Rocky Mountain tailed frog (Ascaphus montanus): COSEWIC assessment and status report

Threatened
2013
Table of Contents
- Document Information
- COSEWIC Assessment Summary
- COSEWIC Executive Summary
- Technical Summary
- Preface
- Wildlife Species Description and Significance
- Distribution
- Habitat
- Biology
- Population Sizes and Trends
- Threats and Limiting Factors
- Protection, Status, and Ranks
- Acknowledgements and Authorities Contacted
- Information Sources
- Biographical Summary of Report Writer(s)
- Collections Examined
List of Figures
- Figure 1. Rocky Mountain Tailed frog, Ascaphus montanus. A) adult male, Bonner County, Idaho. B) tadpole, Idaho County, Idaho. Photos: Gary Nafis
- Figure 2. Mitochondrial DNA genetic variation within the Rocky Mountain Tailed Frog. A) Maximum-likelihood tree estimated from cytochrome b sequence data under the HKY+Γ model of sequence evolution, using Coastal Tailed Frog sequences as the outgroup. Letters indicate different mtDNA haplotypes. Numbers above branches are maximum-likelihood bootstrap values (100 replicates); those below branches are Bayesian estimates of nodal support (4 chains of 107 generations each). B) Distribution of mtDNA haplotypes within the range of the Rocky Mountain Tailed Frog, indicating the northern and southern clades. Source: after Nielson et al. (2006)
- Figure 3. Distribution of Ascaphus montanus in North America. The U.S. locations indicated on the map are occurrences of the species at the level of county (Idaho and Montana) or subcounty (Washington and Oregon). Adapted from Green et al. (in press)
- Figure 4. Range of Ascaphus montanus in Canada. Localities (= element occurrences) are indicated as red dots (Source: British Columbia Conservation Data Centre 2012). Open symbols show approximate locations of unconfirmed records from Montana electrofishing surveys 2008 – 2012 (modifications to map by Ian Adams)
- Figure 5. Frog observations during fish sampling (electrofishing) by Montana Dept. of Fish, Wildlife and Parks personnel in the Canadian Flathead River watershed, 2008 – 2012. If validated, the two southeastern and the far northern records (red symbols) increase the known distribution of the species within the Flathead (Map source: Amber Steed, Montana Dept. of Fish, Wildlife and Parks)
- Figure 6. Estimates of A) area of range extent within occupied drainages and B) index of area of occupancy for the Rocky Mountain Tailed Frog in Canada. Source: BC Conservation Data Centre (2012). The extent of occurrence using a minimum convex polygon and including the intervening unoccupied habitat is 1,900 km² (or 3,300 km² including recent unconfirmed Montana electrofishing records, not shown)
- Figure 7. Rocky Mountain Tailed Frog tadpole and adult distribution in A) the Yahk River watershed and B) the Flathead River watershed based on data from timed searches in 2001 (Yahk) and 2003 (Flathead) during late summer. Source: Dupuis and Friele (2006)
- Figure 8. Distribution of Rocky Mountain Tailed Frog habitat in A) the Yahk River watershed and B) the Flathead River watershed in southeast British Columbia (source: adapted from Cordilleran Geoscience and ESSA Technologies 2010).
- Figure 9. Anticipated effects of climate change on ecosystem distribution in southern British Columbia. The maps show a progressive loss of the Engelmann Spruce/Subalpine Fir (ESSF) ecological zone and its replacement by the Interior Cedar/Hemlock Zone (ICH) ecological zone in the mountains of the extreme southeast, as well as the spread of the Bunch Grass (BG) ecological zone in the southern Rocky Mountain Trench. The ecological zones are: CDF, Coastal Douglas-fir; CWH, Coastal Western Hemlock; BG, Bunchgrass; PP, Ponderosa Pine; IDF, Interior Douglas-fir; ICH, Interior Cedar–Hemlock; SBPS, Sub-boreal Pine and Spruce; SBS, Sub-boreal Spruce; BWBS, Boreal White and Back Spruce; MH, Mountain Hemlock; ESSF, Engelmann Spruce–Subapline Fir; MS, Montane Spruce; SWB, Spruce–Willow–Birch; AT, Alpine Tundra. After Hamann and Wang (2006).
- Figure 10. Wildlife Habitat Areas (WHAs) for Rocky Mountain Tailed Frogs established in A) the Yahk River watershed (purple stream sections) and B) the Flathead River watershed (red stream sections). Source: Ascaphus Consulting (2005).
Document Information

Committee on the Status of Endangered Wildlife in Canada (COSEWIC) status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2013. COSEWIC assessment and status report on the Rocky Mountain Tailed Frog Ascaphus montanus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 46 pp.
Previous report(s):
COSEWIC. 2000. COSEWIC assessment and status report on the Rocky Mountain Tailed Frog Ascaphus montanus and the Coast Tailed Frog Ascaphus truei in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 29 pp.
Dupuis, L.A. 2000. COSEWIC assessment and status report on the on the Rocky Mountain Tailed Frog Ascaphus montanus and the Coast Tailed Frog Ascaphus truei in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-29 pp.
Production note:
COSEWIC would like to acknowledge David M. Green for writing the update status report on the Rocky Mountain Tailed Frog (Ascaphus montanus) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Kristiina Ovaska, Co-chair of the COSEWIC Amphibians and Reptiles Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-953-3215
Fax: 819-994-3684
COSEWIC E-mail
COSEWIC web site
Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur la Grenouille-à-queue des Rocheuses (Ascaphus montanus) au Canada.
Cover illustration/photo:
Rocky Mountain Tailed Frog - Reproduced with permission by Bureau of Fisheries, New York State Department of Environmental Conservation.
©Her Majesty the Queen in Right of Canada, 2014.
Catalogue No. CW69-14/195-2014E-PDF
ISBN 978-1-100-23536-3

COSEWIC Assessment Summary
Assessment Summary - November 2013
- Common name
- Rocky Mountain Tailed Frog
- Scientific name
- Ascaphus montanus
- Status
- Threatened
- Reason for designation
- In Canada, this unusual stream-breeding frog is restricted to two unconnected watersheds, where it relies on small, forested fast-flowing streams. Habitat damage from sedimentation due primarily to roads, logging, and fires, and loss of terrestrial dispersal habitat from logging and wood harvesting are key threats. The total population is small, consisting of approximately 3000 adults, which increases the vulnerability of the population to environmental perturbations. Increases in habitat protection and a moratorium on mining in the Flathead River portion of the range resulted in a change of status from Endangered.
- Occurrence
- British Columbia
- Status history
- Designated Endangered in May 2000. Status re-examined and designated Threatened in November 2013.
COSEWIC Executive Summary
Rocky Mountain Tailed Frog
Ascaphus montanus
Wildlife Species Description and Significance
Adult Rocky Mountain Tailed Frogs are small frogs with a large head, a vertical pupil, broad and flattened outer hind toes and no ear drum. They vary in colour from tan or brown to olive green or red, and there is often a distinct, dark-edged copper bar between the eyes. Males have a short, conical extension of the cloaca, the source of the name “tailed frog”, which is used for copulation. The tadpoles possess an oral disc modified into a sucker for clinging to rocks in swift currents. They are mottled black and tan with a prominent, black-bordered white spot at the tip of the tail.
The two species of tailed frogs, genus Ascaphus, are among the most primitive living frogs in the world and are specialized for life in fast-flowing streams. Rocky Mountain Tailed Frogs are also one of the longest lived of all North American frogs and the slowest to develop, spending 3 years as tadpoles and not attaining sexual maturity until 7 – 8 years of age.
Distribution
Rocky Mountain Tailed Frogs occur from extreme southeastern British Columbia south through western Montana and Idaho north of the Snake River Plain to the Wallowa Mountains of northeastern Oregon and Blue Mountains of extreme southeastern Washington. In Canada, Rocky Mountain Tailed Frogs are restricted to two disjunct mountainous localities, the Flathead River watershed and the Yahk River watershed, separated by the Rocky Mountain Trench.
Habitat
Rocky Mountain Tailed Frogs are restricted to small, permanently flowing, middle elevation creeks in coniferous forest. They are most often associated with rapidly flowing, step-pool streams with streambeds composed largely of smooth rocks, cobbles and boulders, rather than silt, sand or pebbles.
Biology
Tailed frogs have low reproductive rates compared to other frogs, laying relatively small clutches of 50 – 85 colourless, pea-sized eggs every other year. They are cold-adapted and can withstand temperatures only as high as 21°C. Adult Rocky Mountain Tailed Frogs are nocturnal and extremely site-specific, generally dispersing no more than 20 m in a year. The tadpoles eat mainly diatoms scraped from submerged rocks, but transformed frogs will eat a wide variety of terrestrial arthropods. Predators of Rocky Mountain Tailed Frogs include American Dipper, Cutthroat Trout, Garter Snakes, and Western Toad.
Population Sizes and Trends
No capture – recapture surveys of Rocky Mountain Tailed Frogs have been attempted and the number of breeding adults associated with each creek is not known with certainty, but the entire Canadian population is estimated to be ca. 3000 individuals. Larval densities in Canada range from 0.06 to 1.8 individuals/m2 of stream. No data are available to assess population trends. Although dispersal movements of Rocky Mountain Tailed Frogs are poorly known, individuals are more likely to move along stream corridors rather than overland and tend not to move very far; thus the potential for rescue from neighbouring populations in the USA is limited.
Threats and Limiting Factors
Major threats to Rocky Mountain Tailed Frogs in Canada include increases in stream sedimentation, alteration of hydrological regimes, loss of riparian forest habitat and headwater linkages, stochastic environmental and demographic fluctuations due to low population size, and climate change resulting in stream habitat contraction. Human activities associated with logging, mining and road building can exacerbate these threats. Wildfires can have a significant, negative, short-term effect on abundances of Rocky Mountain Tailed Frog tadpoles; however, this species may be able to recover from wildfire within a decade. Epizootic chytridiomycosis disease caused by the fungus Batrachochytrium dendrobatidis has been identified as a major threat to amphibian populations around the world, but at present there is no evidence of significant infection or disease among Rocky Mountain Tailed Frogs. A ban on mining exploration and development under the Flathead Watershed Area Conservation Act has reduced threats in the Flathead portion of the species’ range.
Protection, Status, and Ranks
As of 2004, the Global Status rank of the Rocky Mountain Tailed Frog is G4 (apparently secure), according to NatureServe. At the national level, as of 2011, its U.S. status is N4 (apparently secure) and its Canadian and British Columbia status is N2 (imperilled).
Habitat protection has increased significantly since the previous COSEWIC status assessment in 2000. Ten Wildlife Habitat Areas (WHAs) for Rocky Mountain Tailed Frogs were established in the Flathead River watershed and another nine in the Yahk River watershed under the Forest and Range Practices Act in 2005. As of 2011, these WHAs are considered to be under the Oil and Gas Activities Act. The WHAs altogether cover 1,239 ha of habitat and are intended to protect all known breeding and adjacent foraging habitats for Rocky Mountain Tailed Frogs in British Columbia. The effectiveness of the protection in reducing chronic siltation from the surrounding landscape remains to be established and is currently monitored using sentinel sites.
Technical Summary
Ascaphus montanus
Rocky Mountain Tailed Frog
Grenouille-à-queue des Rocheuses
- Range of occurrence in Canada (province/territory/ocean):
- British Columbia
Demographic Information
Generation time As age of maturity is 7 – 8 years post-hatching, and longevity is up to 14 yrs, then average age of adults is likely to be 9 – 11 yrs
9 – 11 yrs
Is there an [observed, inferred, or projected] continuing decline in number of mature individuals? A decline is inferred from habitat trends, based mainly on chronic sedimentation associated with roads, wildfires, and landslides in both Yahk and Flathead drainages, and logging in the Yahk drainage.
Yes
Estimated percent of continuing decline in total number of mature individuals within [5 years or 2 generations]
No data
[Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over the last [10 years, or 3 generations].
No data
[Projected or suspected] percent [reduction or increase] in total number of mature individuals over the next [10 years, or 3 generations]. Suspected reduction based on threats, mainly chronic sedimentation from various sources, as indicated by the IUCN threats calculator results.
≥10%
[Observed, estimated, inferred, or suspected] percent [reduction or increase] in total number of mature individuals over any [10 years, or 3 generations] period, over a time period including both the past and the future.
No data (but see above)
Are the causes of the decline clearly reversible and understood and ceased?
No
Are there extreme fluctuations in number of mature individuals?
No
Extent and Occupancy Information
Estimated extent of occurrence
Total EO encompassing all confirmed occurrences but excluding uninhabited region between the Yahk and Flathead watersheds:
331 km2
1,900 km2
Total EO encompassing all confirmed occurrences:
3,300 km2
Total EO encompassing all confirmed and recent, unconfirmed, occurrences:
Index of area of occupancy (IAO, based on 2x2 km grid):
IAO encompassing all confirmed occurrences:
296 km2
IAO encompassing all confirmed and recent, unconfirmed, occurrences:
308 km2
Is the population severely fragmented? The frogs inhabit streams that that are physiographically isolated from each other, with connections only at lower reaches in habitats that are uninhabitable by the frogs or tadpoles. Connectivity between these subpopulations is likely maintained by overland dispersal via comparatively rare and difficult to document long-distance movements. Curtailment of these movements will isolate subpopulations resulting in fragmentation of metapopulations. Viability of subpopulations is unknown.
Possible, but supporting data are lacking.
Number of locationsFootnote∗
Unknown but probably <10
Is there an observed, inferred, or projected continuing decline in extent of occurrence?
No
Is there a projected continuing decline in index of area of occupancy?
Unknown
Is there an observed, inferred, or projected continuing decline in number of populations?
No
Is there an observed, inferred, or projected continuing decline in number of locationsFootnote∗?
No
Is there a projected continuing decline in area, extent and/or quality of habitat?
Yes
Are there extreme fluctuations in number of populations?
No
Are there extreme fluctuations in number of locationsFootnote∗?
No
Are there extreme fluctuations in extent of occurrence?
No
Are there extreme fluctuations in index of area of occupancy?
No
| Population (based on area-constrained and time-constrained searches) | Number of Mature Individuals |
|---|---|
| Yahk ¬River Watershed | 1,000 - 2,500 |
| Flathead River Watershed | 500 – 2,000 |
| Total | ca. 3,000 |
Threats (actual or imminent, to populations or habitats)
Main threats: Stream sedimentation from roads, logging, and fires; loss of terrestrial dispersal habitat from logging and wood harvesting.
Additional threats: fire and fire suppression, mining and quarrying, recreational ATV traffic, and drought, stream warming and habitat alteration associated with climate change.
Rescue Effect (immigration from outside Canada)
Status of outside population(s)?
Oregon: S2
Washington: S2
Idaho: S3,
Montana: S4
Is immigration known or possible?
Not known; possible only in limited areas near the border in the Flathead drainage
Would immigrants be adapted to survive in Canada?
Yes
Is there sufficient habitat for immigrants in Canada?
Possibly within already occupied drainages
Is rescue from outside populations likely?
Possible, but limited to areas near the border in the Flathead drainage
Data-Sensitive Species
- Is this a data-sensitive species?
- No
Status History
- COSEWIC:
- Designated Endangered in May 2000. Status re-examined and designated Threatened in November 2013.
Status and Reasons for Designation
- Status:
- Threatened
- Alpha-numeric code:
- C1+2a(i)
- Reason for Designation:
- In Canada, this unusual stream-breeding frog is restricted to two unconnected watersheds, where it relies on small, forested fast-flowing streams. Habitat damage from sedimentation due primarily to roads, logging, and fires, and loss of terrestrial dispersal habitat from logging and wood harvesting are key threats. The total population is small, consisting of approximately 3000 adults, which increases the vulnerability of the population to environmental perturbations. Increases in habitat protection and a moratorium on mining in the Flathead River portion of the range resulted in a change of status from Endangered.
- Criterion A (Decline in Total Number of Mature Individuals):
- Numbers might be declining, especially in the Yahk drainage due to ongoing threats, but there are no accurate data on population trends. A decline of 10% of more is suspected over the next 10 years, based on threats, mainly chronic sedimentation from various sources, as indicated by IUCN threats calculator results.
- Criterion B (Small Distribution Range and Decline or Fluctuation):
- Not applicable. Although the EO and IAO are below the threshold for Endangered, there may be more than 10 locations, the population is not severely fragmented within either of the two occupied drainages, and there is no evidence of severe fluctuations.
- Criterion C (Small and Declining Number of Mature Individuals):
- Meets Threatened under C1 because the number of mature individuals is less than 10,000 adults, and there is an inferred continuing decline in the number of mature individuals greater than 10% based on habitat trends, particularly in the Yahk drainage. Also meets Threatened C2a(i) because no subpopulation is estimated to contain more than 1000 mature individuals (subpopulations are frogs within 8 subdrainages in the Yahk and Flathead drainages).
- Criterion D (Very Small or Restricted Population):
- Does not meet criterion.
- Criterion E (Quantitative Analysis):
- Not enough information is available for analysis.
Preface
The Rocky Mountain Tailed Frog was last assessed by COSEWIC in 2000 along with the Coastal Tailed Frog in a single report as Ascaphus truei. Since then, genetic studies have shown that the Coastal and Rocky Mountain populations of tailed frogs have diverged significantly and represent separate species, now known as the Coastal Tailed Frog (Ascaphus truei) and the Rocky Mountain Tailed Frog (A. montanus). Recent work by Spear and Storfer (2010) has clarified some of the biological differences between Rocky Mountain Tailed Frogs and Coastal Tailed Frogs.
Since the 2000 assessment, extensive surveys for Rocky Mountain Tailed frogs in southeastern British Columbia by Ascaphus Consulting (2002, 2005) and Dupuis and Friele (2006) better defined the distribution of the species but uncovered no additional populations or inhabited watersheds. Montana electrofishing surveys from 2008 to 2012 reported the species from three new localities to the east and north of the previous records in the Flathead drainage, increasing the extent of occurrence from 1,900 km2 to 3,300 km2 . The frogs are patchily distributed within both the Yahk and Flathead drainages at the northern limits of the species' range in Canada. Ascaphus Consulting (2005) concluded that Rocky Mountain Tailed Frogs are limited to basins between 0.3 km² – 100 km² in extent. Dupuis (2007) identified the threats to populations in Canada to include stream sedimentation, alteration of hydrological regimes, loss of forest structure and cover (riparian habitat and headwater linkages), and climate change (through stream habitat contraction). New surveys using time- and area-constrained searches have added baseline data on relative abundance of tadpoles (Cordilleran Geoscience and ESSA Technologies 2010).
Habitat protection has increased significantly since the previous status assessment with the establishment of 19 Wildlife Habitat Areas, which cover 1,239 ha of habitat, for Rocky Mountain Tailed Frogs. The effectiveness of the protection remains to be established and is currently monitored. A ban on mining exploration and development under the Flathead Watershed Area Conservation Act has eliminated threats from these sources in the Flathead portion of the species' range.
No Aboriginal traditional knowledge is available at this time.

COSEWIC History
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
COSEWIC Mandate
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC Membership
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
Definitions (2013)
- Wildlife Species
- A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
- Extinct (X)
- A wildlife species that no longer exists.
- Extirpated (XT)
- A wildlife species no longer existing in the wild in Canada, but occurring elsewhere.
- Endangered (E)
- A wildlife species facing imminent extirpation or extinction.
- Threatened (T)
- A wildlife species likely to become endangered if limiting factors are not reversed.
- Special Concern (SC)Footnote*
- A wildlife species that may become a threatened or an endangered species because of a combination of biological characteristics and identified threats.
- Not at Risk (NAR)Footnote**
- A wildlife species that has been evaluated and found to be not at risk of extinction given the current circumstances.
- Data Deficient (DD)Footnote***
- A category that applies when the available information is insufficient (a) to resolve a species’ eligibility for assessment or (b) to permit an assessment of the species’ risk of extinction.
The Canadian Wildlife Service, Environment Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Wildlife Species Description and Significance
Name and Classification
Until fairly recently, the tailed frogs of the genus Ascaphus were considered to comprise a single species, A. truei. Mittleman and Myers (1949) were the first to propose that the Rocky Mountain populations of tailed frogs were sufficiently distinct from coastal populations morphologically to warrant taxonomic distinction, and named them a subspecies, A. t. montanus. Although Metter (1964) dismissed such a taxonomic distinction, the identity of these populations as a distinct species has been confirmed based on differences in allozymes and mitochondrial DNA (Nielson et al. 2001, 2006), skin secretions (Conlon et al. 2007), and oviposition behaviour (Karraker et al. 2006). The Rocky Mountain Tailed Frog, A. montanus, and Coastal Tailed Frog, A. truei, are now accepted as two distinct, valid species (Crother 2012).
In the original description of the genus Ascaphus, Stejneger (1899) placed the tailed frogs in the family Discoglossidae. Later, Fejérváry (1923) considered it to be a monotypic family, Ascaphidae, but Noble (1931) thereafter placed it with the New Zealand genus Leiopelma in Leiopelmatidae because of shared primitive traits such as the presence of nine presacral vertebrae, free ribs, inguinal amplexus, and vestigial "tail-wagging" muscles. Although Green and Cannatella (1993) argued that the two genera should be in separate families as they have virtually no shared, derived morphological characters, recently San Mauro et al. (2005) and Frost et al. (2006) have again placed them together in Leiopelmatidae based on molecular DNA evidence.
The accepted French name is Grenouille-à-queue des Rocheuses (Green 2012).
Morphological Description
Adult Rocky Mountain Tailed Frogs are small frogs measuring 2.2 – 5.1 cm snout-vent length, with a large head, a vertical pupil, broad and flattened outer hind toes and no tympanum (Matsuda et al. 2006; Figure 1). Adult Rocky Mountain Tailed Frogs vary in colour from tan or brown to olive green or red. Indistinct dark blotches can be seen on paler individuals (Leonard et al. 1993; Corkran and Thoms 1996). There is often a distinct, dark-edged copper bar between the eyes. Numerous epidermal tubercles make the skin appear granular; in males the tubercles on the back and the legs increase in size in the fall (Metter 1964). Males have a short, conical extension of the cloaca which is used for copulation. This led to the name "tailed frog".
Figure 1. Rocky Mountain Tailed frog, Ascaphus montanus. A) adult male, Bonner County, Idaho. B) tadpole, Idaho County, Idaho. Photos: Gary Nafis.

Long description for Figure 1
Two photos showing the adult and tadpole stages of the Rocky Mountain Tailed Frog, Ascaphus montanus. Photo A shows an adult male (back and right side). The frog has a large head, a vertical pupil, and broad and flattened outer hind toes. It is brown to olive green, with numerous epidermal tubercles that make the skin appear granular. Photo B shows a tadpole in water (back and left side). It has an enlarged oral disc, a ventrally flattened body and a laterally compressed tail. The tadpole is mottled black and tan with a prominent, black-bordered white spot at the tip of the tail.
Rocky Mountain Tailed Frog tadpoles measure 2 – 6 cm in total length and possess an enlarged oral disc that is modified into an adhesive sucker for clinging to rocks in swift currents. They have a ventrally flattened body and a laterally compressed tail bordered by a low, straight or tapered dorsal fin. They are mottled black and tan with a prominent, black-bordered white spot at the tip of the tail, which is thought to deter or distract potential predators (Altig and Channing 1993). The tadpoles wag their tails vertically when positioned on channel substrate surfaces.
Rocky Mountain Tailed Frog adults have distinct, dense, fine black speckling on the dorsal and ventral surfaces, which distinguishes them from Coastal Tailed Frogs (Matsuda et al. 2006). As well, the copper markings on transformed individuals generally have green rather than orange undertones (Dupuis 2000), and the webbing of the hindfoot is more extensive (Metter 1964). The mottled dorsal colouration of Rocky Mountain Tailed Frog tadpoles is also distinct from the generally uniform slate grey of Coastal Tailed Frog tadpoles.
Population Spatial Structure and Variability
Rocky Mountain Tailed Frogs in Canada are found in two, entirely discrete mountainous regions, in the Yahk and Flathead drainages, with no possibility that frogs may disperse between them. Within each of the drainages, the population appears to be fragmented, but whether severe fragmentation, as defined by COSEWIC, applies is unknown (i.e., >50% of population is in habitat fragments smaller than can support a viable population). Dupuis and Friele (2004) found that the breeding distribution of the Rocky Mountain Tailed Frogs was highly clustered, resulting in fragmentation of the population into relatively isolated subpopulations. Occasional dispersal among subpopulations may be needed to maintain population viability.
The frogs and tadpoles tend to be sedentary (Daugherty and Sheldon 1982b; Metter 1964) and may move only tens of metres at most. However, mark-recapture studies within constrained areas, such as these, are routinely unable to detect long-distance movements in anurans (Smith and Green 2005). The unexpectedly rapid recolonization of Mount St. Helens by Coastal Tailed Frogs (Crisafulli et al. 2005) demonstrates that Ascaphus are capable of long-distance overland dispersal movements. The frogs inhabit streams that are physiographically isolated from each other and are without connections in areas that are habitable by the frogs or tadpoles in their lower reaches. Therefore, overland movements through forest, even at low frequency, are necessary for maintaining metapopulation structure and ensuring survival of the subpopulations that inhabit these streams. It is possible that a considerable proportion of the total population of Rocky Mountain Tailed Frogs in Canada exists in subpopulations dependent upon dispersal for their continued survival. Although narrow Wildlife Habitat Areas (WHAs; Maxcy 2004) have been established for Rocky Mountain Tailed Frogs along inhabited streams (Figure 8), the terrain between streams over which the animals may disperse remains unprotected and may be logged. Wahbe et al. (2004) showed evidence of reduced dispersal among adult Coastal Tailed Frogs in forested areas that had been clearcut. Degradation, due to logging or other disturbances, of this dispersal habitat that lies between streams is therefore a source of habitat fragmentation likely to bring increased isolation of populations.
Designatable Units
Habitat conditions within the two parts of the Canadian range, Yahk and Flathead river drainages, appear to be similar and the streams in which they occur all lie within the same Engelmann Spruce-Subalpine Fir eco-geographic zone (Cordilleran Geoscience and ESSA Technologies 2010). Canadian populations of Rocky Mountain Tailed Frogs have not been examined genetically, but analysis of genetic variation in U.S. populations using allozymes and mtDNA sequences (Figure 2) revealed two clades, a northern and a southern, with a high degree of genetic uniformity within the northern clade compared to the southern clade (Nielson et al. 2006). It is unlikely, therefore, that the two Canadian populations, as northward extensions of a single northern U.S. clade, are highly differentiated genetically from each other. Rocky Mountain Tailed Frogs in Canada, in light of current knowledge, may best be considered a single designatable unit.
Figure 2 (A&B). Mitochondrial DNA genetic variation within the Rocky Mountain Tailed Frog.
Figure 2A. Maximum-likelihood tree estimated from cytochrome b sequence data under the HKY+Γ model of sequence evolution, using Coastal Tailed Frog sequences as the outgroup. Letters indicate different mtDNA haplotypes. Numbers above branches are maximum-likelihood bootstrap values (100 replicates); those below branches are Bayesian estimates of nodal support (4 chains of 107 generations each).

Figure 2B. Distribution of mtDNA haplotypes within the range of the Rocky Mountain Tailed Frog, indicating the northern and southern clades. Source: after Nielson et al. (2006)
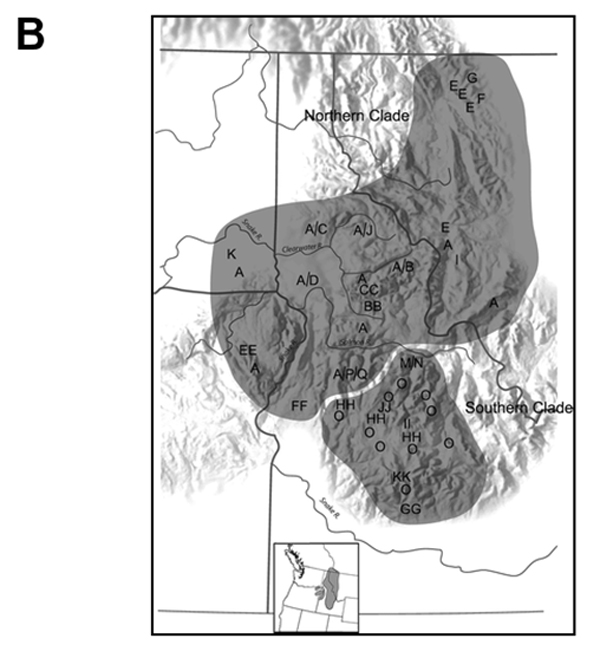
Long description for Figure 2 (A&B)
Two images relating to mitochondrial DNA genetic variation within the Rocky Mountain Tailed Frog. Image A is a maximum-likelihood tree diagram based on analysis of genetic variation in U.S. populations using allozymes and mtDNA sequences. The diagram illustrates two clades, a northern and a southern, with a high degree of genetic uniformity within the northern clade compared to the southern. Letters indicate different mtDNA haplotypes. Numbers above branches are maximum-likelihood bootstrap values; those below branches are Bayesian estimates of nodal support. Image B is a map of the distribution of mtDNA haplotypes within the range of the Rocky Mountain Tailed Frog. The areas occupied by the northern and southern clades are shaded.
Special Significance
The genus Ascaphus is unique among North American frogs. The two species of Ascaphus and the 4 endemic New Zealand species of Leiopelma are universally regarded as the most primitive living frogs in the world (Green and Cannatella 1993; Green 2003). Rocky Mountain Tailed Frogs and Coastal Tailed Frogs are the most specialized of North American frogs for life in fast-flowing streams, with such adaptations as a suctorial oral disc in the tadpole, internal fertilization, and an absence of vocalization accompanied by the absence of a tympanum and middle ear bones (Brown 1975; Leonard et al. 1993; Adams 2005).
Rocky Mountain Tailed Frogs are among the longest lived of all North American frogs and the slowest to develop. They spend 3 – 4 years as tadpoles, do not attain sexual maturity until 7 – 8 years of age (i.e., about 4 years following metamorphosis), and may live up to 14 years in the wild (Daugherty and Sheldon 1982a). They are often the only aquatic vertebrate in the headwater streams where they occur, and thus may play an important role as grazers in these systems and as a source of prey for larger terrestrial vertebrates (Bull and Carter 1996a).
Distribution
Global Range
Rocky Mountain Tailed Frogs occur in extreme southeastern British Columbia south through western Montana and Idaho north of the Snake River Plain to the Wallowa Mountains of northeastern Oregon and Blue Mountains of extreme southeastern Washington (Leonard et al. 1993; Bull 1994; Nielson et al. 2001; Green et al. 2013; Figure 3). They occur at elevations as low as 550 m in British Columbia and up to 2,134 m in the Wallowa Mountains (Leonard et al. 1993).
Figure 3. Distribution of Ascaphus montanus in North America. The U.S. locations indicated on the map are occurrences of the species at the level of county (Idaho and Montana) or subcounty (Washington and Oregon). Adapted from Green et al. (in press).

Long description for Figure 3
Map of the distribution of the Rocky Mountain Tailed Frog in North America, with dots indicating localities. The species occurs in extreme southeastern British Columbia south through western Montana and Idaho north of the Snake River Plain to the Wallowa Mountains of northeastern Oregon and Blue Mountains of extreme southeastern Washington.
Canadian Range
In Canada, Rocky Mountain Tailed Frogs are restricted to two disjunct, mountainous areas in British Columbia, separated by the Rocky Mountain Trench (Figure 4). Within the Flathead River watershed, Rocky Mountain Tailed Frogs are found in the Macdonald Range in the area bounded on the west by Inverted Ridge, on the east by the Flathead River and on the north by the Leslie/Twentynine-Mile Creek divide approximately 21 km north of the Canada/U.S. border (B.C. Conservation Data Centre 2013). Specific drainages in this area occupied by Rocky Mountain Tailed Frogs include Couldrey Creek, Burnham Creek, Cabin Creek, Storm Creek, Leslie Creek and the North Fork of Bighorn Creek (Dupuis and Friele 2004). In the Yahk River watershed in the MacGillivray Range, Rocky Mountain Tailed Frogs occur in the Screw Creek, Boyd Creek, Sprucetree Creek, Malpass Creek, Norge Creek, and Upper Yahk River drainages (B.C. Conservation Data Centre 2012). In total, 14 element occurrences have been mapped for this species in British Columbia (B.C. Conservation Data Centre 2012).
Figure 4. Range of Ascaphus montanus in Canada. Localities (= element occurrences) are indicated as red dots (Source: British Columbia Conservation Data Centre 2012). Open symbols show approximate locations of unconfirmed records from Montana electrofishing surveys 2008 – 2012 (modifications to map by Ian Adams).

Long description for Figure 4
Map of the range of the Rocky Mountain Tailed Frog in Canada, with 14 dots indicating localities. Open symbols show approximate locations of unconfirmed records from electrofishing surveys undertaken from 2008 to 2012. The species is restricted to two disjunct, mountainous areas in British Columbia, separated by the Rocky Mountain Trench.
Electrofishing surveys in Canada conducted from 2008 to 2012 by Montana Fish, Wildlife and Parks personnel (Figure 5) have reported the presence of Rocky Mountain Tailed Frog tadpoles on the east side of the Flathead River watershed in Elder Creek and at the far north end of the watershed in McEvoy Creek (Adams pers. comm. 2013; Steed pers. comm. 2013). Amber Steed (pers. comm. 2013) aptly referred to the tadpoles as "sucker frogs". These anecdotal reports appear to constitute significant range extensions for the species and increase the number of element occurrences for the species in British Columbia to at least 17.
Figure 5. Frog observations during fish sampling (electrofishing) by Montana Dept. of Fish, Wildlife and Parks personnel in the Canadian Flathead River watershed, 2008 – 2012. If validated, the two southeastern and the far northern records (red symbols) increase the known distribution of the species within the Flathead (Map source: Amber Steed, Montana Dept. of Fish, Wildlife and Parks).
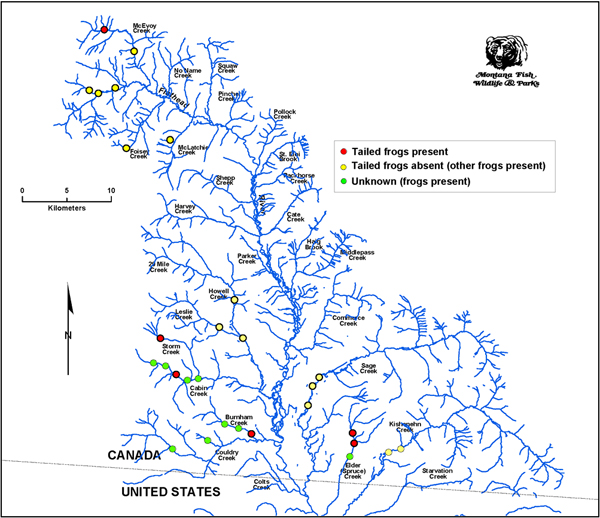
Long description for Figure 5
Map showing frog observations from electrofishing surveys in Canada conducted from 2008 to 2012 by Montana Fish, Wildlife and Parks. Dots indicate the presence of tailed frogs, the absence of tailed frogs with other frogs present, and the presence of unknown frogs. If validated, the two southeastern and the far northern records of tailed frogs (on the east side of the Flathead River watershed in Elder Creek and at the far north end of the watershed in McEvoy Creek) would increase the known distribution of the species and bring the number of element occurrences for the species in British Columbia to at least 17.
Russell and Bauer (2000) speculated that Rocky Mountain Tailed Frogs might exist in Alberta in Waterton Lakes National Park and near the Castle River, but there is no evidence that this is so.
Extent of Occurrence and Area of Occupancy
According to the B.C. Conservation Data Centre (2013), the extent of occurrence (EO) of Rocky Mountain Tailed Frogs in British Columbia is 331 km2 (Figure 6), exclusive of the region between the Yahk River watershed and the Flathead River watersheds that is uninhabited by the species. If all confirmed records of Rocky Mountain Tailed Frogs are contained within a single minimum convex polygon, the inscribed area is 1,900 km2. This increases to 3,300 km2 if the recent, unconfirmed records by Montana Fish, Wildlife and Parks personnel (Figure 5) are also included.
The index of area of occupancy (IAO) for Rocky Mountain Tailed Frogs in British Columbia, calculated by the B.C. Conservation Data Centre (2013) based on a 2 km x 2 km grid mapped onto inhabited streams is 296 km2 (Figure 6). Including the records by Montana Fish, Wildlife and Parks personnel (Figure 5) increases the IAO to 308 km2.
Figure 6. Estimates of (A) area of range extent within occupied drainages and (B) index of area of occupancy for the Rocky Mountain Tailed Frog in Canada. Source: BC Conservation Data Centre (2012). The extent of occurrence using a minimum convex polygon and including the intervening unoccupied habitat is 1,900 km² (or 3,300 km² including recent unconfirmed Montana electrofishing records, not shown).
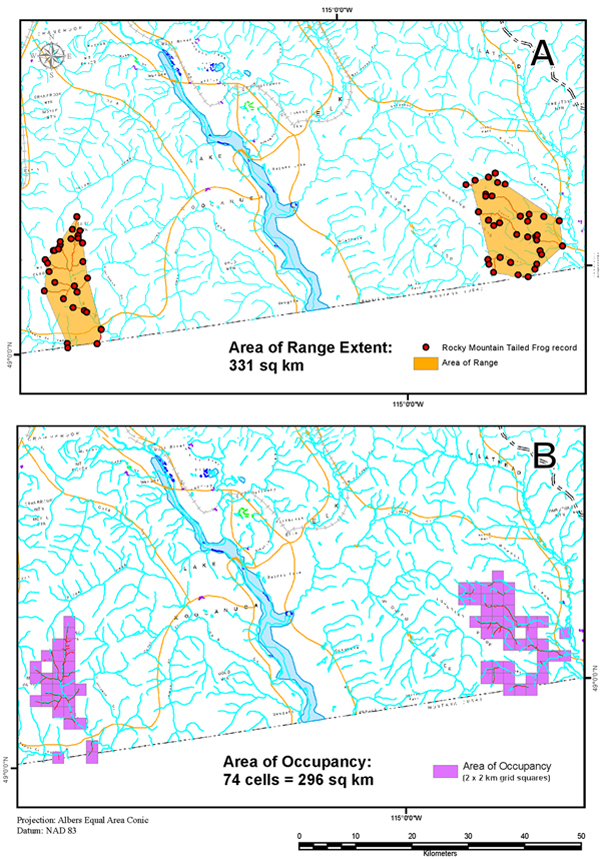
Long description for Figure 6
Two map panels showing estimates of area of range extent (Panel A) within occupied drainages and index of area of occupancy (Panel B) for the Rocky Mountain Tailed Frog in Canada. Panel A shows two minimum convex polygons (shaded area) with a combined area of 331 square kilometres (excludes uninhabited region between the Yahk and Flathead river watersheds). Panel B shows the grid blocks from which the index of area of occupancy (IAO) was calculated. IAO is 296 square kilometres.
Search Effort
Rocky Mountain Tailed Frogs occur in British Columbia in areas that are mountainous, remote from major highways, and difficult to access. Tailed frogs were not known from southeastern British Columbia until Grant (1958) recorded an adult female near the headwaters of Storm Creek, a tributary of Cabin Creek in the Flathead Drainage, at approximately 1,770 m elevation. Stan Orchard and Crispin Guppy later collected a series of individuals (RBCM Nos. 1797-1804) from this locality for the Royal British Columbia Museum in 1989, but extensive surveys for Rocky Mountain Tailed Frogs in southeastern British Columbia were not conducted until the late 1990s, when Dupuis and Bunnell (1997) and Dupuis and Wilson (1999) systematically searched 162 watercourses in the vicinity of the original confirmed sighting. More recent intensive surveys were conducted in both the Yahk and Flathead drainages in 2001 and 2003 (Ascaphus Consulting 2002, 2005; Dupuis and Friele 2004; Figure 7). These surveys uncovered no additional populations or inhabited streams, and according to Dupuis (2007) it is unlikely that additional occurrences will be found, as the vast majority of creeks in both the MacDonald Range and the McGillivray Range are unstable or ephemeral, and thus unsuitable for Rocky Mountain Tailed Frogs. More recent surveys were conducted from 2007 to 2009 by Cordilleran Geoscience (2009) and Cordilleran Geoscience and ESSA Technologies (2010), but these were designed to collect baseline data on Rocky Mountain Tailed Frog habitat preferences, refine sampling methodology, and characterize abiotic conditions of streams and climate in relation to tadpole and frog abundance and size in known sites, rather than to uncover new sites. Montana Fish and Wildlife personnel have lately engaged in extensive electrofishing in the Canadian Flathead River drainage (Adams pers. comm. 2013). Though the purpose of these surveys was to study fish ecology in the headwaters of the Flathead River, evidence of Rocky Mountain Tailed Frogs, if present, was also recorded (Steed pers. comm. 2013).
Figure 7.Rocky Mountain Tailed Frog tadpole and adult distribution in A) the Yahk River watershed and B) the Flathead River watershed based on data from timed searches in 2001 (Yahk) and 2003 (Flathead) during late summer. Source: Dupuis and Friele (2006).

Long description for Figure 7
Two map panels showing the distribution of Rocky Mountain Tailed Frog tadpoles and adults in the Yahk River watershed (Panel A) and the Flathead River watershed (Panel B) based on data from timed searches in 2001 (Yahk drainage) and 2003 (Flathead drainage) during late summer. Circles of varying sizes indicate the number of individuals found during 30-minute searches.
Habitat
In British Columbia, the Rocky Mountain Tailed Frog’s range corresponds to the Engelmann Spruce-Subalpine Fir biogeoclimatic zone, which has a relatively cold continental climate with frozen soils in winter (Meidinger and Pojar 1991; Demarchi 2011). Dupuis et al. (2000) suggest that Rocky Mountain Tailed Frogs are limited to Engelmann Spruce, Picea engelmannii, forests with winter precipitation levels high enough to blanket creeks with snow and thereby buffer them from freezing conditions. The species appears to be linked to moist, mid-elevation forests and low- to mid-gradient creeks (Dupuis and Wilson 1999). The lack of occurrences in steeper headwaters is likely influenced by the availability of permanent creeks in these relatively dry biogeoclimatic zones and by channel instability resulting from fragility of the underlying bedrock, which is prone to breakage (Dupuis and Wilson 1999).
Habitat Requirements
Rocky Mountain Tailed Frogs are restricted to small, permanently flowing, middle elevation creeks < 4 m wide with an average gradient of 4% (Franz and Lee 1970; Dupuis and Bunnell 1997; Dupuis and Wilson 1999). Steeper headwaters in the region are ephemeral and/or characterized by fracturing, unstable channels, and are generally uninhabited (Dupuis and Wilson 1999). As Rocky Mountain Tailed Frogs at all life stages have low tolerance for high temperatures, they can exist only in creeks that remain cool in summer. Tailed frog eggs require temperatures of 5ºC – 18.5ºC for survival, the narrowest range and lowest maximum of all North American frogs (Metter 1966; Claussen 1973; Brown 1975). Low summer stream temperatures are associated with areas of deep snowpack and prolonged snow melt. Heavy snow also has the benefit of buffering creeks from freezing during the winter months. The lack of anchored ice is critical because tadpoles and adults are known to overwinter aquatically under rocks (Brown 1990) or remain at the surface (Bull and Carter 1996a), rather than burrow into the stream substrate. Daugherty and Sheldon (1982b) captured adult Rocky Mountain Tailed Frogs swimming in March, when streams were snowbound. Furthermore, the non-filamentous algae that Rocky Mountain Tailed Frog tadpoles graze on grow best in shaded, fast-flowing streams (Murphy and Meehan 1991). Franz and Lee (1970) suggested that water chemistry may also influence population distributions in Montana as tadpoles were found only in streams with pH < 7.7 and dissolved oxygen levels > 8.2 ppm.
Rocky Mountain Tailed Frog tadpoles are typically associated with streambeds composed largely of smooth rocks, cobbles and boulders, rather than of silt, sand or pebbles, which do not provide tadpoles with refuge sites against floods, debris flows, predators, or elevated temperatures (Altig and Brodie 1972; Dupuis and Friele 1996). Tadpole densities are low in creeks with large amounts of fine sediment (Franz and Lee 1970; Welsh 1993; Welsh and Ollivier 1998). Large boulders and cobbles also provide a diversity of microhabitats necessary for the various stages of tadpole development. Younger tadpoles are more commonly found in shallow or deep pools whereas large tadpoles tend to frequent riffles (Wahbe 1996). Metamorphosing tadpoles are most strongly associated with pools containing large boulders (Dupuis 2000).
Rocky Mountain Tailed Frog adults will forage in upland forest habitat during cool, wet weather, and some individuals may overwinter on land (Nussbaum et al. 1983). In the Yahk River area, adult abundance was highest in areas with the greatest percent cover of mature forest (Ascaphus Consulting 2002).
Habitat Trends
By the early 1990s, roughly 75% of British Columbia’s watersheds had been at least partially altered (Bunnell and Dupuis 1993) due to human activities, including logging. At the same time, Engelmann Spruce and Subalpine Fir, Abies lasiocarpa, forests were also decreasing in extent and continuity (Hogan et al. 1994). The subsequent establishment of Wildlife Habitat Areas (Maxcy 2004) may have significantly stemmed the decline in suitable habitat for Rocky Mountain Tailed Frogs. However, virtually all identified threats would result in continuing deterioration of stream habitats(Appendix 1) . Thus, although streams occupied by Rocky Mountain Tailed Frogs may be currently protected from the direct effects of logging within Wildlife Management Areas, no drainage can be considered entirely secure or its condition considered stable in the long term. Of particular concern are impacts of activities occurring upstream of the Wildlife Habitat Areas that could potentially increase siltation or effects of storm surges on stream morphology.
There are many additional probable agents of stream degradation within the range of Rocky Mountain Tailed Frogs in British Columbia (Appendix 1). Large forest fires in the East Kootenay region will certainly occur in the future. Aside from the obvious damage to forest overstorey and underbrush, ash, dirt and the flame retardant dropped onto the fires can enter and degrade streams. Furthermore, the sumps installed in streams for use by helicopters in firefighting can themselves lead to extensive erosion. Although livestock farming and ranching currently account for less than 1% of land use within the range of Rocky Mountain Tailed Frogs, this might increase in the future, magnifying the risk of turbid streams with increased nutrient loading. A recently announced moratorium on mining in the Flathead watershed may have reduced the immediate risk these activities pose for the populations of Rocky Mountain Tailed Frogs in that part of the range. However, the mines and, particularly, the quarries that remain may continue to be sources of materials entering streams inhabited by the species. The recreational use of all-terrain vehicles, which continues in the region, is likely to result in ongoing erosion of trails, resulting in siltation of streams. There are also roads and skid trails in the area that are being used but are not being maintained due to the reduction of mining and timber harvesting. While some of these will brush-in and stabilize, others can be expected to fail and wash out, further adding to the silt loading of streams. As forest stands age and become harvestable outside the WHAs in the next 10 years, increased logging is expected, creating silt-laden runoff that will degrade stream quality. Furthermore, some climate change models predict warmer drier summers in the region (Hamann and Wang 2006; Gayton 2008), which may reduce the extent of permanently flowing streams, increase stream temperature and threaten the health of the surrounding forests (Woods 2011). Besides directly decreasing the quality of stream habitats for tailed frogs, changes in climate may trigger increasing numbers of landslides or increase the effect of naturally occurring slides.
Biology
There is a fairly large literature on the biology of tailed frogs because of their distinctive nature. However, literature sources prior to the early 2000s did not always distinguish between the two species. Consequently, it is necessary to review each information source to determine if the species of tailed frog discussed is the Rocky Mountain Tailed Frog or Coastal Tailed Frog, as their biologies differ (Spear and Storfer 2010).
Life Cycle and Reproduction
Tailed frogs have low reproductive rates compared to other anurans. Rocky Mountain Tailed Frogs attain sexual maturity at 7 – 8 years of age (Daugherty and Sheldon 1982a), and females lay eggs every other year (Metter 1964; Nussbaum et al. 1983). Courtship and mating take place in the water (Noble and Putnam 1931) from late August to early October. During copulation, which normally lasts 24 to 30 hours, the “tail” of the male (Figure 1) becomes engorged with blood and is inserted into the female’s cloaca and sperm is transferred (Nussbaum et al. 1983). The sperm remain viable within the female's oviducts until egg laying in June or early July the following year (Nussbaum et al. 1983; Leonard et al. 1993).
Tailed frog females produce a double-strand of colourless, pea-sized ova, which are attached to the underside of a large cobble or boulder in a stream (Nussbaum et al. 1983; Karraker et al. 2006). Clutches consist of 50 – 85 eggs (Metter 1964; Franz 1970a). Tailed frog eggs are the largest of all North American frogs (Wright and Wright 1949), and they have the longest embryonic period (Brown 1975), from 4 – 6 weeks (Metter 1964; Franz 1970a; Brown 1975). Hatchlings remain in situ until their suctorial mouth is fully developed and their yolk sac is depleted (Metter 1964; Brown 1990). Rocky Mountain Tailed Frogs in Montana metamorphose after spending 3 years as larvae (Daugherty and Sheldon 1982a) but elevation, correlated with water temperature, may influence duration of the larval period (Leonard et al. 1993). Survivorship rates of adults are unknown, but they may live to the age of 14 (Daugherty and Sheldon 1982a; Brown 1990).
Physiology and Adaptability
Tailed frogs are cold-adapted (Green 2003; Adams 2005). They usually do not tolerate temperatures above 16°C, although Rocky Mountain Tailed Frogs can withstand temperatures as high as 21°C (Dunham et al. 2007). Both Rocky Mountain Tailed Frogs and Coastal Tailed Frogs have among the lowest tolerances for desiccation among anurans (Claussen 1973; Brown 1975).
Dispersal and Migration
Adult Rocky Mountain Tailed Frogs are active nocturnally under suitable ambient air temperature and humidity conditions (Metter 1964; Daugherty and Sheldon 1982b). They are also extremely philopatric. Daugherty and Sheldon (1982b) reported a maximum movement of 20 m (per year and between years) for 50% of the reproductively mature individuals in a population in western Montana; males and females exhibited similar movement patterns. Metter (1964) found individuals no more than 12 m from the banks of creeks off the North Fork of the Palouse River in northern Idaho. This sedentary lifestyle may be advantageous for securing food, mates and shelter in an otherwise dry, inhospitable environment (Daugherty and Sheldon 1982b).
Newly metamorphosed froglets tend to be sedentary, but juveniles aged 4 to 7 years old appear to exhibit a greater level of movement than do sexually mature individuals (Dupuis 2000). Daugherty and Sheldon (1982a) recorded a much lower recapture rate for juveniles (0 – 33%) compared to adults (39 – 73%) in Montana, although this may also reflect a higher mortality rate among juveniles than adults. A juvenile female moved 360 m over a period of 1 year (Daugherty and Sheldon 1982b). Dispersal capabilities of Rocky Mountain Tailed Frog tadpoles are not known, but Coastal Tailed Frog tadpoles are known to disperse or drift downstream up to 65 m in streams in old-growth forests devoid of log jams and slash piles (Jenkins and Ormerod 1996; Wahbe 1996). Overland movements between streams by adults and juveniles have not been studied but are highly likely to occur. The surprisingly rapid recolonization of Mt. St. Helens by Coastal Tailed Frogs following the 1980 eruption (Crisafulli et al. 2005) may indicate that Rocky Mountain Tailed Frogs also are capable of occasional, long-range dispersal that cannot be inferred from home range studies.
Interspecific Interactions
The diet of Rocky Mountain Tailed Frog tadpoles is limited largely to diatoms, which are scraped from submerged rocks by means of numerous rows of small, black, labial teeth (Metter 1964; Franz 1970b). However, juvenile and adult Rocky Mountain Tailed Frogs will eat a wide variety of food items. They feed primarily on terrestrial arthropods (Metter 1964), and spiders appear to be a favoured food item (Held 1985). They will also prey on snails, ticks, mites, collembolans, flies, moths, ants, mayflies, crickets and lacewings (Metter 1964). In turn, Rocky Mountain Tailed Frogs are preyed upon by American Dippers (Cinclus americanus), Cutthroat Trout (Salmo clarki), garter snakes (Thamnophis spp.), and Western Toads (Anaxyrus boreas) (Daugherty and Sheldon 1982a; Jenkins and Ormerod 1996; Dupuis 2000).
Population Sizes and Trends
Sampling Effort and Methods
Most survey efforts have focused on tadpoles. Adult Rocky Mountain Tailed Frogs are much more difficult to find than are tadpoles because they are both less abundant and less visible. Adults are active largely at night, but conducting night-time surveys in the steep creeks they inhabit is a treacherous undertaking. The most recent surveys for Rocky Mountain Tailed Frogs in British Columbia consist of time- and area-constrained searches conducted from 2007 to 2009 by Cordilleran Geoscience (2009) and Cordilleran Geoscience and ESSA Technologies (2010). No capture – recapture surveys have been attempted, and the number of breeding adults associated with each creek is unknown. Recently, Goldberg et al. (2012) and Flores et al. (2013) showed that the presence of tailed frogs can be detected by assaying for environmental DNA (eDNA) in water samples, but the method has not yet been systematically applied to surveying for Rocky Mountain Tailed Frogs.
Abundance
There are approximately 3,000 adult Rocky Mountain Tailed Frogs in Canada, clustered into isolated breeding areas within the Yahk River and Flathead River watersheds. This is a ballpark estimate derived from the estimated total length of habitable streams where breeding may occur, coupled with estimates of the density of tadpoles in such streams and estimates of the relative numbers of tadpoles vs. juvenile frogs vs. adult frogs. The recent discoveries of the species in new areas of the Flathead drainage based on Montana electrofishing surveys would add to the estimates presented below, but the increases in adult population are probably slight in light of the patchy distribution of the species in the north and east.
Ascaphus Consulting (2005) estimated there to be approximately 294 km of streams in British Columbia habitable by Rocky Mountain Tailed Frogs, 123 km in the Yahk River watershed and 171 km in the Flathead River Watershed. Of this total, 98.2 km, consisting of 49.7 km in the Yahk River watershed and 48.5 km in the Flathead River Watershed, were considered to be “core” breeding habitat (Figure 8). Cordilleran Geoscience (2009) conducted area-constrained surveys along 100 m stretches of six inhabited streams in 2008 and found there to be, on average, 0.47 tadpoles/m of stream in the Yahk River Watershed and 0.89 tadpoles/m of stream in the Flathead River Watershed. Dupuis and Wilson (1999) searched 10 m transects along seven inhabited streams in 1998 and, though they reported Rocky Mountain Tailed frog densities in terms of animals of all life stages per m2 of stream, also provided data sufficient to calculate that there were, on average, 0.43 tadpoles/m of stream in the Yahk River Watershed and 0.92 tadpoles/m of stream in the Flathead River Watershed. Averaging these estimates yields 0.45 tadpoles/m of stream (=450 tadpoles/km) in the Yahk River Watershed and 0.905 tadpoles/m of stream (=905 tadpoles/km) in the Flathead River Watershed. Metamorphosed frogs, both juveniles and adults, were found to be about 1/10 as numerous as tadpoles by Cordilleran Geoscience (2009) and 1/12 as numerous by Dupuis and Wilson (1999). Finally, considering that metamorphosed individuals spend about 4 years as juveniles and live to an age of ca. 14 years (Daugherty and Sheldon 1982a), and taking mortality rate into consideration, the ratio of juveniles to adults may be conservatively estimated as 1:1. If so, tadpoles should outnumber adults by a factor of 22. With this information, the following calculations can be made:
- Yahk - 49.7 km habitat × 450 tadpoles/km ÷ 22 tadpoles/adult = 1,017 adults;
- Flathead – 48.5 km habitat × 905 tadpoles/km ÷ 22 tadpoles/adult = 1,995 adults.
These two estimates, added together, come to 3,012 adults, in total.
Figure 8. Distribution of Rocky Mountain Tailed Frog habitat in A) the Yahk River watershed and B) the Flathead River watershed in southeast British Columbia (source: adapted from Cordilleran Geoscience and ESSA Technologies 2010).
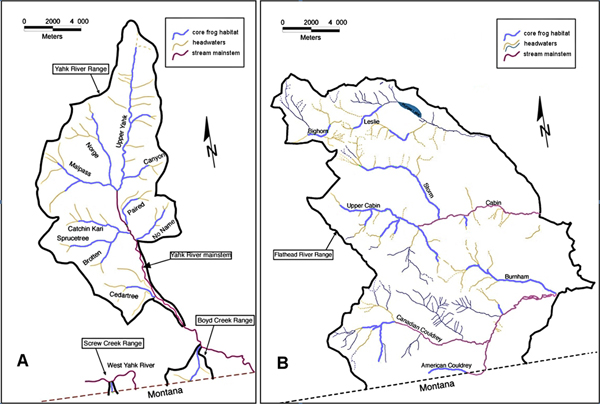
Long description for Figure 8
Two map panels showing the distribution of Rocky Mountain Tailed Frog habitat in the Yahk River watershed (Panel A) and the Flathead River watershed (Panel B). Watercourses are coloured to represent core frog habitat, headwaters, and stream mainstem.
Another ballpark estimate may be based on time-constrained search results and habitat availability. Ascaphus Consulting (2002), in sampling the extent of the Rocky Mountain Tailed Frog population in the Yahk River watershed, estimated the total length of perennial creek habitat to be 59 km, roughly 65% of which (= 38,350 m) was core breeding habitat. They also found a mean abundance of 0.8 females per 30-minute search of 25 m of stream. This yields an estimated 1,230 adult females. Assuming a 1:1 sex ratio yields an estimated 2,460 adult Rocky Mountain Tailed Frogs. For the Flathead River watershed, Ascaphus Consulting (2002) estimated there to be 50 km of breeding streams and 0.124 female / 25 m, which gives 250 females, or 500 adults. These two estimates, added together, come to 2,960 adults in total.
The BC Conservation Data Centre (2013) estimates the population size as 1,000 – 2,500 adults according to their latest review on 10 December 2010. This estimate is based on larval densities and the clustered distribution of breeding areas.
There are clearly many problems with crude estimates. Abundances vary over time, streams are highly heterogeneous along their lengths, differing survey methods can yield differing results, abundance estimates may be inexact without intensive capture/recapture data, and tadpole abundances may not necessarily reflect adult abundances. Even after extensive sampling, Cordilleran Geoscience and ESSA Technologies (2010) declined to extrapolate abundance data from area-constrained searches to encompass the entire network of streams occupied by the frogs. They argued that hydrographic heterogeneity among streams and the resulting variance in tadpole abundance along their full lengths precluded accurate estimation of the population size of Rocky Mountain Tailed Frogs. The level of agreement between the results of the area-constrained searches conducted by Cordilleran Geoscience (2009), Dupuis and Wilson (1999) and Ascaphus Consulting (2002) and the time-constrained searches by Ascaphus Consulting (2002) provides some measure of confidence in an overall ballpark estimate of ca. 3,000 adults. Nevertheless, although the data presented by Cordillera Geoscience (2009) and Dupuis and Wilson (1999) show approximately twice as many Rocky Mountain Tailed Frogs in the Flathead River Watershed as in the Yahk River Watershed, the time-constrained searches by Ascaphus Consulting (2002) found that frogs in the Flathead River watershed outnumbered frogs in the Yahk River watershed by 4:1. It may be that this discrepancy reflects population fluctuations but, nevertheless, more accurate estimates of total abundance may not possible at this time.
Fluctuations and Trends
Few data are available on which to ascertain population trends in Rocky Mountain Tailed Frogs. Evidence from area-constrained searches for 2005 and 2007 to 2009 indicate higher variance in tadpole abundance in the Yahk watershed compared to the Flathead watershed (Cordilleran Geoscience and ESSA Technologies 2010), but there are no comparable estimates of adult abundance. However, short-term losses of tadpoles are to be expected immediately following stream siltation due to logging (Bull and Carter 1996b), followed by recovery in stabilized logged streams, provided adults are still present. This decline in abundance followed by recovery may be related to greater light penetration and increased algal growth in the logged streams, as has been reported for Coastal Tailed Frogs. More likely, though, it may be due to higher survival rates of the young tadpoles hatching from eggs lain after the disturbance due to the eradication of older cohorts of tadpoles, that would act as competitors, as well as reduction of aquatic predators (Corn and Bury 1989; Richardson and Neil 1995).
Rescue Effect
The Yahk watershed population of Rocky Mountain Tailed Frogs in the McGillivray Range is evidently isolated from U.S. populations, but populations in the Canadian Flathead watershed may have downstream connections with populations in northwestern Montana. The MacDonald Range where the species occurs in British Columbia is the northward continuation of the Whitefish Ranges in Montana. Rocky Mountain Tailed Frogs have been found in Dutch Creek and Sprague Creek in Montana, both of which are tributaries to the north fork of the Flathead River (Franz and Lee 1970). However, rescue from Montana would probably be limited, as adults normally exhibit limited movements. In Montana, no reproductively mature adults were observed to move more than 40 m from one year to the next (Daugherty and Sheldon 1982b). Adult frogs were found only to make seasonal migrations to avoid high water temperatures (Adams and Frissell 2001). Adults and juveniles are more likely to move along stream corridors rather than overland (Spear and Storfer 2010).
Threats and Limiting Factors
The conservation status and threats to the Rocky Mountain Tailed Frog have been reviewed by Dupuis (2002, 2004, 2007) and Ascaphus Consulting (2005) for Canada, and by Adams (2005) for the U.S. The draft Recovery Strategy for Rocky Mountain Tailed Frogs in Canada (Dupuis 2007) identified increases in stream sedimentation, alteration of hydrological regimes, loss of riparian forest habitat and headwater linkages, stochastic environmental and demographic fluctuations due to reduced population size, and climate change resulting in stream habitat contraction as major proximate threats. Numerous additional effects associated with logging, road building and other human activities that may be operating within the species’ range can exacerbate these threats.
Assessment of threats to Rocky Mountain Tailed Frogs is somewhat complicated by literature comparisons to Coastal Tailed Frogs. Ecological and physiological differences between the two species are only slowly becoming clear but indicate that the two species may respond differently to certain threats despite their similar morphologies (Dunham et al. 2007; Spear and Storfer 2010). Rocky Mountain Tailed Frogs maintain levels of genetic connectivity equivalent to Coastal Tailed Frogs despite inhabiting a harsher climate, perhaps due to a better ability to disperse via streams (Spear and Storfer 2010) and/or a better ability of tadpoles to survive at higher temperatures (Dunham et al. 2007).
An IUCN threats calculator assessment identified one high-medium threat (pollution, primarily sedimentation), one medium-low threat (natural systems modification) and several low-impact threats (biological resource use [logging], recreational use, geological events, and climate change) that were projected to affect the population over the next 10 years, with the overall threat impact rated as high (Appendix 1). Specific major threats (Salafsky et al. 2008) to Rocky Mountain Tailed Frogs are discussed below in order of severity:
Agricultural and forestry effluents (Threat code 9.3)
Sedimentation of streams derived from logging activities, eroding and/or heavily used roads, fires and, to a lesser extent, cattle grazing can seriously damage stream habitats used by Rocky Mountain Tailed Frogs. The erosion of road surfaces, ditches and cutbanks during and following logging is a significant source of sediments in streams (Murphy et al. 1981; Beschta 1983; Hawkins et al. 1983). Heavily used logging roads produce up to 130 times more sediment than abandoned roads (Reid and Dunne 1984). The Yahk River watershed and Flathead River watershed, though remote, are riddled with roads that may be potential chronic sources of stream sedimentation. The risk of road failures and the high number of older roads and skid trails that are not being maintained makes this a lasting threat. If such roads re-vegetate, they could stabilize but any amount of vehicular or ATV use can counteract this regrowth and render roads chronically unstable. Sedimentation in the Flathead River system is chronic and has a depressive effect on larval densities. There are many documented cases of local declines of tailed frogs in response to acute sedimentation events, but impacts of chronic sedimentation are poorly understood and largely undocumented.
Logging and wood harvesting (Threat code 5.3)
Stream habitats for Rocky Mountain Tailed Frogs can be adversely affected by timber harvesting. Most of the Yahk River watershed has been heavily impacted by fire and forestry (Ascaphus Consulting 2002) and active logging still occurs in the Flathead watershed (B.C. Conservation Data Centre 2013). The occurrence of Coastal Tailed Frog adults and tadpoles is significantly lower in disturbed drainages subjected to logging than in undisturbed drainages (Corn and Bury 1989; Richardson and Neil 1995; Dupuis and Steventon 1999) and this is likely true also for Rocky Mountain Tailed Frogs. The main negative effect of timber harvesting and associated road-building operations is to increase the frequency and magnitude of sediment inputs to channel beds. Woody debris in stream channels can increase the risk of log jams, which trap fine sediments and alter a channel's substrate composition. Such disturbances negatively affect tailed frogs (Dupuis and Steventon 1999), though the vulnerability of tadpoles depends to some extent on the geological makeup of the creek bed and the amounts of fine sediments entering streams following disturbance (Dupuis and Friele 1996). Tailed frogs have been documented to recolonize previously disturbed creeks within a few years if nearby populations persist (Richardson and Neil 1995; Dupuis and Friele 1996). Logging can also alter the hydrological regime of a watershed and accentuate both peak discharges and low summer flows. Lohman (2002) found that severe flooding can eliminate populations of Rocky Mountain Tailed Frog tadpoles from streams in northern Idaho. As Rocky Mountain Tailed Frogs reside primarily in cold headwater streams and remain tadpoles for over 3 years, disturbances that increase temperature can also result in mortality of tadpoles and subsequent population declines (Corn et al. 2003). Clearcutting significantly raises stream temperatures (Brown and Krygier 1970).
Fire and fire suppression (Threat code 7.1)
Since 1987 approximately 24% of the U.S. range of Rocky Mountain Tailed Frogs has burned (Hossack and Pilliod 2011). Summer maximum water temperatures can remain significantly elevated for at least a decade following wildfire, particularly in streams with severe channel reorganization (Dunham et al. 2007). Hossack et al. (2006) found that wildfire had a significant short-term negative effect on abundances of Rocky Mountain Tailed Frog tadpoles in Montana. However, even significant changes to channel structure after wildfire did not affect the long-term distribution or abundance of Rocky Mountain Tailed Frog larvae. Dunham et al. (2007) found recovery of the number of Rocky Mountain Tailed Frog tadpoles in six streams 11 years after burning, suggesting that the frogs may be more resistant to wildfire, or resilient afterwards, than previously thought. Thus, despite the apparent short-term effect on tadpoles, Hossack et al. (2006) did not consider wildfires to be a threat leading to extirpation of populations of Rocky Mountain Tailed Frogs. Following the 2003 Ram-Cabin fire, which burned over much of the Cabin Creek, Storm Creek and Leslie Creek catchments in the Flathead watershed, there was no significant difference in channel substrate or Rocky Mountain Tailed Frog abundance in 2005 (Cordilleran Geoscience and ESSA Technologies 2010). In this case, the most intense area of burn did not impinge upon the core habitat of the frogs and extreme sedimentation did not occur. Fires on steeper slopes and in denser forest closer to frog-inhabited streams would be expected to result in direct mortality of frogs and greater indirect effects through sedimentation in streams.
Fire suppression is potentially a more serious threat to Rocky Mountain Tailed Frogs than fires. Firebreaks, vehicle access roads and sumps installed for helicopter firefighting in streams can cause extensive erosion and silting of streams. Flame retardant chemicals sprayed to control fires will also contaminate streams and surrounding forests.
Drought (Threat code 11.2)
Drought is a potential threat in the context of global climate change (Hamann and Wang 2006; Gayton 2008; Lundy 2008; Schnorbus et al. 2012). Anticipatory climate models indicate a high likelihood of change from the montane Engelmann Spruce–Subalpine Fir forests inhabited by Rocky Mountain Tailed Frogs to Interior Cedar–Hemlock forests over the next half century in British Columbia (Figure 9).
Figure 9. Anticipated effects of climate change on ecosystem distribution in southern British Columbia. The maps show a progressive loss of the Engelmann Spruce/Subalpine Fir (ESSF) ecological zone and its replacement by the Interior Cedar/Hemlock Zone (ICH) ecological zone in the mountains of the extreme southeast, as well as the spread of the Bunch Grass (BG) ecological zone in the southern Rocky Mountain Trench. The ecological zones are: CDF, Coastal Douglas-fir; CWH, Coastal Western Hemlock; BG, Bunchgrass; PP, Ponderosa Pine; IDF, Interior Douglas-fir; ICH, Interior Cedar–Hemlock; SBPS, Sub-boreal Pine and Spruce; SBS, Sub-boreal Spruce; BWBS, Boreal White and Back Spruce; MH, Mountain Hemlock; ESSF, Engelmann Spruce–Subapline Fir; MS, Montane Spruce; SWB, Spruce–Willow–Birch; AT, Alpine Tundra. After Hamann and Wang (2006).

Long description for Figure 9
Four maps showing anticipated effects of climate change on ecosystem distribution in southern British Columbia. Maps are provided for 2005, 2025, 2055, and 2085, and show a progressive loss of the Engelmann Spruce/Subalpine Fir (ESSF) ecological zone and its replacement by the Interior Cedar/Hemlock Zone (ICH) ecological zone in the mountains of the extreme southeast, as well as the spread of the Bunch Grass (BG) ecological zone in the southern Rocky Mountain Trench. Ecozones are distinguished by different coloured shading and include: CDF, Coastal Douglas-fir; CWH, Coastal Western Hemlock; BG, Bunchgrass; PP, Ponderosa Pine; IDF, Interior Douglas-fir; ICH, Interior Cedar–Hemlock; SBPS, Sub-boreal Pine and Spruce; SBS, Sub-boreal Spruce; BWBS, Boreal White and Back Spruce; MH, Mountain Hemlock; ESSF, Engelmann Spruce–Subapline Fir; MS, Montane Spruce; SWB, Spruce–Willow–Birch; AT, Alpine Tundra.
Invasive non-native/alien species and genes (Threat code 8.1)
Epizootic chytridiomycosis disease caused by the fungus Batrachochytrium dendrobatidis has been identified as a major threat to amphibian populations around the world (Skerratt et al. 2007) and has been considered especially prevalent among amphibians inhabiting stream habitats, particularly in the tropics (Woodhams and Alford 2005). However, Hossack et al. (2010) found no evidence of B. dendrobatidis infection among 198 larvae and 28 adult Rocky Mountain Tailed Frogs in nine streams in Idaho and Montana. In B.C., 35 adult, 8 juvenile and 14 metamorphic Rocky Mountain Tailed Frogs all tested negative for B. dendrobatidis infection (Purnima Govindarajulu, unpubl. data). This lack of infection may be related to species-specific variation in susceptibility to chytridiomycosis and/or to characteristics of the frogs' habitat (Conlon 2011). The skin secretions of amphibians are an important part of their immune system (Conlon et al. 2009) and both Rocky Mountain Tailed Frogs and Coastal Tailed Frogs secrete skin peptides, termed ascaphins, with broad spectrum antimicrobial activity (Conlon et al. 2007). Furthermore, the headwater streams inhabited by Rocky Mountain Tailed Frogs are usually too cold for too much of the year to allow growth of B. dendrobatidis (Piotrowski et al. 2004).
Other, minor threats (Appendix 1) include (2.3) livestock farming and ranching, (4.1) roads and railroads, (6.1) recreational activities, (6.3) work and other activities, particularly electrofishing, (10.3) avalanches and landslides, (11.3) temperature extremes and (11.4) storms and flooding.
The introduction of non-native fishes via fish stocking, and the possible invasion of native fishes that are predatory on tadpoles due to habitat change, appear to be negligible as direct threats to Rocky Mountain Tailed Frogs. The tadpoles inhabit steep, torrential streams and small headwaters that are largely inaccessible or inhospitable to any fishes than may prey on them. Presence of fish curtails dispersal to the mainstem of the river, adding to isolation of upper stream subpopulations.
Limiting Factors
Tailed frogs have a longer larval stage, slower rate of maturation, lower fecundity, narrower temperature tolerance range and lower dispersal rate than other North American frogs (Green 2003; Adams 2005; Ascaphus Consulting 2005; Spear and Storfer 2010). In British Columbia, Rocky Mountain Tailed Frogs are at their northern range limit and their distribution is confined to only two, entirely disconnected watersheds where populations are limited to permanent, cool mountain streams with distinctive features regarding elevation, gradient, width and streambed substrates (Gyug 2001; Ascaphus Consulting 2005). Xeric conditions in the valley floors and low temperatures at high elevations severely limit the frogs' ability to disperse between headwater streams (B.C. Conservation Data Centre 2013).
Number of Locations
Exhaustive surveys confirmed the presence of Rocky Mountain Tailed Frogs in at least six discrete drainages within two separate areas within the species' range in southeastern British Columbia (Figure 7). Within the Yahk River watershed, they occur in Screw Creek, Boyd Creek and the upper Yahk River drainage. In the Flathead River watershed, they occur in Leslie Creek and its tributaries, Cabin Creek and its tributaries and Couldrey Creek and its tributaries, but not in the Flathead River itself. In addition, Rocky Mountain Tailed Frogs appear to be present in Elder Creek on the east side of the Flathead River watershed and in McEvoy Creek at the far north end of the Flathead River watershed (Figure 5). Each of these eight drainages could conceivably be affected independently by a single, large threatening event such as a major landslide and therefore may be considered separate locations. However, the extent of some of these drainages and diverse topography suggest that there might be many more locations.
Protection, Status, and Ranks
Legal Protection and Status
!Rocky Mountain Tailed Frogs are listed under Schedule 1 of the Species at Risk Act as Endangered, but management of the land where they live is under the jurisdiction of the Province of British Columbia. Rocky Mountain Tailed Frogs are listed as "Wildlife" (Schedule A) under the British Columbia Wildlife Act and so are protected from intentional harm, collection, transport, or trafficking, and are also listed in the Identified Wildlife Management Strategy under the Forest and Range Practices Act and Oil and Gas Activities Act (B.C. Conservation Data Centre 2013). There are no national or provincial parks, ecological reserves or First Nations Reserves in regions of either the Flathead or Yahk River watersheds inhabited by the species (Ascaphus Consulting 2005).
Non-Legal Status and Ranks
NatureServe (2012) lists the Global Status of the Rocky Mountain Tailed Frog as G4 (Apparently Secure), as of 2004, United States National Status as N4 and Canadian National Status as N2 (Imperilled), as of 2011. In British Columbia, Oregon and Washington its NatureServe status is S2 (Imperilled), in Idaho it is S3 (Vulnerable), and in Montana it is S4 (Apparently Secure).
A draft Recovery Strategy (Dupuis 2007) is in the process of being updated (Adams pers. comm. 2013) but as of September 2013 has yet to be finalized or approved.
Habitat Protection and Ownership
Nineteen Wildlife Habitat Areas (WHAs; Maxcy 2004) for Rocky Mountain Tailed Frogs have been established under the Forest and Range Practices Act (Figure 10). There are 10 WHAs in the Flathead watershed, covering 614 ha, and nine in the Yahk watershed, covering 625 ha. The WHAs are 50 m wide forested buffers on either side of streams occupied by Rocky Mountain Tailed Frogs (Antifeau pers. comm. 2010; B.C. Ministry of Environment 2013) to protect aquatic habitat used by tadpoles and adjacent aquatic and terrestrial foraging habitats used by transformed Rocky Mountain Tailed Frogs. If the entire non-harvestable land base is considered, (including the aforementioned locations), Rocky Mountain Tailed Frog WHA reserve zones, riparian reserve zones, old growth and mature management areas, wildlife tree patches and ungulate winter ranges, then 68% of combined aquatic and terrestrial Rocky Mountain Tailed Frog habitat (i.e., 50 m wide forest buffers on either side of streams) in the Yahk River watershed and 63% of combined breeding and non-breeding habitat in the Flathead River Watershed is presumably protected (Ascaphus Consulting 2005). Integrated management areas, including WHA management zones, ungulate winter ranges and riparian management zones, provide partial protection for an additional 7% of combined breeding and non-breeding Rocky Mountain Tailed Frog habitat in the Yahk River watershed and 2% in the Flathead River watershed (Ascaphus Consulting 2005).
Figure 10. Wildlife Habitat Areas (WHAs) for Rocky Mountain Tailed Frogs established in A) the Yahk River watershed (purple stream sections) and B) the Flathead River watershed (red stream sections). Source: Ascaphus Consulting (2005).

Long description for Figure 10
Two map panels showing Wildlife Habitat Areas (WHAs) for Rocky Mountain Tailed Frogs established under the Forest and Range Practices Act. Panel A shows the stream sections covered by the 9 WHAs in the Yahk River watershed. Panel B shows the stream sections covered by the 10 WHAs in the Flathead River watershed.
The protected areas for Rocky Mountain Tailed Frogs, however, do not encompass most of the intervening forest which, though not continuously inhabited by the frogs, nevertheless significantly influences the integrity of inhabited streams. Furthermore, the 50 m forested buffer in the WHAs actually consists of only a 30 m wide zone where logging is not allowed while the remaining 20 m is a so-called special management zone where limited logging is permitted. Finally, WHAs apply only to forestry and range activities in almost all cases. Linear feature developments such as transmission lines, IPPs, and pipelines are not regulated by WHAs. The effectiveness of the WHAs in protecting Rocky Mountain Tailed Frogs is currently being assessed.
Acknowledgements and Authorities Contacted
The Rocky Mountain Tailed Frog draft Status Appraisal Summary was sent to the following jurisdictions for review in 2010 and their comments were incorporated into the present report:
- Canadian Wildlife Service
- Parks Canada Agency
- Department of Fisheries and Oceans
- Province of British Columbia
Consultations
- Ian Adams, Senior Wildlife Biologist, Vast Resource Solutions, Cranbrook, British Columbia
- Ted Antifeau, Co-chair of Rocky Mountain Tailed Frog Recovery Team, British Columbia
- COSEWIC Amphibian & Reptile Subcommittee Memebers
- Patrick T. Gregory, University of Victoria, British Columbia
- Tom Herman, Acadia University, Nova Scotia
- Jacqueline D. Litzgus, Laurentian University, Ontario
- Kristiina Ovaska, Research Associate, Royal British Columbia Museum, Victoria, British Columbia
- Cindy Paszkowski, University of Alberta, Edmonton, Alberta
- Linda Dupuis, Ecologist, Brackendale, British Columbia
- David F. Fraser, Scientific Authority Assessment, Ecosystems Protection & Sustainability Branch, Species and Ecosystems at Risk Section, Ministry of Environment, Government of British Columbia, Victoria, British Columbia
- Pierre Friele, Cordilleran Geosciences, Squamish, British Columbia
- Purnima Govindarajulu, Amphibian/Reptile/Small Mammal Specialist, Conservation Science Section, Ecosystems Branch, Ministry of Environment, Government of British Columbia, Victoria, British Columbia
- Neil Jones, Scientific Project Officer & ATK Coordinator, COSEWIC Secretariat, Canadian Wildlife Service, Environment Canada.
- Lea Gelling, B.C. Conservation Data Centre, Victoria, British Columbia
- Kathy Paige, Ecosystems Branch, British Columbia Ministry of Environment, Victoria, British Columbia
- Amber Steed, Fisheries Biologist, Montana Fish, Wildlife and Parks
- Melissa Todd, Research Wildlife Habitat Ecologist, B.C. Ministry of Forests, Lands and Natural Resource Operations, Victoria, British Columbia
Information Sources
- Adams, I., pers. comm. 2013. Email correspondence to David Green and Kristiina Ovaska. June 2013. Senior Wildlife Biologist, Vast Resource Solutions, Cranbrook, British ColumbiaBritish Columbia.
- Adams, M.J., 2005, Ascaphus montanus Nielson, Lohman, and Sullivan, 2001, Montana (Rocky Mountain) Tailed Frog, in Lannoo, M.J., ed., Amphibian declines--The conservation status of United States species. University of California Press, Berkeley, California, 382 pp.
- Adams, S.B., and C.A. Frissell. 2001. Thermal habitat use and evidence of seasonal migration by Rocky Mountain Tailed Frogs, Ascaphus montanus, in Montana. Canadian Field-Naturalist 115:251–256.
- Altig, R., and A. Channing. 1993. Hypothesis: Functional significance of colour and pattern of anuran tadpoles. Herpetological Journal 3:73-75.
- Altig, R., and E.D. Brodie. 1972. Laboratory behavior of Ascaphus truei tadpoles. Journal of Herpetology 6:21-24.
- Antifeau, T.D., pers. comm. 2010. Email correspondence to R.J.Brooks. Feb. 4, 2010. Co-chair of Rocky Mountain Tailed Frog Recovery Team BC Ministry of the Environment.
- Ascaphus Consulting. 2002. Distribution of Ascaphus montanus in the Yahk River and neighbouring watersheds. Unpubl. report to Tembec Industries, Cranbrook, B.C., and Columbia Basin Fish and Wildlife Compensation Program, Nelson, B.C. 36 pp.
- Ascaphus Consulting. 2005. Rocky Mountain Tailed Frog - Conservation Analysis. Project No. 4055T10. Final Report. Report to Ministry of Forests, Forest Practices Branch, Victoria, B.C. 37 pp.
- Beschta, R.L. 1983. Long-term patterns of sediment production following road construction and logging in the Oregon Coast Range. Water Resource. Research 14:1011-1016.
- B.C. Conservation Data Centre. 2013. Conservation Status Report: Ascaphus montanus. B.C. Ministry of Environment. Web site: http://a100.gov.bc.ca/pub/eswp/ [accessed May 22, 2012].
- B.C. Ministry of Environment. 2013. Approved Wildlife Habitat Areas (WHAs). [accessed Sept. 24, 2013]
- Brown, H.A. 1975. Temperature and development of the tailed frog, Ascaphus truei. Comparative Biochemical Physiology 50A:397-405.
- Brown, H.A. 1990. Morphological variation and age-class determination in overwintering tadpoles of the tailed frog Ascaphus truei. Journal of Zoology (London) 220:171-184.
- Brown, G.W., and J.T. Krygier. 1970. Effects of clear-cutting on stream temperature. Water Resources Research 6:1133-1139.
- Bull, E. 1994. Tailed frogs in the Blue Mountains. Northwest Sciences 68(2):23.
- Bull, E., and B. Carter. 1996a. Winter observations of tailed frogs in Northeastern Oregon. Northwest Naturalist 77:45-47.
- Bull, E., and B. Carter 1996b. Tailed Frogs: Distribution, ecology and association with timber harvest in northeastern Oregon. U.S. Forest Service, Pacific Northwest Research Station. General Technical Report No. 497.
- Bunnell, F., and L. Dupuis. 1993. Riparian habitats in British Columbia: their nature and role. Pp 7 - 21, in K. Morgan and M. Lashmar (eds.). Riparian Habitat Management and Research. Fraser River Action Plan. Special publication.
- Claussen, D.L. 1973. The thermal relations of the tailed frog, Ascaphus truei, and the Pacific treefrog, Hyla regilla. Pp137-153, in Comparative Biochemical Physiology. Permagon Press, Great Britain.
- Conlon, J.M. 2011. The contribution of skin antimicrobial peptides to the system of innate immunity in anurans. Cell and Tissue Research 343:201-212.
- Conlon, J.M., C.R. Bevier, L. Coquet, J. Leprince, T. Jouenne, H. Vaudry, and B.R. Hossack, 2007. Peptidomic analysis of skin secretions supports separate species status for the tailed frogs, Ascaphus truei and Ascaphus montanus, Comparative Biochemistry and Physiology D: Genomics and Proteomics 2:121-125.
- Conlon, J.M., S. Iwamuro, and J.D. King. 2009. Dermal cytolytic peptides and the system of innate immunity in anurans. Annals of the New York Academy of Sciences 1163:75–82.
- Cordilleran Geoscience. 2009. Report on 2008 tailed frog monitoring results, Flathead and Yahk Rivers, Nelson Forest Region, near Cranbrook, British Columbia. Final report. Unpubl. report to Kathy Paige, Biodiversity Branch, Environmental Stewardship Division, British Columbia Ministry of Water, Land and Air Protection, Victoria, B.C. 27 pp.
- Cordilleran Geoscience and ESSA Technologies. 2010. FREP Wildlife Resource Value Team – Rocky Mountain Tailed Frog. Final Report for the Monitoring Pilot Project, 2005, 2007-2009. Unpubl. report to Pauline Hubregtse, Ecosystems Monitoring Specialist, Ecosystems Branch, British Columbia Ministry of Environment, Victoria, B.C.
- Corkran, C., and C. Thoms. 1996. Amphibians of Oregon, Washington, and British Columbia. A Field Identification Guide. Lone Pine Publishing, Vancouver. 175 pp.
- Corn, P.S., and R.B. Bury. 1989. Logging in western Oregon: responses of headwater habitats and stream amphibians. Forest Ecology and Management 29:39-57.
- Corn, P.S., R.B. Bury, and E.J. Hyde. 2003. Conservation of North American stream amphibians. Pp. 24–36, in R. D. Semlitsch (ed.), Amphibian Conservation. Smithsonian Books, Washington, DC.
- Crisafulli, C.M., L.S. Trippe, C.P. Hawkins, and J.A. MacMahon. 2005. Amphibian responses to the 1980 eruption of Mount St. Helens. Pp. 183-197, in V.H. Dale, F.J. Swanson, and C.M. Crisafulli (eds.), Ecological responses to the 1980 eruption of Mount St. Helens. Springer, New York.
- Crother, B.I. 2012. Scientific and standard English names of amphibians and reptiles of North America north of Mexico, with comments regarding confidence in our understanding. 7th Edition. SSAR Herpetological Circulars No. 39:1-92.
- Daugherty, C.H., and A.L. Sheldon. 1982a. Age determination, growth, and life history of a Montana population of the tailed frog (Ascaphus truei). Herpetologica 38:461-468.
- Daugherty, C.H., and A.L. Sheldon. 1982b. Age-specific movement patterns of the frog Ascaphus truei. Herpetologica 38:468-474.
- Demarchi, D.A.. 2011.The British Columbia Ecoregion Classification. Third Edition. Ecosystem Information Section. Ministry of Environment, Victoria, British Columbia.
- Dunham, J.B., A.E. Rosenberger, C.H. Luce, and B.E.Rieman. 2007. Influences of wildfire and channel reorganization on spatial and temporal variation in stream temperature and the distribution of fish and amphibians. Ecosystems 10:335–346.
- Dupuis, L.A. 2000. COSEWIC assessment and status report on the on the Rocky MountainTailed Frog, Ascaphus montanus, and the Coast Tailed Frog, Ascaphus truei, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 26 pp.
- Dupuis, L. 2002. Inland Tailed Frog, Ascaphus montanus. Pp 67-79, in K. Paige (technical ed.). Standards for Managing Identified Wildlife, Version 2. Draft for technical review. Ministry of Water, Land and Air Protection, Biodiversity Branch, Victoria, British Columbia. 492pp.
- Dupuis, L. 2004. Rocky Mountain Tailed Frog. Accounts and measures for managing identified wildlife. British Columbia Ministry of Water, Land and Air Protection, Victoria, British Columbia. 12pp.
- Dupuis, L. 2007. Rocky Mountain Tailed Frog (Ascaphus montanus) Recovery Strategy, Draft, April 2007. Prepared on behalf of the Rocky Mountain Tailed Frog Recovery Team. 41 pp.
- Dupuis, L.A., and F.B. Bunnell. 1997. Status and distribution of the tailed frog in British Columbia. Unpubl. report prepared for Forest Renewal British Columbia.
- Dupuis, L.A., and P.A. Friele. 1996. Riparian management and the tailed frog. Unpubl. report to the Ministry of Forests, Prince Rupert Region, British Columbia. 25 pp.
- Dupuis, L., and P. Friele. 2004. Implications of the river continuum concept to conservation and management efforts: the case of the Tailed Frog. In T.D. Hooper, ed. Proceedings of the Species at Risk 2004 Pathways to Recovery Conference March 2-6, 2004, Victoria, B.C. Species at Risk 2004 Pathways to Recovery Conference Organizing Committee, Victoria, British Columbia. 3pp.
- Dupuis, L., and P. Friele. 2006 The distribution of the Rocky Mountain tailed frog (Ascaphus montanus) in relation to the fluvial system: implications for management and conservation. Ecological Research 21:489-502
- Dupuis, L., and D. Steventon. 1999. Riparian management and the tailed frog in northern coastal forests. Forest Ecology and Management 124:35-43.
- Dupuis, L., and K. Wilson. 1999. Status, distribution and management needs of the tailed frog in the East Kootenays. Unpubl. report to Forestry Renewal British Columbia. British Columbia Ministry of Environment Lands and Parks, Wildlife Program, Kootenay Region, Nelson, B.C.
- Dupuis, L., F. Bunnell, and P. Friele. 2000. Determinants of the tailed frog's range in British Columbia, Canada. Journal of Northwest Science 2:109-115.
- Dupuis, L., T.Wahbe, and F. Bunnell. 1995. The importance of stream and riparian habitats to amphibians in natural and altered landscapes. Ministry of Forests, Victoria, B.C. Interim report.
- Fejérváry, G.J. de. 1923. Ascaphidae, a new family of the tailless batrachians. Annales historico-naturales Musei nationalis hungarici 20:178-181.
- Flores, A.-M., B. Murray, C. Johnson, M. Todd, and D. Steventon. 2013. Environmental DNA Analysis of tailed frogs (Ascaphus truei) in northwestern British Columbia. Canadian Society of Evolution and Ecology, Annual Meeting. Kelowna, British Columbia. (poster).
- Franz, R. 1970a. Egg development of the tailed frog under natural conditions. Bulletin of the Maryland Herpetological Society 6:27-30.
- Franz, R. 1970b. Food of larval tailed frogs. Bulletin of the Maryland Herpetological Society 6:49-51.
- Franz, R., and D. Lee. 1970. The ecological and biogeographical distribution of the tailed frog, Ascaphus truei, in the Flathead River drainage of northwestern Montana. Bulletin of the Maryland Herpetological Society 6:62-73.
- Frost, D.R., T. Grant, J. Faivovich, R. Bain, A. Haas, C.F.B. Haddad, R. de Sá, A. Channing, M. Wilkinson, S.C. Donnellan, C. Raxworthy, J.A. Campbell, B.L. Blotto, P. Moler, R.C. Drewes, R.A. Nussbaum, J.D. Lynch, D.M. Green, and W. Wheeler. 2006. The amphibian tree of life. Bulletin of the American Museum of Natural History 297:1-370.
- Gayton, D. 2008. Impacts of climate change on British Columbia's Diversity: A literature review. Forrex Forest Research Extension Partnership, Kamloops, British Columbia. Forrex Series 23. Web site: http://www.forrex.org/publications/forrexseries/fs23.pdf
- Goldberg, C., D. Pilliod, R. Arkle, and L. Waits. 2012. Detection of stream-breeding amphibians using environmental DNA. 7th World Congress of Herpetology, Vancouver, B.C. (Abstract).
- Govindarajulu, P., C. Nelson, J. LeBlanc, W. Hintz, and H. Schwantje. 2013. Batrachochytrium dendrobatidis surveillance in British Columbia 2008-2009, Canada. Unpubl. report prepared by the Ministry of Environment, Victoria, B.C. Web site: http://www.env.gov.bc.ca/ecocat/ [accessed November 2013].
- Govindarajulu, P. 2013. (Amphibian/Reptile/Small Mammal Specialist, British Columbia Ministry of Environment). Personal communication by email. Feb. 25, 2013.
- Grant, J. 1958. The tailed toad in southeastern British Columbia. Canadian Field Naturalist 75:165.
- Green, D.M. 2003. Tailed frogs. Pp. 77-82, in M. Hutchins, W. E. Duellman, and N. Schlager (eds.). Grzimek's Animal Life Encyclopedia, 2nd ed. Vol. 6. Amphibians. Thompson and Gale, Farmington Hills, Michigan.
- Green, D.M. (ed.). 2012. Noms français standardisés des amphibiens et des reptiles d'Amérique du Nord au nord du Méxique. SSAR Herpetological Circulars 40. 63 pp.
- Green, D. M., L. A. Weir, G. S. Casper and M. J. Lannoo. 2013. North American Amphibians: Distribution and Diversity. University of California Press, Berkeley.
- Green, D.M., and D.C. Cannatella. 1993. Phylogenetic significance of the amphicoelous frogs, Ascaphidae and Leiopelmatidae. Ecol. Ethol. Evol. 5:233-245.
- Green, D.M., L. Weir, G.S. Casper, and M.J. Lannoo. 2013. North American Amphibians: Distribution and Diversity. University of California Press, Berkeley.
- Gyug, L. 2001. Tailed frog inventory, Merritt Forest District. Unpubl. report to Ministry of Water, Land and Air Protection, Kamloops, British Columbia. 30 pp.
- Hamann, A., and T. Wang. 2006. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 87:2773–2786.
- Hawkins, C.P., Murphy, M.L., Anderson, N.H., and M.A. Wilzbach. 1983. Density of fish and salamanders in relation to canopy and physical habitat in streams of the northwestern United States. Can. J. Fish. Aquatic Science 40:1173-1185.
- Held, S.P. 1985. Maintenance, exhibition, and breeding of the tailed frog, Ascaphus truei, in a zoological park. Herpetological Review 16:48-51.
- Hogan, D.L., T.P. Rollerson, and S.C. Chatwin. 1994. Gully assessment procedure for British Columbia (interim methods). Ministry of Environment, Lands and Parks and Ministry of Forests. Watershed Restoration Technical Circular No. 5. 46 pp.
- Hossack, B.R., and D.S. Pilliod. 2011. Amphibian responses to wildfire in the western United States: emerging patterns from short-term studies. Fire Ecology 7:129-143.
- Hossack, B.R., P.S. Corn, and D.B. Fagre. 2006. Divergent patterns of abundance and age-class structure of headwater stream tadpoles in burned and unburned watersheds. Canadian Journal of Zoology 84:1482-1488.
- Hossack, B.R., M.J. Adams, E.H.C. Grant, C.A. Pearl, J.B. Bettaso, W.J. Barichivich, W.H. Lowe, K. True, J.L. Ware, and P.S. Corn. 2010. Low prevalence of chytrid fungus (Batrachochytrium dendrobatidis) in amphibians of US headwater streams. Journal of Herpetology 44:253-260.
- Jenkins, R.K.B., and S.J. Ormerod. 1996. The influence of a river bird, the dipper (Cinclus cinclus), on the behaviour and drift of its invertebrate prey. Freshwater Biology 35:45-56.
- Karraker, N.E, D.S. Pilliod, M.J. Adams, E.L. Bull, P.S. Corn, L.V. Diller, L.A. Dupuis, M.P. Hayes, B.R. Hossack, G.R. Hodgson, E.J. Hyde, K. Lohman, B.R. Norman, L.M. Ollivier, C.A Pearl, and C.R. Peterson. 2006. Taxonomic variation in oviposition by tailed frogs (Ascaphus spp). Northwestern Naturalist 87:87–97.
- Leonard, B.P., K.R. McAllister, L. Jones, H. Brown, and R.M. Storm. 1993. Amphibians of Washington and Oregon. Seattle Audubon Society, Seattle. 168 pp.
- Lohman, K. 2002. Annual variation in the density of stream tadpoles in a northern Idaho (USA) watershed. Verhandlungen de Internationalen Vereinigung für Theoretische und Angewandte Limnologie 28:1-5.
- Lundy, K.N. 2008. Climate change and endangered species in Canada: A screening level impact assessment and analysis of species at risk management and policy. Masters Thesis (Environmental Studies in Geography), University of Waterloo, Ontario.
- Matsuda, B., D.M. Green, and P.T. Gregory. 2006. The Amphibians and Reptiles of British Columbia. Royal British Columbia Museum, Victoria, B.C. vi + 266 pp.
- Maxcy, K. 2004 Indicators and methods for effectiveness monitoring of Tailed Frog Wildlife Habitat Areas. Unpublish. report for BC Ministry of Water, Land, and Air Protection, Victoria, B.C.
- Meidinger, D., and J. Pojar (eds.) 1991. Ecosystems of British Columbia. Special Report Series No. 6. Ministry of Forests, Victoria, B.C. 330 pp.
- Metter, D.E. 1964. A morphological and ecological comparison of two populations of the tailed frog, Ascaphus truei Stejneger. Copeia 1964:181-204.
- Metter, D.E. 1966. Some temperature and salinity tolerances of Ascaphus truei Stejneger. J. Idaho Academic Science 4:44-47.
- Mittleman, M. B., and G. S. Myers. 1949. Geographical variation in the ribbed frog Ascaphus truei. Proceedings of the Biological Society of Washington 62:57-68.
- Murphy, M.L. and W.R. Meehan. 1991. Stream ecosystems. Pp 17-46, in W.R. Meehan (ed.), Influences of Forest and Rangeland Management on Salmonid Fishes and their Habitats. American Fisheries Society. Special Publication 19.
- Murphy, M.L., C.P. Hawkins, and N.H. Anderson. 1981. Effects of canopy modification and accumulated sediment on stream communities. Transactions of the American Fisheries Society 110:469-478.
- NatureServe. 2012. Ascaphus montanus - Mittleman and Myers, 1949. Rocky Mountain Tailed Frog. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. Web site: http://www.natureserve.org/explorer. [Accessed: December 30, 2012].
- Nielson, M., K. Lohman, and J. Sullivan. 2001. Phylogeography of the tailed frog (Ascaphus truei): Implications for the biogeography of the Pacific Northwest. Evolution 55:147-160.
- Nielson, M., K. Lohman, C.H. Daugherty, F.W. Allendorf, K.L. Knudsen, J. Sullivan, D.J. Ellis, B.T. Firth, and I. Belan. 2006. Allozyme and mitochondrial DNA variation in the Tailed Frog (Anura: Ascaphus): the influence of geography and gene flow. Herpetologica 62:235-258.
- Noble, G.K. 1931. The Biology of the Amphibia. McGraw-Hill Book Co., New York.
- Noble, G.K., and P.G. Putnam. 1931. Observations on the life history of Ascaphus truei Stejneger. Copeia 1931:97-101.
- Nussbaum, R.A., Brodie, E.D., and R.M. Storm. 1983. Amphibians and Reptiles of the Pacific Northwest. University of Idaho Press. Moscow. 332 pp.
- Piotrowski, J.S., S.L. Annis, and J.E. Longcore. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15.
- Reid, L.M., and T. Dunne. 1984. Sediment production from forest road surfaces. Water Resources Research 20:1753-1761.
- Richardson, J., and B. Neil. 1995. Distribution patterns of two montane stream amphibians and the effects of forest harvest: the Pacific giant salamander and tailed frog in southwestern British Columbia. Draft Report. Habitat Conservation Fund, Resource Inventory Committee, Skagit Environmental Endowment Commission, and B.C. Ministry of Environment. B.C. Ministry of Environment, Victoria, British Columbia. 42 pp.
- Russell, A.P., and A.M. Bauer . 2000. The Amphibians and Reptiles of Alberta: A Field Guide and Primer of Boreal Herpetology. University of Calgary Press, Calgary, Alberta .
- Salafsky, N., D. Salzer, A.J. Stattersfield, C. Hilton-Taylor, R. Neugarten, S.H.M. Butchart, B. Collen, N. Cox, L.L. Master, S. O'Connor, and D. Wilkie. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology 22: 897-911.
- San Mauro, D., M. Vences, M. Alcobendas, R. Zardoya, and A. Meyer. 2005. Initial diversification of living amphibians predated the breakup of Pangaea. American Naturalist 165:590–599.
- Schnorbus, M., A. Werner, and K. Bennett. 2012. Impacts of climate change in three hydrologic regimes in British Columbia, Canada. Hydrological Processes. DOI: 10.1002/hyp.9661.
- Skerratt, L.F., L. Berger, S. Speare, S. Cashins, K.R. Mcdonald, A.D. Phillott, H.B. Hines, and N. Kenyon. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134.
- Smith, M.A, and D.M. Green. 2005. Are all amphibian populations metapopulations? Dispersal and the metapopulation paradigm in amphibian ecology. Ecography 28:110-128.
- Spear, S.F., and A. Storfer. 2010. Anthropogenic and natural disturbance lead to differing patterns of gene flow in the Rocky Mountain tailed frog, Ascaphus montanus. Biological Conservation 143:778-786.
- Steed, A. 2013. (Fisheries Biologist, Montana Fish, Wildlife and Parks). Personal communication by email. May 21, 2013.
- Stejneger L. 1899. Description of a new genus and species of discoglossid toad from North America. Proceedings of the United States National Museum21: 899-901.
- Wahbe, T. 1996. Tailed frogs (Ascaphus truei, Stejneger) in natural and managed coastal temperate rainforests of southwestern British Columbia, Canada. 49 pp.
- Wahbe, T.R., F.L. Bunnell, and R.. Bury. 2004. Terrestrial movements of juvenile and adult tailed frogs in relation to timber harvest in coastal British Columbia. Canadian Journal of Forest Research 34: 2455-2466.
- Wahbe, T., H. Yueh, R.B. Bury, H. Welsh, K. Ritland, and C. Ritland. 2012. Tailed Frogs in the Pacific Northwest: Genetic variation, isolation, and population status. 7th World Congress of Herpetology, Vancouver, B.C. (Abstract).
- Welsh, H.H., Jr. 1993. A hierarchical analysis of the niche relationships of four amphibians from forested habitats of northwestern California. Ph.D. Dissertation. University of California, Berkeley, California. 202 pp.
- Welsh, H.H., Jr., and L.M. Ollivier. 1998. Stream amphibians as indicators of ecosystem stress: a case study from California's Redwoods. Ecological Applications 8:118-1132.
- Woodhams, D.C., and R.A. Alford. 2005. Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conservation Biology 19:1449–1459.
- Woods, A. 2011. Is the health of British Columbia's forests being influenced by climate change? If so, was this predictable? Canadian Journal of Plant Pathology, 33:117-126.
- Wright, A.H., and A.A. Wright. 1949. Handbook of Frogs and Toads of the United States and Canada. Comstock Publishing Company, Inc. New York.
Biographical Summary of Report Writer(s)
Prof. David M. Green obtained his B.Sc. in Zoology from the University of British Columbia and his M.Sc. and Ph.D., both also in Zoology, from the University of Guelph. He came to the Redpath Museum of McGill University in 1986 and is now a Full Professor and the Director of the Museum.
Prof. Green was Chair of the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) and served as co-chair of COSEWIC’s Amphibians and Reptiles Subcommittee for 14 years. He was a member of the Science Advisory Council of Fisheries and Oceans Canada and the Council of Canadian Academies’ Panel on the State and Trends of Biodiversity Science in Canada. He is an Associate Editor for the journal Diversity and Distributions and the Zoological Journal of the Linnean Society, and is a Fellow of the Linnean Society of London.
Prof. Green’s research concerns the ecology, genetics, and evolution of amphibians. He has particular interests in species at risk, including the determinants of species’ ranges and population declines, population dynamics, dispersal and recruitment in amphibians, and declining amphibian populations. He has authored over 120 refereed publications and book chapters, and more than 100 miscellaneous other publications and reports. True to his calling, few of his publications fail to mention amphibians in some manner. His most recent book is “North American Amphibians. Diversity and Distribution” (University of California Press, 2013) with co-authors Linda A. Weir, Gary S. Casper and Michael J. Lannoo.
Collections Examined
No collections were re-examined specifically for this report.
Aboriginal Tradional Knowledge (ATK)
No ATK relevant to Rocky Mountain Tailed Frog was available at the time of drafting this report.
Appendix 1. Threats Calculator for the Rocky Mountain Tailed Frog (source: Govindarajulu pers. comm. 2013).
Threats Assessment Worksheet
- Species or Ecosystem
- Ascaphus montanus, Rocky Mountain Tailed Frog
- Date:
- 20, Feb 2013
- Assessor(s):
- David Green, Melissa Todd, Dave Fraser, Purnima Govindarajulu, Ted Antifeau, Ian Adams, Kristiina Ovaska, Lea Gelling
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts high range |
low range |
|---|---|---|---|
| A | Very High | 0 | 0 |
| B | High | 1 | 0 |
| C | Medium | 1 | 0 |
| D | Low | 4 | 5 |
| - | Calculated Overall Threat Impact: | High | High |
| Number | Threat | Impact (calculated) Criterion |
Impact (calculated) |
Scope (next 10 Yrs) |
Severity (10 Yrs or 3 Gen.) |
Timing | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Residential & commercial development | - | - | - | - | - | - |
| 1.1 | Housing & urban areas | - | - | - | - | - | It is probably intolerant of persistent turbidity and excessive siltation, both consequences of agricultural and urbanization activities (Scott and Crossman 1973) |
| 1.2 | Commercial & industrial areas | - | - | - | - | - | - |
| 1.3 | Tourism & recreation areas | - | - | - | - | - | - |
| 2 | Agriculture & aquaculture | - | - | - | - | - | - |
| 2.1 | Annual & perennial non-timber crops | - | - | - | - | - | - |
| 2.2 | Wood & pulp plantations | - | - | - | - | - | - |
| 2.3 | Livestock farming & ranching | - | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | 2013: The only range licence covers a small portion of the Yahk (Screw Creek, which flows into the Yahk). Cattle activity has been noted, and there is potential for damage to creek habitat if cattle walk through the streams; however, this is considered minimal. Guide outfitters (one licence at this time) may run horses on the range in the Flathead portion of the range. |
| 2.4 | Marine & freshwater aquaculture | - | - | - | - | - | - |
| 3 | Energy production & mining | - | - | - | - | - | - |
| 3.1 | Oil & gas drilling | - | - | - | - | - | There was significant drilling in the Flathead; however, that has ceased as a result of the Flathead Conservation Act – no oil & gas drilling is permitted within the Flathead portion of the species’ range. |
| 3.2 | Mining & quarrying | - | - | - | - | - | Recently announced moratorium on mining in the Flathead has reduced the risk (Flathead Conservation Act). There are mineral tenures in the upper portion of Yahk (above WHAs), but no evidence of their activation; a proposal for a large mine did not go ahead. Mining activity is not considered a threat for any populations. Residual effects from previous mining: there may be residue from one gas head on NCC property (on the east side of Flathead) but not considered a threat in the current range of the species; there are yearly checks for off-gassing. |
| 3.3 | Renewable energy | - | - | - | - | - | There are no known independent power projects planned for this area, and upper reaches occupied by tailed frogs may not be suitable for them in any case. Occasional captures of tailed frogs have been noted in main stems, but it is unknown whether this is important habitat (there may be a sampling/detectability bias in favour of small reaches). |
| 4 | Transportation & service corridors | Negligible | Large (31-70%) | Negligible (<1%) | High (Continuing) | - | |
| 4.1 | Roads & railroads | - | Negligible | Large (31-70%) | Negligible (<1%) | High (Continuing) | This category records impacts to tailed frogs from new road construction (habitat loss) and roadkill. Road mortality is low because of nocturnal habits and low traffic volumes at night. New logging roads are likely not an issue for this threat category, as not many are being built under the current logging plans. |
| 4.2 | Utility & service lines | - | - | - | - | - | - |
| 4.3 | Shipping lanes | - | - | - | - | - | - |
| 4.4 | Flight paths | - | - | - | - | - | - |
| 5 | Biological resource use | D | Low | Restricted - Small (1-30%) | Moderate - Slight (1-30%) | High (Continuing) | - |
| 5.1 | Hunting & collecting terrestrial animals | - | - | - | - | - | - |
| 5.2 | Gathering terrestrial plants | - | - | - | - | - | - |
| 5.3 | Logging & wood harvesting | D | Low | Restricted - Small (1-30%) | Moderate - Slight (1-30%) | High (Continuing) | Average size of cutblocks in this area is approximately 50 ha (Antifeau pers. obs.). Most stream reaches with tailed frogs are protected with buffers, although in no case is the entire drainage area protected, so there is some threat from this land use. Some increased logging is expected in the next ten years as forest stands age and become harvestable, and some of the newer stands will mature, presumably improving habitat. A small amount of logging is slated in the next 10 years. There is some uncertainty in scope, because plans may change any time. Severity score includes effects on frogs from removal of trees and hydrology impacts (associated sedimentation and roads are dealt with elsewhere). Effects of logging on this species are not clearly established; professional judgment in the past has leant towards high impacts, but there are no data (part of the problem is high variability in tadpole abundance in time and space; tadpole abundance may be a poor indicator of population changes). The impacts are reduced, if logging companies adhere to the standards as they appear to be doing (CanFor, the major logging company in the area, has "Sustainable logging" certification that requires them to adhere to standards and also to monitor impacts; Adams pers. comm.), and if WHAs will function as expected. It is uncertain how logging outside WHAs affects populations. Monitoring at sentinel sites is expected to clarify logging impacts in the future. There is a fair amount of hydrologic recovery (from old logging), but not a lot of new logging. Current GIS work (Pierre Friele and Linda Dupuis) on Sentinel sites may have better spatial data to update the numbers of roads. |
| 5.4 | Fishing & harvesting aquatic resources | - | - | - | - | - | - |
| 6 | Human intrusions & disturbance | D | Low | Large (31-70%) | Slight (1-10%) | High (Continuing) | - |
| 6.1 | Recreational activities | D | Low | Large (31-70%) | Slight (1-10%) | High (Continuing) | ATV use is ongoing and additional to the impact of roads and the scope is large basically in all areas where there are roads (hence same as roads). Flathead guide outfitters sometimes run horses through streams. ATV use and horse outfitters are still an issue (extent of horses probably low). Sometimes ATV clubs armour the exits to stream crossings, reducing the impact to the stream banks. Some roads get brushed in, reducing access to ATVs. Impacts to tailed frogs are from habitat disturbance and direct mortality (low probability). |
| 6.2 | War, civil unrest & military exercises | - | - | - | - | - | - |
| 6.3 | Work & other activities | - | Negligible | Unknown | Negligible (<1%) | High (Continuing) | Electrofishing for fish surveys may affect tailed frogs. Extent of electrofishing is uncertain; there may be some in the Flathead. Severity of impact is unknown but thought to be negligible because tailed frogs may not be predominantly in fish habitat. However, it should be noted that due to volume of water in these fish habitats it is difficult to visually search for tailed frogs and hence their use of larger streams may be underestimated. There is anecdotal evidence that electrofishing in larger streams regularly turns up tailed frog tadpoles (pers. comm. Ron Ptolemy to Dave Fraser). |
| 7 | Natural system modifications | - | - | - | - | - | - |
| 7.1 | Fire & fire suppression | CD | Medium - Low | Restricted (11-30%) | Moderate - Slight (1-30%) | High (Continuing) | Large forest fires in the East Kootenays have occurred and likely will occur in the future; the largest fires exceed the range of the species in BC even though it exists in two disjunct populations (largest fire in BC history covered 617 km2). Sumps installed for helicopter firefighting in streams can cause extensive erosion; these streams have been identified to firefighting agencies, so they can minimize the building of sumps and flyovers with retardant. Fire could burn into riparian areas/WHAs. Stream temperatures won't go up to lethal levels in the long term. Recovery from fire of tailed frog populations may be quite rapid (recolonization of streams by Coastal Tailed Frogs after Mt St Helens eruption). |
| 7.2 | Dams & water management/use | - | - | - | - | - | - |
| 7.3 | Other ecosystem modifications | - | Unknown | Unknown | Unknown | Unknown | Forest loss to Pine Beetle - riparian area doesn't tend to have Lodgepole Pine; however, there could be watershed level effects. Need more info on beetle effects. |
| 8 | Invasive & other problematic species & genes | - | Unknown | Unknown | Unknown | Unknown | - |
| 8.1 | Invasive non-native/alien species | - | Unknown | Unknown | Unknown | Unknown | Batrachochytrium dendrobatidis mortality has not been noted for this species; 35 adults, 8 juveniles and 14 metamorphs were tested for Bd and all were negative (manuscript submitted Govindarajulu, pers. comm.). Anaxyrus boreas shares the range, and there is overlap of habitat; toads can carry Bd and so Bd may already be present in the range. Bd is widespread in B.C. (cite EcoCat publication, Govindarajulu et al. 2013). Cold water habitat may be below optimum for Bd resulting in low detection of Bd in tailed frogs, but it should be noted that 3 out of 38 post-metamorphic Coastal Tailed Frogs in the Coastal/Cascades range in Oregon tested positive for Bd (Hossack et al. 2010). |
| 8.2 | Problematic native species | - | Unknown | Pervasive (71-100%) | Unknown | High (Continuing) | Common Shiner; although cited in previous report, this threat is considered speculative |
| 8.3 | Introduced genetic material | - | - | - | - | - | - |
| 9 | Pollution | BC | Large (31-70%) | Large (31-70%) | Serious - Moderate (11-70%) | High (Continuing) | - |
| 9.1 | Household sewage & urban waste water | D | Low | Small (1-10%) | Serious (31-70%) | Moderate - Low | It is probably intolerant of persistent turbidity and excessive siltation, both consequences of agricultural and urbanization activities (Scott and Crossman 1973) |
| 9.2 | Industrial & military effluents | - | - | - | - | - | - |
| 9.3 | Agricultural & forestry effluents | BC | High - Medium | Large (31-70%) | Serious - Moderate (11-70%) | High (Continuing) | Pollution sources include sedimentation from logging, roads (new and eroding & heavily used old roads), fires, and cattle grazing (minor source because of small scope). Both the Yahk and Flathead areas are riddled with roads despite their remoteness from human habitations (important for showing potential sources for chronic sedimentation). Risk of road failures and number of older roads and skid trails that are not being maintained makes this a chronic threat, but if the roads revegetate, they could become more stable. Flathead sedimentation issues are chronic (having a depressive effect on larval densities). Severity of impact was discussed at length, and there are many unknowns. There are many documented cases of local declines of tailed frogs (both species) in response to acute sedimentation events, but impacts of chronic sedimentation are poorly understood and largely undocumented. It is hoped that monitoring at sentinel sites at WHAs will clarify this issue in the future, but we just don't have the data at this point. Range in severity rating reflects this uncertainty. Recovery of Coastal Tailed Frogs after huge sedimentation event from volcanic eruption of Mt St Helens was discussed: apparently, the depopulated streams were flushed clean by massive spring freshets over a short period, and repopulation was by adult dispersal - doesn't clarify the problem of chronic sedimentation over large numbers of streams. |
| 9.4 | Garbage & solid waste | - | - | - | - | - | - |
| 9.5 | Air-borne pollutants | - | - | - | - | - | - |
| 9.6 | Excess energy | - | - | - | - | - | - |
| 10 | Geological events | D | Low | Small (1-10%) | Serious (31-70%) | High (Continuing) | - |
| 10.1 | Volcanoes | - | - | - | - | - | - |
| 10.2 | Earthquakes/tsunamis | - | - | - | - | - | - |
| 10.3 | Avalanches/landslides | D | Low | Small (1-10%) | Serious (31-70%) | High (Continuing) | 2013: Large number of landslides in 2012 (1 documented case where local tailed frog habitat destroyed by a landslide). |
| 11 | Climate change & severe weather | D | Low | Small (1-10%) | Moderate (11-30%) | High - Moderate | - |
| 11.1 | Habitat shifting & alteration | - |
|
|
Unknown | Unknown | - |
| 11.2 | Droughts | D | Low | Small (1-10%) | Moderate (11-30%) | High - Moderate | Severity estimated over the next 3 generations, 30 years. Climate change models predict warmer, drier summers; however, these changes are not anticipated to result in elimination of breeding streams (tailed frogs appear to be rather resilient to low water levels and persist in moist patches or in water under the cobble until favourable conditions return, pers. observation M. Todd). Populations with the smallest snow packs are likely to be most affected because the streams may be the most vulnerable to drying out during droughts; hence scoring of the scope as small, but variability in the basin geomorphology and hydrology will also contribute to whether a basin is vulnerable. Over the longer term, this could be a much higher threat. Scoring scope of climate change impacts for 10 years is inappropriate, as the threat operates over longer time-frames and cumulative effects should be considered (not just 10 years at a time, which obscures seriousness of the threat due to shifting baseline). |
| 11.3 | Temperature extremes | - | Unknown | Unknown | Unknown | High - Moderate | Higher temperatures contribute to droughts. Temperatures are unlikely to exceed lethal limits in next 10 years, especially considering that the species exists at northern limits in B.C. and frogs could benefit if their distribution is limited by low water temps in B.C. Over the longer term, depending on how high the temperature spikes are and this could become an issue. |
| 11.4 | Storms & flooding | D | Low | Small (1-10%) | Moderate (11 -30%) | High - Moderate | Higher winter precipitation leading to greater and earlier spring freshets, scouring of streams (with negative effects on channel morphology and substrates), and flushing of tadpoles downstream, but if such effects will happen within the species’ range is unknown. |
Classification of Threats adopted from IUCN-CMP, Salafsky et al. (2008).
Page details
- Date modified: