmonella are generally self-limiting, and treatment involves fluid and electrolyte replacement (Percival and Williams, 2014g). Antibiotics can be prescribed in severe cases where there is increased risk of infection spread (Sanchez-Vargas et al., 2011; Percival and Williams, 2014g). No human vaccines are currently available for non-typhoidal Salmonella infections (Sanchez-Vargas et al., 2011). The CDC, WHO and PHAC have categorized non-typhoidal Salmonella resistant to ciprofloxacin, ceftriaxone or multiple classes (e.g., >3) of drugs as priority to high-level threats (CDC 2013a; WHO, 2017, PHAC, 2018a). In developed countries, antibiotic resistance trends have generally followed trends in the use of antimicrobials in food producing animals, with increased rates observed for older antimicrobials (McDermott et al., 2018). Declines in resistance rates for critical drugs for animals and humans (3rd generation beta-lactam antibiotics, ciprofloxacin) have been reported in the US and Canada and are consistent with policies limiting their use in agriculture (McDermott et al., 2018; PHAC, 2018a).
B.1.4.3 Sources and exposure
Non-typhoidal Salmonella are zoonotic pathogens. Chicken, pigs, turkey and cattle are considered the most important reservoirs (Graziani et al., 2017). Other animals (dogs, birds, rodents, reptiles) and humans (infected individuals and asymptomatic carriers) are also recognized as sources (Percival and Williams, 2014g; Graziani et al., 2017). Humans are the only known reservoir for the typhoidal Salmonella serotypes (Percival and Williams, 2014g).
Salmonella are spread by fecal-oral transmission. For the non-typhoidal serotypes, contaminated food is the most common pathway for infection; and person-to-person contact and direct contact with animals are significant exposure pathways (Percival and Williams, 2014g; Graziani et al., 2017). Ingestion of contaminated water is a recognized route for infection (Graziani et al., 2017). For sources of contamination important to drinking water, see Campylobacter spp. (see B.1.1). Non-typhoidal Salmonella are very rarely linked to drinking water outbreaks (CDC, 2004, 2006, 2008, 2011, 2013c, 2015, 2017d; Hrudey and Hrudey, 2004).
B.1.5 Yersinia spp.
B.1.5.1 Description
The genus Yersinia (Class: Gammaproteobacteria, Family: Enterobacteriaceae) contains approximately 20 bacterial species, with only 3 recognized as human pathogens. Two species (Yersinia enterocolitica, Yersinia paratuberculosis) are recognized as food or waterborne enteropathogens that can cause acute gastroenteritis of mild to high severity (Percival and Williams, 2014i; Fredriksson-Ahomaa, 2015). Yersinia pestis is the bacteria responsible for the plague that is transmitted from animals to humans by fleas or in aerosols (Fredriksson-Ahomaa, 2015). Yersinia enterocolitica can be divided into 6 biotypes differentiated by physiochemical and biochemical tests, and into more than 30 serotypes based on variations in their surface O antigens (Sabina et al., 2011; Fredriksson-Ahomaa, 2015). Human infections have traditionally been associated with certain biotype and serotype combinations. Types 1b:O8, 2:O5,27, 2:O9, 3:O3, 4:O3 are most commonly associated with human disease worldwide (Todd, 2014; Fredriksson-Ahomaa, 2015, 2017). Y. paratuberculosis is more closely related to the plague bacteria (Yersinia pestis) than Y. enterocolitica, and is a less common cause of human infections (Todd, 2014). For Y. paratuberculosis there are over 20 serotypes based on O antigen variations, all of which are pathogenic (Percival and Williams, 2014i).
Members of the genus Yersinia are Gram-negative, motile, facultatively anaerobic, rod to coccobacilli-shaped cells that are able to grow at temperatures over the range of 4-43°C (optimum: 28-30°C) (Todd, 2014; Fredriksson-Ahomaa, 2015).
B.1.5.2 Health effects
Enteropathogenic Yersinia are enteroinvasive organisms which colonize and invade colon epithelial cells, causing diarrhea and inflammatory reactions (Percival and Williams, 2014i; Todd, 2014). Symptoms of infection can differ depending on the age and immunity of the person infected, the strain involved and the infective dose (Todd, 2014). Common symptoms in children are diarrhea (often bloody), fever and abdominal pain (Todd, 2014; Fredriksson-Ahomaa, 2015). Diarrhea is less frequently observed in infections with Y. paratuberculosis (Todd, 2014). In older children and adults, fever and abdominal pain which mimics the symptoms of appendicitis are the most common (Todd, 2014; Fredriksson-Ahomaa, 2015). Symptoms occur 1 to 11 days after exposure and can persist 1 to 3 days or longer (Todd, 2014; Fredriksson-Ahomaa, 2015). Asymptomatic infections with Y. enterocolitica and Y. paratuberculosis have been reported; and the pathogens can continue to be shed in feces for weeks after symptoms have ceased (Todd, 2014). Occasionally in severe cases, the bacteria can enter the lymph nodes and infection can be further spread by the bloodstream (Percival and Williams, 2014i; Fredriksson-Ahomaa, 2015). Complications from infections are uncommon, but can include joint pain (reactive arthritis) and skin rash (Percival and Williams, 2014i; Fredriksson-Ahomaa, 2015). Other symptoms less frequently associated with enteropathogenic Yersinia infection are various inflammatory reactions resulting from infection spread to other parts of the body (e.g., liver, spleen, lung, heart, brain, bones) (Percival and Williams, 2014i, Todd, 2014). Young children are more likely to become ill from infection with enteropathogenic Yersinia (Todd, 2014; PHAC, 2018c). Severe infections and fatal cases are rare and are typically observed in the elderly and immunosuppressed individuals (Todd, 2014). The infective dose is estimated to range between 10 thousand and 1 billion organisms for both Y. enterocolitica and Y. paratuberculosis (Todd, 2014); however, the dose is likely lower for immunosuppressed individuals (Fredriksson-Ahomaa, 2017).
Yersinia is a significant cause of bacterial gastrointestinal illness in Canada, the US and Europe (PHAC 2018c; CDC 2018; ECCDC, 2018b). National incidence data is not available as yersiniosis is not a notifiable disease in Canada. The majority of cases of Yersinia-associated illness are caused by Y. enterocolitica and are linked to the consumption of contaminated food (Todd, 2014; Fredriksson-Ahomaa, 2015; PHAC, 2018c). Generally, Yersinia infections are more frequently observed during the winter months (Todd, 2014; Fredriksson-Ahomaa, 2015).
Infections with Y. enterocolitica or Y. paratuberculosis are normally self-limiting, with treatment provided only in severe cases involving systemic infection or bacteremia (Todd, 2014; Fredriksson-Ahomaa, 2015). No human vaccines are available.
B.1.5.3 Sources and exposure
Pathogenic and non-pathogenic Yersinia spp. can be found in the gut of a variety of wild and domestic animals (Percival and Williams, 2014i; Fredriksson-Ahomaa, 2015). Pigs are the major reservoir of pathogenic strains of Y. enterocolitica; and ruminants (e.g., cattle, sheep, goats), dogs and cats are also important sources of the pathogen (Todd, 2014; Fredriksson-Ahomaa, 2015). Rodents and birds are considered the major reservoirs for Y. paratuberculosis (Todd, 2014; Fredriksson-Ahomaa, 2015). Pathogenic Yersinia spp. are zoonotic; thus can be transmitted from animals to human via the fecal-oral route (Fredriksson-Ahomaa, 2015). Contaminated food sources are the most significant pathway for infections (Todd, 2014; Fredriksson-Ahomaa, 2015). Consumption of contaminated water and direct contact with animals are also important infection pathways (Todd, 2014; Fredriksson-Ahomaa, 2015). Person-to-person transmission is possible, but rare (Todd, 2014; Fredriksson-Ahomaa, 2015).
In most studies, it is the non-pathogenic species or strains that are more frequently isolated (Brennhovd et al., 1992; Cheyne et al., 2009; Schaffter and Parriaux, 2002). The low isolation frequency in environmental samples may be due to limitations in the sensitivity of culture-based methods (Fredriksson-Ahomaa and Korkeala, 2003). Cheyne et al. (2010) found that using PCR methods, Yersinia virulence genes were detected in 21-38% of samples taken from a heavily impacted river watershed that was used as a source for a drinking water treatment system.
Yersinia spp. have very rarely been linked to drinking water outbreaks. According to US data for 2001-2014 (the last year for which data is available), Yersinia enterocolitica was associated with one drinking water outbreak as a co-occurring agent with Campylobacter jejuni (CDC, 2004, 2006, 2008, 2011, 2013c 2015, 2017d). A contaminated untreated non-community groundwater supply was identified as the cause of the outbreak (CDC, 2004).
B.1.6 Analytical Methods
Standard methods are available for the detection of Campylobacter spp., pathogenic E. coli /Shigella spp., Salmonella spp. and Yersinia spp. in drinking water (APHA et al., 2017; ISO, 2019). Procedures for isolating and identifying these bacteria commonly involve steps such as enrichment and/or separation, plating, colony screening and identification using biochemical tests, serological techniques, molecular methods or commercial kits (e.g., for toxins) (APHA et al., 2017).
No standard methods for the detection of viable Helicobacter spp. in water have been established (Percival and Williams, 2014d, APHA et al., 2017). Methods for the detection of H. pylori in water environments involve the use of culture-independent molecular techniques such as PCR or fluorescent in-situ hybridization. The literature can be consulted for details on specific methods (Watson et al., 2004; Percival and Williams, 2014d; Santiago et al., 2015; Richards et al., 2018).
B.1.7 Treatment considerations
When properly designed and operated, physical removal technologies—chemically-assisted, slow sand, diatomaceous earth and membrane filtration or an alternative proven technology-and disinfection methods-chlorine, chloramines/monochloramine, chlorine dioxide, ozone and ultraviolet (UV) light-commonly used in drinking water treatment are very effective in reducing or inactivating the enteric bacteria described in the preceding sections (LeChevallier and Au., 2004). The CT (concentration x time) requirements for chemical disinfectant inactivation of these bacteria are comparable to those for E. coli and less than those required for enteric protozoa and enteric viruses (Sobsey, 1989; Lund, 1996; Johnson et al., 1997; Rice et al., 1999; Baker et al., 2002; LeChevallier and Au, 2004; Rose et al., 2007; Wojcicka et al., 2007; Chauret et al., 2008; Rasheed et al., 2016; Jamil et al., 2017; Health Canada, 2019c, 2019d, 2019e). The dose requirements for UV inactivation of these organisms are similarly comparable to those for E. coli and enteric protozoa, and less than those needed for many enteric viruses (Sommer et al., 2000; Zimmer and Slawson, 2002; Smeets et al., 2006; Hayes et al., 2006; Zimmer-Thomas et al., 2007; Hijnen et al., 2011; Health Canada, 2019c, 2019e).
General operational and maintenance practises for the control of microbial growth and survival in drinking water distribution and plumbing systems are outlined in Part A (LeChevallier and Au, 2004; Friedman et al., 2017). These are necessary to manage biofilms which can provide a habitat for the survival of fecal pathogens that may have passed through drinking water treatment barriers or entered the distribution system directly via an integrity breach (Leclerc, 2003).
For residential-scale systems and private wells, regular physical inspection to identify deficiencies and testing of the water system (e.g., for E. coli and total coliforms) to confirm the microbiological quality of the water are important. General guidance on well construction, maintenance, protection and testing is typically available from provincial/territorial jurisdictions. Well owners can also consult the Be Well Aware series for information (Health Canada, 2019a). Where treatment is necessary, Health Canada recommends that consumers use devices certified by an accredited certification body as meeting the appropriate NSF International (NSF)/American National Standards Institute (ANSI) drinking water treatment unit standards (NSF/ANSI, 2018, 2019a, 2019b). Certification organizations provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). An up-to-date list of accredited certification organizations can be obtained from the SCC (2020).
B.1.8 International considerations
No drinking water guidelines for the enteric bacterial pathogens Campylobacter spp., enteric pathogenic E. coli/Shigella spp., Helicobacter pylori, Salmonella spp. and Yersinia spp. have been established by the WHO, the EU, the US EPA or the Australian National Health and Medical Research Council. Similar to Health Canada's guidance document, the WHO and Australian drinking water guidelines contain fact sheets that provide information on waterborne pathogens of concern.
B.2 Naturally-occurring pathogens
B.2.1 Bacteria
B.2.1.1 Aeromonas spp.
B.2.1.1.1 Description
The bacterial genus Aeromonas (Class: Gammaproteobacteria) has a complex taxonomy. Around 30 species have been associated with the genus and potential new species continue to be described, although not all have been universally accepted (Janda and Abbott, 2010; Percival and Williams, 2014a; LPSN, 2019). The difficulties with Aeromonas identification arises from the lack of clear-cut phenotypic characteristics and the absence of a consistent typing scheme for distinguishing species. As a result, the use of biochemical and molecular methods is required for an accurate classification. Clinically relevant Aeromonas spp. are opportunistic pathogens that have been linked to a variety of intestinal and extra-intestinal diseases and syndromes (Janda and Abbott, 2010, Liu, 2015). Fourteen species have been implicated in human illness, however most human infections (85%) are caused by strains of four species: A. hydrophila, A. caviae, A. veronii (biotype sobria) and A. trota (Percival and Williams, 2014a; Liu, 2015; Bhowmick and Battacharjee, 2018).
Aeromonads are Gram-negative, facultatively anaerobic, non-spore-forming rod-shaped bacteria (Janda and Abbott, 2010; Percival and Williams, 2014a). Strains associated with human infections grow optimally at temperatures of 35-37°C, although many strains can grow in 4-42°C (Janda and Abbott, 2010; Percival and Williams, 2014a; Liu, 2015).
B.2.1.1.2 Health effects
Gastroenteritis is the most commonly encountered disease associated with Aeromonas infection (Janda and Abbott, 2010). Forms of the disease range from a watery enteritis accompanied by low-grade fever, vomiting and abdominal pain (most common) to a dysenteric form involving bloody stools (rare), to a cholera-like illness (very rare) (Janda and Abbott, 2010, Liu, 2015). Aeromonas spp. are an infrequent cause of traveller's diarrhea and they can also be associated with a subacute or chronic intestinal infection (Janda and Abbott, 2010, Liu, 2015).
The time between infection and symptom onset is one to two days for Aeromonas-associated traveller's diarrhea (Janda and Abbott, 2010). Subacute cases of diarrhea are defined as those lasting from two weeks to two months, whereas chronic cases persist for longer periods (Janda and Abbot, 2010). Complications that have been associated with more severe cases of Aeromonas gastroenteritis include ulcerative colitis, haemolytic uremic syndrome and inflammatory bowel disease (Janda and Abbott, 2010, Liu, 2015). The dose of Aeromonas spp. necessary to cause gastrointestinal infection is not clear. The only published challenge study showed that only 2/5 strains produced infection (14/57 individuals) and diarrhea (2/57 individuals) at high concentrations (ten thousand to ten billion colony forming units (CFU)) (Morgan et al., 1985). Data provided from foodborne outbreaks that have been observed suggests that the concentration required to cause infection could be orders of magnitude lower for some Aeromonas strains (Teunis and Figueras, 2016).
Skin and soft tissue infections are the second most common forms of Aeromonas-related disease. Aeromonas spp. can be associated with a variety of infections ranging from mild irritations (e.g., pus-filled lesions) to serious or life-threatening infections such as cellulitis or flesh-eating disease (Janda and Abbott, 2010; Bhowmick and Battacharjee, 2018). Aeromonads have also been implicated in blood-borne infections, which largely arise through the transfer of bacteria from gastrointestinal tract or wound infections. Common features associated with these infections are fever, jaundice, abdominal pain and septic shock (Janda and Abbott, 2010). Other less frequent diseases linked to Aeromonas infection include respiratory tract, urogenital tract and ocular infections (Janda and Abbott, 2010). High mortality rates have been observed with Aeromonas septicemia and severe wound infections in high at-risk individuals (Janda and Abbot, 2010; Liu, 2015).
Aeromonas-associated diarrhea has been encountered worldwide in healthy persons across all age groups (Janda and Abbot, 2010; Percival and Williams, 2014a; Teunis and Figueras, 2016). Still, given that Aeromonas spp. are widely encountered in food and water, illness is observed in relatively few individuals who are exposed to the bacteria (Janda and Abbott, 2010). Gastrointestinal infections are more prevalent in developing countries (Ghenghesh et al., 2008). Susceptible groups include infants, young children, the elderly and persons with lowered immune status or underlying disease such as liver disease and malignant illnesses (Ghenghesh et al., 2008; Liu, 2015). Skin and soft tissue infections are often the result of trauma or penetrating injury and occur more frequently in adults than children (Janda and Abbot, 2010). For Aeromonas-associated bacteremia, the vast majority of cases are in immunocompromised individuals (Janda and Abbot, 2010). Antibiotics can be prescribed in severe cases where there is increased risk of infection spread (Percival and Williams, 2014a; Liu et al., 2015). PHAC has categorized drug-resistant Aeromonas spp. as a low priority for research and surveillance compared to other antimicrobial resistant pathogens (Garner et al., 2015). No human vaccines are currently available for Aeromonas infections (Liu et al., 2015).
Aeromonas—associated infections are not reportable illnesses in North America or in most countries worldwide. Case reports and outbreaks of illness have mostly been tied to food, hospital exposures, travel, non-water environments or unknown causes (Teunis and Figueras, 2016).Infections are more frequently observed during the warmer months (Janda and Abbot, 2010; Bhowmick and Battacharjee, 2018).
B.2.1.1.3 Sources and exposure
Aeromonas spp. can exist in virtually every ecosystem niche, including aquatic habitats, soils, vertebrate and invertebrate animal species, insects and foods (Janda and Abbot, 2010; Percival and Williams, 2014a). They are found in water and aquatic environments (e.g., lakes, rivers, groundwater, seawater, potable water supplies, wastewater and sewage) in all but the most extreme conditions of pH, temperature and salinity (Janda and Abbot, 2010). Members of the genus are found in the gastrointestinal tract of cold-blooded and warm-blooded animals including fish, birds, reptiles and domestic livestock. Aeromonas spp. can be isolated from the feces of healthy humans as a result of consumption of food and water containing the organisms (Percival and Williams, 2014a). They can be found in high concentrations in wastewater (Janda and Abbott, 2010; Percival and Williams, 2014a). Aeromonads grow optimally at elevated temperatures, thus concentrations in water are at their highest during the warmer months (LeChevallier et al., 1982; Gavriel et al., 2008; Chauret et al., 2001; Egorov et al., 2011).
Ingestion of contaminated food and water are considered the main routes of transmission for Aeromonas-associated gastroenteritis. Direct body contact with contaminated water is the primary method of transmission for Aeromonas spp. in water-related skin and soft tissue infections. Contaminated floodwaters in natural disaster settings have been identified as an important vehicle for these types of illnesses (Tempark et al., 2013). Person-to-person transfer is not considered a risk with Aeromonas infections.
Aeromonads are not commonly detected in the bulk water in municipal distribution systems with a disinfectant residual (Chauret et al., 2001; Egorov et al., 2011). In a survey of 293 public water systems in the US, Aeromonas spp. were detected by culture methods at 42 systems (14.3%), with concentrations ranging from 0.2 to 880 (median 1.6) CFU per 100 mL (Egorov et al., 2011). Groundwater is expected to have lower numbers of Aeromonads than surface waters, but drinking water wells can become colonized by the bacteria (Borchardt et al., 2003; Percival and Williams, 2014a; Katz et al. 2015). Aeromonads are capable of growth and persistence in distribution system biofilms and this can contribute to an increased recovery of the organisms from drinking water supplies (Gavriel et al., 1998; Chauret et al., 2001).
The importance of drinking water as a route of transmission for Aeromonas-related gastrointestinal illness is not clearly understood. Species of Aeromonas possessing multiple virulence genes have been detected in drinking water supplies in North America and in other countries (Handfield et al., 1996; Kühn et al., 1997; Sen and Rogers, 2004; Robertson et al., 2014b). Some investigations attempting to link strains recovered from drinking water supplies to patient isolates have been unsuccessful (Havelaar et al., 1992; Borchardt et al., 2003). Other studies have presented evidence of an epidemiological link between Aeromonas in clinical samples and drinking water as a source of infection (Khajanchi et al., 2010; Katz et al., 2015). It is generally accepted that only a subset of Aeromonas strains can cause gastrointestinal illness in humans (Teunis and Figueras, 2016). Furthermore, it is believed that infection is a complex process involving the virulence of the Aeromonas strain, its interaction with other microbes present in the gut (as co-infecting pathogens or as part of the natural microbiota) and the health status of the host (Teunis and Figueras, 2016). As a result, the presence of Aeromonas spp. in drinking water on its own is not sufficient to signify that a health risk exists (Edberg et al., 2007). More work is needed to determine the specific combination of host, environment and pathogen factors that lead to the occurrence of gastrointestinal illness associated with Aeromonas infections (Teunis and Figueras, 2016). No known drinking water outbreaks associated with Aeromonas have been recorded (Janda and Abbot, 2010; Teunis and Figueras, 2016).
B.2.1.1.4 Analytical methods
Standard methods for the detection of Aeromonas in drinking water are available (US EPA, 2001; APHA et al., 2017). However, there is no established universally-accepted culture-based method capable of detecting all Aeromonads in water samples (APHA et al., 2017). Aeromonas spp. are heterotrophic bacteria and are detected by heterotrophic plate count (HPC) tests; however, no direct correlation between HPC counts and Aeromonas concentrations exists.
B.2.1.1.5 Treatment considerations
When properly designed and operated, physical removal—chemically—assisted, slow sand, diatomaceous earth and membrane filtration or an alternative proven technology—and disinfection methods—chlorine, chloramines/monochloramine, chlorine dioxide, ozone and UV—commonly used in drinking water treatment are very effective in reducing or inactivating Aeromonas spp. (Chauret et al., 2001; WHO, 2002; US EPA, 2006a; Yu et al., 2008). However, use of granulated activated carbon (GAC) in water treatment may provide nutrient sources for aeromonads which can contribute to their presence and survival in drinking water distribution systems (WHO, 2002; US EPA, 2006a).
Aeromonas are as sensitive to chemical disinfectants as E. coli and other waterborne bacteria (Knøchel, 1991; Medema et al., 1991; Sisti et al., 1998; WHO, 2002; US EPA, 2006a). The CT requirements for inactivation of Aeromonas spp. by chemical disinfectants are less than those required for numerous enteric viruses. The UV dose requirements are comparable to other waterborne enteric bacterial pathogens and the enteric protozoa Giardia and Cryptosporidium and are less than those needed for many enteric viruses (Massa et al., 1999; Gerba et al., 2003; US EPA, 2006a; Health Canada, 2019c, 2019d).
General operational and maintenance practises for managing microbial survival and growth in drinking water distribution and plumbing systems as outlined in Part A are important for the control of Aeromonas spp. (Chauret et al., 2001; WHO, 2002; Percival and Williams, 2014a). Control strategies include maintaining free chlorine and chloramine residuals above 0.2 mg/L and 0.4 mg/L, respectively (Gavriel et al., 1998; Chauret et al., 2001; Pablos et al., 2009).
For residential-scale systems and private wells, regular physical inspection to identify deficiencies and testing of the water system (e.g., for E. coli and total coliforms) to confirm the microbiological quality of the water, are important. Where problems with the microbiological quality of the drinking water are suspected, it may be useful to include additional parameters (e.g., HPC) in the analysis. Specific guidance on construction, operation, maintenance and testing should be obtained from the responsible drinking water authority in the affected jurisdiction.
B.2.1.1.6 International considerations
No guideline for Aeromonas spp. in drinking water has been established by the WHO, the EU, the US EPA or the Australian National Health and Medical Research Council. In the Netherlands, Dutch drinking water legislation specifies a monitoring requirement for Aeromonas as an operational parameter with a target limit of < 1000 CFU/100 mL (Smeets et al., 2009). This target is based on treatment achievability and not on public health significance (WHO, 2002).
B.2.1.2 Legionella spp.
B.2.1.2.1 Description
The bacterial genus Legionella (Class: Gammaproteobacteria) comprises 61 species and 3 subspecies (LPSN, 2019). Some 30 species have been known to cause human infection (Cuhna et al., 2016; Burillo et al., 2017). Pathogenic Legionella spp. are opportunistic pathogens that cause respiratory illness in two main forms: Legionnaires’ disease and Pontiac fever (Percival and Williams, 2014e). Illnesses caused by Legionella spp. are collectively known as legionellosis. Legionella pneumophila (mainly serogroup 1) is the most common and virulent pathogen of the genus, responsible for 65-90% of all cases of Legionnaires’ disease (Fields et al., 2002; Edelstein and Roy, 2015; Percival and Williams, 2014e, Prussin II et al., 2017). Infections involving other species are far less frequent and have been mainly caused by L. micdadei, L. bozmanae, L. dumoffii and L. longbeachae (Edelstein and Roy, 2015; Percival and Williams, 2014e; Cuhna et al., 2016).
The bacteria are Gram-negative, obligately aerobic, predominantly motile, short rod-shaped cells that require specific nutrients (L-cystine and iron) for growth (Percival and Williams, 2014e). During its life cycle, Legionella can adapt to fluctuating conditions by differentiating into cell types that vary in their infectivity and resistance to disinfection (Robertson et al., 2014a; NAS, 2019).
B.2.1.2.2 Health effects
Legionnaires’ disease is a severe respiratory illness involving pneumonia. Other features include fever, cough, chills, neurological aspects (confusion), muscle pain, headache and gastrointestinal problems (diarrhea, nausea, vomiting) (Castillo et al., 2016, Cunha et al., 2016; Edelstein and Roy, 2015). Symptom onset generally occurs 2 to 14 days after becoming infected (NAS, 2019), and the disease can persist for weeks to several months (Palusińska-Szysz and Cendrowska-Pinkosz, 2009). Although many individuals are exposed to Legionella bacteria, few develop illness (Castillo et al., 2016). Legionnaires’ disease has a low attack rate, affecting less than 1-5% of the general population and less than 1-14% of hospital patients who are exposed to the bacteria during outbreaks (Hornei et al., 2007; Edelstein and Roy, 2015; Leoni et al., 2018). Legionnaires’ disease is more likely to occur in older adults or immunocompromised individuals, however healthy individuals can acquire Legionnaires’ disease if they are exposed to a high enough concentration of the bacteria (Springston and Yocavitch, 2017). Reports of Legionnaires’ disease in healthy children are extremely rare (McDonough et al., 2007, Greenberg et al., 2006). Factors associated with an increased susceptibility to Legionnaires’ disease following exposure are male gender, age beginning at 40-50 years, smoking, chronic heart or lung disease, diabetes, chronic renal failure, immunosuppression, organ transplantation and some forms of cancer (Fields et al., 2002; Edelstein and Roy, 2015, Castillo et al., 2016; Cuhna et al., 2016; NAS, 2019). The case fatality rate associated with Legionnaires’ disease depends on the underlying health of the patients, how quickly therapy is delivered and whether the cases are sporadic, hospital-acquired or outbreak-related (Edelstein and Roy, 2015). Mortality is estimated at less than 10-15% for community acquired cases, but can be higher than 25% for hospital acquired cases (Benin et al., 2002; Howden et al., 2003; Dominguez et al., 2009; Soda et al., 2017; Leoni et al., 2018).
Pontiac fever is a milder, flu-like, self-limiting and non-pneumonic disease associated with exposure to Legionella. The disease has mainly been diagnosed in outbreaks where individuals have flu-like symptoms and share exposure to aerosols from a common source (Lüttichau et al., 1998). How Pontiac fever develops is poorly understood and why some persons develop this disease while others develop Legionnaires’ disease is not known (Fields, et al., 2001; Edelstein, 2007). It has been proposed that Pontiac fever may be due to exposure to some combination of live and dead microorganisms (either Legionella species or coexisting microorganisms) and their products (including endotoxins) (Edelstein, 2007). Pontiac fever has a high attack rate, affecting as high as 80-90% of exposed individuals during outbreaks (Leoni et al., 2018). Symptoms appear from five hours to three days after infection and last for two to seven days. Long-term complications are not observed and the disease is not fatal (Tossa et al., 2006; Edelstein and Roy, 2015). There appear to be no predisposing host factors for Pontiac fever (Edelstein and Roy, 2015). Cases of Pontiac fever in children have been reported during outbreaks of the disease (Lüttichau et al., 1998; Goldberg et al., 1989, Jones et al., 2003; Burnsed et al., 2007).
Dose-response models have been developed for a few specific Legionella strains, derived from animal experiments (NAS, 2019). Results of QMRA conducted to estimate the risk of Legionella exposure during a single showering event suggest that over a million viable cells per litre in the bulk water may be necessary to attain a delivered infectious dose of 1-100 CFU (Schoen and Ashbolt, 2011). No expert consensus exists on whether there is a threshold for detectable Legionella below which there is no risk of infection (NAS, 2019).
Legionella is the major cause of waterborne illness in the US (Neil and Berkleman, 2008; CDC, 2017d; Friedman et al., 2017). Large Legionella outbreaks receive the most attention given their substantial health impact. However, it is estimated that less than 20% of all reported legionellosis cases are outbreak-related (Fields et al., 2002; Neil and Berkleman, 2008; Burillo et al., 2017). Legionellosis follows a distinct seasonal pattern, with the peak number of cases occurring during summer and fall (Prussin II et al., 2017, Cuhna et al., 2016). In Canada, reported rates of legionellosis in 2006-2016 (the latest year for which data have been published) were 0.37-1.39 (median: 0.71) per 100,000 population (PHAC, 2019). Reported rates from the US were 1.0-1.89 (median 1.18) per 100,000 population over the same period (Adams et al., 2016, 2017). As legionellosis is underdiagnosed and underreported, the actual number of cases is expected to be much higher (Castillo et al., 2016; PHAC, 2018d). The yearly incidences of legionellosis in Canada and the US are on the rise (Adams et al., 2016, 2017; PHAC, 2019). Factors contributing to the increases include a true increase in the number of cases, greater use of diagnostic testing, and increased reporting (Burillo et al., 2017).
As Legionella are intracellular pathogens, treating Legionnaires’ disease requires the use of antibiotic agents capable of reaching therapeutic concentrations within human cells (Fields et al., 2002; Edelstein and Roy, 2015, Castillo et al., 2016; Wilson et al., 2018). No human vaccine for the disease exists (Edelstein and Roy, 2015). Most individuals with Pontiac fever do not become ill enough to seek medical attention, and antibiotic treatment is generally not required (Edelstein and Roy, 2015, Castillo et al., 2016). Trends in antibiotic resistance in Legionella spp. are not well understood (Wilson et al., 2018). Data on resistance of clinical isolates to antibiotics is not well documented due to the absence of easily performed tests (Wilson et al., 2018).
B.2.1.2.3 Sources and exposure
Legionella has two habitats—a primary reservoir in the natural environment and a secondary habitat in engineered water systems (NAS, 2019). Current understanding is that the primary environment for Legionella growth in these habitats is within free-living protozoa that reside within biofilms (Devos et al., 2005; NAS, 2019). Legionella bacteria are naturally encountered worldwide in freshwater and soil environments including lakes, rivers, sediments and groundwater (Fields et al., 2002; Percival and Williams, 2014e, Burillo et al., 2017; NAS, 2019). Human and animal feces are not considered a source of Legionella, although the organism can be detected in the feces of infected individuals experiencing diarrhea symptoms. Animals can be infected by Legionella, but zoonotic transmission of the organism has not been documented (Surman-Lee et al., 2007; Edelstein and Roy, 2015).
Legionella parasitize and multiply inside numerous species of freshwater protozoa (e.g., amoebae and ciliates) that are found in biofilms in natural waters and engineered water systems, including Acanthamoeba (see B.3.2.1), Hartmanella, Naegleria (see B.3.2.2), Valkampfia, Vermamoeba (formerly Hartmanella), Echinamoeba and Tetrahymena (Fields et al., 2002; Lau and Ashbolt, 2009; Buse et al., 2012, Percival and Williams, 2014e; NAS, 2019). Survival within these protozoa provides a source of nutrients, a protective environment against disinfectants and other adverse conditions (such as elevated temperatures) and a means of transport (Percival and Williams, 2014e, Buse et al., 2012; NAS, 2019). Legionella are also capable of persisting in biofilms in the absence of host protozoa (NAS, 2019).
With the right conditions in engineered environments, Legionella may grow to reach high concentrations. Engineered environments identified as reservoirs for Legionella include large complex premise plumbing systems (such as those found in hospitals, hotels, apartment buildings, community centres, industrial buildings and cruise ships), cooling towers or evaporative condensers in buildings and industry, and drinking water distribution systems. Legionella has a water-to-air transmission, therefore, sites in these systems with both biofilm growth and the potential to generate aerosols are most commonly implicated as the source of exposure (NAS, 2019). These include components of heating, ventilation and air conditioning (HVAC) systems such as cooling towers/air condensing units, plumbing devices (e.g., showerheads, faucets), hot tubs/spas/jacuzzis, and humidifiers and nebulizers. Other water systems that produce aerosols from stored or stagnating warm water sources have been sources of Legionella exposure, including car washes, decorative fountains and supermarket produce misters (NAS, 2019).
Legionella grows at temperatures between 25 and 45°C (optimum: 25-35°C), therefore water supplies with temperatures in this range support the highest levels of growth of this organism (NAS, 2019). They are also thermotolerant, capable of surviving at temperatures between 55 and 70°C (Allegra et al., 2008; Cervero-Aragó, 2015; 2019). Survival of Legionella in protozoan cysts following exposure to 80°C has been demonstrated (NAS, 2019). Within plumbing systems, the hot water supply system is commonly identified as the origin of Legionella contamination. Besides having more favourable temperatures for Legionella growth, hot water systems typically have lower disinfectant residual concentrations than cold water systems. Cold water supplies held at temperatures above 25°C can also have an increased risk of Legionella colonization (Donohue et al., 2014; Schwake et al. 2016).
Data on the detection of Legionella spp. in the distribution systems of drinking water utilities in North America is sparse. Studies have reported the detection of Legionella spp. and L. pneumophila by PCR in 57-83% and 6-14% of water samples, respectively (Wang et al., 2012a; Lu et al., 2015a). Lu et al., (2015b) detected L. pneumophila by PCR in 38% (7/18) of municipal drinking water storage tank sediments from locations across the US The methods used in these studies are not capable of distinguishing between living and dead organisms. The body of evidence including data from international jurisdictions suggests that L. pneumophila can be detected at various rates in distribution system samples (e.g., water, biofilms, loose deposits) from drinking water utilities (Wullings et al., 2011; Wang et al., 2012a; Whiley et al., 2014; Lu et al., 2015a; 2015b). Higher rates of detection of Legionella and L. pneumophila, can be encountered in cooling towers and large building plumbing systems (Stout et al., 2007, Christina et al., 2014, Llewellyn et al., 2017). Stout et al. (2007) reported that Legionella pneumophila was detected in plumbing systems at 70% (14/20) of hospitals tested. Llewellyn et al. (2017) detected Legionella spp. by PCR in 84 % (164/196) of cooling tower samples collected from various regions across the US L. pneumophila was detected by culture methods in 32% (53/164) of the PCR-positive samples (Llewellyn et al., 2017). Based on the analysis of available data from Legionella occurrence studies, the National Academies of Sciences (NAS) Committee on Management of Legionella in Water Systems propose that a concentration of 5 x 104 CFU/L be considered as an action level, for water samples in engineered water systems (NAS, 2019). This action level reflects a concentration high enough to warrant concern and serve as a trigger for remedial action (NAS, 2019). A lower action level may be necessary to protect individuals at higher risk for legionellosis such as hospital patients (NAS, 2019).
Relatively little is known about the environmental sources of community-acquired sporadic cases of legionellosis. Domestic household water systems may be a potential source of Legionella (Collins et al., 2017, Mathys et al., 2008). In various studies conducted in the US and Europe, clinically-relevant Legionella were found using culture methods at distal outlets (e.g., faucets, showerheads) of domestic hot water systems supplied by hot water storage tanks at 6-21% of the households that were sampled (Alary and Joly, 1991; Stout et al., 1992; Bates et al., 2000; Mathys et al., 2008; Dilger et al., 2016; Collins et al., 2017). Studies have reported on the greater prevalence of Legionella spp. in samples from hot water systems supplied by thermally-stratified (i.e., electrically-heated) water heaters and on the absence of Legionella spp.in hot water systems supplied by gas or oil-heated water heaters supplying temperatures greater than 60°C at the water outlet or distal sites (Alary and Joly, 1991, Mathys et al., 2008). The importance of domestic water systems as a source of Legionella infection for susceptible individuals is not clear (Bates et al., 2000; Prussin II et al., 2017). In general, the risk of acquiring Legionnaires’ disease from exposure to the bacterium in residential water systems is considered to be low; however, some individuals, because of their low health status, are at greater risk (Mathys et al., 2008; Prussin II et al., 2017).
Transmission of Legionella occurs mainly through inhalation of aerosols (size 2-10 µm) containing the bacteria (Percival and Williams, 2014e; Castillo et al., 2016). Consumption of drinking water is not a recognized route of Legionella transmission (Percival and Williams, 2014e; Prussin II et al., 2017). It is hypothesized that microaspiration that occurs during drinking, or is associated with certain clinical conditions or procedures, is a potential source of exposure (NAS, 2019). Inoculation of surgical wounds is another less common route of infection (Cuhna et al., 2016; Burillo et al., 2017). Person-to-person transmission generally does not occur (Percival and Williams, 2014e; Edelstein and Roy, 2015), but one probable case has been reported (Correia et al., 2016).
Precipitation and high humidity have been positively associated with the occurrence of disease (Fisman et al., 2005; Beauté et al., 2016). The report of a community-associated outbreak of Legionnaires’ disease in Calgary in November and December of 2012 suggests that Legionella transmission can occur during the late fall and winter months in North America (Knox et al., 2017). Climate change, and its associated temperature increases may be a factor in contributing to conditions which facilitate Legionella growth (Cuhna and Cuhna, 2017; MacIntyre et al., 2018).
Notable outbreaks of Legionnaires’ disease in North America include Brooklyn, New York (2015: 138 cases, 16 deaths); Genessee County, Michigan (2014/2015: 87 cases, 12 deaths), Quebec City, Quebec (2012: 182 cases, 13 deaths) and Scarborough, Ontario (2005: 112 cases, 23 deaths) (Gilmour et al., 2007, Levesque et al., 2014, MDHHS, 2016, Weiss et al., 2017).
B.2.1.2.4 Analytical methods
Detection of Legionella bacteria is technically difficult and requires specialized laboratory facilities. Standard methods for the detection of Legionella in drinking water are available (APHA et al., 2017; ISO 2019). Other methods may be approved for use in other jurisdictions (CEAEQ, 2019). The literature can also be consulted for details on specific methods (Mercante and Winchell, 2015; Wang et al., 2017, Petricek and Hall, 2018). Standards and approved codes of practise that have been developed for the control of Legionella in building water systems recommend site-specific risk assessments be used to inform decisions on the needs for environmental monitoring at individual facilities (HSE 2013c; PWGSC, 2016). In general, monitoring programs consist of routine monitoring of general microbiological quality, as an indication of system control, in conjunction with testing for Legionella at regular time intervals using a combination of culture-based and PCR methods (HSE 2013a; 2013b; 2019; PWGSC, 2016). For epidemiological investigations, sequence-based typing methods are the current gold standard for comparing environmental and patient isolates of Legionella (Gaia et al., 2005; Mercante and Winchell, 2015; APHA et al., 2017).
B.2.1.2.5 Treatment considerations
When properly designed and operated, physical removal technologies—chemically—assisted, slow sand, diatomaceous earth and membrane filtration or an alternative proven technology—will reduce the number of Legionella present in drinking water (US EPA 1989a, 2006b; Hijnen and Medema, 2010). The CT values for inactivation of Legionella pneumophila by chlorine, chloramines (monochloramine), chlorine dioxide and ozone are greater than those required for Giardia, but are less than or equal to those required for Cryptosporidium (Jacangelo et al., 2002; Health Canada, 2019d). Research data indicates that dose requirements for UV are greater than those needed for Giardia and Cryptosporidium, but are less than those needed for many enteric viruses (Hijnen et al., 2011; Health Canada, 2019c, 2019d). Providing effective control of free-living protozoa in drinking water (e.g., Acanthamoeba, Naegleria — see B.3.2) is also necessary for reducing Legionella populations (Loret and Greub, 2010; Thomas and Ashbolt, 2011; NAS, 2019). The general operational and maintenance practises for managing microbial survival and growth in drinking water distribution and plumbing systems outlined in Part A are important for the control of Legionella spp. (Falkinham et al., 2015b). Data from laboratory studies suggest that a free chlorine concentration of greater than 0.5 mg/L is required for inactivation of Legionella in bulk water and that higher concentrations (greater than 2 mg/L) are necessary for effective disinfection of Legionella present within biofilms or free-living amoebae (Miyamoto et al., 2000; Storey et al., 2004; Cooper and Hanlon, 2010; Dupuy et al., 2011). There is evidence that monochloramine provides better control of Legionella in building water systems compared to free chlorine (Pryor et al., 2004; Flannery et al., 2006; Weintraub et al., 2008; NAS, 2019).
The 2014-2015 outbreaks of Legionnaires’ disease in Genesee County, Michigan which coincided with the Flint water crisis, provide an example of the unintentional consequences that can accompany changes in drinking water systems operations. A shift in the source water used by the Flint Water Treatment Plant to the more corrosive Flint River combined with the lack of corrosion control resulted in conditions (unstable chlorine residual, elevated temperatures, increased iron) conducive to Legionella growth in drinking water systems (Masten et al., 2016).
Many resources are available which address measures for reducing the risk of exposure to Legionella in building water systems. The National Building Code of Canada (NRCC, 2015a) and the National Plumbing Code of Canada (NPC) (NRCC 2015b) set out standards and technical provisions for the design and installation of HVAC systems and plumbing systems in buildings, respectively. Both contain provisions dealing with Legionella in building systems. The American National Standards Institute/American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ANSI/ASHRAE) Standard 188 (ASHRAE, 2018) establishes minimum Legionella risk management requirements for building water systems intended for use by those involved in design, construction, installation, commissioning, operation, maintenance and service of centralized building water systems and components. Guidance documents recommend the use of water management/water safety plans for the management of Legionella in building water systems. Healthcare and long-term care facilities and buildings with cooling towers are identified as buildings with a particular need for water management programs to reduce the risk of Legionella growth and spread (WHO, 2007, HSE 2013a, CDC, 2017a). Publications to assist building managers in developing water management/water safety plans are available (WHO, 2007, 2011; HSE, 2013a, 2013b, 2013c; PWGSC, 2016; CDC, 2017a; ASHRAE, 2018). In general, the NAS Committee on Management of Legionella in Water Systems recommends requirements for water management plans in all public buildings and establishing cooling tower registries as two policy initiatives that can improve public health protection from exposure to Legionella (NAS, 2019).
The Province of Quebec enacted building safety legislation in 2013 which included regulations for the maintenance and operation of cooling towers (Government of Quebec, 2020). The regulations outline the requirements for owners that include registering their system with the regulator, implementing a water management plan and conducting regular testing for Legionella pneumophila. The cities of Hamilton and Vancouver have put similar requirements for cooling towers, evaporative condensers or decorative water features into their bylaws (City of Hamilton, 2019; City of Vancouver, 2020).
For plumbing systems, temperature management, i.e., the use of control measures to keep the hot and cold water systems outside the organism's growth range of 25-43°C, is a fundamental aspect of a Legionella control strategy (Bédard et al., 2016a; Boppe et al., 2016; NAS, 2019). Maintaining a minimum hot water tank temperature of 60°C is a key threshold for reducing positive detection of Legionella in buildings (WHO, 2011; HSE, 2013b; NRCC 2015b, NAS, 2019). The NPC specifies that storing hot water at temperatures below 60°C in hot water tanks and delivery systems may lead to growth of Legionella bacteria. The NPC further specifies that electric storage-type water heaters should be pre-set to a temperature of 60°C as a result of the temperature stratification that can occur with this type of heater. Temperature stratification is not a concern for other types of water heaters with different designs that use different fuels (NRCC, 2015b). Adjusting temperature regimes to achieve temperature greater than 55°C at distal points in the system has also been recommended as an effective measure for reducing Legionella colonization (WHO, 2011; HSE, 2013b, NAS, 2019). The hot water temperatures required to prevent Legionella growth are associated with a higher scalding risk (NRCC 2015b, NAS, 2019). Applications of temperature management strategies should operate in accordance with regulations in place regarding maximum allowable temperatures at the tap. The NPC specifies that water valves supplying showerheads and bathtubs should be capable of maintaining a water outlet temperature that does not exceed 49°C in order to reduce the risk of scalding (NRCC, 2015b). Temporarily elevating the water temperature, or heat shock (e.g., a stringent thermal shock of 70°C for 30 minutes) has been utilized as a control measure in building systems. However, the efficacy of this procedure is controversial, and it is considered an extreme remediation measure (NAS, 2019). The use of on-site disinfection technologies is also considered an integral part of a Legionella control strategy in large building water systems (Bartram et al., 2007; US EPA, 2016; NAS, 2019). Various disinfection technologies (free chlorine, monochloramine, chlorine dioxide, copper-silver ionization, UV light, ozone, point-of-use (POU)/point-of-entry (POE) filtration technologies) have demonstrated some level of effectiveness against Legionella (Bentham et al., 2007, Exner et al., 2007; US EPA, 2016; Springston and Yocavitch, 2017, NAS, 2019). Guidance for building plumbing systems recommend maintaining a residual concentration of 0.2 mg/L or greater for free chlorine and a minimum of 1.5 mg/L for monochloramine (Moore and Shelton, 2004; WHO, 2007; HSE, 2014). Applying any supplemental control strategy requires a detailed understanding of the complexity of the system and the composition of the water and system materials (Bartram et al., 2007; US EPA, 2016; NAS, 2019).
For homeowners, recommendations for the control of Legionella in household plumbing systems involve maintaining a minimum hot water tank temperature of 60°C, consistent with NPC specifications (NRCC 2015b; WHO, 2017; NAS, 2019). Educating immunocompromised individuals on the potential risks from in-home devices that create aerosols and may support Legionella growth (e.g., humidifiers, nebulizers) is a useful component of Legionella risk management in the home (NAS, 2019).
The increasing demand for conservation of energy, water and materials can have unplanned impacts on microbiological risks. This is important for Legionella in particular but also has relevance for other opportunistic pathogens that can be problematic in premise plumbing. Changes to water systems operations or features that involve the use of alternative water sources (e.g., reclaimed water, harvested rainwater), increased water age, reduced flows and changes to water temperatures in hot and cold building systems can have the unintended consequences of increasing the potential for growth of these pathogens (Bédard et al., 2016; Rhoads et al., 2016; NAS, 2019). There is a need for more research into engineering options that harmonize conservation efforts and the control of microbiological risks (Rhoads et al., 2016; NAS, 2019).
B.2.1.2.6 International Considerations
No drinking water guideline for Legionella has been established by the WHO, the EU, the US EPA or the Australian National Health and Medical Research Council. Guidelines or standards that have been developed for Legionella spp. in Canada and the US and other countries worldwide relate to control of the organism in building water systems outside of municipal distribution system networks.
B.2.1.3 Mycobacterium spp.
B.2.1.3.1 Description
The genus Mycobacterium (Class: Actinobacteria) contains over 200 recognized species. Bacteria belonging to this genus are diverse in their ability to cause disease in humans. Some are strict pathogens, whereas others cause opportunistic infections or are non-pathogenic. Tuberculosis and leprosy are two illnesses caused by Mycobacterium species; however, these particular species are not relevant to drinking water. The mycobacteria of concern for drinking water providers are the species collectively referred to as the non-tuberculous mycobacteria (NTM).
NTM are a group of over 150 distinct species that are considered to be opportunistic human pathogens (Falkinham, 2016a, b). Members of the M. avium complex—which includes M. avium and its subspecies M. intracellulare and M. chimaera—are the organisms most frequently associated with human illness. Other medically-relevant species include M. abscessus, M. chelonae, M. fortuitum, M. gordonae, M. kansasii, M. malmoense and M. xenopi (Nichols et al., 2004; Hoefsloot et al., 2013; Falkinham, 2016a).
Mycobacteria are Gram-negative, aerobic to microaerophilic, non-motile, non-spore-forming rod-shaped bacteria. Species are categorized as either rapid growers or slow growers based on the time required to produce colonies on growth media (Cangelosi et al., 2004; Falkinham et al., 2015b). Most of the pathogenic mycobacteria are slow-growers (Cangelosi et al., 2004). Mycobacteria can grow at temperatures of 20-45°C (Cangelosi et al., 2004; Kaur, 2014). Optimal growth temperatures for individual species vary within the range of 30-45°C (De Groote, 2004; Stinear et al., 2004). The bacteria are relatively heat-resistant, capable of surviving at temperatures greater than 50°C (Schulze-Robbecke and Buchholtz, 1992; Falkinham, 2016a). Mycobacteria can utilize many substances as nutrient sources and are able survive on very simple substrates (Kaur, 2014). All mycobacteria possess a thick and lipid-rich cell wall that makes the organisms relatively impermeable to hydrophilic compounds. This provides the bacteria with increased resistance to acid/alkaline conditions, disinfectants and antibiotics.
B.2.1.3.2 Health effects
NTM species cause a number of different diseases in humans including pulmonary infections, cervical lymphadenitis (i.e., infection of the lymph nodes of the neck) and infections of skin and soft tissues, the bloodstream and the gastrointestinal tract. NTM rarely cause disease in healthy humans. Infections occur largely in individuals who have weakened or suppressed immune status or persons with underlying respiratory conditions.
Pulmonary disease is the most common form of NTM-associated illness. Features include persistent cough, weakness and night sweats. The attack rate and time to onset of symptoms are not known. Infection can be difficult to diagnose from general respiratory illness and patients may have a long history of symptoms (e.g., months to years) before a diagnosis of mycobacterial disease is made (Falkinham et al., 2015b). Groups having increased risk for NTM-pulmonary infection include older men with a history of smoking, alcoholism or lung damage from occupational exposure to dusts; older slender, taller women lacking any notable risk factors; and persons with chronic disease conditions that affect the lungs (e.g., cystic fibrosis, lung cancer, chronic obstructive pulmonary disease) (Falkinham, 2015c; Adjemian et al., 2018).
Cervical lymphadenitis caused by NTM is marked by swollen lymph nodes in the head or neck, with the majority of cases being observed in children ranging in age from 18 months to 5 years (Falkinham, 2015c). In immunodeficient individuals, NTM infections can spread to other parts of the body including joints, the liver and the brain. NTM-associated bacteremia is a common and life-threatening infection in individuals with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS) (Falkinham, 2015c). Skin and soft tissue infections caused by NTM range from localized skin lesions or nodules to widespread ulcerative or necrotizing disease (Percival and Williams, 2014f). The NTM member M. avium subspecies paratuberculosis, is hypothesized to be a cause of Crohn's disease, though evidence of an association is inconclusive (Waddell et al., 2015; 2016). Risk factors for non-pulmonary NTM diseases typically include other comorbidities that result in a compromised immune system, such as underlying immunological disorders and HIV infection.
The infective doses for NTM species are not known (Stout et al., 2016; Hamilton et al., 2017; Adjemian et al., 2018). Mortality rates associated with cases of NTM illness are not well understood (Adjemian et al., 2018). In the US, the overall mortality burden associated with NTM disease has been estimated at 2.3 deaths per 1,000,000 person years (Vinnard et al., 2016).
The prevalence of illness associated with non-tuberculous mycobacteria in Canada is not known, as cases of illness are not reportable. In Ontario, the annual rate of NTM disease was estimated at 9.7-10.7 cases per 100,000 population in 2006-2010 (Marras et al., 2013). NTM-associated infections are a reportable condition in only a small number of US states (Donohue and Wymer, 2016; Adjemian et al., 2018). The average annual rate of NTM cases across five states reporting illness (Maryland, Mississippi, Missouri, Ohio, and Wisconsin) were 8.7-13.9 cases per 100,000 population in 2008-2013 (Donohue and Wymer, 2016). Data suggests the prevalence of NTM disease is continually increasing in North America and worldwide (Donohue and Wymer, 2016; Stout et al., 2016, Adjemian et al., 2018). Data on non-pulmonary NTM disease is comparatively more limited. Estimates of the incidence of non-pulmonary disease in the US are 1.5-1.9 cases per 100,000 population (Henkle et al., 2017, Adjemian et al., 2018). Little information is available on the impacts of seasonal- or climate-related factors on infections (Falkinham, 2004; Adjemian et al., 2018).
NTM are resistant to many commonly used antibiotics. Treatment typically requires a combination of antimicrobial antibiotics including clarithromycin, arithromycin, rifampin and others (Percival and Williams, 2014f; Falkinham, 2015c, Halstrom et al., 2015).
B.2.1.3.3 Sources and exposure
Non-tuberculous mycobacteria are found in the environment in soil and in water habitats such as marine waters, lakes, rivers, streams, groundwater and swamps. Soils, in particular, acidic, peat-rich soils, are the primary reservoir (Falkinham, 2016b). Wastewater and sewage sludge can contain high numbers of these organisms (Radomski et al., 2011; Percival and Williams, 2014f). Important habitats for NTM are engineered water distribution systems (drinking water distribution and plumbing systems) and downstream facilities or water-using devices that provide nutrient, temperature and disinfection protection conditions that allow the bacteria to increase to high numbers. Characteristics of mycobacteria that make them ideally suited to life in these environments include the ability to grow under low nutrient and oxygen conditions, disinfectant resistance, thermal tolerance and survival and growth in biofilms and free-living protozoa (Falkinham, 2015a). Pathogenic mycobacteria have a water-air transmission, similar to Legionella spp. (Percival and Williams, 2014f; Falkinham 2015c). Therefore, water-using features or devices that generate aerosols present a potential source for human exposure through inhalation (Falkinham, 2015c; 2016b). NTM have been isolated from many water systems and features, including hospital and residential plumbing systems (hot and cold water systems), faucets, showerheads, hot tubs/spas, ice machines, heated nebulizers and swimming pools (Percival and Williams, 2014f; Nichols et al., 2004).
Inhalation of aerosols containing mycobacteria is the primary route of transmission of NTM-associated pulmonary disease (Percival and Williams, 2014f; Falkinham, 2015c; Halstrom et al., 2015). Contact with or ingestion of contaminated water are also recognized routes of infection for other water-related NTM-associated diseases (Percival and Williams, 2014f; Falkinham, 2015c; Falkinham et al., 2015a). While non-tuberculous mycobacteria are primarily acquired from environmentally-contaminated sources, it has been proposed that indirect person-to-person transfer (i.e., via contaminated objects) may be a relevant route of transmission for persons with cystic fibrosis (Bryant et al., 2013; Bryant et al., 2016, Sood and Parrish, 2017). M. avium ssp. paratuberculosis has a fecal-oral route of transmission in cattle; however, the pathogenic role of this organism in human disease and potential sources of infection are topics of significant debate (Harris and Barrletta, 2001; Waddell et al., 2016).
Groundwater generally contains lower numbers of non-tuberculous mycobacteria than surface waters (Falkinham, 2015c). However, the occurrence and concentrations of the organisms in municipal distribution systems using groundwater or surface water can be similar (Covert et al., 1999; Lu et al., 2015b). Studies of the presence of non-tuberculous mycobacteria in water samples from chlorinated or and chloraminated distribution systems detected organisms at 40-100% of sampling sites using PCR methods (Wang et al., 2012a; Thomson et al., 2013; Whiley et al., 2014; Lu et al., 2015a).
Non-tuberculous mycobacteria are frequently detected in biofilm and tap water samples from household plumbing fed by municipal drinking water systems (Feazel et al., 2009; Holinger et al., 2014). Premise plumbing can harbour higher mycobacterial populations in the bulk water and biofilms than are found in the main water distribution system (Hilborn et al., 2006). Data suggests the organisms are less abundant in homes receiving water from private wells (Gebert et al., 2018). Studies of homes supplied by municipal water reported Mycobacterium spp. and M. avium detected in 70%-85% and 7-57% of biofilm samples collected from taps and showerheads, respectively (Feazel et al., 2009; Wang et al., 2012a; Lande et al., 2019). Higher rates of non-tuberculous mycobacteria contamination are found in buildings with recirculating hot water systems (e.g., hospitals, condominiums, apartment buildings) compared to private residences (Falkinham, 2015b; Li et al., 2017).
A wide diversity of non-tuberculous mycobacteria can be isolated from drinking water supplies. However, the clinical significance of individual species varies and can differ in different parts of the world. Establishing epidemiological links to environmental reservoirs is difficult, and few studies have been successful in matching patient isolates with strains found in drinking water (Halstrom et al., 2015; Li et al., 2017). Lande et al. (2019) used advanced molecular techniques to match strains of M. avium from respiratory and household plumbing samples from patients with M. avium pulmonary disease, providing strong evidence that household plumbing can be a source of infection for susceptible individuals. NTM are ubiquitous in the environment and the risks of human infection are determined by the interaction of several environmental microbial and host factors (Halstrom et al., 2015). Current science indicates that the presence of non-tuberculous mycobacteria in domestic water systems does not in itself prove that residents are at particular risk for disease (Halstrom et al., 2015; Adjemian et al., 2018).
Outbreaks of NTM-associated disease in North America have been largely associated with recreational water and health care exposures (Hlavsa et al., 2018; Sood and Parrish, 2017). No drinking water outbreaks of disease have been reported in Canada or the US (CDC, 2013c 2015; 2017d).
B.2.1.3.4 Analytical methods
Methods for isolation and culture-based detection of Mycobacterium spp. in drinking water have been described; however, there is presently no standardized approach to testing (APHA et al., 2017). Isolates can be identified to the genus and species level using PCR or DNA-sequencing methods (Stinear et al., 2004; Falkinham, 2015c). Identification to the subspecies or strain level requires more advanced molecular techniques (Stinear et al., 2004; Falkinham, 2015c). The literature can be consulted for details on specific methods (Stinear et al., 2004; Wang et al., 2017).
B.2.1.3.5 Treatment considerations
When properly designed and operated, physical removal technologies—chemically—assisted, slow sand, diatomaceous earth and membrane filtration or an alternative proven technology—are capable of reducing the number of mycobacteria in drinking water (LeChevallier et al., 2001; Le Dantec et al., 2002; LeChevallier, 2004). However, specific features of mycobacteria such as hydrophobicity and surface charge affect treatment processes in different ways (LeChevallier, 2004; Wong and Shin, 2014). Due to their highly hydrophobic cell wall, mycobacteria have an increased tendency to attach to particles (LeChevallier, 2004). Correlations between turbidity removal and removal of mycobacteria have been demonstrated (Falkinham et al., 2001; Wong and Shin, 2014). The use of GAC filters can provide conditions (accumulated nutrients, neutralized disinfectant residuals) which support the growth of mycobacteria (Le Dantec et al., 2002; LeChevallier, 2004).
Mycobacteria are very resistant to commonly used chemical disinfectants. In order to inactivate non-tuberculous mycobacteria species(including Mycobacterium avium) (Taylor et al., 2000; Jacangelo et al., 2002; Le Dantec et al., 2002):
- the CT values for chlorine may be greater than those needed for Giardia, but are less than for Cryptosporidium; and
- the CT values for chloramines (monochloramine) chlorine dioxide and ozone are less than those required for both Giardia and Cryptosporidium.
Individual species and strains show significant variations in disinfectant sensitivity (Taylor et al., 2000; WHO, 2004); and reported CT values differ among investigators (WHO, 2004). Jacangelo et al. (2002) observed that the inactivation of Mycobacterium fortuitum required CT values for ozone equal to or greater than those required for Cryptosporidium.
The UV dose required to inactivate Mycobacterium avium complex organisms can be greater than those needed for Giardia and Cryptosporidium and comparable to that required for some enteric viruses. It has been reported that the inactivation of some strains of M. avium and M. fortuitum require UV doses comparable to those required for adenovirus (Gerba et. al., 2003; Schiavano et al., 2018).
Even with effective treatment and disinfection in place, non-tuberculous mycobacteria have a strong tolerance for disinfection and can pass into distribution and plumbing systems in low numbers. Full-scale studies suggest that free chlorine is more successful than monochloramine as a secondary disinfectant for controlling mycobacteria colonization in building plumbing systems (Pryor et al., 2004; Wang et al., 2012a; Rhoads et al., 2017). Monochloramine may provide better control in biofilms on certain pipe materials such as corroded iron surfaces (Norton et al., 2004).
The general operational and maintenance practises for managing microbial survival and growth in drinking water distribution and plumbing systems outlined in Part A are important for the control of Mycobacterium spp. (Falkinham et al., 2015a, 2015b). Guidance documents recommend the use of water management/water safety plans for the management of mycobacteria in building water systems (WHO, 2007). Resources are available to provide information for building managers (WHO, 2007; 2011). In health care facilities, control of mycobacteria will be achieved in part through management plans designed to reduce risk from Legionella (Ford et al., 2004). However, it should be recognized that mycobacteria and Legionella have differing sensitivity to drinking water disinfectants (Jacangelo et al., 2002, Pryor et al., 2004; Moore et al., 2006b).
Supplemental strategies described for control in hospital and health care facilities have included superheat and flush disinfection with hot water to temperatures above 50-70°C, the use of various disinfection strategies (free chlorine hyperchlorination, chlorine dioxide) and the use of POU membrane filtration technologies (LeChevallier, 2004; Sebakova et al., 2008; Williams et al., 2011; Hsu et al., 2016). Additional actions recommended as part of a water safety plan include regular cleaning and maintenance of water features and water using devices that generate aerosols (faucets, showerheads, hot tubs/spas, cooling towers) (Ford et al., 2004).
For homeowners, maintaining hot water tank temperature consistent with NPC specifications for the control of Legionella (i.e., minimum of 60°C) (NRCC 2015b) has been recommended as part of a general strategy for minimizing the risks of exposure to opportunistic premise plumbing pathogens in the home (WHO, 2011; Falkinham et al., 2015a, 2015b; NAS, 2019).
B.2.1.3.6 International considerations
No drinking water guideline for Mycobacterium spp. has been established by the WHO, the EU, the US EPA or the Australian National Health and Medical Research Council.
B.2.1.4 Pseudomonas spp.
B.2.1.4.1 Description
The bacterial genus Pseudomonas (Class: Gammaproteobacteria) includes over 30 species (Chakravarty and Anderson, 2015). Pseudomonas aeruginosa is the most clinically relevant species and is an opportunistic pathogen capable of causing of a variety of infections in humans (Chakravarty and Anderson, 2015; Daniels and Gregory, 2015). Other species (P. fluorescens, P. putida, P. stutzeri) have been infrequently reported in human infections (Chakravarty and Anderson, 2015).
Pseudomonas spp. are Gram-negative, strictly aerobic, motile, straight or slightly curved rod-shaped bacteria that grow over the range of 4-42°C (optimum: 28-37°C) (Moore et al., 2006a; Chakravarty and Anderson, 2015). They are metabolically versatile, capable of utilizing numerous substances as nutrient sources and surviving under low nutrient conditions (Chakravarty and Anderson, 2015; Falkinham et al., 2015a). Pseudomonas spp. are also significant due to their capacity to join or form biofilms in water environments (Bédard et al., 2016b).
B.2.1.4.2 Health effects
P. aeruginosa causes disease following colonization in patients where some predisposing factor (e.g., reduced immunity, underlying disease, traumatic injury or medical procedure) has made them more vulnerable to infection (Chakravarty and Anderson, 2015). The respiratory tract is the most common site of human infections. Symptoms typically include fever, chills, cough and laboured breathing; the onset can be sudden and severe (Daniels and Gregory, 2015). Cystic fibrosis patients are particularly prone to respiratory infection with P. aeruginosa, and the organism is a leading cause of morbidity and mortality in these individuals (Chakravarty and Anderson, 2015). P. aeruginosa is an important cause of infections involving the skin, eyes, ears and urinary tract (Chakravarty and Anderson, 2015; Daniels and Gregory, 2015). Bloodstream infections resulting from lung, skin or urinary tract infections can result in spread of the organism to other parts of the body. High mortality rates have been observed with P. aeruginosa septicaemia in high risk individuals (Chakravarty and Anderson, 2015; Daniels and Gregory, 2015). Individuals at higher risk for infections include those that have lowered immune status (e.g., patients with low neutrophil counts or HIV/AIDS); have underlying diseases (cystic fibrosis, diabetes, chronic pulmonary disease); are undergoing procedures with invasive devices (vascular and urinary catheters, ventilator, endotracheal tubes); or have breaches in host defenses as a result of burns or penetrating trauma (surgical incisions, wounds) (Daniels and Gregory, 2015). The doses of P. aeruginosa required to cause infection via the various transmission pathways are not well understood (Roser et al., 2014). P. aeruginosa infections in healthy individuals are rare.
Infections caused by Pseudomonas are not reportable illnesses in North America or in most countries worldwide. The vast majority of cases or outbreaks of P. aeruginosa-related illness have been linked to hospitals or recreational water use at treated water facilities (e.g., hot tubs/spas, swimming pools) (CDC, 2013b; Falkinham et al., 2015a; Hlavsa et al., 2018).
Treatment of P. aeruginosa infections is difficult as a result of increasing antibiotic resistance (Falkinham et al., 2015a). Some strains have been found to be resistant to nearly all or all antibiotics including later generation beta-lactam antibiotics, fluoroquinolones and carbapenems (CDC, 2013a). Multidrug-resistant P. aeruginosa has been categorized as a serious threat by the CDC, and a priority for risk management attention by PHAC (CDC, 2013a; Garner et al., 2015). Carbapenem-resistant P. aeruginosa in particular have been identified by the WHO as a critical priority for developing new antibiotic strategies (WHO, 2017).
B.2.1.4.3 Sources and exposure
Pseudomonas spp. are ubiquitous bacteria, found in a wide variety of habitats including soil, aquatic environments (fresh and marine surface waters, groundwater, potable water supplies) and vegetation (Falkinham et al., 2015a; Degnan, 2006). Human and animal feces are not a significant source; however, the organisms can be found in large numbers in sewage and wastewater (Degnan, 2006). Habitats for P. aeruginosa are engineered water systems and downstream facilities or devices that provide conditions (nutrients, temperature, protection from disinfectants) allowing for amplification of the bacteria (Bédard et al., 2016b). Water supply systems in hospitals and other health-care settings are important sources of the bacteria (Bédard et al., 2016b). Confirmed reservoirs in these settings include potable water faucets, sink and shower drains, humidifiers, water baths, hydrotherapy pools and bathing basins (Falkinham, 2015a; Bédard et al., 2016b). In community settings, hot tubs/spas and swimming pools can also be important sources of infections (Bédard et al., 2016b).
P. aeruginosa can be transmitted through person-to-person contact or through direct contact with contaminated objects or water (Falkinham, 2015a; Bédard et al., 2016b). Consumption of drinking water is not a recognized route of infection (Bédard et al., 2016b).
Studies involving both culture-based and molecular detection methods have found that P. aeruginosa is sporadically detected in water and sediment samples from drinking water distribution systems (Wingender and Flemming, 2004; Van der Wielen and van der Kooij, 2013; Lu et al., 2015a, 2015b) and can be more frequently detected in samples collected from premise plumbing systems (Reuter et al., 2002; Rogues et al., 2007; Lavenir et al., 2008; Van der Wielen and van der Kooij, 2013; Charron et al., 2015). The amplification of P. aeruginosa populations within biofilms in premise plumbing or POU devices is proposed as the reason for the increased detection in these samples (Bédard et al., 2016b). Ingestion by free-living amoebae such as Acanthamoeba spp. (see B.3.2.1) can also contribute to the survival and growth of P. aeruginosa in biofilms in drinking water distribution and premise-plumbing systems (Bédard et al., 2016b). No known drinking water outbreaks associated with P. aeruginosa have been recorded (CDC, 2004, 2006, 2008, 2011, 2013c, 2015, 2017d).
B.2.1.4.4 Analytical methods
Standard methods for the detection of Pseudomonas spp. in drinking water are available (APHA et al., 2017; ISO, 2019). The literature can also be consulted for details on specific methods (Wang et al., 2017). Pseudomonas spp. are heterotrophic bacteria and are detected by HPC tests; however, no direct correlation between HPC counts and P. aeruginosa concentrations exists.
B.2.1.4.5 Treatment considerations
When properly designed and operated, physical removal technologies—chemically—assisted, slow sand, diatomaceous earth and membrane filtration or an alternative proven technology—and disinfection methods—chlorine, chloramines/monochloramine, chlorine dioxide, ozone and UV—commonly used in drinking water treatment are effective at removing or inactivating P. aeruginosa (LeChevallier and Au., 2004; Clauβ, 2006; Xue et al., 2013; Behnke and Camper, 2012, Zuma et al., 2009; Garvey et al., 2014; Zhang et al., 2015). For the inactivation of P. aeruginosa, the CT requirements for chlorine and monochloramine (chloramine) are less than those required for the inactivation of many enteric viruses and the UV dose requirements are less than those required for the enteric protozoa Giardia and Cryptosporidium (Clauβ, 2006; Xue et al., 2013; Health Canada, 2019c).
The general operational and maintenance practises for managing microbial survival and growth in drinking water distribution and plumbing systems outlined in Part A are important for the control of Pseudomonas spp. (Falkinham et al., 2015a; Bédard et al., 2016b). Resistance to chlorination will vary depending on the strain and the protective effects provided by biofilms (Bédard et al., 2016; Mao et al., 2018). Laboratory-scale and pilot-scale studies suggest that maintaining free chlorine residuals above 0.3 mg/L is useful for control of Pseudomonas spp. in bulk water (Wang et al., 2012b; Mao et al., 2018). Mao et al., (2018) highlighted that long-term, continuous exposure to an effective chlorine residual is important in order to prevent regrowth of Pseudomonas and the selection of resistant strains. Further research on the effects of chlorine-based disinfectants on P. aeruginosa in premise plumbing water and biofilms is needed (Bédard et al., 2016).
Water management/water safety plans are recommended for the management of Pseudomonas aeruginosa in building water systems (WHO, 2011). Supplemental strategies described as control measures in hospital and health care facilities have included superheat and flush disinfection with hot water to temperatures above 50-70°C, and the use of POU membrane filtration technologies (Falkinham et al., 2015a; Bédard et al., 2016b).
For homeowners, no specific actions have been identified as necessary to reduce their risk of P. aeruginosa infections. However, homeowners can minimize their risk of exposure to opportunistic waterborne pathogens by maintaining the temperature of their hot water tank at a minimum of 60°C (WHO, 2011; Falkinham et al., 2015a, 2015b).
B.2.1.5.6 International considerations
No drinking water guideline for P. aeruginosa has been established by the WHO, the EU, the US EPA or the Australian National Health and Medical Research Council. Guidelines or standards developed for Pseudomonas spp. in Canada and the US and other countries worldwide relate to control of the organism in building water systems outside of municipal distribution system networks.
B.2.2 Protozoa
B.2.2.1 Acanthamoeba spp.
B.2.2.1.1 Description
Acanthamoeba spp. are free-living amoebae commonly found in soil and aquatic environments. They are opportunistic pathogens that can cause rare but severe human diseases affecting the eye, skin, lungs, brain and central nervous system (Visvesvara et al., 2007; Chalmers, 2014a). Species of Acanthamoeba were originally classified based on differences in life stage (e.g., cyst — see below) morphology; however, genotyping is currently used to classify members of the genus (Visvesvara et al., 2007; Juárez et al., 2018). Approximately 20 different genotypes of Acanthamoeba have been identified based on gene sequence differences (Juárez et al., 2018). Acanthamoeba genotype T4 is the predominant type encountered in cases of illness and in the environment; however, other genotypes have also been associated with disease (Chalmers, 2014a; Juárez et al., 2018). Acanthamoeba spp. are also significant due to their ability to act as hosts for certain pathogenic microorganisms within drinking water systems.
Acanthamoeba spp. have low nutrient requirements and grow over the range of 12-45°C (optimum 30°C) (Chalmers, 2014a). Their lifecycle consists of two stages: a feeding trophozoite (25-40 µm) and resistant cyst (10-30 µm) that can withstand temperatures of -20°C-56°C and provide resistance to desiccation and disinfection (Chalmers, 2014a; Juárez et al., 2018).
B.2.2.1.2 Health effects
Acanthamoeba infections are rare in the general population (Visvesvara et al., 2007; Juárez et al., 2018). Acanthamoeba keratitis (AK) is the most common form of illness (Juárez et al., 2018). Early symptoms of AK include blurred vision, intense pain and photosensitivity, usually in one eye (Chalmers, 2014a; Juárez et al., 2018). In advanced and severe cases, symptoms include ulceration, swelling, glaucoma, cataract and blindness (Juárez et al., 2018). AK has a slow onset, taking days to several weeks to develop after infection, and the disease has a slow but severe progression (Köhsler et al., 2016, Juárez et al., 2018). In developed countries, AK primarily occurs among individuals who wear contact lenses (Chalmers, 2014a). Persons at increased risk of exposure include those who use unsterile tap water to store, wash or disinfect contact lenses; and persons who swim, use hot tubs or showers while wearing contact lenses (Chalmers, 2014a; Juárez et al., 2018). In the minority of AK cases that are not associated with contact lenses, the infections are generally associated with ocular trauma or environmental contamination (Chalmers, 2014a).
Other expressions of Acanthamoeba-associated disease are disseminated infections originating in the skin or lungs that can spread to areas such as the kidneys and adrenal glands; and granulomatous amoebic encephalitis (GAE), a fatal disease which occurs when infection spreads to the brain and central nervous system (Visvesvara et al., 2007; Chalmers, 2014a). These are very rare forms of illness and primarily affect individuals who have weakened or suppressed immune status or underlying disease (e.g., persons with HIV/AIDS, cancer, diabetes, liver disease or who are undergoing chemotherapy or organ transplantation) (Visvesvara et al., 2007; Chalmers, 2014a; Guimaraes et al., 2016). The numbers of Acanthamoeba spp. necessary to cause infections are not known.
Despite the widespread occurrence of the organism in environmental waters, the number of cases of illness caused by Acanthamoeba spp. is low. The estimated incidence of AK in developed countries is one to 33 cases per million contact lens wearers (CDC, 2017b). Treatment of AK is difficult, as the cysts are resistant to most antimicrobials at concentrations tolerated by the human eye (Juárez et al., 2018). Prolonged treatment with a combination of drugs is needed (Visvesvara et al., 2007).
B.2.2.1.3 Sources and exposure
Acanthamoeba spp. are ubiquitous in soil and water worldwide; and are one of the most common free-living amoebae occurring in the environment (Visvesvara et al., 2007; Juárez et al., 2018). The amoebae have been isolated from an abundance of natural and man-made environments including soil, mud, fresh and brackish waters, swimming pools, hot tubs/spas, cooling towers, humidifiers, heating, ventilation and air conditioning equipment, drinking water and airborne dust (Visvesvara et al., 2007; Chalmers, 2014a).
The relative importance of water as a pathway for infection is unclear. The ubiquitous presence of Acanthamoeba in the environment makes it difficult to determine sources of infection. Drinking and inhalation of contaminated water are not considered routes of infection (Chalmers, 2014a). No outbreaks of AK as a result of exposure to drinking water have been reported in North America (Kilvington et al., 2004; Craun et al., 2010; Yoder et al., 2012b). Cases of AK have been associated with the use of nonsterile tap water in the preparation of contact-lens solutions (Visvesvara et al., 2007). Disseminated infections and GAE caused by Acanthamoeba spp. are not thought to be waterborne (Chalmers, 2014a).
Acanthamoeba spp. can be commonly detected in drinking water distribution systems in North America and internationally (Magnet et al., 2012; Lu et al., 2015a, 2015b; Qin et al., 2017). Regrowth of Acanthamoeba spp. can occur in biofilms and loose deposits in drinking water distribution and premise plumbing systems (Thomas and Ashbolt, 2011; Wang et al., 2012a; Qin et al., 2017).In the U.S., Acanthamoeba spp. have been detected by PCR in 40-63% of municipal storage tank sediments (Lu et al., 2015b; Qin et al., 2017).
Acanthamoeba spp. may serve as hosts for pathogenic amoebae-resisting microorganisms, providing conditions (nutrients, protection from environmental stresses) critical for the survival, amplification and transport of these organisms (Thomas and Ashbolt, 2011). It has been proposed the virulence of amoebae-resisting microorganisms is enhanced by infecting protozoa (Visvesvara et al., 2007; Thomas and Ashbolt, 2011; Chalmers, 2014a). Pathogenic bacteria isolated from Acanthamoeba spp. include Legionella pneumophila, Mycobacterium avium, Helicobacter pylori, Escherichia coli serotype O157, Listeria monocytogenes, Pseudomonas spp. and Vibrio cholerae (Visvesvara et al., 2007; Juárez et al., 2018). Acanthamoeba spp. are also able to harbour protozoa, fungi and viruses (Köhsler et al., 2016; Juárez et al., 2018). More research is needed to determine the implications of the interactions between free-living amoeba species and pathogenic amoeba-resisting microorganisms in drinking water (Thomas and Ashbolt, 2011).
B.2.2.1.4 Analytical methods
No standardized methods have been established for the detection and identification of Acanthamoeba spp. in drinking water. Procedures for the isolation of Acanthamoeba in water samples involve concentration by membrane filtration or centrifugation; plaque screening and identification using molecular methods (Chalmers, 2014a). The literature can be consulted for details on specific methods (Wang et al., 2017).
B.2.2.1.5 Treatment considerations
Acanthamoeba cysts are larger than Giardia cysts and Cryptosporidium oocysts (Chalmers, 2014a; Health Canada, 2019d), thus physical removal mechanisms used during drinking water treatment are expected to remove these cysts. The cysts are very resistant to commonly used chemical disinfectants and UV (Loret et al., 2008; Hijnen et al., 2011). For the inactivation of cysts of Acanthamoeba spp., the CT value for free chlorine is greater than required for Giardia, but less than that reported for Cryptosporidium, and the CT values for chlorine dioxide and ozone are greater than those reported for both organisms (Loret et al., 2008). The UV dose requirements for inactivation of cysts of Acanthamoeba spp. are similar to those required for adenovirus (Hijnen et al., 2011; Health Canada, 2019c).
The general operational and maintenance practises important for the control of waterborne pathogens, including Acanthamoeba spp., in drinking water distribution and plumbing systems are outlined in Part A (Chalmers, 2014a; Ashbolt, 2015). As part of a general facility water management plan, building system managers may implement regular cleaning and maintenance of water features and water-using devices (e.g., faucets, showerheads, hot tubs/spas, cooling towers). Control of Acanthamoeba spp. may be particularly important in some specialised uses of water such as emergency eye-wash stations (Chalmers, 2014a).
No specific homeowner actions are necessary. However, homeowners can minimize risks of exposure to opportunistic waterborne microorganisms in the home by maintaining the temperature of their hot water tank at a minimum of 60°C (WHO, 2011). Individuals in the home who wear contact lenses should also follow guidance from their eye care providers on proper lens handling, cleaning and wear (CDC, 2017b).
B.2.2.1.6 International considerations
No drinking water guideline for Acanthamoeba spp. has been established by the WHO, the EU, the US EPA or the Australian National Health and Medical Research Council.
B.2.2.2 Naegleria fowleri
B.2.2.2.1 Description
Naegleria fowleri is a pathogenic free‐living amoeba that causes primary amebic meningoencephalitis (PAM) in humans, a rare but almost always fatal disease. Over 40 species of Naegleria spp. have been identified. However, to date, only N. fowleri has proven to be pathogenic to humans (Marciano‐Cabral & Cabral et al. 2007; Yoder et al. 2010). Eight known N. fowleri genotypes have been found worldwide, and all are suspected to be pathogenic to humans (Bartrand et al., 2014; Chalmers, 2014b). N. fowleri are thermophilic, grow well at 25-40°C (optimum: 37°C) and can tolerate temperatures exceeding 50-60°C (Hallenbeck & Brenniman, 1989; Visvesvara et al., 2007; Zaongo et al., 2018). There are three distinct phases in the N. fowleri life cycle: a feeding and infectious trophozoite stage, an intermediate flagellate stage and a resistant cyst stage (Bartrand et al., 2014, Chalmers, 2014b). Cysts are the most resistant form of the organism and can survive under adverse environmental conditions such as cold temperatures and low food/nutrient environments (Bartrand et al., 2014; Chalmers, 2014b).
B.2.2.2.2 Health effects
Symptoms of PAM are clinically similar to bacterial or viral meningitis, beginning with headache, fever, nausea and vomiting and then moving to stiff neck, altered mental status, occasional hallucinations, seizures and coma (Visvesvara et al., 2007; Chalmers, 2014b). Onset of symptoms occurs within one to seven days of exposure and the disease progresses rapidly, with death generally occurring within five days (Visvesvara et al., 2007; Chalmers, 2014b). PAM has an extremely high fatality rate (greater than 97%) (De Jonckheere, 2011; Capewell et al., 2015). Among documented cases in the US, there have been only five known survivors (Capewell et al., 2015). Infection occurs when water containing N. fowleri enters the nasal passages. The amoebae invade the mucous membranes and travel along the olfactory nerve to the brain where they consume nerve and blood cells, causing inflammation and cell damage leading to death (Chalmers, 2014b; Siddiqui et al., 2016). The dose of N. fowleri necessary to cause infection is not well understood (Bartrand et al., 2014).
Despite the widespread occurrence of N. fowleri in environmental waters, PAM occurs infrequently. As of 2011, only 235 cases of PAM had been reported globally, with the majority occurring in the US (De Jonckheere, 2011). Cases of PAM largely occur during the hot summer months. Illness occurs more frequently in children and young adults, as these are age groups that are more energetic during aquatic activities (Visvesvara et al., 2007).
PAM is difficult to detect, with most cases progressing so rapidly that diagnosis is made following death (Chalmers, 2014b). Several drugs have demonstrated effectiveness against N. fowleri in the laboratory, but effective treatment remains unclear (Capewell et al., 2015; Siddiqui et al., 2016). Survival of infection has been demonstrated in two cases using a combination of antimicrobial agents and aggressive management of brain-swelling (CDC, 2019) Continued testing of therapies is necessary (Capewell et al., 2015; Siddiqui et al., 2016). No human vaccines for PAM exists (Siddiqui et al., 2016).
B.2.2.2.3 Sources and exposure
N. fowleri are naturally found in warm freshwater environments and soils worldwide (Chalmers, 2014b). The organisms have been isolated from a wide variety of natural and human-made warm water sources, including lakes, rivers, ponds, hot springs, geothermal groundwater, water receiving thermal discharges from power plants or industrial facilities and poorly maintained swimming pools (Chalmers, 2014b, Bartrand et al., 2014). In the US, N. fowleri has been most commonly detected in natural waters in southern-tier states. Detection in northern waters is less common; however, the pathogen has been encountered in lake water in states as far north as Minnesota (Yoder et al., 2010; 2012a). N. fowleri has been isolated from drinking water and premise plumbing supplies in Australia and in Arizona and Louisiana in the US (Bartrand et al., 2014). Increasing ambient temperatures as a result of climate change may increase the geographical range of N. fowleri (Bartrand et al., 2014; Chalmers, 2014b).
Transmission of N. fowleri occurs through the intranasal route. Most infections have been associated with recreational activities (e.g., swimming, diving) in warm, fresh recreational waters or contaminated pools. In very rare cases, infections have been linked to contaminated drinking water supplies, through activities such as nasal cleansing, bathing or recreational water uses (Yoder et al., 2010, 2012a, Bartrand et al., 2014). Drinking contaminated water is not a route of infection.
There is very little data on the occurrence of N. fowleri in North American drinking water distribution and premise plumbing systems (Bartrand et al., 2014). N. fowleri is widespread in environmental reservoirs but at low numbers, unless the environment provides conditions for amplification such as optimal growth temperatures, availability of nutrients and the absence of a disinfectant residual (Bartrand et al., 2014). Drinking water systems vulnerable to N. fowleri contamination are those where the temperature of the water supply continually exceeds 25°C and where adequate disinfectant residuals are not maintained (Bartrand et al., 2014). Long-term survival of the cysts at temperatures below optimal growth temperatures is possible, and N. fowleri has the ability to survive over winter in lakes in subtropical and temperate regions (Bartrand et al., 2014). Laboratory-scale and full-scale studies have demonstrated that N. fowleri can persist and grow in distribution system and premise plumbing biofilms (Bartrand et al., 2014).
N. fowleri can act as a reservoir for amoeba-resisting microorganisms (Thomas and Ashbolt, 2011; Bartrand et al., 2014). Naegleria species are regarded as a host for L. pneumophila and can provide conditions which permit the replication, protection and distribution of this pathogen in the environment (Thomas and Ashbolt, 2011; Bartrand et al., 2014; Siddiqui et al., 2016). Significant research is needed in order to understand the interactions between free-living amoeba species and pathogenic amoeba-resisting microorganisms in order to quantify any risk to human health (Thomas and Ashbolt, 2011). Only six of 132 US cases of N. fowleri reported between 1962 and 2013 have resulted from exposures related to drinking water (Yoder et al. 2010; CDC, 2017c). Three of the cases were linked to nasal cleansing (Louisiana (2): 2011, US Virgin Islands (1), 2012), two were related to bathing (Arizona, 2002), and one was related to tap water exposure on an outdoor play slide (Louisiana, 2013) (Yoder et al. 2010, 2012a; Bartrand et al., 2014). The two Louisiana cases represent the first time disinfected tap water has been implicated in N. fowleri infection in the US (Yoder et al., 2012a). To date, there have been no known cases of PAM documented in Canada.
B.2.2.2.4 Analytical methods
The detection and identification of N. fowleri in drinking water requires highly specialized laboratories (Bartrand et al., 2014, Chalmers, 2014b). No standardized methods have been established. Procedures for the isolation of N. fowleri involve concentration (membrane filtration or centrifugation) or separation; plaque screening and identification using immunofluorescence or molecular assays (Bartrand et al., 2014; Chalmers, 2014b, Wang et al., 2017). The literature can be consulted for details on specific methods (Bartrand et al., 2017; Wang et al., 2017).
B.2.2.2.5 Treatment considerations
When properly designed and operated, physical removal technologies—chemically—assisted, slow sand, diatomaceous earth and membrane filtration or an alternative proven technology—commonly used in drinking water treatment are expected to remove N. fowleri. The cysts are very resistant to commonly used disinfectants—chlorine, chloramines/monochloramine and UV. N. fowleri cysts arevery similar in size to Giardia cysts (Chalmers, 2014b, Health Canada, 2019d). For the inactivation of cysts of Naegleria spp. the CT requirements for free chlorine and chloramine (monochloramine) are less than those needed for Giardia and Cryptosporidium, whereas the dose requirements for UV are greater than those needed for these protozoa but less than those needed for adenovirus (Sarkar and Gerba, 2012; Goudot et al., 2014; Health Canada, 2019c, 2019d).
The general operational and maintenance practises important for the control of waterborne pathogens, including Naegleria spp., in drinking water distribution and plumbing systems are outlined in Part A (Bartrand et al., 2014). Maintaining minimum free chlorine or chloramine residuals of 0.5 mg/L throughout the distribution system is recommended for the control of N. fowleri in vulnerable drinking water systems (Robinson and Christy, 1984; Trolio et al., 2008; Bartrand et al., 2014; NHMRC, NRMMC, 2011).
N. fowleri is not an immediate risk for drinking water systems in Canada. However, homeowners should ensure that they conduct nasal rinses using water that has been boiled and cooled, or distilled water.
B.2.2.2.6 International considerations
No drinking water guideline for N. fowleri has been established by the WHO, the EU, the US EPA or the Australian National Health and Medical Research Council.
Part C. References
- Adams, D.A., Thomas, K.R., Jajosky, R.A., Foster, L., Sharp, P., Onweh, D.H., Schley, A.W. and Anderson, W.J. (2016). Summary of notifiable infectious diseases and conditions—United States, 2014. MMWR Morb. Mortal. Wkly. Rep., 63(54): 1-152.
- Adams, D.A., Thomas, K.R., Jajosky, R.A., Foster, L., Baroi, G., Sharp, P., Onweh, D.H., Schley, A.W. and Anderson, W.J. (2017). Summary of notifiable infectious diseases and conditions—United States, 2015. MMWR Morb. Mortal. Wkly. Rep., 64(53): 1-143.
- Adjemian, J., Daniel-Wayman, S., Ricotta, E. and Prevots, D.R. (2018). Epidemiology of nontuberculous mycobacteriosis. Seminars in Respiratory and Critical Care Medicine, 39(3): 325-335.
- Alary, M. and Joly, J.R. (1991). Risk factors for contamination of domestic hot water systems by Legionellae. Appl. Environ. Microbiol., 57(8): 2360-2367.
- Allegra, S., Berger, F., Berthelot, P., Grattard, F., Pozzetto, B. and Riffard, S. (2008). Use of flow cytometry to monitor Legionella viability. Appl. Environ. Microbiol., 74 (24):7813-7816.
- APHA, AWWA and WEF. (2017). Standard methods for the examination of water and wastewater. 23rd edition. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC.
- Ashbolt, N.J. (2015). Environmental (saprozoic) pathogens of engineered water systems: Understanding their ecology for risk assessment and management. Pathogens, 4(2): 390-405.
- ASHRAE. (2018). ANSI/ASHRAE Standard 188-2018, Legionellosis: Risk Management for Building Water Systems. American Society of Heating, Refridgerating and Air Conditioning Engineers. Atlanta, GA. www.ashrae.org.
- Backert, S., Tegtmeyer, N., Cróinín, T.Ó, Boehm, M. and Heimesaat, M.M. (2016). Human campylobacteriosis. In Campylobacter: Features, detection, and prevention of foodborne disease. Klein, G (ed.). Academic Press. Cambridge, USA. pp. 1-25.
- Baker, K.H. and Hegarty, J.P. (2001). Presence of Helicobacter pylori in drinking water is associated with clinical infection (2001) Scand. J. Infect. Dis., 33 (10):744-746.
- Baker, K.H., Hegarty, J.P., Redmond, B., Reed, N.A. and Herson, D.S. (2002). Effect of oxidizing disinfectants (chlorine, monochloramine, and ozone) on Helicobacter pylori. Appl. Environ. Microbiol., 68(2): 981-984.
- Bartram, J., Bentham, R., Briand, E., Callan, P., Crespi, S., Lee, J.V. and Surman-Lee, S. (2007). Chapter 3 approaches to risk management. In Legionella and the prevention of legionellosis. Bartram, J. (ed.). World Health Organization, Geneva, Switzerland. pp. 39-56.
- Bartrand, T.A., Causey, J.J. and Clancy, J.L. (2014). Naegleria fowleri: An emerging drinking water pathogen. J. Am. Water Works Assoc., 106(10): E418-E432.
- Bates, M.N., Maas, E., Martin, T., Harte, D., Grubner, M. and Margolin, T. (2000). Investigation of the prevalence of Legionella species in domestic hot water systems. New Zealand Med. J., 113(1111): 218-220.
- Beauté, J., Sandin, S., Uldum, S.A., Rota, M.C., Brandsema, P., Giesecke, J. and Sparén, P. (2016). Short-term effects of atmospheric pressure, temperature, and rainfall on notification rate of community-acquired Legionnaires’ disease in four European countries. Epidemiol. Infect., 144(16): 3483-3493.
- Bédard, E., Boppe, I., Kouamé, S., Martin, P., Pinsonneault, L., Valiquette, L., Racine, J. and Prévost, M. (2016a). Combination of heat shock and enhanced thermal regime to control the growth of a persistent Legionella pneumophila strain. Pathogens, 5(2): 35.
- Bédard, E., Prévost, M. and Déziel, E. (2016b). Pseudomonas aeruginosa in premise plumbing of large buildings. MicrobiologyOpen, 5(6): 937-956.
- Behnke, S. and Camper, A.K. (2012). Chlorine dioxide disinfection of single and dual species biofilms, Benin, A.L., Benson, R.F. and Besser, R.E. (2002). Trends in Legionnaires’ disease, 1980–1998: Declining mortality and new patterns of diagnosis. Clin. Infect. Dis., 35(9): 1039-1046.
- Bentham, R., Surman-Lee, S., Lee, J.V., Briand, E. and Van de Kooj, D. (2007). Chapter 4: Potable water and in-building distribution systems. In Legionella and the prevention of legionellosis. Bartram, J. et al. (eds.). World Health Organization, Geneva, Switzerland. pp. 57-68.
- Bernstein, C.N., McKeown, I., Embil, J.M., Blanchard, J.F., Dawood, M., Kabani, A., Kliewer, E., Smart, G., Coghlan, G., MacDonald, S., Cook, C., Orr, P. (1999). Seroprevalence of Helicobacter pylori, incidence of gastric cancer, and peptic ulcer-associated hospitalizations in a Canadian Indian population. Digest. Dis. Sci., 44 (4): 668-674.
- Bhowmick, U.D. and Bhattacharjee, S. (2018). Bacteriological, clinical and virulence aspects of Aeromonas-associated diseases in humans. Pol. J. Microbiol., 67(2): 137-149.
- Boppe, I., Bédard, E., Taillandier, C., Lecellier, D., Nantel-Gauvin, M. A., Villion, M., Laferrière, C. and Prevost, M. (2016). Investigative approach to improve hot water system hydraulics through temperature monitoring to reduce building environmental quality hazard associated to Legionella. Build. Environ., 108, 230-239.
- Borchardt, M.A., Stemper, M.E. and Standridge, J.H. (2003). Aeromonas isolates from human diarrheic stool and groundwater compared by pulsed-field gel electrophoresis. Emerg. Infect. Dis., 9(2): 224-228.
- Bragança, S.M., Azevedo, N.F., Simöes, L.C., Keevil, C.W. and Vieira, M.J. (2007). Use of fluorescent in situ hybridisation for the visualisation of Helicobacter pylori in real drinking water biofilms. Water Sci. Technol., 55(8-9):387-393.
- Brennhovd, O., Kapperud, G. and Langeland, G. (1992). Survey of thermotolerant Campylobacter spp. and Yersinia spp. in three surface water sources in Norway. Int. J. Food Microbiol., 15(3-4): 327-338.
- Brown, L.M. (2000). Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol. Rev., 22 (2): 283-297.
- Bryant, J.M. et al. (2016). Population-level genomics identifies the emergence and global spread of a human transmissible multidrug-resistant nontuberculous Mycobacterium. Science, 354(6313): 751-757.
- Bryant, J.M., Grogono, D.M., Greaves, D., Foweraker, J., Roddick, I., Inns, T., Reacher, M., Haworth, C.S., Curran, M.D., Harris, S.R., Peacock, S.J., Parkhill, J. and Floto, R.A. (2013). Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: A retrospective cohort study. Lancet, 381(9877): 1551-1560.
- Burillo, A., Pedro-Botet, M.L. and Bouza, E. (2017). Microbiology and epidemiology of Legionnaires’ disease. Infect. Dis. Clin. North Am., 31(1): 7-27.
- Burnsed, L.J., Hicks, L.A., Smithee, L.M.K., Fields, B.S., Bradley, K.K., Pascoe, N., Richards, S.M., Mallonee, S., Littrell, L., Benson, R.F. and Moore, M.R. (2007). A large, travel-associated outbreak of legionellosis among hotel guests: Utility of the urine antigen assay in confirming Pontiac fever. Clin Infect Dis, 44(2): 222-228.
- Buse, H.Y., Schoen, M.E. and Ashbolt, N.J. (2012). Legionellae in engineered systems and use of quantitative microbial risk assessment to predict exposure. Water Res., 46 (4): 921-933.
- Butler, A.J., Pintar, K.D.M. and Thomas, M.K. (2016). Estimating the Relative Role of Various Subcategories of Food, Water, and Animal Contact Transmission of 28 Enteric Diseases in Canada. Foodborne Pathog. Dis., 13 (2): 57-64.
- Cangelosi, G., Clark-Curtiss, J., Behr, M., Bull, T. and Stinear, T. (2004). Biology of waterborne pathogenic mycobacteria. In Pathogenic mycobacteria in water—A guide to public health consequences, monitoring and management. IWA Publishing. World Health Organization. Geneva, Switzerland. pp. 39-54.
- Capewell, L.G., Harris, A.M., Yoder, J.S., Cope, J.R., Eddy, B.A., Roy, S.L., Visvesvara, G.S., Fox, L.M. and Beach, M.J. (2015). Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937-2013. J. Pediatric Infect. Dis. Soc., 4(4): e68-e75.
- Castillo, N.E., Rajasekaran, A. and Ali, S.K. (2016). Legionnaires’ disease: A review. Infect. Dis. Clin. Pract., 24(5): 248-253.
- CDC. (2004). Surveillance for Waterborne-Disease Outbreaks Associated with Recreational Water—United States, 2001–2002 and Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water—United States, 2001–2002. In Surveillance Summaries, October 22, 2004. MMWR 2004:53(no. SS-8).
- CDC. (2006). Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking--United States, 2003-2004. MMWR Surveill Summ, 55(12): 31-65.
- CDC. (2008). Surveillance for Waterborne Disease and Outbreaks Associated with Recreational Water use and Other Aquatic Facility-Associated Health Events—United States, 2005–2006 and Surveillance for Waterborne Disease and Outbreaks Associated with Drinking Water and Water Not Intended for Drinking—United States, 2005–2006. Surveillance Summaries. MMWR 2008;57(no. SS-9).
- CDC. (2011). Surveillance for waterborne disease outbreaks associated with drinking water---United States, 2007--2008. MMWR Surveill. Summ., 60(12): 38-68.
- CDC. (2013a). Antibiotic Resistance Threats in the United States, 2013.
- CDC. (2013b). Pseudomonas aeruginosa in healthcare settings. Centers for Disease Control and Prevention. Atlanta, GA. https://www.cdc.gov/hai/organisms/pseudomonas.html.
- CDC. (2013c). Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water and Other Nonrecreational Water—United States, 2009–2010. Surveillance Summaries. MMWR 2013;62(no. 35).
- CDC. (2015). Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water—United States, 2011–2012. Surveillance Summaries. MMWR 2015;64 (no. 31).
- CDC. (2017a). Developing a water management program to reduce Legionella growth & spread in buildings. A practical guide to implementing industry standards. Version 1.1. Centers for Disease Control and Prevention. Atlanta, GA. Accessed November 2019 from: https://www.cdc.gov/legionella/wmp/toolkit/index.html
- CDC. (2017b). Parasites—Acanthamoeba—Granulomatous Amebic Encephalitis (GAE); Keratitis. Centers for Disease Control and Prevention. Atlanta, GA. Accessed July 2019 from: https://www.cdc.gov/parasites/acanthamoeba/index.html
- CDC. (2017c). Parasites—Naegleria fowleri—Primary Amebic Meningoencephalitis (PAM)—Amebic Encephalitis. Naegleria fowleri in Louisiana Public Water Systems. Centers for Disease Control and Prevention. Atlanta, GA. Accessed July 2019 from:
https://www.cdc.gov/parasites/naegleria/public-water-systems-louisiana.html - CDC. (2017d). Surveillance for waterborne disease outbreaks associated with drinking water -United States, 2013-2014. MMWR., 2017; 66(44): 1216-1221.
- CDC. (2018). Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System, 2017 Annual Tables of Infectious Disease Data. Atlanta, GA. CDC Division of Health Informatics and Surveillance, 2018. Available at
https://wonder.cdc.gov/nndss/nndss_annual_tables_menu.asp - CDC. (2019). Parasites—Naegleria fowleri—Primary Amebic Meningoencephalitis (PAM)—Amebic Encephalitis. General Information. Centers for Disease Control and Prevention. Atlanta, GA. Accessed July 2019 from: https://www.cdc.gov/parasites/naegleria/general.html
- CEAEQ. (2018). Centre d'Expertise en Analyse Environmentale du Québec. Méthodes d'analyses (analyses biologie, microbiologie et toxicologie). Ministère du Développement durable, de l'Environnement et de la Lutte contre les changements climatiques. Accessed November 2019 from:
http://www.ceaeq.gouv.qc.ca/methodes/bio_toxico_micro.htm . - Cervero-Aragó, S., Rodríguez-Martínez, S., Puertas-Bennasar, A. and Araujo, R.M. (2015). Effect of common drinking water disinfectants, chlorine and heat, on free Legionella and amoebae-associated Legionella. PLoS ONE, 10 (8), art. no. e0134726.
- Cervero-Aragó, S., Schrammel, B., Dietersdorfer, E., Sommer, R., Lück, C., Walochnik, J. and Kirschner, A. (2019). Viability and infectivity of viable but nonculturable Legionella pneumophila strains induced at high temperatures. Water Res., 158: 268-279.
- Chakravarty, S. and Anderson, G.G. (2015). The genus Pseudomonas. In Practical handbook of microbiology. Goldman, E. and Green, L.H. (eds.). Third. CRC Press, Taylor & Francis Group, Boca Raton, pp. 321-343.
- Chalmers, R.M. (2014a). Acanthamoeba. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd. Oxford, UK pp. 263-276.
- Chalmers, R.M. (2014b). Naegleria. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd. Oxford, UK pp. 407-416.
- Charron, D., Bédard, E., Lalancette, C., Laferrière, C. and Prévost, M. (2015). Impact of electronic faucets and water quality on the occurrence of Pseudomonas aeruginosa in water: A multi-hospital study. Infect. Control Hosp. Epidemiol., 36(3): 311-319.
- Chauret, C., Volk, C., Creason, R., Jarosh, J., Robinson, J. and Warnes, C. (2001). Detection of Aeromonas hydrophila in a drinking-water distribution system: A field and pilot study. Can. J. Microbiol., 47(8): 782-786.
- Chauret, C., Smith, C. and Baribeau, H. (2008). Inactivation of Nitrosomonas europaea and pathogenic Escherichia coli by chlorine and monochloramine. J. Water Health, 6(3): 315-322.
- Cheyne, B.M., Van Dyke, M.I., Anderson, W.B. and Huck, P.M. (2009). An evaluation of methods for the isolation of Yersinia enterocolitica from surface waters in the grand river watershed. J. Water Health, 7(3): 392-403.
- Cheyne, B.M., Van Dyke, M.I., Anderson, W.B. and Huck, P.M. (2010). The detection of Yersinia enterocolitica in surface water by quantitative PCR amplification of the ail and yadA genes. J. Water Health, 8(3): 487-499.
- Christenson, J.C. (2013). Salmonella infections. Pediatr Rev, 34(9): 375-383.
- City of Hamilton. (2019). Accessed February, 2020 from: https://www.hamilton.ca/operating-business/health-requirements-inspections/cooling-towers
- City of Vancouver (2020). Vancouver, Canada. Accessed February, 2020 from: https://vancouver.ca/home-property-development/operating-permit.aspx#review-operating-permit
- Cristina, M.L., Spagnolo, A.M., Casini, B., Baggiani, A., Del Giudice, P., Brusaferro, S., Poscia, A., Moscato, U., Perdelli, F. and Orlando, P. (2014). The impact of aerators on water contamination by emerging gram-negative opportunists in at-risk hospital departments. Infect. Cont. Hosp. Epi., 35 (2): 122-129.
- Clauß, M. (2006). Higher effectiveness of photoinactivation of bacterial spores, UV resistant vegetative bacteria and mold spores with 222 nm compared to 254 nm wavelength. Acta Hydrochim. Hydrobiol., 34(6): 525-532.
- Collins, S., Stevenson, D., Bennett, A. and Walker, J. (2017). Occurrence of Legionella in UK household showers. Int. J. Hyg. Environ. Health, 220(2): 401-406.
- Cooper, I.R. and Hanlon, G.W. (2010). Resistance of Legionella pneumophila serotype 1 biofilms to chlorine-based disinfection. J. Hosp. Infect., 74(2): 152-159.
- Correia, A.M., Gonçalves, J. and Gomes, J.P. (2016). Probable person-to-person transmission of legionnaires’ disease. New Engl. J. Med., 374(5): 497-498.
- Covert, T.C., Rodgers, M.R., Reyes, A.L. and Stelma Jr., G.N. (1999). Occurrence of nontuberculous mycobacteria in environmental samples. Appl. Environ. Microbiol., 65(6): 2492-2496.
- Craun, G.F., Brunkard, J.M., Yoder, J.S., Roberts, V.A., Carpenter, J., Wade, T., Calderon, R.L., Roberts, J.M., Beach, M.J. and Roy, S.L. (2010). Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clin. Microbiol. Rev., 23(3): 507-528.
- Croxen, M.A., Law, R.J., Scholz, R., Keeney, K.M., Wlodarska, M. and Finlay, B.B. (2013). Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev., 26(4): 822-880.
- Cunha, B.A., Burillo, A. and Bouza, E. (2016). Legionnaires’ disease. Lancet, 387(10016): 376-385.
- Cunha, C.B. and Cunha, B.A. (2017). Legionnaires’ disease since Philadelphia: Lessons learned and continued progress. Infect. Dis. Clin. North Am., 31(1): 1-5.
- Daniels, T.L. and Gregory, D.W. (2015). Pseudomonas, Stenotrophomonas and Burkholderia. In Clinical infectious disease. Schlossberg, D. (ed.). Second edition. Cambridge University Press, Cambridge, pp. 966-974.
- De Groote, M.A. (2004). Disease resulting from contaminated equipment and invasive procedures. In Pathogenic mycobacteria in water—A guide to public health consequences, monitoring and management. Pedley, S. et al., (eds). World Health Organization, Geneva, Switzerland. pp: 131-142.
- De Jonckheere, J.F. (2011). Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect. Genet. Evol., 11(7): 1520-1528.
- Degnan, A.J. (2006). Chapter 16. Pseudomonas. In AWWA manual of water supply practices—M48: Waterborne pathogens. 2nd edition. American Water Works Association, Denver, CO, pp. 131-134.
- Dekker, J.P. and Frank, K.M. (2015). Salmonella, Shigella, and Yersinia. Clin. Lab. Med., 35(2): 225-246.
- Devos, L., Boon, N. and Verstraete, W. (2005). Legionella pneumophila in the environment: The occurrence of a fastidious bacterium in oligotrophic conditions. Rev. Environ. Sci. Bio., 4 (1-2): 61-74.
- Dilger, T., Melzl, H. and Gessner, A. (2016). Legionella contamination in warm water systems in Germany—influence of maximal temperature and temperature during sampling. GWF Wasser Abwasser, 157(3): 262-271.
- Dominguez, A., Alvarez, J., Sabria, M., Carmona, G., Torner, N., Oviedo, M., Cayla, J., Minguell, S., Barrabeig, I., Sala, M., Godoy, P. and Camps, N. (2009). Factors influencing the case-fatality rate of Legionnaires’ disease. Int. J. Tuberc. Lung Dis., 13(3): 407-412.
- Donohue, M.J., O'Connell, K., Vesper, S.J., Mistry, J.H., King, D., Kostich, M. and Pfaller, S. (2014). Widespread molecular detection of Legionella pneumophila serogroup 1 in cold water taps across the United States. Environ. Sci. Technol., 48(6): 3145-3152.
- Donohue, M.J., Mistry, J.H., Donohue, J.M., Oconnell, K., King, D., Byran, J., Covert, T. and Pfaller, S. (2015). Increased frequency of nontuberculous mycobacteria detection at potable water taps within the United States. Environ. Sci. Technol., 49(10): 6127-6133.
- Donohue, M.J. and Wymer, L. (2016). Increasing prevalence rate of nontuberculous mycobacteria infections in five states, 2008-2013. Annals of the American Thoracic Society, 13(12): 2143-2150.
- Dupuy, M., Mazoua, S., Berne, F., Bodet, C., Garrec, N., Herbelin, P., Ménard-Szczebara, F., Oberti, S., Rodier, M.-., Soreau, S., Wallet, F. and Héchard, Y. (2011). Efficiency of water disinfectants against Legionella pneumophila and Acanthamoeba. Water Res., 45(3): 1087-1094.
- ECCDC. (2018a). European Centre for Disease Prevention and Control. Shigellosis. In ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2018.
- ECCDC. (2018b). European Centre for Disease Prevention and Control. Yersiniosis. In ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2018.
- ECCDC. (2019). European Centre for Disease Prevention and Control. Salmonellosis. In ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2019.
- Edberg, S.C., Browne, F.A. and Allen, M.J. (2007). Issues for microbial regulation: Aeromonas as a model. Crit. Rev. Microbiol., 33(1): 89-100.
- Edelstein, P.H. and Roy, C.R. (2015). Legionnaires’ disease and Pontiac fever. In Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Bennett, J.E. et al. (eds.). Saunders. Philadelphia, USA. pp. 2633-2644.
- Edelstein, P.H. (2007). Urine antigen tests positive for Pontiac fever: Implications for diagnosis and pathogenesis. Clin Infect Dis, 44(2): 229-231.
- Egorov, A.I., Best, J.M., Frebis, C.P. and Karapondo, M.S. (2011). Occurrence of Aeromonas spp. in a random sample of drinking water distribution systems in the USA. J. Water. Health., 9(4): 785-798.
- Exner, M., Hartemann, P. and Lajoie, L (2007). Chapter 6. Health-care facilities. In Legionella and the prevention of legionellosis. Bartram, J. et al. (eds.). World Health Organization, Geneva, Switzerland. pp. 89-102.
- Fagan-Garcia, K., Geary, J., Chang, H.-J., McAlpine, L., Walker, E., Colquhoun, A., Van Zanten, S.V., Girgis, S., Archie, B., Hanley, B., Corriveau, A., Morse, J., Munday, R., Goodman, K.J. (2019). Burden of disease from Helicobacter pylori infection in western Canadian Arctic communities. BMC Public Health, 19 (1), art. no. 730.
- Falkinham III, J.O., Norton, C.D. and Lechevallier, M.W. (2001). Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol., 67(3): 1225-1231.
- Falkinham, J.O. (2004). Environmental sources of Mycobacterium avium linked to routes of exposure. In Pathogenic mycobacteria in water A guide to public health consequences, monitoring and management. Pedley, S. et al (eds.). IWA Publishing. World Health Organization, Geneva, Switzerland. pp. 26-38.
- Falkinham, J.O.,3rd (2015a). Common features of opportunistic premise plumbing pathogens. Int. J. Environ. Res. Public. Health., 12(5): 4533-4545.
- Falkinham, J.O., III (2015b). Environmental sources of nontuberculous mycobacteria. Clinics in Chest Medicine, 36(1): 35-41.
- Falkinham, J.O. (2015c). The Mycobacterium avium complex and slowly growing mycobacteria. In Molecular medical microbiology: Second edition. Elsevier Ltd, London, UK. pp. 1669-1678.
- Falkinham, J.O. III, Hilborn, E.D., Arduino, M.J., Pruden, A. and Edwards, M.A. (2015a). Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect., 123(8): 749-758.
- Falkinham, J.O., Pruden, A. and Edwards, M. (2015b). Opportunistic premise plumbing pathogens: Increasingly important pathogens in drinking water. Pathogens, 4(2): 373-386.
- Falkinham, J.O.,3rd (2016a). Current epidemiologic trends of the nontuberculous mycobacteria (NTM). Curr Environ Health Rep, 3(2): 161-167.
- Falkinham, J.O. (2016b). Nontuberculous mycobacteria: Community and nosocomial waterborne opportunistic pathogens. Clin. Microbiol. Newsl., 38(1): 1-7.
- Feazel, L.M., Baumgartner, L.K., Peterson, K.L., Frank, D.N., Harris, J.K. and Pace, N.R. (2009). Opportunistic pathogens enriched in showerhead biofilms. Proceedings of the National Academy of Sciences of the United States of America, 106(38): 16393-16398.
- Fields, B.S., Haupt, T., Davis, J.P., Arduino, M.J., Miller, P.H. and Butler, J.C. (2001). Pontiac fever due to Legionella micdadei from a whirlpool spa: Possible role of bacterial endotoxin. J. Infect. Dis., 184(10): 1289-1292.
- Fields, B.S., Benson, R.F. and Besser, R.E. (2002). Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev., 15(3): 506-526.
- Fisman, D.N., Lim, S., Wellenius, G.A., Johnson, C., Britz, P., Gaskins, M., Maher, J., Mittleman, M.A., Spain, C.V., Haas, C.N. and Newbern, C. (2005). It's not the heat, it's the humidity: Wet weather increases legionellosis risk in the greater Philadelphia metropolitan area. J. Infect. Dis., 192(12): 2066-2073.
- Flannery, B., Gelling, L.B., Vugia, D.J., Weintraub, J.M., Salerno, J.J., Conroy, M.J., Stevens, V.A., Rose, C.E., Moore, M.R., Fields, B.S. and Besser, R.E. (2006). Reducing Legionella colonization of water systems with monochloramine. Emerg. Infect. Dis., 12(4): 588-596.
- Fleury, M., Charron, D.F., Holt, J.D., Allen, O.B. and Maarouf, A.R. (2006). A time series analysis of the relationship of ambient temperature and common bacterial enteric infections in two Canadian provinces. Int. J. Biometeorol., 50 (6): 385-391.
- Ford, T., Hermon-Taylor, J., Nichols, G., Cangelosi, G. and Bartram, J. (2004). Approaches to risk management in priority setting. In Pathogenic mycobacteria in Water—A guide to public health consequences, monitoring and management. Pedley, S. et al., (eds). World Health Organization, Geneva, Switzerland. pp. 169-178.
- Fredriksson-Ahomaa, M. and Korkeala, H. (2003). Low occurrence of pathogenic Yersinia enterocolitica in clinical, food, and environmental samples: A methodological problem. Clin. Microbiol. Rev., 16(2): 220-229.
- Fredriksson-Ahomaa, M. (2015). Enteropathogenic Yersinia spp. In Zoonoses-infections affecting humans and animals: Focus on public health aspects. Sing, A. (ed.). Springer. Dordrecht. pp. 213-234.
- Fredriksson-Ahomaa, M. (2017). Yersinia enterocolitica. In Foodborne diseases: Third edition. Yersinia enterocolitica. Dodd, C. et al. (eds.). Academic Press. Cambridge, USA. pp. 223-233.
- Friedman, M., Ashbolt, J., Hanson, A., Meteer, L. and Ureta, A. (2017). Chapter 3: Understanding and managing biofilm, coliform occurrence, and the microbial community. Manual of water supply practices M68– Water quality in distribution systems. American Water Works Association, Denver, CO., pp. 39-80.
- Gaia, V., Fry, N.K., Afshar, B., Lück, P.C., Meugnier, H., Etienne, J., Peduzzi, R. and Harrison, T.G. (2005). Consensus sequence-based scheme for epidemiological typing of clinical and environmental isolates of Legionella pneumophila. J. Clin. Microbiol., 43 (5):2047-2052.
- Galanis, E., Mak, S., Otterstatter, M., Taylor, M., Zubel, M., Takaro, T.K., Kuo, M. and Michel, P. (2014). The association between campylobacteriosis, agriculture and drinking water: A case-case study in a region of British Columbia, Canada, 2005-2009. Epidemiol. Infect., 142 (10): 2075-2084.
- Gargano, J.W., Adam, E.A., Collier, S.A., Fullerton, K.E., Feinman, S.J. and Beach, M.J. (2017). Mortality from selected diseases that can be transmitted by water—United States, 2003-2009. J. Water Health, 15(3): 438-450.
- Garvey, M., Thokala, N. and Rowan, N. (2014). A comparative study on the pulsed UV and the low-pressure UV inactivation of a range of microbial species in water. Water Environ. Res., 86(12): 2317-2324.
- Gavriel, A.A., Landre, J.P.B. and Lamb, A.J. (1998). Incidence of mesophilic Aeromonas within a public drinking water supply in north-east Scotland. J. Appl. Microbiol., 84(3): 383-392.
- Gebert, M.J., Delgado-Baquerizo, M., Oliverio, A.M., Webster, T.M., Nichols, L.M., Honda, J.R., Chan, E.D., Adjemian, J., Dunn, R.R. and Fierer, N. (2018). Ecological analyses of mycobacteria in showerhead biofilms and their relevance to human health. mBio, 9(5).
- Gerba, C.P., Nwachuku, N. and Riley, K.R. (2003). Disinfection resistance of waterborne pathogens on the United States Environmental Protection Agency's Contaminant candidate list (CCL). J. Water Supply Res. T., 52(2): 81-94.
- Ghenghesh, K.S., Ahmed, S.F., El-Khalek, R.A., Al-Gendy, A. and Klena, J. (2008). Aeromonas-associated infections in developing countries. J. Infect. Dev. Countr., 2(2): 81-98.
- Gilmour, M.W., Bernard, K., Tracz, D.M., Olson, A.B., Corbett, C.R., Burdz, T., Ng, B., Wiebe, D., Broukhanski, G., Boleszczuk, P., Tang, P., Jamieson, F., Van Domselaar, G., Plummer, F.A. and Berry, J.D. (2007). Molecular typing of a Legionella pneumophila outbreak in Ontario, Canada. J. Med. Microbiol., 56(3): 336-341.
- Goldberg, D., Collier, P., Fallon, R., Mckay, T., Markwick, T., Wrench, J., Emslie, J., Forbes, G., Macpherson, A. and Reid, D. (1989). Lochgoilhead fever: Outbreak of non-pneumonic legionellosis due to Legionella micdadei. Lancet, 333(8633): 316-318.
- Goudot, S., Herbelin, P., Mathieu, L., Soreau, S., Banas, S. and Jorand, F.P.A. (2014). Biocidal efficacy of monochloramine against planktonic and biofilm-associated Naegleria fowleri cells. J. Appl. Microbiol., 116(4): 1055-1065.
- Government Inquiry into Havelock North Drinking Water. (2017). Havelock North drinking water inquiry: Stage 2. May 2017, Auckland, New Zealand. ISBN: 978-0-473-39743-2. Available at https://www.dia.govt.nz/stage-1-of-the-water-inquiry.
- Government of Quebec. (2020). Régie du Bâtiment Québec. Quebec, Canada. Accessed February, 2020 from: https://www.rbq.gouv.qc.ca/domaines-dintervention/batiment/interpretation-directives-techniques-et-administratives/chapitre-batiment-du-code-de-securite/tours-de-refroidissement-a-leau-obligations-du-proprietaire.html
- Graham, D.Y., Opekun, A.R., Osato, M.S., El-Zimaity, H.M.T., Lee, C.K., Yamaoka, Y., Qureshi, W.A., Cadoz, M. and Monath, T.P. (2004). Challenge model for Helicobacter pylori infection in human volunteers. Gut, 53(9): 1235-1243.
- Graziani, C., Losasso, C., Luzzi, I., Ricci, A., Scavia, G. and Pasquali, P. (2017). Salmonella. In Foodborne diseases: Third edition. Dodd, C. et al. (eds.). Academic Press. Cambridge, USA. pp. 133-169.
- Greenberg, D., Chiou, C.C., Famigilleti, R., Lee, T.C. and Yu, V.L. (2006). Problem pathogens: Paediatric legionellosis-implications for improved diagnosis. Lancet Infect. Dis., 6(8): 529-535.
- Grimont, P.A.D. and Weill, F-X. (2007). Antigenic formulae of the Salmonella serovars. 9th edition. WHO Collaborating Centre for Reference and Research on Salmonella. Institut Pasteur, Paris, France. Accessed January 2020 from: https://www.pasteur.fr/sites/default/files/veng_0.pdf
- Guimaraes, A.J., Gomes, K.X., Cortines, J.R., Peralta, J.M. and Peralta, R.H.S. (2016). Acanthamoeba spp. as a universal host for pathogenic microorganisms: One bridge from environment to host virulence. Microbiol. Res., 193: 30-38.
- Hallenbeck, W.H. and Brenniman, G.R. (1989). Risk of fatal amebic meningoencephalitis from waterborne Naegleria fowleri. Environ. Manage., 13(2): 227-232.
- Halstrom, S., Price, P. and Thomson, R. (2015). Review: Environmental mycobacteria as a cause of human infection. Int. J. Mycobacteriology, 4(2): 81-91.
- Hamilton, K.A., Weir, M.H. and Haas, C.N. (2017). Dose response models and a quantitative microbial risk assessment framework for the Mycobacterium avium complex that account for recent developments in molecular biology, taxonomy, and epidemiology. Water Res., 109: 310-326.
- Handfield, M., Simard, P., Couillard, M. and Letarte, R. (1996). Aeromonas hydrophila isolated from food and drinking water: Hemagglutination, hemolysis, and cytotoxicity for a human intestinal cell line (HT-29). Appl. Environ. Microbiol., 62(9): 3459-3461.
- Harris, N.B. and Barletta, R.G. (2001). Mycobacterium avium subsp. paratuberculosis in Veterinary Medicine. Clin. Microbiol. Rev., 14 (3):489-512
- Havelaar, A.H., Schets, F.M., van Silfhout, A., Jansen, W.H., Wieten, G. and van der Kooij, D. (1992). Typing of Aeromonas strains from patients with diarrhoea and from drinking water. J. Appl. Bacteriol., 72(5): 435-444.
- Hayes, S.L., White, K.M. and Rodgers, M.R. (2006). Assessment of the effectiveness of low-pressure UV light for inactivation of Helicobacter pylori. Appl. Environ. Microbiol., 72(5): 3763-3765.
- Hayes, S.L., Sivaganesan, M., White, K.M. and Pfaller, S.L. (2008). Assessing the effectiveness of low-pressure ultraviolet light for inactivating Mycobacterium avium complex (MAC) micro-organisms. Lett. Appl. Microbiol., 47(5): 386-392.
- Health Canada. (2018). Guidelines for Canadian drinking water quality: Guideline technical document – chloramines – in preparation. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Canada.
- Health Canada. (2019a). Be Well Aware—Information for private well owners. Healthy Environments and Consumer Safety Branch, Health Canada. Ottawa, Ontario. Accessed February 2020 from: https://www.canada.ca/en/health-canada/services/publications/healthy-living/water-talk-information-private-well-owners.html
- Health Canada. (2019a). Guidance on Monitoring the Biological Stability of Drinking Water in Distribution Systems—in preparation. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada. Ottawa, Ontario.
- Health Canada. (2019c). Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Enteric Viruses. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
- Health Canada. (2019d). Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Enteric protozoa: Giardia and Cryptosporidium. Water, and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
- Health Canada. (2019e). Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Escherichia coli in Drinking Water – in preparation. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
- Henkle, E., Hedberg, K., Schafer, S.D. and Winthrop, K.L. (2017). Surveillance of extrapulmonary nontuberculous mycobacteria infections, Oregon, USA, 2007–2012. Emerg. Infect. Dis., 23(10): 1627-1630.
- Hermon-Taylor., J. and El-Zaatari, F.A.K. (2004). The Mycobacterium avium subspecies paratuberculosis problem and its relation to the causation of Crohn's disease. In Pathogenic mycobacteria in Water—A guide to public health consequences, monitoring and management. Pedley, S. et al., (eds). World Health Organization, Geneva, Switzerland. pp: 74-94.
- Hijnen, W.A.M. and Medema, G.J. (2010). Elimination of micro-organisms by drinking water treatment processes. KWR Watercycle Research Institute. IWA Publishing, London, United Kingdom. Pp. 1-102.
- Hijnen, W.A.M., Beerendonk, E.F. and Medema, G.J. (2011). Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review.). In Quantitative methods to assess capacity of water treatment to eliminate microorganisms. Hijnen, W.A.M ed.; KWR Watercycle Research Institute. IWA Publishing., London, United Kingdom.
- Hilborn, E.D., Covert, T.C., Yakrus, M.A., Harris, S.I., Donnelly, S.F., Rice, E.W., Toney, S., Bailey, S.A. and Stelma Jr., G.N. (2006). Persistence of nontuberculous mycobacteria in a drinking water system after addition of filtration treatment. Appl. Environ. Microbiol. , 72(9): 5864-5869.
- Hlavsa, M.C., Cikesh, B.L., Roberts, V.A., Kahler, A.M., Vigar, M., Hilborn, E.D., Wade, T.J., Roellig, D.M., Murphy, J.L., Xiao, L., Yates, K.M., Kunz, J.M., Arduino, M.J., Reddy, S.C., Fullerton, K.E., Cooley, L.A., Beach, M.J., Hill, V.R. and Yoder, J.S. (2018). Outbreaks associated with treated recreational water—United States, 2000–2014. Am. J. Transplant., 18(7): 1815-1819.
- Hoefsloot, W. et al. (2013). The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. European Respiratory Journal, 42(6): 1604-1613.
- Holinger, E.P., Ross, K.A., Robertson, C.E., Stevens, M.J., Harris, J.K. and Pace, N.R. (2014). Molecular analysis of point-of-use municipal drinking water microbiology. Water Res., 49: 225-235.
- Hooi, J.K.Y., Lai, W.Y., Ng, W.K., Suen, M.M.Y., Underwood, F.E., Tanyingoh, D., Malfertheiner, P., Graham, D.Y., Wong, V.W.S., Wu, J.C.Y., Chan, F.K.L., Sung, J.J.Y., Kaplan, G.G. and Ng, S.C. (2017). Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology, 153 (2): 420-429.
- Hornei, B., Ewig, S., Exner, M., Tartakovsky, I., Lajoie, L., Dangendorf, F., Surman-Lee, S. and Fields, B. (2007). Chapter 1: Legionellosis. In Legionella and the prevention of legionellosis. Bartram, J. et. al. (eds.). World Health Organization, Geneva, Switzerland. pp. 1-27.
- Howden, B.P., Stuart, R.L., Tallis, G., Bailey, M. and Johnson, P.D.R. (2003). Treatment and outcome of 104 hospitalized patients with legionnaires’ disease. Internal Medicine Journal, 33(11): 484-488.
- Hrudey, S. E. and Hrudey, E. (2004). Safe drinking water: Lessons from recent outbreaks in affluent nations. Safe drinking water: Lessons from recent outbreaks in affluent nations. IWA Publishing, London.
- HSE. (2013a). Legionnaires’ Disease: Technical Guidance. HSG274 Part 1: The Control of Legionella Bacteria in Evaporative Cooling Systems. Health and Safety Executive, Government of the United Kingdom. London, UK. Pp. 1-57. Accessed December 2018 from: http://www.hse.gov.uk/pubns/books/hsg274.htm
- HSE. (2013b). Legionnaires’ Disease: Technical Guidance. HSG274 Part 2: The Control of Legionella Bacteria in Hot and Cold Water Systems. Health and Safety Executive, Government of the United Kingdom. London, UK. Pp. 1-65. Accessed December 2018 from: http://www.hse.gov.uk/pubns/books/hsg274.htm
- HSE. (2013c). Legionnaires’ Disease. The Control of Legionella Bacteria in Water Systems—Approved Code of Practice and Guidance. L8 (Fourth Edition). Health and Safety Executive, Government of the United Kingdom. London, UK. Pp. 1-28. Accessed November 2018 from: http://www.hse.gov.uk/pubns/books/l8.htm
- HSE. (2019). Legionella and Legionnaires’ Disease – Frequently asked questions. Health and Safety Executive, Government of the United Kingdom. London, UK. Accessed November 2018 from: http://www.hse.gov.uk/legionnaires/faqs.htm#Testing-monitoring
- Hsu, M.-S., Wu, M.-Y., Huang, Y.-T. and Liao, C.-H. (2016). Efficacy of chlorine dioxide disinfection to non-fermentative gram-negative bacilli and non-tuberculous mycobacteria in a hospital water system. Journal of Hospital Infection, 93(1): 22-28.
- Huang, H., Brooks, B.W., Lowman, R. and Carrillo, C.D. (2015). Campylobacter species in animal, food, and environmental sources, and relevant testing programs in Canada. Can. J. Microbiol., 61(10): 701-721.
- IARC Helicobacter pylori Working Group. (2014). Helicobacter pylori eradication as a strategy for preventing gastric cancer. International Agency for Research on Cancer, IARC Working Group Reports, No. 8, Lyon, France.
- Ishii, S. and Sadowsky, M.J. (2008). Escherichia coli in the environment: Implications for water quality and human health. Microbes Environ., 23(2): 101-108.
- ISO. (2019). ISO ICS 07.100.20 – Microbiology of Water. International Organization for Standardization. Geneva, Switzerland. Accessed from: www.iso.org/Ics/07.100.20/X/.
- Jacangelo, J.G., Patania, N.L., Trussell, R.R., Haas, C.N. and Gerba, C. (2002). Inactivation of waterborne emerging pathogens by selected disinfectants. AWWA Research Foundation and American Water Works Association, Denver, CO.
- Jamil, A., Farooq, S. and Hashmi, I. (2017). Ozone disinfection efficiency for indicator microorganisms at different pH values and temperatures. Ozone Sci. Eng., 39(6): 407-416.
- Janda, J.M. and Abbott, S.L. (2010). The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clinical Microbiology Reviews, 23(1): 35-73.
- Johnson, C.H., Rice, E.W. and Reasoner, D.J. (1997). Inactivation of Helicobacter pylori by chlorination. Appl. Environ. Microbiol., 63(12): 4969-4970.
- Jones, T.F., Benson, R.F., Brown, E.W., Rowland, J.R., Crosier, S.C. and Schaffner, W. (2003). Epidemiologic investigation of a restaurant-associated outbreak of pontiac fever. Clin Infect Dis, 37(10): 1292-1297.
- Juárez, M.M., Tártara, L.I., Cid, A.G., Real, J.P., Bermúdez, J.M., Rajal, V.B. and Palma, S.D. (2018). Acanthamoeba in the eye, can the parasite hide even more? latest developments on the disease. Contact Lens and Anterior Eye, 41(3): 245-251.
- Kaakoush, N.O., Castaño-Rodríguez, N., Mitchell, H.M. and Man, S.M. (2015). Global epidemiology of Campylobacter infection. Clinical Microbiology Reviews, 28(3): 687-720.
- Katz, M.J., Parrish, N.M., Belani, A. and Shah, M. (2015). Recurrent Aeromonas bacteremia due to contaminated well water. Open Forum Infectious Diseases, 2(4).
- Kaur, S. (2014). Chapter 9: Pathogenic mycobacteria and water. In Water and health. Singh, P.P. and Sharma, V. (eds.) Springer. India. pp. 137-153.
- Keithlin, J., Sargeant, J., Thomas, M.K. and Fazil, A. (2014a). Chronic sequelae of E. coli O157: Systematic review and meta-analysis of the proportion of E. coli O157 cases that develop chronic sequelae. Foodborne Pathog. Dis., 11 (2): 79-95.
- Keithlin, J., Sargeant, J., Thomas, M.K. and Fazil, A. (2014b). Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health, 14 (1), art. no. 1203: 1-19.
- Keithlin, J., Sargeant, J.M., Thomas, M.K. and Fazil, A. (2015). Systematic review and meta-analysis of the proportion of non-typhoidal Salmonella cases that develop chronic sequelae. Epidemiol. Infect., 143: 1333–1351.
- Khajanchi, B.K., Fadl, A.A., Borchardt, M.A., Berg, R.L., Horneman, A.J., Stemper, M.E., Joseph, S.W., Moyer, N.P., Sha, J. and Chopra, A.K. (2010). Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples: Suggestive evidence of water-to-human transmission. Appl. Environ. Microbiol., 76(7): 2313-2325.
- Kilvington, S., Gray, T., Dart, J., Morlet, N., Beeching, J.R., Frazer, D.G. and Matheson, M. (2004). Acanthamoeba keratitis: The role of domestic tap water contamination in the United Kingdom. Invest. Ophth. Vis. Sci., 45(1): 165-169.
- Knøchel, S. (1991). Chlorine resistance of motile Aeromonas spp. Water Sci. Technol., 24(2): 327-330.
- Knox, N.C., Weedmark, K.A., Conly, J., Ensminger, A.W., Hosein, F.S. and Drews, S.J. (2017). Unusual Legionnaires’ outbreak in cool, dry western Canada: An investigation using genomic epidemiology. Epidemiol. Infect., 145(2): 254-265.
- Köhsler, M., Mrva, M. and Walochnik, J. (2016). Acanthamoeba. In Molecular parasitology: Protozoan parasites and their molecules. Walochnik, J. and Duchêne, M. (eds.). Springer. Vienna. pp. 285-324.
- Kothary, M.H. and Babu, US (2001). Infective dose of foodborne pathogens in volunteers: A review. J. Food Safety, 21(1): 49-73.
- Kühn, I., Allestam, G., Huys, G., Janssen, P., Kersters, K., Krovacek, K. and Stenström, T.-. (1997). Diversity, persistence, and virulence of Aeromonas strains isolated from drinking water distribution systems in Sweden. Appl. Environ. Microbiol., 63(7): 2708-2715.
- Kvalsvig, A., Baker, M.G., Sears, A. and French, N. (2013). Bacteria: Campylobacter. In Encyclopedia of food safety. Motarjemi, Y., Moy, G. and Todd, E. (eds.). Academic Press. Cambridge, USA. pp. 369-380.
- Lal, A., Hales, S., French, N., and Baker, M.G. (2012). Seasonality in human zoonotic enteric diseases: A systematic review. PLoS ONE, 7 (4), art. no. e31883.
- Lande, L., Alexander, D.C., Wallace, R.J., Jr., Kwait, R., Iakhiaeva, E., Williams, M., Cameron, A.D.S., Olshefsky, S., Devon, R., Vasireddy, R., Peterson, D.D. and Falkinham, J.O., III (2019). Mycobacterium avium in community and household water, suburban Philadelphia, Pennsylvania, USA, 2010-2012. Emerg. Infect. Dis., 25(3): 473-481.
- Langenberg, W., Rauws, E.A.J., Oudbier, J.H. and Tytgat, G.N.J. (1990). Patient-to-patient transmission of Campylobacter pylori infection by fiber optic gastroduodenoscopy and biopsy. J. Infect. Dis., 161(3): 507-511.
- Lau, H.Y. and Ashbolt, N.J. (2009). The role of biofilms and protozoa in Legionella pathogenesis: Implications for drinking water. J. Appl. Microbiol., 107 (2): 368-378.
- Lavenir, R., Sanroma, M., Gibert, S., Crouzet, O., Laurent, F., Kravtsoff, J., Mazoyer, M.-. and Cournoyer, B. (2008). Spatio-temporal analysis of infra-specific genetic variations among a Pseudomonas aeruginosa water network hospital population: Invasion and selection of clonal complexes. J. Appl. Microbiol., 105(5):1491-1501.
- Le Dantec, C., Duguet, J.-., Montiel, A., Dumoutier, N., Dubrou, S. and Vincent, V. (2002). Occurrence of mycobacteria in water treatment lines and in water distribution systems. Appl. Environ. Microbiol., 68(11): 5318-5325.
- LeChevallier, M.W. (2004). Control, treatment and disinfection of Mycobacterium avium complex in drinking water. In Pathogenic mycobacteria in water—A guide to public health consequences, monitoring and management. Pedley, S. et al., (eds). World Health Organization, Geneva, Switzerland. pp: 143-168.
- LeChevallier, M.W., Evans, T.M., Seidler, R.J., Daily, O.P., Merrell, B.R., Rollins, D.M. and Joseph, S.W. (1982). Aeromonas sobria in chlorinated drinking water supplies. Microb. Ecol., 8(4): 325-333.
- LeChevallier, M.W. and Au, K.K. (2004). Water treatment and pathogen control: Process efficiency in achieving safe drinking water. IWA Publishing, London, UK, on behalf of the World Health Organization, Geneva, Switzerland.
- LeChevallier, M.W., Norton, C.D., Falkinham, III, J.O., Williams, M.D., Taylor, R.H. and Cowan, H.E. (2001). Occurrence and control of Mycobacterium avium complex. AWWA Research Foundation and American Water Works Association., Denver. CO.
- Leclerc, H. (2003). Relationships between common water bacteria and pathogens in drinking-water. In Heterotrophic plate counts and drinking-water safety. The significance of HPCs for water quality and human health. Bartram, J., Cotruvo, J., Exner, M., Fricker, C. and Glasmacher, A. (eds.). IWA Publishing, London, UK. pp. 80–118.
- Leoni, E., Catalani, F., Marini, S. and Dallolio, L. (2018). Legionellosis associated with recreational waters: A systematic review of cases and outbreaks in swimming pools, spa pools, and similar environments. Int. J. Env. Res. Pub. He., 15 (8), art. no. 1612.
- Lévesque, S., Plante, P.-., Mendis, N., Cantin, P., Marchand, G., Charest, H., Raymond, F., Huot, C., Goupil-Sormany, I., Desbiens, F., Faucher, S.P., Corbeil, J. and Tremblay, C. (2014). Genomic characterization of a large outbreak of Legionella pneumophila serogroup 1 strains in Quebec City, 2012. PLoS ONE, 9(8).
- Li, T., Abebe, L.S., Cronk, R. and Bartram, J. (2017). A systematic review of waterborne infections from nontuberculous mycobacteria in health care facility water systems. Int. J. Hyg. Envir. Heal., 220(3): 611-620.
- Liu, D. (2014). Aeromonas. In Molecular medical microbiology: Second edition. Tang, Y-W. et al., eds. Elsevier Ltd. London, UK. pp. 1099-1110.
- Llewellyn, A.C., Lucas, C.E., Roberts, S.E., Brown, E.W., Nayak, B.S., Raphael, B.H. and Winchell, J.M. (2017). Distribution of Legionella and bacterial community composition among regionally diverse US cooling towers. PLoS ONE, 12 (12).
- Loret, J.-. and Greub, G. (2010). Free-living amoebae: Biological by-passes in water treatment. Int. J. Hyg. Envir. Heal., 213(3): 167-175.
- Loret, J.-., Jousset, M., Robert, S., Anselme, C., Saucedo, G., Ribas, F., Martinez, L. and Catalan, V. (2008). Elimination of free-living amoebae by drinking water treatment processes. Journal Europeen D'Hydrologie, 39(1): 37-50.
- LPSN. (2019). List of prokaryote names with standing in nomenclature. Retrieved June 2019, from www.bacterio.net.
- Lu, J., Struewing, I., Vereen, E., Kirby, A.E., Levy, K., Moe, C. and Ashbolt, N. (2015a). Molecular detection of Legionella spp. and their associations with Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in a drinking water distribution system. J. Appl. Microbiol., 120(2): 509-521.
- Lu, J., Struewing, I., Yelton, S. and Ashbolt, N. (2015b). Molecular survey of occurrence and quantity of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in municipal drinking water storage tank sediments. J. Appl. Microbiol., 119(1): 278-288.
- Lund, V. (1996). Evaluation of E. coli as an indicator for the presence of Campylobacter jejuni and Yersinia enterocolitica in chlorinated and untreated oligotrophic lake water. Water Res., 30(6): 1528-1534.
- Lüttichau, H.R., Vinther, C., Uldum, S.A., Møller, J., Faber, M. and Jensen, J.S. (1998). An outbreak of Pontiac fever among children following use of a whirlpool. Clin. Infect. Dis., 26(6): 1374-1378.
- MacIntyre, C.R., Dyda, A., Bui, C.M. and Chughtai, A.A. (2018). Rolling epidemic of Legionnaires’ disease outbreaks in small geographic areas. Emerg. Microbes Infect., 7(1): 36.
- Magnet, A., Galván, A.L., Fenoy, S., Izquierdo, F., Rueda, C., Fernandez Vadillo, C., Pérez-Irezábal, J., Bandyopadhyay, K., Visvesvara, G.S., Da Silva, A.J. and Del Aguila, C. (2012). Molecular characterization of Acanthamoeba isolated in water treatment plants and comparison with clinical isolates. Parasitol. Res., 111(1): 383-392.
- Mao, G., Song, Y., Bartlam, M. and Wang, Y. (2018). Long-term effects of residual chlorine on Pseudomonas aeruginosa in simulated drinking water fed with low AOC medium. Front. Microbiol., 9(MAY).
- Marciano-Cabral, F. and Cabral, G.A. (2007). The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol. Med. Mic., 51(2): 243-259.
- Marras, T.K., Mendelson, D., Marchand-Austin, A., May, K. and Jamieson, F.B. (2013). Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998-2010. Emerg. Infect. Dis., 19(11): 1889-1891.
- Massa, S., Armuzzi, R., Tosques, M., Canganella, F. and Trovatelli, L.D. (1999). Note: Susceptibility to chlorine of Aeromonas hydrophila strains. J. Appl. Microbiol. 86 (1):169-173.
- Masten, S.J., Davies, S.H. and McElmurry, S.P. (2016). Flint water crisis: What happened and why? J. Am. Water Works Assoc., 108(12): 22-34.
- Mathys, W., Stanke, J., Harmuth, M. and Junge-Mathys, E. (2008). Occurrence of Legionella in hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating. Int. J. Hyg. Environ. Heal., 211 (1-2): 179-185.
- Matysiak-Budnik, T., Briet, F., Heyman, M. and Mégraud, F. (1995). Laboratory-acquired Helicobacter pylori infection. Lancet, 346(8988): 1489-1490.
- McDermott, P.F., Zhao, S. and Tate, H. (2018). Antimicrobial Resistance in Nontyphoidal Salmonella. Microbiology spectrum, American Society for Microbiology. 6(4):1-26.
- McDonough, E.A., Metzgar, D., Hansen, C.J., Myers, C.A. and Russell, K.L. (2007). A cluster of Legionella-associated pneumonia cases in a population of military recruits. J. Clin. Microbiol., 45(6): 2075-2077.
- MDHHS. (2016). Legionellosis outbreak-Genesee County, June, 2014 – March, 2015. Full analysis. Michigan Department of Health and Human Services, MI. Accessed November 2018 from www.michigan.gov.
- Medema, G.J., Wondergem, E., Van Dijk-Looyaard, A.M. and Havelaar, A.H. (1991). Effectivity of chlorine dioxide to control Aeromonas in drinking water distribution systems. Water Sci. Technol., 24(2): 325-326.
- Mégraud, F. and Lehours, P. (2007). Helicobacter pylori detection and antimicrobial susceptibility testing (2007) Clin. Microbiol. Rev., 20 (2): 280-322.
- Mercante, J.W. and Winchell, J.M. (2015). Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin. Microbiol. Rev., 28(1): 95-133.
- Mir, R.A. and Kudva, I.T. (2019). Antibiotic-resistant Shiga toxin-producing Escherichia coli: An overview of prevalence and intervention strategies. Zoonoses Public Hlth., 66 (1): 1-13.
- Missouri Department of Health and Senior Services (2010). Communicable Disease Surveillance 2010 Annual Report.
- Miyamoto, M., Yamaguchi, Y., and Sastsu, M. (2000). Disinfectant effects of hot water, ultraviolet light, silver ions and chlorine on stains of Legionella and nontuberculous mycobacteria. Microbios. 101(398):7-13.
- Moore, E.R.B., Tindall, B.J., Martins Dos Santos, V.A.P., Pieper, D.H., Ramos, J-L. and Palleroni, N.J. (2006a). Nonmedical: Pseudomonas. In The Prokaryotes—A handbook on the biology of bacteria. Third edition. Volume 4: Bacteria: Firmicutes, Cyanobacteria. Dworkin, M. et al., eds. Springer, Singapore. pp. 646-703.
- Moore, M.R., Pryor, M., Fields, B., Lucas, C., Phelan, M. and Besser, R.E. (2006b). Introduction of monochloramine into a municipal water system: Impact on colonization of buildings by Legionella spp. Appl. Env. Microbiol. 72 (1):378-383.
- Moreira, N.A. and Bondelind, M. (2017). Safe drinking water and waterborne outbreaks. J. Water. Health., 15(1): 83-96.
- Moreno, Y., Piqueres, P., Alonso, J.L., Jiménez, A., González, A. and Ferrús, M.A. (2007). Survival and viability of Helicobacter pylori after inoculation into chlorinated drinking water. Water Res., 41(15): 3490-3496.
- Morgan, D.R., Johnson, P.C., DuPont, H.L., Satterwhite, T.K. and Wood, L.V. (1985). Lack of correlation between known virulence properties of Aeromonas hydrophila and enteropathogenicity for humans. Infect. Immun., 50(1): 62-65.
- Mraz, A.L. and Weir, M.H. (2018). Knowledge to predict pathogens: Legionella pneumophila lifecycle critical review Part I Uptake into host cells. Water (Switzerland), 10(2), 132.
- Murphy, H.M., Thomas, M.K., Schmidt, P.J., Medeiros, D.T., McFadyen, S. and Pintar, K.D.M. (2016). Estimating the burden of acute gastrointestinal illness due to Giardia, Cryptosporidium, Campylobacter, E. coli O157 and norovirus associated with private wells and small water systems in Canada. Epidemiol. Infect., 144 (7): 1355-1370.
- Naja, F., Kreiger, N. and Sullivan, T. (2007). Helicobacter pylori infection in Ontario: Prevalence and risk factors. Can. J. Gastroenterol., 21 (8):501-506.
- NAS. (2019). Management of Legionella in Water Systems. National Academies of Sciences, Engineering, and Medicine. Washington, DC. The National Academies Press. https://doi.org/10.17226/25474.
- Neil, K. and Berkelman, R. (2008). Increasing incidence of legionellosis in the United States, 1990-2005: Changing epidemiologic trends. Clin. Infect. Dis., 47(5): 591-599.
- NHMRC, NRMMC. (2011). Australian drinking water guidelines 6—national water quality management strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra.
- Nichols, G., Ford, T., Bartram, J., Dufour, A. and Portaels, F. (2004). Introduction. In Pathogenic mycobacteria in water—A guide to public health consequences, monitoring and management. Pedley, S. et al., (eds). World Health Organization, Geneva, Switzerland. pp. 1-14.
- Norton, C.D., LeChevallier, M.W. and Falkinham III, J.O. (2004). Survival of Mycobacterium avium in a model distribution system. Water Res., 38(6): 1457-1466.
- NRCC. (2015a). National building code. National Research Council of Canada, Ottawa, Ontario. Available at https://nrc.canada.ca/en/certifications-evaluations-standards/codes-canada/codes-canada-publications/national-building-code-canada-2015
- NRCC. (2015b). National plumbing code of Canada. National Research Council of Canada, Ottawa, Ontario. Available at https://nrc.canada.ca/en/certifications-evaluations-standards/codes-canada/codes-canada-publications/national-plumbing-code-canada-2015
- NSF/ANSI. (2018). Standard 244—Drinking Water Treatment Units-Supplemental Microbiological Water Treatment Systems-Filtration. NSF International, Ann Arbor, Michigan
- NSF/ANSI. (2019a). Standard 55—Ultraviolet microbiological water treatment systems. NSF International, Ann Arbor, Michigan.
- NSF/ANSI. (2019b). Standard 60- Drinking water treatment chemicals: Health effects. NSF International, Ann Arbor, Michigan.
- O’Connor, D.R. (2002). Report of the Walkerton inquiry, Part one: The events of May 2000 and related issues. Ontario Ministry of the Attorney General (ISBN: 0-7794-2559-6).
- Orta de Velásquez, M. T., Yáñez Noguez, I., Casasola Rodríguez, B. and Román Román, P.I. (2017). Effects of ozone and chlorine disinfection on VBNC Helicobacter pylori by molecular techniques and FESEM images. Environ. Technol., 38(6): 744-753.
- O’Ryan, M., Vidal, R., Del Canto, F., Salazar, J.C. and Montero, D. (2015). Vaccines for viral and bacterial pathogens causing acute gastroenteritis: Part II: Vaccines for Shigella, Salmonella, enterotoxigenic E. coli (ETEC) enterohemorragic E. coli (EHEC) and Campylobacter jejuni. Hum. Vacc. and Immunother., 11(3): 601-619.
- Pablos, M., Rodríguez-Calleja, J.M., Santos, J.A., Otero, A. and García-López, M.-. (2009). Occurrence of motile Aeromonas in municipal drinking water and distribution of genes encoding virulence factors. Int. J. Food Microbiol., 135(2): 158-164.
- Palusińska-Szysz, M. and Cendrowska-Pinkosz, M. (2009). Pathogenicity of the family Legionellaceae. Arch. Immunol. Ther. Ex., 57(4): 279-290.
- Park, J., Kim, J.S., Kim, S., Shin, E., Oh, K.-., Kim, Y., Kim, C.H., Hwang, M.A., Jin, C.M., Na, K., Lee, J., Cho, E., Kang, B.-., Kwak, H.-., Seong, W.K. and Kim, J. (2018). A waterborne outbreak of multiple diarrhoeagenic Escherichia coli infections associated with drinking water at a school camp. Int. J. Infect. Dis., 66: 45-50.
- Park, S.R., Mackay, W.G. and Reid, D.C. (2001). Helicobacter sp. recovered from drinking water biofilm sampled from a water distribution system. Water Res., 35(6): 1624-1626.
- Percival, S.L. and Williams, D.W. (2014a). Aeromonas. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd. Oxford, UK. pp. 49-64.
- Percival, S.L. and Williams, D.W. (2014b). Campylobacter. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd. Oxford, UK. pp. 65-78.
- Percival, S.L. and Williams, D.W. (2014c). Escherichia coli. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd. Oxford, UK. pp. 89-117.
- Percival, S.L. and Williams, D.W. (2014d). Helicobacter pylori. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd., Oxford, UK. pp. 119-154.
- Percival, S.L. and Williams, D.W. (2014e). Legionella. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd. Oxford, UK. pp. 155-175.
- Percival, S.L. and Williams, D.W. (2014f). Mycobacterium. In Microbiology of waterborne diseases. Second edition. Elsevier Ltd., Oxford, UK, pp. 177-207.
- Percival, S.L. and Williams, D.W. (2014g). Salmonella. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd. Oxford, UK. pp. 209-222.
- Percival, S.L. and Williams, D.W. (2014h). Shigella. In Microbiology of waterborne diseases: Microbiological aspects and risks: Second edition. Elsevier Ltd. Oxford, UK. pp. 223-236.
- Percival, S.L. and Williams, D.W. (2014i). Yersinia. In Microbiology of waterborne diseases. Second edition. Elsevier Ltd., Oxford, UK, pp. 249-259.
- Petrisek, R. and Hall, J. (2018). Evaluation of a most probable number method for the enumeration of Legionella pneumophila from North American potable and nonpotable water samples. J. Water Health, 16 (1), pp. 57-69.
- PHAC. (2010). Pathogen Safety Data Sheets: Infectious Substances – Shigella spp. Public Health Agency of Canada, Ottawa, Canada. Accessed September 2019 from: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/shigella.html
- PHAC. (2011). Pathogen Safety Data Sheets: Infectious Substances – Salmonella enterica spp. Public Health Agency of Canada, Ottawa, Canada. Accessed September 2019 from: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/salmonella-enterica.html
- PHAC. (2018a). Canadian antimicrobial resistance surveillance system. Update 2018. Public Health Agency of Canada. Ottawa, Canada. Accessed October 2019 from: https://www.canada.ca/en/services/health/publications/drugs-health-products.html
- PHAC. (2018b). FoodNet Canada Annual Report 2016. Public Health Agency of Canada, Ottawa, Canada.
- PHAC. (2018c). FoodNet Canada Annual Report 2017. Public Health Agency of Canada, Ottawa, Canada.
- PHAC. (2018d). Legionella. Public Health Agency of Canada, Ottawa, Canada. Accessed June 2019 from:
https://www.canada.ca/en/public-health/services/infectious-diseases/legionella.html. - PHAC. (2019). Notifiable diseases on-line. Reported cases from 1924 to 2016 in Canada. Public Health Agency of Canada, Ottawa, Canada. Accessed September 2019 from: http://diseases.canada.ca/ndis/charts-list.
- Posteraro, B., Posteraro, P. and Sanguinetti, M. (2015). Helicobacter pylori. In Molecular medical microbiology: Second edition. Elsevier Ltd, London, UK. pp. 1237-1258.
- Prussin, A.J., II, Schwake, D.O. and Marr, L.C. (2017). Ten questions concerning the aerosolization and transmission of Legionella in the built environment. Build. Environ., 123: 684-695.
- Pryor, M., Springthorpe, S., Riffard, S., Brooks, T., Huo, Y., Davis, G. and Sattar, S.A. (2004). Investigation of opportunistic pathogens in municipal drinking water under different supply and treatment regimes. Water Sci. Technol., 50(1): 83-90.
- PWGSC. (2016). MD 15161 – 2013 Control of Legionella in Mechanical Systems. Standard for Building Owners, Design Professionals, and Maintenance Personnel. Public Works and Government Services Canada. Gatineau, Quebec. Accessed June 2019 from: https://www.tpsgc-pwgsc.gc.ca/biens-property/legionella/index-eng.html
- Qin, W., Li, W.G., Gong, X.J., Huang, X.F., Fan, W.B., Zhang, D., Yao, P., Wang, X.J. and Song, Y. (2017). Seasonal-related effects on ammonium removal in activated carbon filter biologically enhanced by heterotrophic nitrifying bacteria for drinking water treatment. Environ. Sci. Pollut. Res. Int. 6(4):54.
- Radomski, N., Betelli, L., Moilleron, R., Haenn, S., Moulin, L., Cambau, E., Rocher, V., Gonçalves, A. and Lucas, F.S. (2011). Mycobacterium behavior in wastewater treatment plant, a bacterial model distinct from Escherichia coli and enterococci. Environ. Sci. Technol., 45(12): 5380-5386.
- Rasheed, S., Hashmi, I. and Campos, L. (2016). Inactivation of Escherichia coli and Salmonella with chlorine in drinking waters at various pH and temperature levels. Proc. Pak. Acad. Sci., 53(2B): 83-92.
- Reuter, S., Sigge, A., Wiedeck, H. and Trautmann, M. (2002). Analysis of transmission pathways of Pseudomonas aeruginosa between patients and tap water outlets. Crit. Care Med., 30(10): 2222-2228.
- Rhoads, W.J., Pruden, A. and Edwards, M.A. (2016). Survey of green building water systems reveals elevated water age and water quality concerns. Environ. Sci.: Wat. Res., 2 (1): 164-173.
- Rhoads, W.J., Garner, E., Ji, P., Zhu, N., Parks, J., Schwake, D.O., Pruden, A. and Edwards, M.A. (2017). Distribution system operational deficiencies coincide with reported Legionnaires’ disease clusters in Flint, Michigan. Environ. Sci. Technol., 51(20): 11986-11995.
- Rice, E.W., Clark, R.M. and Johnson, C.H. (1999). Chlorine inactivation of Escherichia coli O157:H7. Emerg. Infect. Dis., 5(3): 461-463.
- Richards, C.L., Broadaway, S.C., Eggers, M.J., Doyle, J., Pyle, B.H., Camper, A.K. and Ford, T.E. (2018). Detection of pathogenic and non-pathogenic bacteria in drinking water and associated biofilms on the Crow Reservation, Montana, USA. Microb. Ecol., 76(1): 52-63.
- Robertson, P., Abdelhady, H. and Garduño, R.A. (2014a). The many forms of a pleomorphic bacterial pathogen-the developmental network of Legionella pneumophila. Front. Microbiol. 5, art. no. 670: 1-20.
- Robertson, B.K., Harden, C., Selvaraju, S.B., Pradhan, S. and Yadav, J.S. (2014b). Molecular detection, quantification, and toxigenicity profiling of Aeromonas spp. in source- and drinking-water. Open Microbiol. J., 8(1): 32-39.
- Robins-Browne, R.M., Holt, K.E., Ingle, D.J., Hocking, D.M., Yang, J. and Tauschek, M. (2016). Are Escherichia coli pathotypes still relevant in the era of whole-genome sequencing? Front. Cell. Infect. Mi., 6 (NOV), art. no. 141.
- Robinson, B.S. and Christy, P.E. (1984). Disinfection of water for control of amoebae. Water, (11): 21-24.
- Rogues, A.-., Boulestreau, H., Lashéras, A., Boyer, A., Gruson, D., Merle, C., Castaing, Y., Bébear, C.M. and Gachie, J.-. (2007). Contribution of tap water to patient colonisation with Pseudomonas aeruginosa in a medical intensive care unit. J. Hosp. Infect., 67(1): 72-78.
- Rohr, U., Weber, S., Michel, R., Selenka, F. and Wilhelm, M. (1998). Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist sanitary areas by identifying genera and determining temperature tolerance. Appl. Environ. Microbiol., 64 (5), pp. 1822-1824.
- Rose, L.J., Rice, E.W., Hodges, L., Peterson, A. and Arduino, M.J. (2007). Monochloramine inactivation of bacterial select agents. Appl. Environ. Microbiol., 73(10): 3437-3439.
- Roser, D.J., Van Den Akker, B., Boase, S., Haas, C.N., Ashbolt, N.J. and Rice, S.A. (2014). Pseudomonas aeruginosa dose response and bathing water infection. Epidemiol. Infect., 142(3): 449-462.
- Sabina, Y., Rahman, A., Ray, R.C. and Montet D. (2011). Yersinia enterocolitica: Mode of transmission, molecular insights of virulence, and pathogenesis of infection. J. Pathog. 2011. Article ID 429069.
- Sanchez-Vargas, F.M., Abu-El-Haija, M.A. and Gomez-Duarte, O.G. (2011). Salmonella infections: An update on epidemiology, management, and prevention. Travel Med. Infect. Dis., 9(6): 263-277.
- Santiago, P., Moreno, Y. and Ferrús, M.A. (2015). Identification of viable Helicobacter pylori in drinking water supplies by cultural and molecular techniques. Helicobacter, 20(4): 252-259.
- Sarkar, P. and Gerba, C.P. (2012). Inactivation of Naegleria fowleri by chlorine and ultraviolet light. J. Am. Water Works Assoc., 104(3): 51-52.
- Saxena, G., Bharagava, R.N., Kaithwas, G. and Raj, A. (2015). Microbial indicators, pathogens and methods for their monitoring in water environment. J. Water Health, 13(2): 319-339.
- Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M., Roy, S. L. and Griffin, P. M. (2011). Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis., 17(1), 7-15. https://dx.doi.org/10.3201/eid1701.p11101.
- SCC. (2019). Directory of Accredited Product, Process and Service Certification Bodies. Standards Council of Canada, Ottawa, Ontario. Available at https://www.scc.ca/en/accreditation/product-process-and-service-certification/directory-of-accredited-clients
- Schaffter, N. and Parriaux, A. (2002). Pathogenic-bacterial water contamination in mountainous catchments. Water Res., 36(1): 131-139.
- Schiavano, G.F., De Santi, M., Sisti, M., Amagliani, G. and Brandi, G. (2018). Disinfection of Mycobacterium avium subspecies hominissuis in drinking tap water using ultraviolet germicidal irradiation. Environ.Technol. (United Kingdom), 39(24): 3221-3227.
- Schoen, M.E. and Ashbolt, N.J. (2011). An in-premise model for Legionella exposure during showering events. Water Res., 45(18): 5826-5836.
- Schulze-Robbecke, R. and Buchholtz, K. (1992). Heat susceptibility of aquatic mycobacteria. Appl. Environ. Microbiol., 58(6): 1869-1873.
- Schwake, D.O., Garner, E., Strom, O.R., Pruden, A. and Edwards, M.A. (2016). Legionella DNA markers in tap water coincident with a spike in Legionnaires’ disease in Flint, MI. Environ. Sci. Technol. Let., 3(9): 311-315.
- Sebakova, H., Kozisek, F., Mudra, R., Kaustova, J., Fiedorova, M., Hanslikova, D., Nachtmannova, H., Kubina, J., Vraspir, P. and Sasek, J. (2008). Incidence of nontuberculous mycobacteria in four hot water systems using various types of disinfection. Can. J. Microbiol., 54(11): 891-898.
- Segal, I., Otley, A., Issenman, R., Armstrong, D., Espinosa, V., Cawdron, R., Morshed, M.G. and Jacobson, K. (2008). Low prevalence of Helicobacter pylori infection in Canadian children: A cross-sectional analysis. Can. J. Gastroenterol., 22 (5):485-489.
- Sen, K. and Rodgers, M. (2004). Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: A PCR identification. J. Appl. Microbiol., 97(5): 1077-1086.
- Sethi, A., Chaudhuri, M., Kelly, L. and Hopman, W. (2013). Prevalence of Helicobacter pylori in a First Nations population in northwestern Ontario. Can. Fam. Physician, 59 (4): e182-e187.
- Siddiqui, R., Ali, I.K.M., Cope, J.R. and Khan, N.A. (2016). Biology and pathogenesis of Naegleria fowleri. Acta Trop., 164: 375-394.
- Sisti, M., Albano, A. and Brandi, G. (1998). Bactericidal effect of chlorine on motile Aeromonas spp. in drinking water supplies and influence of temperature on disinfection efficacy. Lett. Appl. Microbiol., 26(5): 347-351.
- Smeets, P., Rietveld, L., Hijnen, W., Medema, G. and Stenstrom, T. (2006). Efficacy of water treatment processes. In MicroRisk—microbiological risk assessment: A scientific basis for managing drinking water safety from source to tap. April 2006. Accessed June 2019 from: www.kwrwater.nl
- Smeets, P. W. M. H., Medema, G.J. and Van Dijk, J.C. (2009). The Dutch secret: How to provide safe drinking water without chlorine in the Netherlands. Drink. Water Eng. Sci., 2(1): 1-14.
- Sobsey, M.D. (1989). Inactivation of health-related microorganisms in water by disinfection processes. Water Sci. Technol., 21(3): 179-195.
- Soda, E.A. (2017). Vital signs: Health Care–Associated Legionnaires’ disease surveillance data from 20 states and a large metropolitan area—United States, 2015. MMWR Morb Mortal Wkly Rep, 66.
- Solnick, J.V., Hansen, L.M., Canfield, D.R. and Parsonnet, J. (2001). Determination of the infectious dose of Helicobacter pylori during primary and secondary infection in rhesus monkeys (Macaca mulatta). Infect. Immun., 69(11): 6887-6892.
- Sommer, R., Lhotsky, M., Haider, T. and Cabaj, A. (2000). UV inactivation, liquid-holding recovery, and photoreactivation of Escherichia coli O157 and other pathogenic Escherichia coli strains in water. J. Food Prot., 63(8): 1015-1020.
- Sood, G. and Parrish, N. (2017). Outbreaks of nontuberculous mycobacteria. Curr. Opin. Infect. Dis., 30(4): 404-409.
- Spinale, J.M., Ruebner, R.L., Copelovitch, L. and Kaplan, B.S. (2013). Long-term outcomes of Shiga toxin hemolytic uremic syndrome. Pediatr. Nephrol., 28 (11):2097-2105.
- Springston, J.P. and Yocavitch, L. (2017). Existence and control of Legionella bacteria in building water systems: A review. J. Occup. Environ. Hyg., 14(2): 124-134.
- Sterk, A., Schijven, J., De Nijs, T. and De Roda Husman, A.M. (2013). Direct and indirect effects of climate change on the risk of infection by water-transmitted pathogens. Environ. Sci. Technol., 47 (22):12648-12660.
- Stinear, T., Ford, T. and Vincent, V. (2004). Analytical methods for the detection of waterborne and environmental pathogenic mycobacteria. In Pathogenic mycobacteria in water. A guide to public health consequences, monitoring and management. Pedley, S. (ed.). IWA Publishing. World Health Organization., Geneva, Switzerland. pp. 55-73.
- Storey, M.V., Winiecka-Krusnell, J., Ashbolt, N.J. and Stenström, T.-. (2004). The efficacy of heat and chlorine treatment against thermotolerant acanthamoebae and legionellae. Scand. J. Infect. Dis., 36(9): 656-662.
- Stout, J.E., YU, V.L., Yee, Y.C., Vaccarello, S., Diven, W. and Lee, T.C. (1992). Legionella pneumophila in residential water supplies: Environmental surveillance with clinical assessment for Legionnaires’ disease. Epidemiol. Infect., 109(1): 49-57.
- Stout, J.E., et al., (2007). Role of environmental surveillance in determining the risk of hospital-acquired legionellosis: A national surveillance study with clinical correlations. Infect. Cont. Hosp. Ep., 28 (7): 818-824.
- Stout, J.E., Koh, W.-. and Yew, W.W. (2016). Update on pulmonary disease due to non-tuberculous mycobacteria. Int. J. Infect. Dis., 45: 123-134.
- Surman-Lee, S., Fields, B., Hornei, B., Ewig, S., Exner, M., Tartakovsky, I., Lajoie, L., Dangendorf, F., Bentham, R., Cabanes, P.A., Fourrier, P., Trouvet, T. and France Wallet, F. (2007). Chapter 2: Ecology and environmental sources of Legionella. In Legionella and the prevention of legionellosis. Bartram, J. et.al. (eds.). World Health Organization, Geneva, Switzerland. pp. 29-38.
- Taylor, R.H., Falkinham III, J.O., Norton, C.D. and LeChevallier, M.W. (2000). Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol., 66(4): 1702-1705.
- Tempark, T., Lueangarun, S., Chatproedprai, S. and Wananukul, S. (2013). Flood-related skin diseases: A literature review. Int. J. Dermatol., 52(10): 1168-1176.
- Teunis, P. and Figueras, M.J. (2016). Reassessment of the enteropathogenicity of mesophilic Aeromonas species. Front. Microbiol., 7(Article 1395):1-12.
- Thomas, M.K., Charron, D.F., Waltner-Toews, D., Schuster, C., Maarouf, A.R. and Holt, J.D. (2006). A role of high impact weather events in waterborne disease outbreaks in Canada, 1975-2001. Int. J. Environ. Heal. R., 16 (3): 167-180.
- Thomas, J.M. and Ashbolt, N.J. (2011). Do free-living amoebae in treated drinking water systems present an emerging health risk? Environ. Sci. Technol., 45(3): 860-869.
- Thomson, R.M., Carter, R., Tolson, C., Coulter, C., Huygens, F. and Hargreaves, M. (2013). Factors associated with the isolation of nontuberculous mycobacteria (NTM) from a large municipal water system in Brisbane, Australia. BMC Microbiol., 13(1).
- Todd, E.C.D. (2014). Bacteria: Yersinia enterocolitica and Yersinia pseudotuberculosis. In Encyclopedia of food safety. Volume 1. Academic Press, Cambridge, USA, pp. 574-580.
- Tossa, P., Deloge-Abarkan, M., Zmirou-Navier, D., Hartemann, P. and Mathieu, L. (2006). Pontiac fever: An operational definition for epidemiological studies. BMC Public Health, 6(1): 112.
- Trolio, R., Bath, A., Gordon, C., Walker, R. and Wyber, A. (2008). Operational management of Naegleria spp. in drinking water supplies in Western Australia. Water Sci. Technol.—W. Supp., 8(2): 207-215.
- US EPA. (1989). National Primary Drinking Water Regulations; Filtration, Disinfection; Turbidity, Giardia lamblia, Viruses, Legionella, and Heterotrophic Bacteria; Final Rule. Part III. Federal Register, 54:124:27486. (June 29, 1989).
- US EPA. (2001). Method 1605: Aeromonas in Finished Water by Membrane Filtration using Ampicillin-Dextrin Agar with Vancomycin (ADA-V). United States Environmental Protection Agency, Office of Water. Washington, DC. EPA-821-R-01-034.
- US EPA. (2006a). Aeromonas: Human Health Criteria Document. United States Environmental Protection Agency, Office of Water. Washington, D.C. March 2006.
- US EPA. (2006b). National primary drinking water regulations: Long term 2 enhanced surface water treatment rule. Final rule. Fed. regist., 71(3): 653–702.
- US EPA. (2016). Technologies for Legionella control in premise plumbing systems: Scientific literature review. United States Environmental Protection Agency, Office of Water. Washington, DC. EPA 810-R-16-001. September 2016.
- van der Wielen, P. W. J. J. and van der Kooij, D. (2013). Nontuberculous mycobacteria, fungi, and opportunistic pathogens in unchlorinated drinking water in the Netherlands. Appl. Environ. Microbiol., 79(3): 825-834.
- Vicuña-Reyes, J.P., Luh, J. and Mariñas, B.J. (2008). Inactivation of Mycobacterium avium with chlorine dioxide. Water Res., 42(6-7): 1531-1538.
- Vinnard, C., Longworth, S., Mezochow, A., Patrawalla, A., Kreiswirth, B.N. and Hamilton, K. (2016). Deaths related to nontuberculous mycobacterial infections in the United States, 1999-2014. Annals of the American Thoracic Society, 13(11): 1951-1955.
- Visvesvara, G.S., Moura, H. and Schuster, F.L. (2007). Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Mic., 50(1): 1-26.
- Waddell, L.A., Rajic, A., Stärk, K.D.C. and McEwen, S.A. (2015). The zoonotic potential of Mycobacterium avium ssp. paratuberculosis: A systematic review and meta-analyses of the evidence. Epidemiol. Infect., 143 (15):3135-3157.
- Waddell, L., Rajić, A., Stärk, K. and McEwen, S.A. (2016). Mycobacterium avium ssp. paratuberculosis detection in animals, food, water and other sources or vehicles of human exposure: A scoping review of the existing evidence. Prev. Vet. Med., 132:32-48.
- Wagenaar, J.A., Newell, D.G., Kalupahana, R.S. and Lapo Mughini-Gras, L. (2015). Campylobacter: Animal reservoirs, human infections, and options for control. In Zoonoses-infections affecting humans and animals: Focus on public health aspects. Sing, A. (ed.). Springer. Dordrecht, Netherlands. pp. 159-177.
- Wang, H., Edwards, M., Falkinham, J.O. and Pruden, A. (2012a). Molecular survey of the occurrence of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa, and amoeba hosts in two chloraminated drinking water distribution systems. Appl. Environ. Microbiol., 78(17): 6285-6294.
- Wang, H., Masters, S., Hong, Y., Stallings, J., Falkinham, J.O.,3rd, Edwards, M.A. and Pruden, A. (2012b). Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and two amoebas. Environ. Sci. Technol., 46(21): 11566-11574.
- Wang, H., Bédard, E., Prévost, M., Camper, A.K., Hill, V.R. and Pruden, A. (2017). Methodological approaches for monitoring opportunistic pathogens in premise plumbing: A review. Water Res., 117: 68-86.
- Watson, C.L., Owen, R.J., Said, B., Lai, S., Lee, J.V., Surman-Lee, S. and Nichols, G. (2004). Detection of Helicobacter pylori by PCR but not culture in water and biofilm samples from drinking water distribution systems in England. J. Appl. Microbiol., 97(4): 690-698.
- Weigel, K.M., Nguyen, F.K., Kearney, M.R., Meschke, J.S. and Cangelosi, G.A. (2017). Molecular viability testing of UV-inactivated bacteria. Appl. Environ. Microbiol., 83(10).
- Weintraub, J.M., Flannery, B., Vugia, D.J., Gelling, L.B., Salerno, J.J., Conroy, M.J., Stevens, V.A., Rose, C.E., Besser, R.E., Fields, B.S. and Moore, M.R. (2008). Legionella reduction after conversion to monochloramine for residual disinfection. J. Am. Water Works Assoc., 100(4): 129-139.
- Weiss, D., Boyd, C., Rakeman, J.L., et al. (2017). A large community outbreak of Legionnaires’ disease associated with a cooling tower in New York City, 2015. Public Health Rep., 132(2): 241-250.
- Whiley, H., van den Akker, B., Giglio, S. and Bentham, R. (2013). The role of environmental reservoirs in human campylobacteriosis. Int. J. Environ. Res. Public Health, 10(11): 5886-5907.
- Whiley, H., Keegan, A., Fallowfield, H. and Bentham, R. (2014). Detection of Legionella, L. pneumophila and Mycobacterium avium complex (MAC) along potable water distribution pipelines. Int. J. Env. Res. Pub. He., 11(7): 7393-7405.
- WHO. (2002). Guidelines for drinking-water quality Second Edition. Addendum: Microbiological agents in drinking water. World Health Organization, Geneva, Switzerland.
- WHO. (2004). Pathogenic Mycobacteria in Water—A Guide to Public Health Consequences, Monitoring and Management. Pedley, S. et al., (eds). World Health Organization, Geneva, Switzerland.
- WHO. (2007). Legionella and the prevention of legionellosis. Bartram, J. (ed.). World Health Organization, Geneva, Switzerland.
- WHO. (2011). Water safety in buildings. World Health Organization. Geneva, Switzerland.
- WHO. (2017). Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. World Health Organization. Geneva, Switzerland. Accessed October 2019 from https://www.who.int/medicines/areas/rational_use/prioritization-of-pathogens/en/
- Williams, M.M., Chen, T., Keane, T., Toney, N., Toney, S., Armbruster, C.R., Ray Butler, R. and Arduino, M.J. (2011). Point-of-use membrane filtration and hyperchlorination to prevent patient exposure to rapidly growing mycobacteria in the potable water supply of a skilled nursing facility. Infect. Con. Hosp. Ep., 32(9): 837-844.
- Wilson, R.E., Hill, R.L.R., Chalker, V.J., Mentasti, M., and Ready, D. (2018). Antibiotic susceptibility of Legionella pneumophila strains isolated in England and Wales 2007–17. J. Antimicrob. Chemoth., 73 (10): 2757-2761.
- Wingender, J. and Flemming, H.-. (2004). Contamination potential of drinking water distribution network biofilms. Water Sci. Technol., 49(11-12): 277-286.
- Winiecka-Krusnell, J., Wreiber, K., Von Euler, A., Engstrand, L. and Linder, E. (2002). Free-living amoebae promote growth and survival of Helicobacter pylori. Scand. J. Infect. Dis., 34(4): 253-256.
- Wojcicka, L., Hofmann, R., Baxter, C., Andrews, R.C., Auvray, I., Lière, J., Miller, T., Chauret, C. and Baribeau, H. (2007). Inactivation of environmental and reference strains of heterotrophic bacteria and Escherichia coli O157:H7 by free chlorine and monochloramine. J. Water Supply Res. Technol. Aqua, 56(2): 137-150.
- Wong, E.A. and Shin, G.-. (2015). Removal of Mycobacterium avium subspecies hominissuis (MAH) from drinking water by coagulation, flocculation and sedimentation processes. Lett. Appl. Microbiol., 60(3): 273-278.
- Wullings, B.A., Bakker, G. and Van, D.K. (2011). Concentration and diversity of uncultured Legionella spp. in two unchlorinated drinking water supplies with different concentrations of natural organic matter. Appl. Environ. Microbiol., 77(2): 634-641.
- Xue, Z., Hessler, C.M., Panmanee, W., Hassett, D.J. and Seo, Y. (2013). Pseudomonas aeruginosa inactivation mechanism is affected by capsular extracellular polymeric substances reactivity with chlorine and monochloramine. FEMS Microbiol. Ecol., 83(1): 101-111.
- Yoder, J., Roberts, V., Craun, G.F., Hill, V., Hicks, L.A., Alexander, N.T., Radke, V., Calderon, R.L., Hlavsa, M.C., Beach, M.J. and Roy, S.L. (2008). Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking—United States, 2005-2006. MMWR Surveill. Summ., 57(9): 39-62.
- Yoder, J.S., Eddy, B.A., Visvesvara, G.S., Capewell, L. and Beach, M.J. (2010). The epidemiology of primary amoebic meningoencephalitis in the USA, 1962-2008. Epidemiol. Infect., 138(7): 968-975.
- Yoder, J.S., Straif-Bourgeois, S., Roy, S.L., Moore, T.A., Visvesvara, G.S., Ratard, R.C., Hill, V.R., Wilson, J.D., Linscott, A.J., Crager, R., Kozak, N.A., Sriram, R., Narayanan, J., Mull, B., Kahler, A.M., Schneeberger, C., Da Silva, A.J., Poudel, M., Baumgarten, K.L., Xiao, L. and Beach, M.J. (2012a). Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin. Infect. Dis., 55(9): e7-e85.
- Yoder, J.S., Verani, J., Heidman, N., Hoppe-Bauer, J., Alfonso, E.C., Miller, D., Jones, D.B., Bruckner, D., Langston, R., Jeng, B.H., Joslin, C.E., Tu, E., Colby, K., Vetter, E., Ritterband, D., Mathers, W., Kowalski, R.P., Acharya, N.R., Limaye, A.P., Leiter, C., Roy, S., Lorick, S., Roberts, J. and Beach, M.J. (2012b). Acanthamoeba keratitis: The persistence of cases following a multistate outbreak. Ophthalmic Epidemiology, 19(4): 221-225.
- Yu, C.-P., Farrell, S.K., Robinson, B. and Chu, K.-. (2008). Development and application of real-time PCR assays for quantifying total and aerolysin gene-containing Aeromonas in source, intermediate, and finished drinking water. Environ. Sci. Technol., 42(4): 1191-1200.
- Zahran, S., McElmurry, S.P., Kilgore, P.E., Mushinski, D., Press, J., Love, N.G., Sadler, R.C. and Swanson, M.S. (2018). Assessment of the Legionnaires’ disease outbreak in Flint, Michigan. Proceedings of the National Academy of Sciences of the United States of America, 115(8): E173-E1739.
- Zamani, M., Ebrahimtabar, F., Zamani, V., Miller, W.H., Alizadeh-Navaei, R., Shokri-Shirvani, J. and Derakhshan, M.H. (2018). Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment. Pharm. Therap., 47 (7):868-876.
- Zaongo, S.D., Shaio, M.-F. and Ji, D.-D. (2018). Effects of culture media on Naegleria fowleri growth at different temperatures. J. Parasitol., 104 (5):451-456.
- Zautner, A.E., Masanta, W.O. (2016). Campylobacter: Health Effects and Toxicity. In Encyclopedia of Food and Health. Caballero, B. et al. (eds.). Academic Press, Cambridge, USA. pp. 596-601.
- Zhang, Y.Q., Wu, Q.P., Zhang, J.M., Yang, X.H. (2015). Effects of ozone on the cytomembrane and ultrastructure of Pseudomonas aeruginosa. Food Sci. Biotechnol., 24(3): 987-993.
- Zimmer, J.L. and Slawson, R.M. (2002). Potential repair of Escherichia coli DNA following exposure to UV radiation from both medium- and low-pressure UV sources used in drinking water treatment. Appl. Environ. Microbiol., 68 (7): 3293-3299.
- Zimmer-Thomas, J.L., Slawson, R.M. and Huck, P.M. (2007). A comparison of DNA repair and survival of Escherichia coli O157:H7 following exposure to both low- and medium- pressure UV irradiation. J. Water Health, 5(3): 407-415.
- Zuma, F.N., Lin, J. and Jonnalagadda, S.B. (2009). Kinetics of inactivation of Pseudomonas aeruginosa in aqueous solutions by ozone aeration. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng., 44(10): 929-935.
Appendix A: List of Acronyms
- AIDS
- acquired immunodeficiency syndrome
- AK
- Acanthamoeba keratitis
- ANSI
- American National Standards Institute
- ASHRAE
- American Society of Heating, Refrigerating, and Air-Conditioning Engineers
- CDC
- Centers for Disease Control and Prevention
- CDW
- Committee on Drinking Water (Federal-Provincial-Territorial)
- CFU
- colony forming units
- CT
- concentration (C) × time (T)
- DAEC
- diffuse adherent Escherichia coli
- DNA
- deoxyribonucleic acid
- EAEC
- enteroaggregative Escherichia coli
- EHEC
- enterohaemorrhagic Escherichia coli
- EIEC
- enteroinvasive Escherichia coli
- EPEC
- enteropathogenic Escherichia coli
- ESBL
- extended spectrum β-lactamase
- ETEC
- enterotoxigenic Escherichia coli
- EU
- European Union
- GAC
- granulated activated carbon
- GAE
- granulomatous amoebic encephalitis
- HIV
- human immunodeficiency virus
- HPC
- heterotrophic plate count
- HUS
- hemolytic uremic syndrome
- HVAC
- heating, ventilation and air conditioning
- IARC
- International Agency for Research on Cancer
- ISO
- International Organization for Standardization
- NAS
- National Academies of Sciences
- NPC
- National Plumbing Code (Canada)
- NSF
- NSF International
- NTM
- non-tuberculous mycobacteria
- PAM
- primary amebic meningoencephalitis
- PCR
- polymerase chain reaction
- PHAC
- Public Health Agency of Canada
- POE
- point-of-entry
- POU
- point-of-use
- QMRA
- quantitative microbial risk assessment
- SCC
- Standards Council of Canada
- spp.
- species
- US EPA
- United States Environmental Protection Agency
- US
- United States
- UV
- ultraviolet
- VBNC
- viable but non-culturable
- VTEC
- verotoxin-producing Escherichia coli
- WHO
- World Health Organization
| Pathogen | Members most frequently associated with human illness | Health Effects | Groups at higher risk for illness. | Main reservoirs | Major route for waterborne transmission | Significance as a drinking water pathogen |
|---|---|---|---|---|---|---|
| Campylobacter spp. | C. jejuni, C. coli | Gastroenteritis (symptoms: watery, profuse and sometimes bloody diarrhea; fever and abdominal pain). | Young children, young adults, the elderly. | Poultry, cattle, sheep, domestic pets. | Ingestion of fecally contaminated water. | Well-documented as a cause of outbreaks associated with contaminated drinking water. |
| Pathogenic Escherichia coli/Shigella spp. | Enterohaemorrhagic E. coli (EHEC) group. E. coli serotype O157:H7 is the most prevalent. | Gastroenteritis (symptoms: watery diarrhea that can be accompanied by blood, nausea, vomiting, abdominal pain, fever). EHEC illness can progress to the hemolytic uremic syndrome (HUS), a potentially life-threatening condition involving decreased blood cell and platelet counts and acute kidney failure. | Young children, the elderly. | Cattle and other ruminants, humans. | Ingestion of fecally-contaminated drinking water. | Well-documented as a cause of outbreaks associated with contaminated drinking water. |
| Shigella spp.: S. sonnei and S. flexneri | Gastroenteritis (symptoms: watery diarrhea that can be accompanied by blood, abdominal pain, fever). | Young children. | Humans. | Ingestion of fecally-contaminated water. | Rarely linked to drinking water outbreaks | |
| Helicobacter pylori | H. pylori | Asymptomatic superficial gastritis. Some infections develop into peptic (e.g., duodenal or gastric) ulcers. | Individuals living in areas with crowded or high density living conditions and/or poor sanitation. | Humans. | Ingestion of fecally-contaminated water suspected as a possible route. | Further research is needed on the importance of drinking water as a source of infection. |
| Salmonella spp. | Non-typhoidal Salmonella group, particularly: S. serotype Enteritidis and S. serotype Typhimurium. | Gastroenteritis (diarrhea, fever and abdominal pain). Severe cases with immunocompromised individuals: can spread to other parts of the body (e.g., blood, urine, joints, brain) | Young children, the elderly, persons with weakened immune systems. | Chicken, pigs, turkey and cattle. | Ingestion of fecally-contaminated water. | Rarely linked to drinking water outbreaks. |
| Yersinia spp. | Y. enterocolitica, Y. paratuberculosis | Gastroenteritis ranging in severity depending on the strain (symptoms: diarrhea that can be accompanied by blood, fever and abdominal pain in children; appendicitis-like symptoms in adults). | Young children, the elderly, persons with weakened immune systems. | Y. enterocolitica: Pigs, ruminants, dogs, cats. Y. paratuberculosis: rodents, birds | Ingestion of fecally-contaminated water. | Rarely linked to drinking water outbreaks. |
| Pathogen | Members most frequently associated with human illness | Health Effects | Groups at higher risk for illness. | Main reservoirs | Major route for waterborne transmission | Significance as a drinking water pathogen |
|---|---|---|---|---|---|---|
| Aeromonas spp. | A. hydrophila, A. caviae, A. veronii (biotype sobria), A. trota | Linked to a variety of intestinal and extra-intestinal diseases and syndromes. Gastroenteritis is the most common disease (symptoms: watery diarrhea along with low grade fever, vomiting and abdominal pain). |
Young children, the elderly, persons with weakened immune systems. | Ubiquitous bacteria, found in a wide variety of habitats, including aquatic habitats, soils, vertebrate and invertebrate animal species, insects and foods. |
Ingestion of contaminated water. | Further research is needed on the importance of drinking water as a source of infection. |
| Legionella spp. | L. pneumophila (mainly serogroup 1) | Legionnaires' disease: severe respiratory illness involving pneumonia. Pontiac Fever: milder, flu-like, self-limiting and non-pneumonic disease. |
The elderly, smokers, persons with weakened immune systems or underlying disease. | Free-living protozoa that can be found within biofilms in the natural environment and in engineered water systems (large plumbing systems, cooling towers, drinking water distribution systems). |
Inhalation of contaminated aerosols generated by devices associated with HVAC systems, plumbing systems and humidification equipment. | Well-documented as a cause of outbreaks associated with water systems (cooling towers, plumbing systems) of large buildings (most commonly hospitals, long-term care facilities, hotels and resorts). |
| Mycobacterium spp. | Non-tuberculous mycobacteria (NTM), group, particularly: M. avium complex (MAC): M. avium and its subspecies, M. intracellulare, and M. chimaera |
Pulmonary disease. Symptoms: persistent cough, weakness and night sweats. Severe cases with immunocompromised individuals, infection can spread to other parts of the body (e.g., joints, liver, brain). Other diseases: cervical lymphadenitis, skin and soft tissue infections. |
Individuals with weakened immune systems or underlying respiratory conditions. | Soils, water habitats, biofilms in engineered water systems (plumbing systems, drinking water distribution systems). |
Inhalation of contaminated aerosols from aerosol -generating devices associated with plumbing systems and humidification equipment. | No reported outbreaks associated with drinking water. Contaminated water can be an important source for infections in specific settings (e.g., health care facilities) for at risk groups. |
| Pseudomonas spp. | P. aeruginosa | Respiratory infections (symptoms: fever, chills, cough and laboured breathing); infections involving the skin, eyes, ears and urinary tract. | Individuals with weakened immune systems or underlying diseases (particularly cystic fibrosis), patients undergoing procedures with invasive medical devices or have burns or penetrating trauma (surgical incisions, wounds). | Ubiquitous bacteria found in a wide variety of habitats, including soil, aquatic environments, vegetation and biofilms in engineered water systems (plumbing systems). |
Direct body contact with contaminated water or devices in contact with water from contaminated engineered water systems. | No reported outbreaks associated with drinking water. Contaminated water can be an important source for infections in specific settings (e.g., health care facilities) for at risk groups. |
| Acanthamoeba spp. | Acanthamoeba genotype T4 | Acanthamoeba keratitis (AK), a vision-threatening disease (symptoms: blurred vision, intense pain and photosensitivity; later, severe cases, ulceration, swelling, glaucoma, cataracts and blindness). | Contact lens wearers. | Ubiquitous in soil and water; also present in biofilms in engineered water systems (plumbing systems, drinking water distribution systems cooling towers) and in airborne dust. |
Eye contact with lenses exposed to water containing the organisms during lens washing, storage or wear. | Incidence of disease is rare. Can act as hosts for pathogenic bacteria including Legionella and non-tuberculous mycobacteria. |
| Naegleria spp. | N. fowleri | Primary Amebic Meningoencephalitis (symptoms similar to viral or bacterial meningitis: headache, fever, nausea and vomiting; later: stiff neck, altered mental status, occasional hallucinations seizures, coma). Infections almost always fatal. | Children and young adults engaging in recreational water activities where organism is prevalent; individuals performing nasal cleansing with non-sterile water. | Warm freshwater environments (lakes, rivers, hot springs) and soils. Can adapt to growth in biofilms in distribution and plumbing systems if conditions are favourable (optimal |
Intranasal contact with contaminated water through diving, swimming, bathing, splashing or nasal cleansing. | Incidence of disease is rare. Infections and isolations from piped water systems have primarily occurred in areas with a subtropical climate. Can act as hosts for pathogenic bacteria including Legionella and non-tuberculous mycobacteria. |
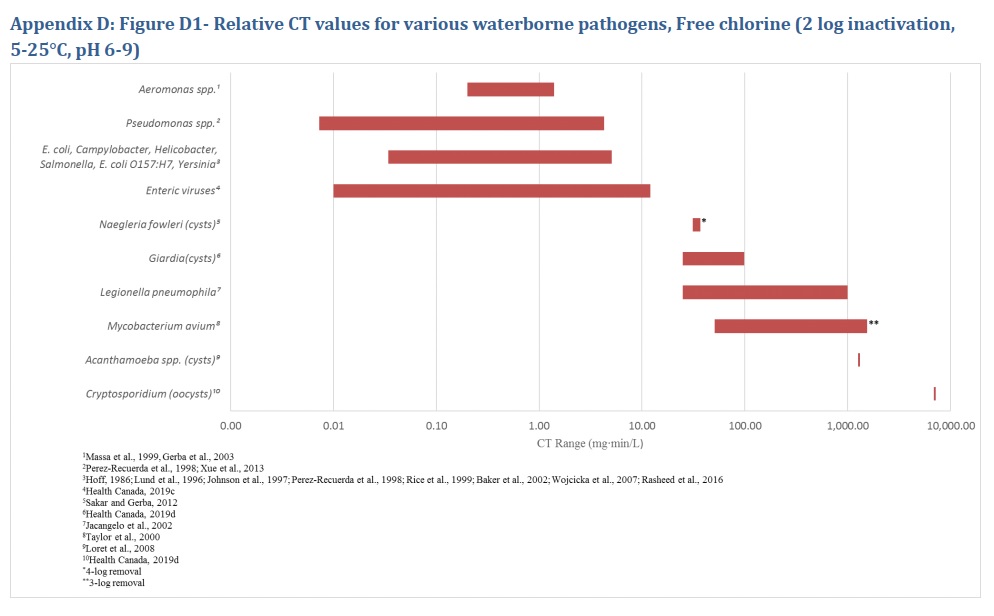
Figure - Text Description
A chart showing the CT values required for achieving a 2 log reduction in numbers for various waterborne pathogens using free chlorine. The x-axis on the chart is the CT values expressed as milligram minutes per Litre. It is presented on the logarithmic scale and the axis values range from 0.001 to 10000. The y-axis displays different pathogens or groups of pathogens. Each pathogen or group has a footnote marker that corresponds to the citations used as the sources of information. The list of footnotes is displayed beneath the chart. In the chart, the CT values are displayed as bars that extend from the lowest value reported in the citations to the highest. The CT values displayed for the pathogens or groups are: 1. Aeromonas spp.: 0.2 to 1.4; 2. Pseudomonas spp.: 0.0073 to 4.3; 3. The group of E. coli, Campylobacter, Helicobacter, Salmonella, E. coli O157:H7 and Yersinia: 0.034 to 5.1; 4. Enteric viruses: 0.01 to 12; 5. Naegleria fowleri cysts: 31 to 37; 6. Giardia cysts: 25 to 99; 7. Legionella pneumophila: 25 to 1000; 8. Mycobacterium avium: 51 to 1552; 9. Acanthamoeba spp. cysts: 1300; 10. Cryptosporidium oocysts: 7200. The chart has two footnote markers – one (*) beside the bar for Naegleria fowleri cysts to show that these values are for a 4 log reduction, and one (**) beside the bar for Mycobacterium avium to show that these values are for a 3 log reduction. The footnote information displayed is: 1. Massa et al., 1999; Gerba et al., 2003; 2. Perez-Recuerda et al., 1998; Xue et al., 2013; 3. Hoff, 1986; Lund et al., 1996; Johnson et al., 1997; Perez-Recuerda et al., 1998; Rice et al., 1999; Baker et al., 2002; Wojcicka et al., 2007; Rasheed et al., 2016; 4. Health Canada, 2019c; 5. Sakar and Gerba, 2012; 6. Health Canada, 2019d; 7. Jacangelo et al., 2002; 8. Taylor et al., 2000; 9. Loret et al., 2008; 10. Health Canada, 2019d; (*) 4 log removal; (**) 3 log removal.

Figure - Text Description
A chart showing the UV dose requirements for achieving a 4 log reduction in numbers for various waterborne pathogens. The x-axis on the chart is the UV dose expressed as millijoules per square centimetre. It is presented on the logarithmic scale and the axis values range from 1 to 1000. The y-axis displays different pathogens or groups of pathogens. Each pathogen or group has a footnote marker that corresponds to the citations used as the sources of information. The list of footnotes is displayed beneath the chart. In the chart, the UV doses are displayed as bars that extend from the lowest value reported in the citations to the highest. The UV doses displayed for the pathogens or groups are: 1. Pseudomonas spp.: 3.1; 2. Aeromonas spp.: 2.5 to 8; 3. Cryptosporidium oocysts: 22; 4. Giardia cysts: 22; 5. Legionella pneumophila: 11 to 30; 6. The group of E. coli, Campylobacter, Helicobacter, Salmonella, E. coli O157:H7 and Yersinia: 5 to 51; 7. The group of Hepatitis A, Coxsackievirus, Poliovirus, Rotavirus: 16.4 to 61; 8. Mycobacterium avium: 12.3 to 64; 9. Naegleria fowleri cysts: 121; 10. Acanthamoeba spp. cysts: 167; 11. Some strains of Mycobacterium spp.: 96 to192; 12. Adenovirus: 51 to 261. The chart has two footnote markers – one (*) beside the bar for Aeromonas spp.: to show that these values are for a 2 log reduction, and one (**) beside the bar for Some strains of Mycobacterium spp.:to show that these values are for a 2 to 5 log reduction. The footnote information displayed is: 1. Clauβ et al., 2006; 2. Gerba et al., 2003; 3. Health Canada, 2019d; 4. Health Canada, 2019d; 5. Hijnen et al., 2011; 6. Zimmer and Slawson, 2002; Hayes et al., 2006; Zimmer-Thomas et al., 2007; Hijnen et al., 2011; 7. Health Canada, 2019c; 8. Hayes et al., 2008; Schiavano et al., 2018; 9. Sakar and Gerba, 2012; 10. Hijnen et al., 2011; 11; Gerba et al., 2003, Schiavano et al., 2018; 12. Health Canada, 2019c; (*) 2 log reduction; (**) 2 to 5 log reduction.
Page details
- Date modified: