COSEWIC Assessment and Status Report on the Gypsy Cuckoo Bumble Bee (Bombus bohemicus) in Canada - 2014
List of Figures
- Figure 1. Lateral image of female Gypsy Cuckoo Bumble Bee (Bombus bohemicus) housed at the Packer Collection, York University, Toronto, ON. Photograph by Sheila Colla.
- Figure 2. Colour patterns for Gypsy Cuckoo Bumble Bee (reproduced with permission from Colla 2012).
- Figure 3. Distribution of Gypsy Cuckoo Bumble Bee in North America with predicted suitability modeled using MaxEnt software (Phillips et al. 2006) based on museum specimens (red circles) (reproduced with permission from Williams et al. 2014).
- Figure 4. Gypsy Cuckoo Bumble Bee records (n=844) in Canada and recent search effort (2000-2012) that shows collection records for all Bombus specimens (reproduced with permission from the dataset of Williams et al. 2014).
- Figure 5. Bumble bee collection points (all dots total 236,260 specimens) for North America from 1892 - 2013. Dots in red represent host bee Western Bumble Bee and dots in blue represent host bee Yellow-banded Bumble Bee (see Wildlife Species Description and Significance). No data exists for areas without points. Note there has been taxonomic debate about Western Bumble Bee and Yellow-banded Bumble Bee, and it is not guaranteed that all specimens used in these maps are correctly identified. These maps should be used as general range maps and outliers further investigated. Map compiled by Leif Richardson November 2013 and used with permission from Sheffield et al. (in prep.).
- Figure 6. Phenology for Gypsy Cuckoo Bumble Bee in southern Ontario (specimens from 1883-2008), earliest spring record April 21 (n= 275 [note additional historic records have been added to Ontario records since this graph was produced, see Table 2]) (reproduced with permission from Colla 2012).
- Figure 7. Comparison of the relative abundance of each bumble bee species collected from 1971-1973 (black) and 2004-2006 (grey) in Guelph and Belwood, Ontario (* indicate P <0.0001) (reproduced with permission from Colla 2012).
- Figure 8. Relative abundance of Gypsy Cuckoo Bumble Bee (GCBB) in YT, NT, BC, AB and SK based on all databased Bombus records in Canada (1882 - 2011). The left Y-axis (shaded portions of bars) indicates GCBB specimens and the right Y-axis (triangles) represents the proportion of GCBB specimens by ten-year intervals. Linear regression was used to examine trends in relative abundance in GCBB over time; the line represents a best fit of the data. See also Table 3. Graphs generated using Minitab ® software.
- Figure 9. Relative abundance of Gypsy Cuckoo Bumble Bee (GCBB) in MB, ON, QC, NB and NS based on all databased Bombus records in Canada (1882 - 2011). The left Y-axis (shaded portions of bars) indicates GCBB specimens and the right Y-axis (triangles) represent the proportion of GCBB specimens by ten-year intervals. Linear regression was used to examine trends in relative abundance in GCBB over time; the line represents a best fit of the data. See also Table 3. Graphs generated using Minitab ® software.
- Figure 10. Relative abundance of Gypsy Cuckoo Bumble Bee (GCBB) in PE and NL based on all databased Bombus records in Canada (1882 - 2011). The left Y-axis (shaded portions of bars) indicates GCBB specimens and the right Y-axis (triangles) represent the proportion of GCBB specimens by ten-year intervals. Linear regression was used to examine trends in Relative abundance in GCBB over time; the line represents a best fit of the data. See also Table 3. Graphs generated using Minitab ® software.
- Figure 11. Total number of databased bumble bee specimens in Canada (1882 - 2011) from each province and territory; triangles represent the number of Gypsy Cuckoo Bumble Bee (GCBB) specimens. Values above each bar represent the percentage of specimens, which are GCBB. See also Table 3. Graphs generated using Minitab ® software.
List of Tables
- Table 1. Number of North American records of selected Bombus species by time period and results of logistic regression on relative abundance [* indicates the species is a social parasite (cuckoo); bold/underlined indicates significant change in relative abundance over time] (from Colla et al. 2012).
- Table 2. Number of Gypsy Cuckoo Bumble Bee records by province. Records compiled for this status report are part of a database that is continuously being updated with new information. More than 70 individuals and institutions contributed to the dataset, and are listed at: www.leifrichardson.org/bbna.html.
- Table 3. Relative abundance of Gypsy Cuckoo Bumble Bee (GCBB) and two of its hosts, Western Bumble Bee (WBB) and Yellow-banded Bumble Bee (YBBB) compared with databased Bombus collection data (1882 - 2011) in Canada. Note the decline in relative abundance of GCBB from 1991 - 2001 and 2002 - 2011 (red). See Figures 8 - 12 for graphical representation of this data. More than 70 individuals and institutions contributed to the dataset, and are listed at: www.leifrichardson.org/bbna.html. Specimens compiled in a dataset for Williams et al. 2014.
- Table 4. The threat classification below is based on the IUCN-CMP (World Conservation Union–Conservation Measures Partnership) unified threats classification system. For a detailed description of the threat classification system, see the Conservation Measures Partnership web site (CMP 2010). For information on how the values are assigned, see Master et al. (2009) and table footnotes for details. Threats for Gypsy Cuckoo Bumble Bee were assessed across the species’ geographic range in Canada. Threats calculator completed by Sheila Colla, Jennifer Heron, Cory Sheffield and Dave Fraser (November 2013). For additional threat information see Threats section.

Committee on the Status of Endangered Wildlife in Canada (COSEWIC) status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows:
COSEWIC. 2014. COSEWIC assessment and status report on the Gypsy Cuckoo Bumble Bee Bombus bohemicus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 56 pp. (Species at Risk Public Registry website).
COSEWIC would like to acknowledge Sheila Colla, Cory Sheffield and Leif Richardson for writing the status report on the Gypsy Cuckoo Bumble Bee (Bombus bohemicus) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Jennifer Heron, Co-chair of the COSEWIC Arthropods Specialist Subcommittee.
For additional copies contact:
COSEWIC Secretariat
c/o Canadian Wildlife Service
Environment Canada
Ottawa, ON
K1A 0H3
Tel.: 819-953-3215
Fax: 819-994-3684
COSEWIC E-mail
COSEWIC web site
Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Psithyre bohémien (Bombus bohemicus) au Canada.
Cover illustration/photo:
Gypsy Cuckoo Bumble Bee - Provided by the authors.
© Her Majesty the Queen in Right of Canada, 2014.
Catalogue No. CW69-14/692-2014E-PDF
ISBN 978-1-100-23931-6

Assessment Summary - May 2014
Gypsy Cuckoo Bumble Bee (Bombus bohemicus) is one of six cuckoo bumble bees (subgenus Psithyrus) occurring in North America. Both sexes are medium-sized (12 – 18 mm length), with a white-tipped abdomen and similar colour pattern. Gypsy Cuckoo Bumble Bee is an obligate social parasite of bumble bees of the subgenus Bombus in North America, including the Rusty-patched Bumble Bee (B. affinis) (assessed Endangered by COSEWIC), Yellow-banded Bumble Bee (B. terricola) and Western Bumble Bee (B. occidentalis) (both currently being assessed by COSEWIC). Cryptic Bumble Bee (B. cruptarum) may also serve as a host. Due to recent analysis of DNA barcode and morphological data, the formerly recognized species Bombus ashtoni was found to be conspecific with the widespread Old World species Bombus bohemicus.
Gypsy Cuckoo Bumble Bee is a holarctic species, occurring throughout most of Europe (except Iceland) and extreme southwestern Europe and parts of north and central Asia. In Canada, Gypsy Cuckoo Bumble Bee has been recorded in every province and territory except Nunavut. Canadian records are from 1883 to 2008, the most recent records being from Pinery Provincial Park in Ontario (2008) and Parc national des Monts-Valin in Quebec (2008). Since 1991, the species has only been recorded from three provinces: Ontario (67 specimens), Quebec (39 specimens) and Nova Scotia (18 specimens). Despite high search effort in recent years (2001 – 2013), only 42 specimens of Gypsy Cuckoo Bumble Bee have been recorded. The species distribution is partially determined by the distribution and abundance of its host bumble bee species.
Gypsy Cuckoo Bumble Bee occurs in diverse habitats, including open meadows, mixed farmlands, urban areas, boreal forest and montane meadows. The species feeds on pollen and nectar from a variety of plant genera. Gypsy Cuckoo Bumble Bee emerges slightly later than host queens, and parasitizes host nests in the spring. Host nests occur in abandoned underground rodent burrows and rotten logs.
Gypsy Cuckoo Bumble Bee is a social parasite, and does not have the typical eusocial colony cycle of other bumble bees, and therefore does not produce workers. Mated females emerge in the spring and look for potential host nests. The female kills or subdues the host queen and lays eggs that the host colony workers tend. In the late summer and autumn, females and males emerge from the host nest and leave to mate with conspecifics. Mated females then select an overwintering site. Like other bumble bees, the males and the egg-laying female of that generation die at the onset of cold weather.
Recent surveys at historically occupied sites have recorded no specimens. Historical abundance data on Gypsy Cuckoo Bumble Bee are available for only a fraction of the species Canadian range (mainly southern Ontario and Manitoba). The species has not been recorded at many sites surveyed within the last four decades, even where its hosts remain present.
The most likely threat to Gypsy Cuckoo Bumble Bee is the decline of two of the host species, especially Rusty-patched Bumble Bee in eastern Canada and Western Bumble Bee in western Canada. The third host, Yellow-banded Bumble Bee, is more widespread although may also be declining in parts of its range. At regional scales, pesticide use, pathogen spillover and habitat loss are probable threats.
Gypsy Cuckoo Bumble Bee is not protected in Canada by any federal or provincial laws. The Canada General Status Rank is undetermined overall in Canada but ‘may be at risk’ in Ontario, Quebec, Nova Scotia, New Brunswick and Newfoundland. The global conservation status rank is possibly extinct (GH).
Given this expansive range of Gypsy Cuckoo Bumble Bee across Canada, many suitable areas of habitat are within protected areas.
Bombus bohemicus (previously Bombus ashtoni)
Demographic Information
Extent and Occupancy Information
| Population | N Mature Individuals |
|---|---|
| Total | Unknown |
Quantitative Analysis
Threats (actual or imminent, to populations or habitats)
Rangewide threats are unclear. At regional scales, primary threats include pesticide use and pathogen spillover. The host bumble bee species have declined in parts of the range of Gypsy Cuckoo Bumble Bee. The Rusty-patched Bumble Bee (B. affinis) is gone from its range in southern Ontario and Quebec; Yellow-banded Bumble Bee (B. terricola) has declined in southern parts of its Canadian range in central and eastern Canada; and Western Bumble Bee occidentalis subspecies (B. occidentalis occidentalis) has declined in southern areas of the western provinces.
Rescue Effect (immigration from outside Canada)
Current Status
COSEWIC: none
Status and Reasons for Designation:
Applicability of Criteria

The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) was created in 1977 as a result of a recommendation at the Federal-Provincial Wildlife Conference held in 1976. It arose from the need for a single, official, scientifically sound, national listing of wildlife species at risk. In 1978, COSEWIC designated its first species and produced its first list of Canadian species at risk. Species designated at meetings of the full committee are added to the list. On June 5, 2003, the Species at Risk Act (SARA) was proclaimed. SARA establishes COSEWIC as an advisory body ensuring that species will continue to be assessed under a rigorous and independent scientific process.
The Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assesses the national status of wild species, subspecies, varieties, or other designatable units that are considered to be at risk in Canada. Designations are made on native species for the following taxonomic groups: mammals, birds, reptiles, amphibians, fishes, arthropods, molluscs, vascular plants, mosses, and lichens.
COSEWIC comprises members from each provincial and territorial government wildlife agency, four federal entities (Canadian Wildlife Service, Parks Canada Agency, Department of Fisheries and Oceans, and the Federal Biodiversity Information Partnership, chaired by the Canadian Museum of Nature), three non-government science members and the co-chairs of the species specialist subcommittees and the Aboriginal Traditional Knowledge subcommittee. The Committee meets to consider status reports on candidate species.
A species, subspecies, variety, or geographically or genetically distinct population of animal, plant or other organism, other than a bacterium or virus, that is wild by nature and is either native to Canada or has extended its range into Canada without human intervention and has been present in Canada for at least 50 years.
The Canadian Wildlife Service, Environment Canada, provides full administrative and financial support to the COSEWIC Secretariat.
Bombus bohemicus was described by Seidl in 1837, and has been considered a valid species in the Old World since that time. In North America, Cresson (1864) described Apathus ashtoni and, until recently, Bombus ashtoni (Cresson) (Ashton’s Cuckoo Bumble Bee) has been the scientific name used for this taxon. However, Williams (1991) questioned the species status of B. ashtoni based on morphological evidence. Later, Cameron et al. (2007), using molecular data (i.e., DNA), also suggested that B. ashtoni may be conspecific with the Old World B. bohemicus. More recently, and using additional genetic data (COI sequences from mitochondrial DNA), the North American B. ashtoni has been synonymized under B. bohemicus, a name which is now applied to this Holarctic species (Williams 2013; Williams et al. 2014).
The morphological description of Gypsy Cuckoo Bumble Bee below is from information in Plath (1934), Mitchell (1962), Colla et al. (2011) (as B. ashtoni) and Williams et al. (2014).
Female: Body length 17 - 18 mm; breadth of abdomen 8 - 8.5 mm. The outer surface of the hind tibia is convex, with dense hair covering the surface, without a corbicula (i.e., pollen basket). The hair on the face and top of the head is typically all black, occasionally with some yellow hairs at the posterior top of the head. The sides of the thorax are predominantly with black hair; hair on the anterior surface of thorax (i.e., in front of wings) is yellow, and varies from yellow to black on the remaining dorsal surface. The first two abdominal segments have black hair, the 3rd to 5th abdominal segments are laterally variable yellowish-white, but usually white at least posteriorly in the middle of the 4th segment (Figure 2). Like all cuckoo bumble bees, the tip of abdomen is strongly recurved ventrally (Figure 1), with the ventral surface with two strong carina (ridges).
Figure 1. Lateral image of female Gypsy Cuckoo Bumble Bee (Bombus bohemicus) housed at the Packer Collection, York University, Toronto, ON. Photograph by Sheila Colla.

Photo: © S. Colla
Long description for Figure 1
Photo of a female Gypsy Cuckoo Bumble Bee specimen, lateral view. For a full description of the colour pattern and characteristic features, see report text under “Morphological Description.”
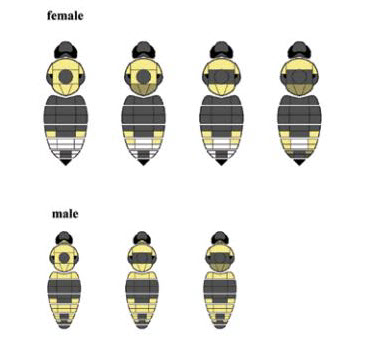
Figure 2. Colour patterns for Gypsy Cuckoo Bumble Bee (reproduced with permission from Colla 2012).
Long description for Figure 2
Illustrations of the colour patterns of the female (four images; top) and male (three images; bottom) Gypsy Cuckoo Bumble Bee.
Female pattern: The hair on the face and top of the head is all black. The hair on the sides of the thorax is predominantly black, whereas the hair on the anterior surface of thorax (in front of the wings) is yellow, and varies from yellow to black on the remaining dorsal surface. The first two abdominal segments have black hair, the third to fifth abdominal segments are laterally variable yellowish-white, but white in the middle of the fourth segment.
Male pattern: The first abdominal segment is yellow; the second segment is entirely black; the third, fifth, and sixth segments are primarily yellow with black hairs present medially; the fourth segment is primarily yellow; and the seventh segment is entirely black.
Gypsy Cuckoo Bumble Bee is frequently misidentified as one of three co-occurring Psithyrus species: B. insularis, B. flavidus, and B. suckleyi. Gypsy Cuckoo Bumble Bee females have predominantly dark hairs on the top of head that differentiate this from other species, which have pale hairs. In addition, the black pleura and carina of the 6th sternum are reliable characters that differentiate females from B. insularis, B. flavidus, and most specimens of Suckley’s Cuckoo Bumble Bee (B. suckleyi). Further, the hairs on the 3rd and 4th tergites of Gypsy Cuckoo Bumble Bee females are usually white, at least posteriorly in the middle of T4. Proper identification of males may require examination of genitalia structures (Williams et al. 2014).
Genetic variability and population structure have not been studied for Gypsy Cuckoo Bumble Bee. The Barcode of Life database (BOLD) (www.barcodeoflife.org) has eight specimens barcoded from four sites in Canada (from British Columbia and Nova Scotia), and the United States (Alaska) (Packer pers. comm. 2011). All sequences are almost identical, and do not vary significantly from samples of Gypsy Cuckoo Bumble Bee from Germany (Cameron et al. 2011; Williams et al. 2012). As such, Gypsy Cuckoo Bumble Bee is now considered a Holarctic species, and B. ashtoni is now considered a junior synonym.
Gypsy Cuckoo Bumble Bee is being assessed as one designatable unit, in the absence of information on discreteness or evolutionary significance among populations. The species spans all COSEWIC (2011) Ecological Areas, except the eastern fringe of the Pacific (just west of the Rockies) and the Arctic (where it has not been reported).
Gypsy Cuckoo Bumble Bee is a social parasite in other bumble bee colonies. The species likely plays a significant ecological role through its effect on host dynamics and distribution (Antonovics and Edwards 2011). In this case, host species include the Rusty-patched Bumble Bee (B. affinis), Western Bumble Bee (both subspecies of B. occidentalis), Yellow-banded Bumble Bee (B. terricola) and possibly the Holarctic Cryptic Bumble Bee (B. cruptarum) (Owen et al. 2012).
The biology of Gypsy Cuckoo Bumble Bee has been studied previously (e.g., Fisher 1983, as B. ashtoni). Its main significance lies in its sensitivity to environmental degradation: bees in general, and cuckoo and social bees in particular, are sensitive to impacts of small population size (Williams et al. 2010) because of their sex determining mechanism (Zayed and Packer 2005) (see Limiting Factors).
Gypsy Cuckoo Bumble Bee is a Holarctic species. In North America, it ranges throughout most of Canada (except Nunavut) and parts of the northern United States (US) (i.e., Alaska, Connecticut, Indiana, Massachusetts, Minnesota, North Dakota, New Hampshire, New Jersey, New York, Ohio, Pennsylvania, Virginia, West Virginia, Wisconsin) (Figure 3). In the Old World the species occurs throughout most of Europe (except Iceland and extreme southwestern Europe) and across Asia.
Figure 3. Distribution of Gypsy Cuckoo Bumble Bee in North America with predicted suitability modeled using MaxEnt software (Phillips et al. 2006) based on museum specimens (red circles) (reproduced with permission from Williams et al. 2014).

Map: © Environment Canada
Long description for Figure 3
Map of the distribution of the Gypsy Cuckoo Bumble Bee in North America based on museum specimens. Areas of predicted suitable habitat are also indicated; four degrees of suitability are shown, ranging from more likely to less likely. The species ranges throughout most of Canada (except Nunavut) and parts of the northern United States (Alaska, Connecticut, Indiana, Massachusetts, Minnesota, North Dakota, New Hampshire, New Jersey, New York, Ohio, Pennsylvania, Virginia, West Virginia, and Wisconsin).
Gypsy Cuckoo Bumble Bee has been recorded from every Canadian province and territory except Nunavut (NU) (Colla and Sheffield 2010) (Figure 4). Canadian records are from 1883 to 2008. Since 1991, the species has been recorded from only three provinces: Ontario (67 specimens), Quebec (39 specimens) and Nova Scotia (18 specimens) (Appendix 1). The most recent are from Pinery Provincial Park in Ontario (2008) and Parc national des Monts-Valin in Quebec (2008). Despite its wide distribution, climate suitability modelling based on collected and museum specimens suggests that southern and central Ontario and Quebec are most suitable for the species (Figure 3), which also corresponds to the most recent records in Canada (2008).
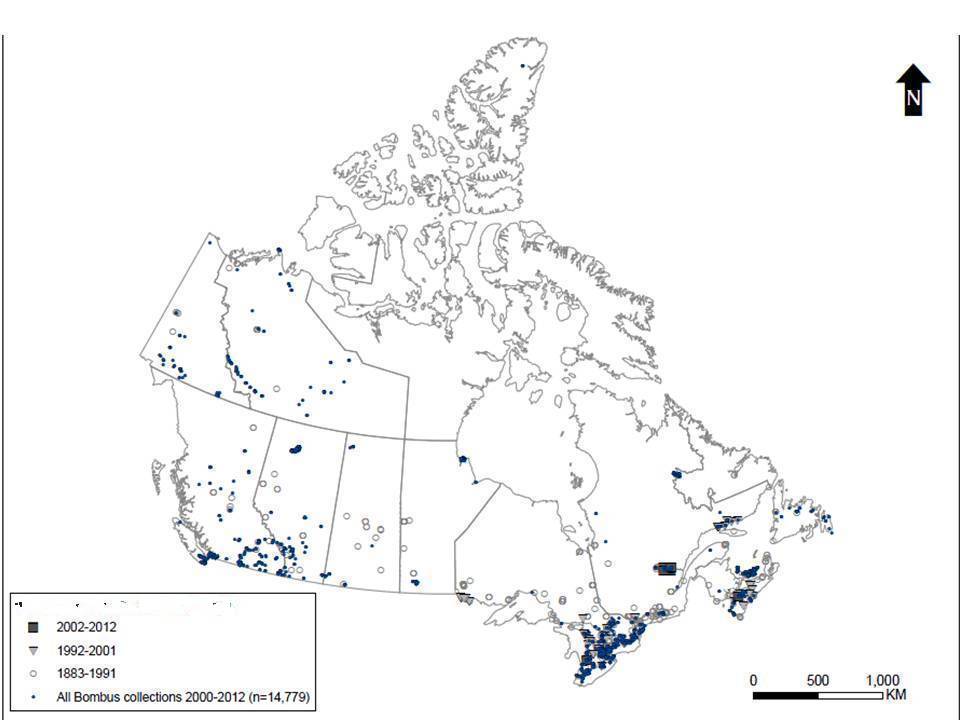
Figure 4. Gypsy Cuckoo Bumble Bee records (n=844) in Canada and recent search effort (2000-2012) that shows collection records for all Bombus specimens (reproduced with permission from the dataset of Williams et al. 2014).
Map: © Environment Canada
Long description for Figure 4
Map indicating the distribution of Gypsy Cuckoo Bumble Bee records in Canada for three periods (2002 to 2012; 1992 to 2001; 1883 to 1991) and recent search effort (2000 to 2012) that includes collection records for all Bombus specimens. Gypsy Cuckoo Bumble Bee has been recorded from every Canadian province and territory except Nunavut.
Each province and territory is listed (Table 2) below (chronologically descending with most recent records first). Information on the range of the host bee within each province is also listed where the data is available. The ranges of host species throughout Canada are:
- Rusty-patched Bumble Bee in southern Ontario (ON) and Quebec (QC);
- Western Bumble Bee (northern and southern subspecies) (Figure 5) in British Columbia (BC), Alberta (AB), southern Saskatchewan (SK), Yukon (YK) and western Northwest Territories (NT);
- Yellow-banded Bumble Bee (Figure 5) from the Rockies in eastern BC through the boreal zone, southern NT and southern half of Canada to Newfoundland (NF);
- Possibly the Holarctic Cryptic Bumble Bee in the YK, NT, BC, AB and SK, with a few unconfirmed records from NU and extreme northern ON (Colla pers. data 2013).
Figure 5. Bumble bee collection points (all dots total 236,260 specimens) for North America from 1892 - 2013. Dots in red represent host bee Western Bumble Bee and dots in blue represent host bee Yellow-banded Bumble Bee (see Wildlife Species Description and Significance). No data exists for areas without points. Note there has been taxonomic debate about Western Bumble Bee and Yellow-banded Bumble Bee, and it is not guaranteed that all specimens used in these maps are correctly identified. These maps should be used as general range maps and outliers further investigated. Map compiled by Leif Richardson November 2013 and used with permission from Sheffield et al. (in prep.).

Map: © Environment Canada
Long description for Figure 5
Map indicating bumble bee collection points in North America from 1892 to 2013. Collection points are colour-coded to distinguish between host bee Western Bumble Bees, host bee Yellow-banded Bumble Bees, and all Bombus collections. In Canada, Western Bumble Bee (northern and southern subspecies) is shown in British Columbia, Alberta, southern Saskatchewan, Yukon, and western Northwest Territories. Yellow-banded Bumble Bee is shown from the Rockies in eastern British Columbia through the boreal zone, southern Northwest Territories, and southern half of Canada to Newfoundland and Labrador.
One of the most recent Canadian records for Gypsy Cuckoo Bumble Bee is from Pinery Provincial Park (2008). Host(s) in Ontario: Yellow-banded Bumble Bee ranges across the Mixedwood Plains and Boreal Shield ecozones of southern Ontario, and there are some in the Hudson Bay lowlands around James Bay. This host species was last collected in southern Ontario in 2013 (e.g., Toronto, Barrie and Ottawa) (Colla pers. comm. 2014). Rusty-patched Bumble Bee is much more uncommon and was last recorded in 2009 from Pinery Provincial Park (COSEWIC 2010).
The most recent Canadian records of Gypsy Cuckoo Bumble Bee are from Quebec at Parc national des Monts-Valin – Chalet La Courtepointe (July 4, 2008) (Savard 2012), Parc national des Monts-Valin – Pied-du-Mont (July 29, 2007) (Savard 2012) and Anticosti Island - Jupiter in the Gulf of St. Lawrence (June 16 to 30, 2007) (Brousseau 2011; Savard pers. comm. 2012). Numerous specimens were collected during surveys from 2000-2001 at Magpie, Aguanish, Baie-Johan-Beetz, and Rivière-Saint-Jean (Buidin pers. comm. 2011) (See Appendix 1). Older records are from southern and central Quebec with unconfirmed records from northern Quebec (Laverty and Harder 1988). Host(s) in Quebec: Yellow-banded Bumble Bee remains sparsely distributed throughout southern Quebec. This host species was formerly common across the Mixedwood Plains and Boreal Shield ecozones of southern Quebec, and there are scattered collections from the Taiga Shield as far north as Schefferville at nearly 55° N. Most recently Yellow-banded Bumble Bee was collected in 2013 by M. Chagnon on farms south of Montreal and Quebec City (Sheffield pers. comm. 2014). The Rusty-patched Bumble Bee has not been recorded in Quebec since the 1970s (COSEWIC 2010).
The most recent Gypsy Cuckoo Bumble Bee records are from near Middleton (2002). The species was recorded from many sites throughout the 1990s including specimens housed at Cape Breton University (most recent from 2001) (McCorquodale pers. comm. 2012). Host(s) in Nova Scotia: Yellow-banded Bumble Bee has been collected over most of Nova Scotia, and was most recently collected in 2013 during resurveys of historic collections made in Lockeport, Greenfield and New Germany (Colla pers. comm. 2014).
The most recent specimen was collected in 1983 (Curley pers. comm. 2011). The University of Prince Edward Island [Entomology] Museum has specimens collected during the 1970s and 1980s from Riverdale, Charlottetown, Cornwall and Vernon River. Host(s) in Prince Edward Island: Yellow-banded Bumble Bee is common at the same survey sites within Riverdale, Charlottetown, Cornwall and Vernon River (Giberson pers. comm. 2011) and most recently recorded in 2013 (Colla pers. comm. 2014).
The species was last collected 40 km south of Quesnel in 1988. The westernmost range extent for this species is within the central interior of BC. The species has not been detected along the coast, or in the southwestern portion of the province (Figure 3). Host(s) in British Columbia: The hosts include Western Bumble Bee and Yellow-banded Bumble Bee, although both these species appear to be declining within British Columbia. Recent surveys for Western Bumble Bee, with a minimum of 281 hours cumulative search effort over more than 104 sites (additional samples still to be processed), were conducted in BC in 2013 (Sheffield et al.; data being used in a manuscript in prep.). These surveys yielded a minimum of 6447 Bombus specimens, 115 specimens (or 1.7% of total examined) were Western Bumble Bee (at 36 of the 104 sites) and 295 specimens (or 4% of total) were Yellow-banded Bumble Bee (Sheffield et al. in prep.). There were no records of Gypsy Cuckoo Bumble Bee within these surveys, despite finding its hosts.
Specimens have been recorded only in central and southern Manitoba. The most recent specimen of Gypsy Cuckoo Bumble Bee is from 1986 (see also Turnock et al. [2007] in Population Sizes and Trends). Host(s) in Manitoba: Historical collections of Yellow-banded Bumble Bee are predominantly from the Boreal Plains and Prairies ecozones in the southern one third of the province, but a few were made as far north as Hudson Bay. The most recent Yellow-banded Bumble Bee collection is at Gillam and York Factory in 2010 (Colla pers. comm. 2014).
The most recent record of Gypsy Cuckoo Bumble Bee is from Lethbridge in 1983. Host(s) in Alberta: Yellow-banded Bumble Bee is found throughout the province, with collections in all ecozones, including southern Prairies, central Boreal Plains, western mountains and northern Taiga Plains. It was observed at Edmonton and Slave Lake in 2013 during resurveys of historical collection locations (Rowe pers. comm. 2013). Additional specimens were collected in a 200 km radius around Edmonton by G. Anweiler (Sheffield pers. comm. 2014). In 2013, surveys resulted in Western Bumble Bee and Yellow-banded Bumble Bee collections, albeit uncommonly from near Dinosaur Provincial Park (near Red Deer), Red Cliff (south of Medicine Hat) and Cypress Hills areas (Sheffield pers. comm. 2014).
The most recent record of Gypsy Cuckoo Bumble Bee is 1979 from Gros Morne National Park. Additional specimens have been collected at Goose Bay, Codroy Valley and Grand Falls. Host(s) in Newfoundland and Labrador: Yellow-banded Bumble Bee has been collected mainly in coastal areas, particularly along the Gulf of St. Lawrence. There are a few undated collections (likely <2004) from Labrador in the Canadian National Collection from the towns of Cartwright and St. Anthony. The most recent Yellow-banded Bumble Bee collections from the province are from 2010 where it remains common (Sheffield pers. comm. 2014).
The species has been collected along the western half of this territory, the most recent specimen collected ‘3 miles southeast’ of Fort Simpson in 1972. Host(s) in Northwest Territories: Yellow-banded Bumble Bee occurs in the central Taiga Plains ecozone of NT, but not the mountainous western parts of the territory. The most recent Yellow-banded Bumble Bee collections were made in Hay River area (2005) and Fort Simpson (2011). Very few records exist for Western Bumble Bee (northern subspecies) in the NT, these from the extreme western part of the jurisdiction. There is only one record from pre-2011 (August 4, 1944 – exact site not given). The remaining eight specimens are from various sites on the South Nahanni River, collected on various dates in August 2011 (Stotyn 2012; Sheffield pers. comm. 2014).
The most recent record of Gypsy Cuckoo Bumble Bee is Meadow Lake (1972), and the species has been recorded from southern and central SK, including Val Marie. Host(s) in Saskatchewan: Yellow-banded Bumble Bee is found on the Boreal Plains and Prairies ecozones in the southern third of the province. Curry (1984) describes Yellow-banded Bumble Bee as being common and widespread in the province from prairies to the northern coniferous forests. In 2013 specimens were collected at Killaly and Prince Albert (Colla pers. comm. 2014) and additional collecting events suggest the species is one of the most common in the Prince Albert, Birch Hills areas as far south as Regina (Sheffield pers. comm. 2014). There are few historical surveys or museum collections of Western Bumble Bee from SK. Recently Western Bumble Bee (i.e., 2012-2013) has been recorded throughout the southern third of SK. (i.e., Grasslands National Park, Saskatchewan Landing Provincial Park, Great Sand Hills, Big Muddy Valley, and Cypress Hills Provincial Park, Eastend, Swift Current and as far east as Regina) (samples are still being processed, Sheffield et al. (in prep.)). This species appears rather uncommon compared to other bumble bees. Prior to these surveys, there are very few historic records databased from these areas (Sheffield pers. comm. 2014).
The most recent record of Gypsy Cuckoo Bumble Bee is from ‘15 miles east of Dawson’ in 1962. Host(s) in Yukon: Western Bumble Bee has been recorded at numerous sites in YT over the past 3 years without detecting B. bohemicus (Cannings pers. comm. 2013; Sheffield pers. comm. 2014). Western Bumble Bee was present at many sites surveyed in 2009, 2010 and 2013 (Cannings pers. comm. 2013; Sheffield pers. comm. 2014). The northern subspecies is still considered common in adjacent Alaska, where it accounted for over 30% of all bumble bees observed (Koch and Strange 2012).
The most recent record of Gypsy Cuckoo Bumble Bee is Coldbrook (1961), near Saint John. Host(s) in New Brunswick: Yellow-banded Bumble Bee is found throughout New Brunswick (Laverty and Harder 1988). The species was a common blueberry pollinator in the 1970s and early 1990s (Sheffield pers. comm. 2014). The most recent specimens were collected during 2013 resurveys of historic collection sites in the towns of Springfield and Norton (Colla pers. comm. 2014). There is one confirmed specimen of Rusty-patched Bumble Bee found in New Brunswick from the 1940s (Klymko pers. comm. 2014).
Much time and effort have been invested (recently and historically) in surveys that focus on bumble bees, including Gypsy Cuckoo Bumble Bee. There are more data available for wild bumble bees than for most other North American insects. A recently compiled dataset (Williams et al. 2014) of approximately 236,260 bumble bee specimens (Figure 5) from museums in Canada and the United States shows the increase in bumble bee specimens collected, particularly in the past decade. This is due to recent studies in the US (e.g., Cameron et al. 2011; Grixti et al. 2009) and Canada (e.g. Colla and Packer 2008; Sheffield pers. data 2013; Williams et al. 2014) showing substantial search effort, accumulating hundreds of person-hours surveying wild bumble bee populations each decade.
Additionally, many recent bumble bee surveys throughout the range of Gypsy Cuckoo Bumble Bee but targeting Rusty-patched Bumble Bee and Western Bumble Bee have not recorded the species. These surveys used opportunistic hand netting and pan trapping as a sampling method (bees were actively searched out while foraging on flowers, captured with an insect net and either released or captured and pinned). Recent bumble bee surveys in YT, BC, AB and SK are summarized (as part of ongoing preparation for the COSEWIC status report for Western Bumble Bee) and surveys in NB and ON (see COSEWIC 2010).
However, there are shortcomings in search effort. Surveys typically have not been systematic or comprehensive over time and across the range of Gypsy Cuckoo Bumble Bee. Thus, there are large areas (e.g., northern halves of most Canadian provinces, and the territories, mainly due to inaccessibility) and time periods where no data are available. Most surveys occurred in the southern parts of Gypsy Cuckoo Bumble Bee’s range, whereas there are numerous historical sites in the boreal forest biome, which need surveys (Figure 4). Also, surveyors often did not document the time and effort invested, making it difficult to accurately quantify and compare search effort. These factors make it difficult to interpret spatial and temporal patterns in Gypsy Cuckoo Bumble Bee records (see Population Sizes and Trends).
During surveys conducted as part of this report, known historical sites were re-sampled for Gypsy Cuckoo Bumble Bee from June – October 2011, using opportunistic hand netting (Colla pers. data 2011). These historical sites in Ontario include: Toronto (5 days), Pinery Provincial Park (8 days), Beamsville (1 day), Guelph (1 day), and in Quebec, Sainte-Anne-de-Bellevue (2 days). Presqu’ile Provincial Park (Quebec) was surveyed (one day) in June 2012. Gypsy Cuckoo Bumble Bee was not recorded during these surveys.
A summary of search effort for each province and territory is below and Bombus collections (general) are shown in Figure 4.
Much of NT has not been sampled for bumble bees. Most recently (July 2011) bumble bees were surveyed at 19 sites along riparian areas of the South Nahanni River from Moose Ponds to the Liard River (Stotyn 2012). Of the 78 individuals collected, none were Gypsy Cuckoo Bumble Bee; however, Western Bumble Bee was recorded (Stotyn 2012).
Despite frequent surveys within the Churchill and surrounding areas, the species has not been recorded. Patenaude (2007) collected over 600 bumble bees throughout his prairie sites in southwestern Manitoba (May to September 2005 and 2006) without recording the species.
Southern ON has been extensively surveyed for bumble bees (n >4000 Bombus) from 2004 – 2012 (Figure 4). One of the most recent Canadian records of Gypsy Cuckoo Bumble Bee is from Pinery Provincial Park (in 2008), the same site where the last known Rusty-patched Bumble Bee (host species) populations are recorded (2009). Pinery Provincial Park has been extensively surveyed from 2008 – 2011 with no new specimens of Gypsy Cuckoo Bumble Bee collected. Some sites in central and northern Ontario (e.g. Mississagi Provincial Park June 13 – 16, 2011) have been surveyed in recent years with no specimens recorded.
Laverty and Harder (1988) report unconfirmed records from Northern QC. Numerous specimens were collected during surveys from 2000 - 2008 at Magpie, Aguanish, Baie-Johan-Beetz, and Rivière-Saint-Jean (Buidin pers. comm. 2011; Savard 2012) (See Appendix 1).
The species was not recorded during surveys in the summer of 2008 near Moncton, Fundy National Park, and Saint John (S. Colla surveyed bumble bees for 4 days in search of Rusty-patched Bumble Bee) (COSEWIC 2010). In 2010 and 2011, 219 bumble bees were collected although none were Gypsy Cuckoo Bumble Bee (Klymko pers. comm. 2012).
This province has been thoroughly surveyed for bumble bees over the past ten years by students and others (Sheffield pers. comm. 2012). Gypsy Cuckoo Bumble Bee was recorded from many sites throughout the 1990s. Cape Breton University has a few specimens from 1986 - 1998 with the most recent from 2001 (McCorquodale pers. comm. 2012). No Gypsy Cuckoo Bumble Bee specimens were collected during 2010 and 2011 surveys (Klymko pers. comm. 2012).
There are no surveys for the species in the past ten years.
Intensive surveys have been completed over 57 sites since 2000 yielding 266 bumble bee specimens and no new records for Gypsy Cuckoo Bumble Bee, with Yellow-banded Bumble Bee common at these sites (Giberson pers. comm. 2011). Gypsy Cuckoo Bumble Bee was not recorded during fieldwork in 2004 – 2005 (MacPhail 2011).
At least 2000 bumble bees have been recorded from numerous sites over the past three years without detecting Gypsy Cuckoo Bumble Bee, although potential host species Western Bumble Bee and Cryptic Bumble Bee remain common (Cannings pers. comm. 2013).
Many sites have been surveyed for bumble bees by biologists from universities and government agencies without detection of Gypsy Cuckoo Bumble Bee (Figure 4). The search effort during the past 10 years has been very high, with 1000s of Bombus individuals collected over hundreds survey hours. For example, a study in Fraser Valley in 2003 and 2004 yielded 4221 Bombus individuals without yielding Gypsy Cuckoo Bumble Bee (Ratti 2006). Recent bumble bee surveys with a minimum of 281 hours cumulative search effort over approximately 104 sites (additional samples still to be processed) were conducted in BC in 2013 (Sheffield et al.; data being used in a manuscript in prep.). These surveys were intensive with a minimum of 30 minutes to one hour per site and collecting all species present. No Gypsy Cuckoo Bumble Bees were recorded in the 104 samples processed to date (Sheffield et al. in prep.).
Many sites have been surveyed for bumble bees by biologists from universities and government agencies without detection of Gypsy Cuckoo Bumble Bee (e.g. Colla pers. data 2010; Owen et al. 2012; Figure 4). The search effort during the past 10 years has been very high, with 1000s of Bombus individuals collected over hundreds survey hours. In 2010, 775 Bombus individuals were collected from southern Alberta without detecting this species (Colla pers. data 2010). Surveys in Cypress Hills (in 2007 and 2013), Dinosaur Provincial Park, Red Cliff (south of Medicine Hat), Edmonton and surrounding areas did not record the species (Sheffield pers. comm. 2014). At least 20 sites (minimum 30 minutes at each site) within a 200 km radius around Edmonton by G. Anweiler did not record Gypsy Cuckoo Bumble Bee (Sheffield et al. in prep).
Recent surveys (i.e., 2011-2013) (i.e., Grasslands National Park, Saskatchewan Landing Provincial Park, Great Sand Hills, Big Muddy Valley, Eastend, Leader, Swift Current, Prince Albert, Cypress Hills Provincial Park and as far east as Regina) and other areas (samples are still being processed, Sheffield et al. (in prep.)) have not recorded the species. Additionally bumble bee surveys in 2011 (vane trapping Page Footnote1.1 for one week by A. Crosby) at Cypress Hills did not yield any specimens (Colla pers. comm. 2013).
Outside Canada, surveys conducted from 2007 - 2009 at 382 sites (n= 16,788 Bombus collected) throughout the continental US failed to detect Gypsy Cuckoo Bumble Bee and found drastic declines in all three occurring host species Yellow-banded Bumble Bee, Western Bumble Bee and Rusty-patched Bumble Bee (Cameron et al. 2011).
Gypsy Cuckoo Bumble Bee is a social parasite or cuckoo in nests of bumble bees in the subgenus Bombus senso stricto Host species select abandoned underground rodent burrows as nest sites (Plath 1934), and have been collected in various habitats such as montane meadows, old fields, mixed farmlands, urban areas and open woodlands.
Gypsy Cuckoo Bumble Bee are generalist foragers, primarily for nectar (see Colla and Dumesh 2010) and are associated with food plants flowering close to wooded areas (Colla and Dumesh 2010) and blueberry fields (Vaccinium spp.) (Sheffield pers. comm. 2011)
Overwintering habitat requirements are unknown but in general, bumble bees overwinter in the ground, in mulch or other decomposing vegetation, and in rotting logs near nesting sites (Macfarlane 1974).
Gypsy Cuckoo Bumble Bee has one of the largest ranges of all bumble bee species in Canada. It is unlikely that specific habitat trends have caused its decline at such large scales, though habitat loss due to urbanization or intensive agriculture may threaten this species (via its hosts) in the southern parts of its range along the international border. Habitat fragmentation, new agricultural development, including the conversion of insect-pollinated crops to wind-pollinated or greenhouse systems), and/or agricultural intensification, possibly in combination with increased pathogen rates have likely contributed to the decline in habitat quality for this species.
Climate change-induced habitat alteration may also negatively impact this species via the effect on its hosts, but more research is required.
Gypsy Cuckoo Bumble Bee is a socially parasitic (or cuckoo) bumble bee, and follows the same life cycle pattern of its host bumble bee species. The generation time is one year. In the spring, females of the subgenus Psithyrus (cuckoo bumble bees) invade nests of social Bombus (true bumble bees) and displace the resident queen (either by killing or injuring her). The daughters (workers) of the host queen are used by the cuckoo to rear their own offspring (Michener 2000) through the use of chemical cues (Zimma et al. 2003). Eggs hatch approximately four days later and the small larvae begin to feed on pollen and nectar. The larval stage of bumble bees has four instars. After almost two weeks of development, larvae spin cocoons and pupate. Pupae develop for another two weeks before hatching as full-sized adults. In total, development takes approximately five weeks but varies with temperature and food supply (Alford 1975). Males and females of Psithyrus emerge (Figure 5) and after mating, males die and females overwinter.
One Rusty-patched Bumble Bee colony dug up by Plath (1934) on August 9th contained the old queen, one-hundred Rusty-patched Bumble Bee workers, three Gypsy Cuckoo Bumble Bee females and six Gypsy Cuckoo Bumble Bee males. The colony was observed until the end of September and produced twenty-nine Gypsy Cuckoo Bumble Bee males and sixty-one Gypsy Cuckoo Bumble Bee females. Although the injured Rusty-patched Bumble Bee queen was seen with a distended abdomen and laying eggs, no further Rusty-patched Bumble Bee males, workers or queens were produced. Fisher (1983) hypothesized that the presence of a live Rusty-patched Bumble Bee queen is required by Gypsy Cuckoo Bumble Bee to suppress ovarian development of the worker caste, but that the Gypsy Cuckoo Bumble Bee female eats the eggs produced by the Rusty-patched Bumble Bee queen to reduce competition with her offspring. Similar details are not known for other hosts/potential hosts in Canada, including Western Bumble Bee Yellow-banded Bumble Bee, and Cryptic Bumble Bee.
Very little is known about Gypsy Cuckoo Bumble Bee mating behaviour. Adults visit flowers, both after emergence (sometime in the autumn) and, females only, prior to nest invasion in the spring (Antonovics and Edwards 2011). Phenology differs with latitude and altitude but generally females emerge approximately one month after the host species (Plath 1934) and are detected until late summer. Males emerge early summer and are detected until late autumn. Figure 6 shows the phenology of the species in southern Ontario, one of the best-sampled regions of Canada. Phenology for Gypsy Cuckoo Bumble Bee likely differs slightly by latitude, elevation and host emergence but similar information is not known for other parts of its range.
Figure 6. Phenology for Gypsy Cuckoo Bumble Bee in southern Ontario (specimens from 1883-2008), earliest spring record April 21 (n= 275 [note additional historic records have been added to Ontario records since this graph was produced, see Table 2]) (reproduced with permission from Colla 2012).

Long description for Figure 6
Line chart illustrating phenology for Gypsy Cuckoo Bumble Bee in southern Ontario (specimens from 1883 to 2008) (frequency on y axis; month on x axis). Lines are given for queens and males. The line for queens runs from April to August (with a peak in June), while the line for males runs from June to October (with a peak in August). For more information, see report text under “Life Cycle and Reproduction.”
| Species | Total records in North America | <1931 | 1931-1960 | 1961-1990 | 1991-2009 | Slope (sign indicates direction of change) | Χ2 | P-value |
|---|---|---|---|---|---|---|---|---|
| Rusty-patched Bumble Bee (B. affinis) | 1563 | 355 | 303 | 812 | 93 | -0.2779 | 0.5281 | 0.4674 |
| Gypsy Cuckoo Bumble Bee (B. bohemicus)* | 941 | 311 | 280 | 267 | 83 | -0.5166 | 13.7488 | 0.0002 |
| Lemon Cuckoo Bumble Bee (B. citrinus*) | 1202 | 222 | 217 | 178 | 585 | 0.1750 | 0.4106 | 0.5217 |
| Fernald’s Cuckoo Bumble Bee (B. fernaldae*) | 474 | 77 | 277 | 97 | 23 | -0.5064 | 1.0955 | 0.2952 |
| Common Eastern Bumble Bee (B. impatiens) | 9111 | 1141 | 851 | 2709 | 4410 | 0.3984 | 6.7176 | 0.0095 |
| Indiscriminate Cuckoo Bumble Bee (B. insularis*) | 1025 | 159 | 361 | 470 | 35 | -0.3099 | 0.5251 | 0.4687 |
| Yellow-banded Bumble Bee (B. terricola) | 3724 | 963 | 456 | 1632 | 673 | -0.1723 | 0.4516 | 0.5016 |
| Variable Cuckoo Bumble Bee (B. variabilis*) | 94 | 76 | 11 | 7 | 0 | -1.7406 | 39.2118 | 0.0000 |
| Total (all species) |
69600 | 12375 | 16093 | 21386 | 19746 | -0.0682 | 1.2002 | 0.2733 |
| Province | Earliest record | Most recent record | Number of records Footnotea | Sites collected |
|---|---|---|---|---|
| Alberta | 1953 | 1983 | 15 | McMurray, Calais, Beaverlodge, Delburne, Fairview, Lethbridge, Jasper |
| British Columbia | 1915 | 1988 | 11 | Revelstoke, Golden, Fort Nelson, Peters Lake, Kenney Dam, 40km South of Quesnel |
| Manitoba | 1924 | 1986 | 84 | Teulon, Aweme, Cormorant Lake, Winnipeg, Carberry, Victoria B[each], The Pas, Wanless, Erickson, Bowsman and specimens in Turnock et al. 1986. |
| New Brunswick | 1914 | 1961 | 8 | Painsec, Fredericton, St. Andrews, Coldbrook |
| Newfoundland and Labrador | 1925 | 1979 | 5 | Grand Falls, Goose Bay, Codroy Valley |
| Nova Scotia | 1910 | 2002 | 45 | Barrington Passage, Digby, Antigonish, Jimtown, Ottawa, Thession, Halifax, South Maitland, Shubernacadie Lake, Truro, Merigomish Harbour, Pleasant River, McNabs Island, Meat Cove, Pleasant Bay, Debert, Belaps Cove, West Dover, Greenfield, Mount Uniacke, Armdale, Whycocomagh, West Black Rock, Middleton, Cheticamp (Cape Breton Island) |
| Northwest Territories | 1948 | 1972 | 38 | Reindeer Depot, Norman Wells, Fort Smith, Fort Simpson, Hay River, Aklavik, Fort McPherson, No Name Creek |
| Yukon | 1916 | 1962 | 4 | Dawson, and non-gazetted sites |
| Saskatchewan | 1938 | 1972 | 34 | Saskatoon, Waskesiu Lake, Love, White Fox, Hudson Bay, Torch River, Estevan, Candle Lake, Greenwater Lake, Emma Lake, Meadow Lake, Val Marie, Melfort, Indian Head |
| Ontario | 1883 | 2008 | 352 | Throughout southern Ontario, including Toronto, Guelph, London, Mica Bay, Merivale, Ottawa, Pinery Provincial Park, Port Franks, Presqu’ile Provincial Park, Speedside, Sudbury and other sites. |
| Quebec | 1913 | 2008 | 121 | Throughout southern Quebec including Lakeside, St.-Hilaire, Shawbridge, Montréal, Lac Jean-Venne, Luskville Falls, Gaspé, Hull, Lanoraie and other sites. |
| Prince Edward Island | 1970 | 1983 | 5 | Riverdale, Vernon River, Charlottetown, Cornwall |
| Total | 722 |
Female Psithyrus are adapted for their parasitic lifestyle and have a thicker, more protective exoskeleton, larger mandibles, greater number of ovarioles and a longer venom gland compared to host females (Fisher and Sampson 1992). They do not have hindleg corbicula (so do not carry pollen) and their abdomens generally have less pile.
In one study, females emerged approximately one month after its host species Rusty-patch Bumble Bee (Plath 1934). Although this host species is only in a small portion of Gypsy Cuckoo Bumble Bee range in Canada, the emergence pattern is probably similar for other host species in other parts of its range.
In general, there is very little information on natural dispersal rates for bumble bees. The ability and rate of dispersal for Gypsy Cuckoo Bumble Bee depends on its hosts’ population dynamics and distribution. The opportunity for dispersal occurs with the movement of reproductive individuals, primarily females in spring that disperse while searching for suitable nest sites (Goulson 2003). Given the patchiness of bumble bee habitat (e.g. Hatfield and LeBuhn 2007) and increased problems associated with small effective population sizes in haplodiploid insects (Zayed and Packer 2005) (see Limiting Factors), dispersal is likely important to survival.
There is some evidence bumble bees are able to disperse long distances. Males of the well-studied Buff-tailed Bumble Bee (B. terrestris, and host to Gypsy Cuckoo Bumble Bee in the Old World) are estimated to fly between 2.6 and 9.9 km from the colony of origin (Kraus et al. 2008). Additionally, Buff-tailed Bumble Bee was introduced to Tasmania in the early 1990s and has since spread at a rate of approximately 10 km per year (Stout and Goulson 2000).
Gypsy Cuckoo Bumble Bee is an obligate social parasite of bumble bees in the subgenus Bombus senso stricto. The species detects its host using chemical cues (Fisher et al. 1993). In the eastern range of Gypsy Cuckoo Bumble Bee, Rusty-patched Bumble Bee was a more common host than Yellow-banded Bumble Bee (at least in New England where both species co-occur) (Plath 1934). In the west, host species are unknown but likely include Cryptic Bumble Bee, Yellow-banded Bumble Bee and Western Bumble Bee. In the Old World, Gypsy Cuckoo Bumble Bee specializes on Old World members of the same subgenus (e.g. B. lucorum and B. terrestris), and potentially the Holarctic Cryptic Bumble Bee.
Four different analysis are used to show declines in relative abundance of Gypsy Cuckoo Bumble Bee. Relative abundance (RA) is the number of individuals of one species (e.g., Gypsy Cuckoo Bumble Bee) divided by the total number of individuals (e.g., Bombus) collected, and is often used as a proxy of abundance when data are not amenable to other analysis. The RA is also used as an index of search effort for Gypsy Cuckoo Bumble Bee, and it is assumed that if the species was within an area during a collection event, that it would likely have been collected. It is noted that measuring the RA of a species may not reflect actual population abundance. Historically, relative abundance data for Gypsy Cuckoo Bumble Bee is estimated at 1-2% of all bumble bees collected (Colla et al. 2012). Although bumble bee surveys have increased in the recent decade in some areas, few Gypsy Cuckoo Bumble Bee individuals have been recorded. For ease of reference between the next sections, these studies are numbered.
- The first study uses a dataset of bumble bees for Canada, with 44,706 museum and observation records from 1882 – 2011 (this dataset does not include data from 2012 and 2013 [e.g., Sheffield et al. in prep.]). The RA of Gypsy Cuckoo Bumble Bee, western host Western Bumble Bee, and one of its eastern hosts Yellow-banded Bumble Bee were analyzed in ten-year increments and for each jurisdiction where found (Table 3; Figure 8 [YT, NT, BC, AB and SK], Figure 9 [MB, ON, QC, NB and NS] and Figure 10 [PE and NL]) and Figure 11 [all collections]).
- Historical relative abundances of Gypsy Cuckoo Bumble Bee in North America were compared in 30 year time periods, from 1864 - 1930, 1931-1960, 1961-1990 and 1991- 2009 (Table 1).
- Indirect results of a study in Manitoba (see Turnock et al. 2007) can be interpreted as data applicable to population trends for Gypsy Cuckoo Bumble Bee. In this study, traps were placed in a Canola (Brassica rapa L.) field to trap Bertha Armyworm (Mamestra configurata Wlk.). Fourteen sites in four regions located in southern MB (Swan River Valley, Western Upland, Manitoba Lowlands and Red River Valley) were sampled every two weeks from mid-June to early August, and yearly from 1986 - 1993 (Turnock et al. 2007).
- A study in Guelph and surrounding areas of southern Ontario, replicated surveys completed from 1971 - 1973 (Macfarlane 1974) again in 2004 - 2006 (Colla and Packer 2008; COSEWIC 2010). Bees were collected opportunistically using insect nets and regularly sampled from April-October for both studies.
- The conservation status (using IUCN [2001] methodology and only considering records within the past 20 years) of 21 native bumble bee species throughout their North American ranges was assessed based on more than 69,000 georeferenced records dating back to 1864 (Colla et al. 2012). Grid cells measuring 50 km x 50 km were resampled for bumble bees.
| Bee Species | 1882-1891 | 1892-1901 | 1902-1911 | 1912-1921 | 1922-1931 | 1932-1941 | 1942-1951 | 1952-1961 | 1962-1971 | 1972-1981 | 1982-1991 | 1992-2001 | 2002-2011 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YT | GCBB | - | - | - | 0.048 | - | - | 0.013 | - | 0.003 | - | - | - | - |
| YT | WBB | - | - | 0.50 | 0.38 | 0.67 | - | 0.18 | 0.12 | 0.69 | 0.36 | 0.35 | 0.50 | 0.63 |
| YT | YBBB | - | - | - | - | - | - | - | - | - | - | - | - | - |
| NT | GCBB | - | - | - | - | - | - | 0.06 | 0.25 | 0.005 | 0.03; 1 specimen |
- | - | - |
| NT | WBB | - | - | - | - | - | - | - | - | - | - | - | - | 0.09 |
| NT | YBBB | - | - | - | - | 0.900 | - | 0.004 | 0.250 | 0.000 | 0.182 | - | 0.167 | 0.221 |
| NU | GCBB | - | - | - | - | - | - | - | - | - | - | - | - | - |
| NU | WBB | - | - | - | - | - | - | - | - | - | - | - | - | - |
| NU | YBBB | - | - | - | - | - | - | - | - | - | - | - | - | - |
| BC | GCBB | - | - | - | 0.003 | - | - | 0.006 | - | 0.01 | - | 0.0044; 1 specimen |
- | - |
| BC | WBB | 0.40 | 0.17 | 0.30 | 0.22 | 0.24 | 0.31 | 0.30 | 0.36 | 0.50 | 0.44 | 0.45 | 0.43 | 0.03 |
| BC | YBBB | - | - | 0.006 | - | 0.003 | - | 0.029 | 0.008 | 0.015 | 0.138 | 0.020 | 0.086 | 0.002 |
| AB | GCBB | - | - | 0.017 | 0.012 | - | - | 0.007 | 0.024 | 0.02 | 0.008; 1 specimen |
0.034; 2 specimens |
- | - |
| AB | WBB | 0.18 | 0.09 | 0.09 | 0.14 | 0.20 | 0.15 | 0.30 | 0.18 | 0.16 | 0.13 | 0.02 | 0.83 | 0.07 |
| AB | YBBB | 0.077 | - | 0.017 | 0.012 | 0.247 | 0.051 | 0.000 | 0.043 | 0.060 | 0.008 | - | - | 0.135 |
| SK | GCBB | - | - | - | - | - | 0.057 | - | - | 0.03 | 0.56; 5 specimens |
- | - | - |
| SK | WBB | - | - | - | - | - | 0.06 | 0.02 | - | 0.01 | - | - | 0.09 | 0.03 |
| SK | YBBB | 0.500 | - | - | 0.065 | - | 0.071 | 0.107 | 0.500 | 0.284 | - | - | 0.364 | - |
| MB | GCBB | - | - | - | 0.018 | 0.045 | - | - | 0.09 | - | 0.32; 6 specimens |
0.035; 5 specimens |
0.14; 1 specimen |
- |
| MB | WBB | - | - | - | - | - | 0.46 | - | - | - | - | 0.01 | - | - |
| MB | YBBB | - | - | 0.200 | 0.464 | 0.727 | 0.224 | 0.795 | 0.351 | 0.256 | 0.316 | 0.716 | 0.429 | 0.019 |
| ON | GCBB | 0.1; 3 specimens |
0.075; 4 specimens |
0.036 | 0.04 | 0.13 | 0.09 | 0.06 | 0.09 | 0.02 | 0.02; 46 specimens |
0.012; 23 specimens |
0.045; 65 specimens |
0.00027; 1 specimen |
| ON | WBB | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| ON | YBBB | 0.100 | 0.170 | 0.325 | 0.028 | 0.292 | 0.148 | 0.113 | 0.078 | 0.393 | 0.416 | 0.542 | 0.310 | 0.009 |
| QC | GCBB | - | - | - | 0.29 | 0.09 | 0.08 | 0.04 | - | 0.12 | 0.34; 12 specimens |
0.47; 45 specimens |
0.28; 29 specimens |
0.0066; 10 specimens |
| QC | WBB | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| QC | YBBB | - | - | 0.077 | 0.018 | 0.233 | 0.032 | 0.115 | 0.725 | 0.207 | 0.171 | 0.292 | 0.288 | 0.021 |
| NB | GCBB | - | - | - | 0.038 | - | - | - | 0.33 | - | 0.02; 7 specimens |
- | - | - |
| NB | WBB | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| NB | YBBB | - | - | - | 0.038 | - | 0.400 | - | 0.667 | 0.391 | 0.356 | 0.818 | 0.399 | 0.200 |
| NS | GCBB | - | - | - | 0.052 | 0.04 | - | - | 0.15 | 0.29 | 0.006; 1 specimen |
0.035; 4 specimens |
0.07; 16 specimens |
0.02; 2 specimens |
| NS | WBB | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| NS | YBBB | - | - | 0.169 | 0.247 | 0.654 | 0.184 | - | 0.135 | 0.419 | 0.479 | 0.281 | 0.158 | 0.173 |
| PE | GCBB | - | - | - | - | - | - | - | - | 0.15 | 0.02; 2 specimens |
2 0.04; specimens |
- | - |
| PE | WBB | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| PE | YBBB | - | - | - | - | - | 0.600 | - | 1.000 | 0.208 | 0.384 | 0.064 | 1.000 | 0.037 |
| NL | GCBB | - | - | - | - | 0.042 | - | 0.07 | - | - | 0.09; 3 specimens |
- | - | - |
| NL | WBB | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| NL | YBBB | - | - | 0.750 | 0.222 | 0.083 | 0.333 | 0.250 | - | - | 0.594 | 0.000 | 0.416 | 0.050 |
| ALL | GCBB | 0.034 | 0.026 | 0.01 | 0.044 | 0.035 | 0.035 | 0.029 | 0.06 | 0.02 | 0.024 | 0.03 | 0.05 | 0.00089 |
| ALL | WBB | 0.15 | 0.08 | 0.13 | 0.09 | 0.14 | 0.12 | 0.10 | 0.09 | 0.13 | 0.05 | 0.05 | 0.02 | 0.02 |
| ALL | YBBB | 0.080 | 0.058 | 0.113 | 0.041 | 0.201 | 0.097 | 0.105 | 0.143 | 0.249 | 0.355 | 0.448 | 0.297 | 0.033 |
| Threat Impact | Threat Impact (descriptions) | Level 1 Threat Impact Counts high range |
low range |
|---|---|---|---|
| A | Very High | 0 | 0 |
| B | High | 0 | 0 |
| C | Medium | 0 | 0 |
| D | Low | 2 | 2 |
| Calculated Overall Threat Impact: | Low | Low |
| # | Threat | Impact Footnotea.1 (calculated) |
Scope Footnoteb (next 10 Yrs) |
Severity Footnotec (10 Yrs or 3 Gen.) |
Timing Footnoted | Comments |
|---|---|---|---|---|---|---|
| 1 | Residential & commercial development | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | Negligible scope because there are large areas of natural habitat where development is not ongoing. Slight severity because cumulative impacts of housing and industrial development surrounding the urban centres of western Canada, specifically in southern regions approximately 200km from the US border, often results in complete loss of habitat High timing because the practice is continuing. |
| 1.1 | Housing & urban areas | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | Another suspected threat to host colony populations is habitat loss as a result of agricultural intensification and increased urbanization. Both Gypsy Cuckoo Bumble Bee and host bumble bees require large amounts of pollen over a long period of time, as reproductives for the next generation are only produced towards the end of the colony cycle. |
| 1.2 | Commercial & industrial areas | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | Another suspected threat to host colony populations is habitat loss as a result of agricultural intensification and increased urbanization. |
| 1.3 | Tourism & recreation areas | N/A; some recreational development may cause bee habitat to be lost, but overall other tangential impacts may affect bee habitat (e.g., pesticide use on golf courses, water diversion, etc.) and captured in other threats. | ||||
| 2 | Agriculture & aquaculture | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | Negligible scope because there are large areas of natural habitat where agricultural practices do not apply; slight severity because there are agricultural areas where bees are abundant and widespread; high timing because the practice is continuing. |
| 2.1 | Annual & perennial non-timber crops | Negligible | Negligible (<1%) | Slight (1-10%) | High (Continuing) | Another suspected threat to host colony populations is habitat loss as a result of agricultural intensification. Both Gypsy Cuckoo Bumble Bee and host bumble bees require large amounts of pollen over a long period of time, as reproductives for the next generation are only produced towards the end of the colony cycle. The increased reliance on intensive agriculture over the past few decades has resulted in decreased quality foraging habitat for bumble bees globally (e.g., Williams 1989; Kosior et al. 2007). Small parts of the Canadian range of Gypsy Cuckoo Bumble Bee (although potentially the most suitable) contain some of the most highly urbanized/ farmed regions of Canada (e.g., southern ON and southern regions of SK and MB). Suitable habitat is possibly in short supply and difficult to find in these regions for this species and its hosts. |
| 4 | Transportation & service corridors | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | Negligible scope because there are large areas of natural habitat where road building and utility/service lines are not planned. Negligible severity because in many cases transportation corridors may leave habitat more open for bees (provided the transportation corridor is not paved). High timing because the practice is continuing. |
| 4.1 | Roads & railroads | Negligible | Negligible (<1%) | Unknown | High (Continuing) | N/A; may temporarily increase habitat adjacent to roadsides |
| 4.2 | Utility & service lines | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | N/A; may temporarily increase habitat adjacent to roadsides |
| 5 | Biological resource use | Not a Threat | Negligible (<1%) | Negligible (<1%) | High (Continuing) | N/A |
| 5.3 | Logging & wood harvesting | Not a Threat | Negligible (<1%) | Negligible (<1%) | High (Continuing) | The threat is considered negligible. Logging may temporarily increase available habitat if there are habitat connections. |
| 6 | Human intrusions & disturbance | Negligible | Negligible (<1%) | Negligible (1-10%) | High (Continuing) | Negligible scope because there are large areas of natural habitat where recreational activities are not ongoing; negligible severity because recreational activities may trample or decrease host nest sites, although every host nest will not likely have Gypsy Cuckoo Bumble Bee; high timing because the practice is continuing. |
| 6.1 | Recreational activities | Negligible | Negligible (<1%) | Negligible (<1%) | High (Continuing) | N/A; some recreational activities may cause local extirpations of nests; overall likely minor. |
| 8 | Invasive & other problematic species & genes | Medium | Small (1-10%) | Extreme (71-100%) | High (Continuing) | Small scope because the spread of invasive species is primarily within the urban and agricultural areas of Canada. The natural habitats do not appear to have non-native bees present. Pathogen spillover and impacts remain unstudied in much of the ssp. range. Extreme severity because these practices impact bees. High timing because these practices are continuing. |
| 8.1 | Invasive non-native/alien species | Medium | Small (1-10%) | Extreme (71-100%) | High (Continuing) | The introduction and use of Common Eastern Bumble Bee (B. impatiens) for pollination services in western Canada may further impact declining host populations of Western Bumble Bee and Yellow-banded Bumble Bee. Common Eastern Bumble Bee may out-compete native bee species for nesting habitat or forage resources, and may serve as a pathogen or disease source. Pathogen spillover is a poorly understood threat for bumble bees. The use of infected commercial bumble bees (e.g., Common Eastern Bumble Bee in Canada) for greenhouse pollination is known to cause pathogen spillover into populations of wild bumble bees foraging nearby (Colla et al. 2006; Otterstatter and Thomson 2008). Lab studies have shown the parasite species Crithidia bombi and Nosema bombi (suspected) have devastating effect on Bombus colony-founding queens, foraging workers and entire nests (Brown et al. 2000, 2003; Otterstatter et al. 2005). The increased use of bumble bees in greenhouse operations in recent decades has been implicated in the decline of members of the subgenus Bombus. |
| 9 | Pollution | Medium | Small (1-10%) | Serious (31-70%) | High (Continuing) | Small scope because there are large areas of natural habitat where the pesticide is not applied; serious severity because of the known impacts of pesticides, and high timing because the practice is continuing. |
| 9.3 | Agricultural & forestry effluents | Medium | Small (1-10%) | Serious (31-70%) | High (Continuing) | Imidacloprid (a neonicotinoid), pose a particular threat to bees (compared to other pesticides) because they are harmful even at concentrations in the parts per billion (ppb) range (Environmental Protection Agency [EPA] 1994; Marletto et al. 2003). These pesticides are systemic and travel throughout the plant. |
| 11 | Climate change & severe weather | Not Calculated | Pervasive(71-100%) | Unknown | High (Continuing) | Pervasive scope because climate change is ongoing across the entire species’ range. Unknown severity because impacts are unstudied at a large scale. High timing because the threat is continuing. |
| 11.2 | Droughts | Not Calculated | Pervasive(71-100%) | Unknown | High (Continuing) | Climate change is another possible threat (Williams and Osborne 2009). Bumble bee species been shown to have narrow climatic tolerances are more vulnerable to extrinsic threats (Williams et al. 2009). Climatic tolerances for Gypsy Cuckoo Bumble Bee are not currently known, but there is evidence one of the species’ hosts (Rusty-patched Bumble Bee) may be negatively impacted by climate change due to the increase in precipitation variability over time (Kerr et al. in revision). |
Figure 7. Comparison of the relative abundance of each bumble bee species collected from 1971-1973 (black) and 2004-2006 (grey) in Guelph and Belwood, Ontario (* indicate P < 0.0001) (reproduced with permission from Colla 2012).

Long description for Figure 7
Bar chart comparing the relative abundance of each of 14 bumble bee species collected from 1971 to 1973 and from 2004 to 2006 in Guelph and Belwood, Ontario. Asterisks indicate p values less than 0.0001. For discussion, see report text, point 4 under “Abundance.”
Figure 8. Relative abundance of Gypsy Cuckoo Bumble Bee (GCBB) in YT, NT, BC, AB and SK based on all databased Bombus records in Canada (1882 - 2011). The left Y-axis (shaded portions of bars) indicates GCBB specimens and the right Y-axis (triangles) represents the proportion of GCBB specimens by ten-year intervals. Linear regression was used to examine trends in relative abundance in GCBB over time; the line represents a best fit of the data. See also Table 3. Graphs generated using Minitab ® software.

Long description for Figure 8
Five chart panels illustrating the relative abundance of Gypsy Cuckoo Bumble Bee based on Bombus records in five western jurisdictions in Canada (1882 to 2011). The panels represent Yukon, Northwest Territories, British Columbia, Alberta, and Saskatchewan. The left y-axis indicates Gypsy Cuckoo Bumble Bee specimens and the right y-axis represents the proportion of specimens by 10-year intervals. Linear regression was used to examine trends in relative abundance over time; a line on the chart represents a best fit of the data. For discussion, see report text under “Abundance.”
Figure 9. Relative abundance of Gypsy Cuckoo Bumble Bee (GCBB) in MB, ON, QC, NB and NS based on all databased Bombus records in Canada (1882 - 2011). The left Y-axis (shaded portions of bars) indicates GCBB specimens and the right Y-axis (triangles) represent the proportion of GCBB specimens by ten-year intervals. Linear regression was used to examine trends in relative abundance in GCBB over time; the line represents a best fit of the data. See also Table 3. Graphs generated using Minitab ® software.

Long description for Figure 9
Five chart panels illustrating the relative abundance of Gypsy Cuckoo Bumble Bee based on Bombus records in five western jurisdictions in Canada (1882 to 2011). The panels represent Yukon, Northwest Territories, British Columbia, Alberta, and Saskatchewan. The left y-axis indicates Gypsy Cuckoo Bumble Bee specimens and the right y-axis represents the proportion of specimens by 10-year intervals. Linear regression was used to examine trends in relative abundance over time; a line on the chart represents a best fit of the data. For discussion, see report text under “Abundance.”
Figure 10. Relative abundance of Gypsy Cuckoo Bumble Bee (GCBB) in PE and NL based on all databased Bombus records in Canada (1882 - 2011). The left Y-axis (shaded portions of bars) indicates GCBB specimens and the right Y-axis (triangles) represent the proportion of GCBB specimens by ten-year intervals. Linear regression was used to examine trends in Relative abundance in GCBB over time; the line represents a best fit of the data. See also Table 3. Graphs generated using Minitab ® software.

Long description for Figure 10
Two chart panels illustrating the relative abundance of Gypsy Cuckoo Bumble Bee based on Bombus records in Prince Edward Island and Newfoundland and Labrador (1882 to 2011). The left Y-axis indicates Gypsy Cuckoo Bumble Bee specimens and the right Y-axis represents the proportion of specimens by 10-year intervals. Linear regression was used to examine trends in relative abundance over time; a line on the chart represents a best fit of the data. For discussion, see report text under “Abundance.”
Figure 11. Total number of databased bumble bee specimens in Canada (1882 - 2011) from each province and territory; triangles represent the number of Gypsy Cuckoo Bumble Bee (GCBB) specimens. Values above each bar represent the percentage of specimens, which are GCBB. See also Table 3. Graphs generated using Minitab ® software.

Long description for Figure 11
Bar chart summarizing total numbers of databased bumble bee specimens in Canada (1882 to 2011) from each province and territory. Symbols in bars indicate the number of Gypsy Cuckoo Bumble Bee specimens. Values above each bar represent the percentage of specimens that are Gypsy Cuckoo Bumble Bees.
- The RA of Gypsy Cuckoo Bumble Bee declines > 90% when the time interval 1992 - 2001 (0.5) is compared with 2002 - 2011 (0.00089) (Table 3; Figures 8 - 10).
The RA of the host species also declined within the past ten years. In BC, RA of Western Bumble Bee declines from approximately 40% (1992 - 2001) to approximately 3% within the last ten-year increment (2002 - 2011). In AB, RA of Western Bumble Bee declines from more than 80% (1992 - 2001) to less than 10% (2002 - 2011). The eastern host, Yellow-banded Bumble Bee, does not exhibit the same declines as the western host. - Historical relative abundances of Gypsy Cuckoo Bumble Bee in North America show a decline (Colla et al. 2010). From 1864 - 1930 RA is 2.5%, 1931-1960 is 7%, 1961-1990 is 1.2 % and 1991- 2009 is 0.4% (n= 69600 Bombus) (Table 1). This decline in relative abundance over these time periods is statistically significant (X2= 13.7488, p-value =0.0002) (Colla et al. 2012).
- The MB study recorded 47 Gypsy Cuckoo Bumble Bee from 1986-1993 in all regions except the Red River Valley site in 1986 (Turnock et al. 2007). In 1986, a total of 398 bumble bees were collected with RA of Gypsy Cuckoo Bumble Bee 1.1% of bees collected (Turnock et al. 2007). Surveys in remaining years (1987-1993) did not record Gypsy Cuckoo Bumble Bee (total of 1891 Bombus collected) (Turnock et al. 2007).
- The Ontario (Guelph and Belwood) study shows that from 1971-1973, the RA of Gypsy Cuckoo Bumble Bee was 1% (n = 3632 specimens), while its hosts Rusty-patched Bumble Bee and Yellow-banded Bumble Bee made up 14% and 4%, respectively (Figure 7; Colla and Packer 2008). Neither Gypsy Cuckoo Bumble Bee nor Rusty-patched Bumble Bee was recorded during surveys from 2004 - 2006 within these areas (n = 1195), and only two specimens of Yellow-banded Bumble Bee were recorded (Colla and Packer 2008).
- The proportion of re-sampled historical (1864-1990) 50 km x 50 km grid cells occupied by Gypsy Cuckoo Bumble Bee in the most recent time period (1991 - 2010) was 0.347 (Colla et al. 2012). In summary, Gypsy Cuckoo Bumble Bee was found in only 35% of those portions of its historical range (209 grid cells) that had been resampled at least once from 1991 - 2010. The species significantly declined throughout its entire range, unlike other cuckoo bee species (e.g., Lemon Cuckoo Bumble Bee [B. citrinus], although this species has different host taxa) (Table 1) (Colla et al. 2012).
Other studies that show declines include a recent study considering over 30,000 museum specimens from 438 bee species (including bumble bees) occurring in the Northeastern US (38 to 45° N latitude & -85 to -70° W longitude); it found Gypsy Cuckoo Bumble Bee to be within the top three species in “rapid and recent decline” within the study region (Bartomeus et al. 2013). The other two species in decline were also bumble bees, including a host species, Rusty-patched Bumble Bee (Bartomeus et al. 2013).
Little is known about the natural fluctuations and trends of bumble bee populations. Despite that surveys have been done over large geographic areas of Canada (e.g., Colla et al. 2012; Cameron et al. 2011; Colla and Packer 2008), there are few studies that have repeatedly surveyed sites over an entire season or several years. For bumble bees (e.g. Colla and Packer 2008) some areas may contain three or four common species and a handful of relatively rare ones. Common species will have fairly stable populations over time (e.g., large effective population sizes), whereas rare species will fluctuate and suffer from local stochastic extinction (e.g., small effective population sizes, may be uncommon members of the local bee fauna or may have more specific habitat requirements. Cuckoo bumble bees have the added complexity of being dependent on the host bee species’ presence, abundance, and subsequent population dynamics.
The low abundance of Gypsy Cuckoo Bumble Bee and the possible declines of its host species make recolonization by rescue effect throughout its historical range in southern Canada unlikely. In the past decade, there have been few confirmed records from the US, all from Alaska (Williams et al. 2014). Immigration from Alaska may be possible if adequate host populations are present in YK. Recent surveys in YK have recorded potential host species (see Search Effort) (Cannings pers. comm. 2013).
The International Union for Conservation of Nature-Conservation Measures Partnership (2006) (IUCN-CMP) threats calculator (Salafsky et al. 2008; Master et al. 2009) was used to classify and list threats to Gypsy Cuckoo Bumble Bee. The calculated overall threat impact is low (Table 3). The scope of most threats is difficult to quantify, mainly because much of the species range has not been surveyed and there appears not to be one unifying threat across the species range. Regardless, the species has not been collected since 2008 within parts of its range where host species are also declining (southern Canada) or are still present and not declining (YK, NT).
The most significant threat to Gypsy Cuckoo Bumble Bee is the decline of host bumble bee populations (primarily Rusty-patched Bumble Bee, Western Bumble Bee and Yellow-banded Bumble Bee) in southern Canada to abundances low enough to cause local extirpations of this cuckoo bee species.
The rapid decline of members of the subgenus Bombus senso stricto appears to have started in the mid-1990s (National Research Council [NRC] 2007). These declines have not yet been attributed to any one cause, but based on the timing of the observed collapse; possible threats have been hypothesized and are discussed below (NRC 2007; Evans et al. 2008). One species in the subgenus, Cryptic Bumble Bee, seems to be increasing its range and in abundance (Owen et al. 2012). Whether or not this species is a suitable host for Gypsy Cuckoo Bumble Bee is unknown.
The introduction and use of the highly successful Common Eastern Bumble Bee (B. impatiens) for pollination services in Canada may further impact declining host populations of Western Bumble Bee and Yellow-banded Bumble Bee in the southern parts of their range. Common Eastern Bumble Bee may out-compete some native bee species for nesting habitat or forage resources, and may serve as a source for pathogen or disease. The status of establishment of wild populations of Common Eastern Bumble Bee in western Canada is unknown, but likely to have a negative impact on native species, as has been documented in other parts of the world (Williams and Osborne 2009).
Pathogen spillover is implicated in the significant declines of many animals (e.g., Morton et al. 2004; Power and Mitchell 2004) but is a poorly understood threat for bumble bees. Pathogen spillover occurs when pathogens spread from a heavily infected ‘reservoir’ host population to a sympatric ‘non-reservoir’ host population (Power and Mitchell 2004). The use of infected commercial bumble bees (e.g., Common Eastern Bumble Bee) in Canada for greenhouse pollination is known to cause pathogen spillover into populations of wild bumble bees foraging nearby (Colla et al. 2006; Otterstatter and Thomson 2008).
Lab studies have shown the parasite species Crithidia bombi and Nosema bombi (suspected) have devastating effect on Bombus colony-founding queens, foraging workers and entire nests (Brown et al. 2000, 2003; Otterstatter et al. 2005). These parasites are found in a variety of bumble bee species (Macfarlane 1974; Macfarlane et al. 1995; Colla et al. 2006), but their virulence in wild bumble bees remains unknown. Nonetheless, the increased use of commercially available bumble bee colonies in greenhouse operations in recent decades has been implicated as a possible contributing factor in the decline of members of the subgenus Bombus, including Rusty-patched Bumble Bee, Yellow-banded Bumble Bee and Western Bumble Bee in southern parts of the country (Thorp and Shepherd 2005; NRC 2007; Evans et al. 2008; COSEWIC 2010; Szabo et al. 2012), though seemingly not members of other subgenera. Hosts in these areas may be the most important for Gypsy Cuckoo Bumble Bee (see Figure 3), though remain relatively common in other areas.
It has long been known that chemicals used in agricultural applications (i.e., pesticides) can have negative impacts on bees (e.g., see Johansen and Mayer 1990; NRC 2007). Around the time when the declines of subgenus Bombus were observed in North America, a new pesticide, Imidacloprid (a neonicotinoid), was registered for use in the United States and Canada (1994 and 1995 respectively: Cox 2001; Pest Management Regulatory Agency [PMRA] 2001). Neonicotinoids can pose a particularly severe threat to bees because they can be harmful even at concentrations in the parts per billion (ppb) range (Environmental Protection Agency [EPA] 1994; Marletto et al. 2003). These pesticides are systemic, travelling throughout plant tissues and integrating with pollen and nectar; they are routinely used on golf courses and agricultural lands (Sur and Stork 2003). The effects of Imidacloprid are not lethal to bumble bees when used as directed (e.g., Tasei et al. 2001), though in North America studies of its effects on bumble bees have been largely tested on one species, the commercially available Common Eastern Bumble Bee, as a representative for all North American species (e.g., Gels et al. 2002; Morandin and Winston 2003). It is unknown if that species serves as an accurate model for all bumble bee species. Additionally, colonial insects (e.g., insects which produce reproductive individuals at the end of the colony cycle) may be negatively impacted by cumulative sub-lethal effects of this and other pesticides. Studies on the lethal and sub-lethal effects of this group of pesticides on other wild bumble bees are lacking at this time.
Another suspected threat to host colony populations is habitat loss as a result of agricultural intensification and increased urbanization. Both Gypsy Cuckoo Bumble Bee and its host bumble bees require large amounts of pollen over a long period of time, as reproductives for the next generation are only produced towards the end of the colony cycle. The increased and continuing reliance on intensive agriculture over the past few decades has resulted in decreased quality foraging habitat for bumble bees globally (e.g., Williams 1989; Kosior et al. 2007), and probably has had similar impact in Canada as much of the area traditionally occupied by Gypsy Cuckoo Bumble Bee and its hosts in Canada has changed significantly (Grant and Javorek 2011). The part of the Canadian range of Gypsy Cuckoo Bumble Bee adjacent to the international border though the most suitable habitat for wildlife, contains some of the most highly urbanized/ farmed regions of Canada (e.g., southern ON and southern regions of SK and MB).
Climate change is another possible threat to bumble bees worldwide (Williams and Osborne 2009). Bumble bee species with narrow climatic tolerances are shown to be more vulnerable to extrinsic threats (Williams et al. 2009). Climatic tolerances of Gypsy Cuckoo Bumble Bee are currently unknown, but there is some evidence that one of the species’ hosts (Rusty-patched Bumble Bee) may be negatively impacted by climate change due to the increase in precipitation variability over time (Kerr et al. in revision). However, the other known hosts (subgenus Bombus senso stricto) are more widespread in Canada, and their climatic tolerances are also unknown. In general, female Psithryrs emerge approximately one month after its host species (Plath 1934), but it is unknown if emergence synchrony of host/parasite could be affected by climate change.
Bumble bees require a constant suite of floral resources to support colony growth: pollen and nectar needs to be constant throughout the growing season. Without these resources, emerging queens, workers and colony growth is limited. Only mated queens overwinter, so lack of abundant early season floral resources will cause colonies to die, or newly emerged queens to disperse. Abundant food resources throughout the colony growth period ensure that local populations will persist.
Bumble bees are haplodiploid organisms with complementary sex determination which makes them extremely susceptible to extinction when effective population sizes are small (Zayed and Packer 2005). This is due to the ‘diploid male extinction vortex’ (Zayed and Packer 2005). Sex in bees, and most other haplodiploids, is determined by genotype at a single “sex locus”: hemizygotes (haploids) are males, heterozygotes are female and homozygotes are diploid males. Diploid males are usually sterile or inviable. The number of sex alleles in a population determines the proportion of diploids that are male and is itself determined primarily by the effective size of the population. This means that as bumble bee populations decrease in size, the frequency of diploid males increases. As diploid males are attempts at female production, their increasing production in smaller populations increases the rate of population decline causing a special case of the extinction vortex: “the diploid male extinction vortex.” This special form of genetic load is the largest known (Hedrick et al., 2006). In practical terms, if a bee population decreases to a few reproducing individuals, it is certain to become locally extirpated even under stable environmental conditions unless its number increases within a few generations.
There are no federal or provincial laws that protect Gypsy Cuckoo Bumble Bee, mitigate threats or protect the species nest sites or habitat.
- Wild Species 2010 General Status Rankings (Canadian Endangered Species Conservation Council 2011)
- Not ranked in YT, BC, AB, SK, NT, MB
- May be at risk in ON, QC, NS, NB, PE, NL, LB
- Undetermined overall in Canada.
- Global Status Rank: GH (Possibly Extinct) (NatureServe 2012).
- International Union for the Conservation of Nature (IUCN) Red list: None
- Provincial/Territorial Sub-national Conservation Status Ranks:
- YT (Status not ranked [SNR]),
- AB (Undetermined),
- ON (S4, apparently secure),
- Atlantic Canada Conservation Data Centre (SNR).
- This species is not listed in Quebec, and is not on the Liste des espèces susceptibles d’être designées menaces ou vulnérables
- Not ranked in other territories or provinces.
Most Gypsy Cuckoo Bumble Bee records are from provincially owned and managed lands. At present, there are approximately 44 Gypsy Cuckoo Bumble Bee records from protected areas in Canada. The provinces and territories of BC, AB, SK, NB, PE, NT, YT and NU have no records within protected areas. In Ontario, the species has been recorded from Algonquin Provincial Park, Presqu’ile Provincial Park, Awenda Provincial Park, McGregor Point Provincial Park and Pinery Provincial Park. In Quebec the species has been recorded from Forillon National Park, Parc nationale des Monts-Valin, Parc nationale du Canada Forillon, Queens Park, Aylmer. In Nova Scotia, the species has been recorded from Cape Breton Highlands National Park, and in Newfoundland the species has been recorded from Gros Morne National Park.
Special thanks to Leif Richardson for producing GIS and MaxEnt maps and for database management figures. Special thanks to Syd Cannings, Jennifer Heron, Andrew Hebda, Elizabeth Elle, Michel Savard, Nathalie Desrosiers, Peter Hallett, Ray Poulin, Matthias Buck, Donna Giberson, Christophe Buidin-Yann Rochepault, Jeffery Ogden, Cory Sheffield, Laurence Packer, and many other collectors for information on bumble bee specimens and surveys.
- Anderson, Robert. 2011. Research Scientist, Canadian Museum of Nature, Ottawa, ON.
- Amirault, Diane. 2011. Biologist, Environment Canada, Bélanger; Ste-Foy, QC.
- Belanger, Luc. 2011. Biologist, Environment Canada, Bélanger; Ste-Foy, QC.
- Blaney, Sean. 2011. Botanist, Atlantic Canada Conservation Data Centre, Sackville, NB.
- Boates, Sherman. 2011. Manager, Biodiversity Wildlife Division Department of Natural Resources, Government of Nova Scotia, Kentville, NS.
- Bromwell, Vivian. 2011. Senior Species at Risk Biologist, Ontario Ministry of Natural Resources, Peterborough, ON.
- Bucknell, Shelagh. 2011. Pacific Wildlife Research Centre, Environment Canada, Delta, BC.
- Carrière, Suzanne. 2011. Biologist, Department of Environment and Natural Resources (ENR), Government of the Northwest Territories, Yellowknife, NT.
- Charlwood, Vanessa. 2011. Environment Canada, Yellowknife, NT.
- Court, Gord. 2011. Provincial Wildlife Status Biologist, Dept. of Sustainable Resource Development, Edmonton, AB.
- Curley, Rosemary. 2011. Conservation Biologist, Department of Agriculture and Forestry, Charlottetown, PE.
- Desmet, Ken. 2011. Species at Risk Biologist, Manitoba Conservation Data Centre, Winnipeg, MB.
- Duncan, Dave. 2011. Biologist, Environment Canada, Edmonton, AB.
- Elder, K.M.F. 2011. Species at Risk Biologist, Department of Natural Resources, Kentville, NS.
- Fournier, Francois. 2011. Direction de la conservation de l’environnement, Environment Canada, Sainte-Foy, QC.
- Fraser, Dave. 2011. Unit Head Scientific Authority Assessment, B.C. Ministry of Environment, Ecosystems Branch, Victoria, BC.
- Gauthier, Isabelle. 2014. Biologiste, Coordonnatrice provinciale, espèces fauniques menacées et vulnérables, Ministère des Forêts, de la Faune et des Parcs, Québec (Québec)
- Giasson, Pascal. 2011. Manager, Species at Risk Program, Fish and Wildlife Branch, Department of Natural Resources, Fredericton, NB.
- Gravel, Mike. 2011. Provincial Species at Risk Biologist, Department of Natural Resources, Kentville, NS.
- Howes, Briar. 2011. Ecological Integrity Branch, Parks Canada Agency, Gatineau, QC.
- Ingstrup, David. 2011. Biologist, Environment Canada, Edmonton. AB.
- Jung, Thomas. 2011. Senior Wildlife Biologist, Environment Yukon, Whitehorse, YT.
- Levesque, Annie. 2011. Biologiste, Coordonnatrice provinciale, espèces fauniques menacées et vulnérables, Ministère des Forêts, de la Faune et des Parcs, Québec (Québec)
- MacDonald, Bruce. 2011. Biologist, Environment Canada, Yellowknife, NT.
- McConnell, Angela. 2011. Biologist, Environment Canada, Toronto, ON.
- Millikin, Rhonda. 2011. A/Head Population Assessment, Pacific Wildlife Research Centre, Environment Canada, Delta, BC.
- Nantel, Patrick. 2011. Conservation Biologist, Parks Canada Agency, Vancouver, B.C.
- Oldman, Michael. 2011. Botanist, Ontario Natural Heritage Information Centre, Perterborough, ON.
- Paquet, Annie. 2011. Biologiste, Coordonnatrice provinciale, espèces fauniques menacées et vulnérables, Ministère des Forêts, de la Faune et des Parcs, Québec (Québec)
- Pardy, Shelley. 2011. Senior Manager, Department of Environment and Conservation, NL.
- Pepper, Jeanette. 2011. Science Panning Section, Saskatoon, SK.
- Pittoello, Gigi. 2011. Habitat Ecologist, Saskatchewan Ministry of Environment, Regina, SK.
- Quinlan, Richard. 2011. Biologist, Alberta Fish and Wildlife, Lethbridge, AB.
- Raillard, Martin. 2011. Manager, Environment Canada, Sackville, NB.
- Sabine, Mary. 2011. Biologist, Department of Natural Resources, Department of Environment and Conservation, Fredericton, NB.
- Squires, Susan. 2011. Ecosystem Management Ecologist, Corner Brook, NL.
- Stipec, Katrina. 2011. Data Management Specialist. B.C. Conservation Data Centre, Victoria, B.C.
- Tuininga, Ken. 2011. Biologist, Environment Canada, Toronto, ON.
- Watkins, William. 2011. Wildlife and Ecosystem Protection Branch, Manitoba Department of Conservation, Winnipeg, MB.
- Alford DV. 1975. Bumble Bees. London: Davis-Poynter. xii+352 pp.
- Antonovics, J. and M. Edwards. 2011. Spatio-temporal dynamics of Bumble Bee nest parasites (Bombus subgenus Psithyrus ssp.) and their hosts (Bombus spp.). Journal of Animal Ecology 80: 999-1011.
- Bartomeus, I., Ascher, J.S., Gibbs, J., Danforth, B.N., Wagner, D.L., Hedtke, S. and Winfree, R. 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proceedings of the National Academy of Sciences. Accessed February 21, 2014
- Brousseau, P.-M. 2011. Impact de la densité de cerfs de Virginie sur les communautés d'insectes de l'île d'Anticosti. Mémoire de maîtrise, Université Laval, Québec. 92 pp.
- Brown M.J.F., R. Loosli and P. Schmid-Hempel. 2000. Condition-dependent expression of virulence in a trypanosome infecting Bumble Bees. Oikos 91: 421–427.
- Brown M.J.F., R. Schmid-Hempel and P. Schmid-Hempel. 2003. Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. Journal of Animal Ecology 72: 994–1002.
- Buidin, C., pers. comm. 2011. Email correspondence to S. Colla. 2011. Association Le Balbuzard de la Minganie, Rivière-Saint-Jean, Québec.
- Cameron, S.A., H.M. Hines, and P.H. Williams. 2007. A comprehensive phylogeny of the Bumble Bees (Bombus), Biological Journal of the Linnean Society 91, 161-188.
- Cameron, S.A., J.D. Lozier, J.P. Strange, J.B. Koch, N. Cordes, L.F. Solter and T. Griswold. 2011. Patterns of widespread decline in North American Bumble Bees. Proceedings of the National Academy of Science 108: 662-667.
- Canadian Endangered Species Conservation Council (CESCC). 2011. Wild Species 2010: The General Status of Species in Canada. National General Status Working Group: 302 pp.
- Cannings, S. pers. comm. 2011-13. Email correspondence to S. Colla and J. Heron. Biologist, Environment Canada, Whitehorse, Yukon.
- Colla, S.R. (2012) The Ecology and Conservation of Eastern North American Bumblebees (Bombus spp.), Ph.D. Dissertation, York University, Toronto, ON. 214 pp.
- Colla, S.R. and S. Dumesh. 2010. Natural history notes for the Bumble Bees of southern Ontario. Journal of the Entomological Society of Ontario 141: 38-67.
- Colla, S.R., F. Gadallah, L. Richardson, D. Wagner and L. Gall (2012) Assessing declines of North American Bumble Bees (Bombus spp.) using museum specimens. Biodiversity and Conservation 21: 3585-3595.
- Colla, S. R., M. C. Otterstatter, R. J. Gegear and J. D. Thomson. 2006. Plight of the Bumble Bee: Pathogen spillover from commercial to wild populations. Biological Conservation 129:461-467.
- Colla, S.R. and L. Packer. 2008. Evidence for decline in eastern North American Bumble Bees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodiversity and Conservation 17:1379-1391.
- Colla, S.R, L. Richardson and P. Williams. 2011. Bumble Bees of the Eastern United States, USDA Forest Service Publication, (PDF, 5.9 Mb), [accessed September 24, 2011]
- Conservation Measures Partnership (CMP). 2010. [accessed February 27, 2013].
- COSEWIC 2010. COSEWIC assessment and status report on the Rusty-patched Bumble Bee Bombus affinis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 34 pp. [Online].
- COSEWIC. 2011. Guidelines for recognizing designatable units. [accessed February 27, 2013].
- Cox, C. 2001. Insecticide factsheet: Imidacloprid. Journal of Pesticide Reform 21:15-22.
- Cresson, E.T. 1864. Descriptions of several new species of North American Apidae. Proceedings of the Entomological Society of Philadelphia 3: 38-43.
- Curley, R., pers. comm. 2011 Email correspondence to S. Colla. 2011. Conservation Biologist, Forests Fish and Wildlife Division, PEI Dept Environment, Energy and Forestry, Charlottetown, Prince Edward Island
- Environmental Protection Agency (EPA), U.S.A. 1994. Pesticide fact sheet: Imidacloprid, Washington, D.C. Mar. 18
- Evans, E., R. Thorp, S. Jepsen and S.H. Black. 2008. Status Review of Three Formerly Common Species of Bumble Bee in the Subgenus Bombus. The Xerces Society for Invertebrate Conservation, Portland, OR.
- Fisher, R.M. 1983. Inability of the social parasite Psithyrus ashtoni to suppress ovarian development in workers of Bombus affinis (Hymenoptera:Apidae). Journal of the Kansas Entomological Society 56:69-73.
- Fisher, R.M., D.R. Greenwood and G.J. Shaw. 1993. Host recognition and the study of a chemical basis for attraction by cuckoo Bumble Bees (Hymenoptera: Apidae). Journal of Chemical Ecology 19: 771-786.
- Fisher, R.M. and B.J. Sampson 1992. Morphological specializations of the Bumble Bee social parasite Psithyrus- Ashtoni (Cresson) (Hymenoptera, Apidae) Canadian Entomologist 124:69-77.
- Gels, J.A., D.W. Held, and D.A. Potter. 2002. Hazards of insecticides to the Bumble Bees Bombus impatiens (Hymenoptera: Apidae) foraging on flowering white clover in turf. Journal of Economic Entomology 95:722-728.
- Giberson, D., 2011. pers. comm. 2011 Email correspondence via L. Packer. 2011. Professor at the University of Prince Edward Island, Charlottetown, Prince Edward Island.
- Goulson, D. 2003. Bumble Bees, Their Behaviour and Ecology. Oxford University Press, Oxford, 235 pp.
- Grixti, J.C., L.T. Wong, S.A. Cameron, and C. Favret. 2009. Decline of Bumble Bees (Bombus) in the North American Midwest. Biological Conservation 142: 75-84.
- Hatfield, R.G. and G. LeBuhn. 2007. Patch and landscape factors shape community assemblages of Bumble Bees, Bombus spp. (Hymenoptera:Apidae), in montane meadows. Biological Conservation 139: 150-158.
- IUCN (International Union of Conservation Networks). 2001. IUCN red list categories and criteria: Version 3.1. IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge, UK. ii + 30 pp.
- Javorek, S.K. and M.C. Grant. 2011. Trends in wildlife habitat capacity on agricultural land in Canada, 1986 – 2006. Canadian Biodiversity: Ecosystem Status and Trends 2010, Technical Thematic Report No. 14. Canadian Councils of Resource Ministers. Ottawa, ON. vi + 46 p.
- Johansen, C.A., and D.F. Mayer. 1990. Pollinator Protection. A Bee and Pesticide Handbook. Cheshire, CT. Wicwas Press.
- Kerr, J., N.Szabo, S.R. Colla, L. Richardson and L. Packer (in Revision). Strongly increasing climatic variation and concurrent near extinction of a historically abundant pollinator.
- Klymko, J. 2012. Personal Communication to S. Colla. Atlantic Canada Conservation Data Centre, Cornerbrook, NL.
- Klymko, J. 2014. Personal Communication to C. Sheffield. Atlantic Canada Conservation Data Centre, Cornerbrook, NL.
- Kosoir, A., W. Celary, P. Olejniczak, J. Fijal, W. Krol, W. Solarz, and P. Plonka. 2007. The decline of the Bumble Bees and cuckoo bees (Hymenoptera:Apidae : Bombini) of Western and Central Europe. Oryx 41:79-88
- Laverty , T.M. and L. Harder. 1988. The Bumble Bees of Eastern Canada. Canadian Entomologist 120: 965-987.
- Macfarlane, R. 1974. Ecology of Bombinae (Hymenoptera: Apidae) of Southern Ontario, with emphasis on their natural enemies and relationships with flowers. PhD, University of Guelph, Guelph, Ontario.
- Macfarlane, R.P., J.J. Lipa, and H.J. Liu. 1995. Bumble Bee pathogens and internal enemies. Bee World 76: 130-148.
- Macfarlane, R.P. and Patten, K.D. 1997. Food sources in the management of Bumble Bee populations around cranberry marshes. In Proceedings of the 7th International Symposium on Pollination. K.W. Richards (ed.) Acta Horticulturae: 239-244.
- MacPhail, V.J. 2007. Pollination Biology of Wild Roses (Rosa spp.) in Eastern Canada. MSC thesis. University of Guelph, Ontario. 174 pp.
- Marletto, F., A. Patetta, and A. Manino. 2003. Laboratory assessment of pesticide toxicity to Bumble Bees. Bulletin of Insectology 56:155-158.
- Master, L., D. Faber-Langendoen, R. Bittman, G.A. Hammerson, B. Heidel, J. Nichols, L. Ramsay, and A. Tomaino. 2009. NatureServe conservation status assessments: factors for assessing extinction risk. NatureServe, Arlington, Virginia, USA. [accessed June 15, 2012].
- McCorquodale, D., pers. comm. 2012 Email correspondence to S. Colla. 2012. Cape Breton University, Sydney, Nova Scotia
- Michener, C.S. 2000. The Bees of the World. The Johns Hopkins University Press, Baltimore, 952 pp.
- Mitchell, T.B. 1962 Bees of the Eastern United States. North Carolina Agricultural Experiment Station Technical Bulletin No. 152.
- Morandin, L.A., and M.L. Winston. 2003. Effects of novel pesticides on bumble bee (Hymenoptera: Apidae) colony health and foraging ability. Community and Ecosystem Ecology 32:555-563.
- Morton, A., Routledge, R.C. Peet, and A. Ladwig. 2004. Sea lice infection rates on juvenile pink and chum salmon in the nearshore environment of British Columbia, Canada. Canadian Journal of Fish and Aquatic Sciences 61:147-158.
- National Research Council (NRC). 2007. Status of Pollinators in North America. The National Academies Press, Washington, DC.
- NatureServe. 2012. www.NatureServe.org/explorer/ranking.htm [accessed January 22, 2013].
- National Research Council (NRC) 2007. Status of pollinators in North America. Committee on the Status of Pollinators in North America, The National Academies Press, Washington, D.C. 312 pp.
- Otterstatter, M.C., R.J. Gegear, S.R. Colla and J.D. Thomson. 2005. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of Bumble Bees. Behavioral Ecology and Sociobiology, 58: 383-389.
- Otterstatter, M.C., and J.D. Thomson. 2008 Does Pathogen Spillover from Commercially Reared Bumble Bees Threaten Wild Pollinators? PLoS One 3: e2771.
- Owen, R., M.C. Otterstatter, R.V. Cartar, A. Farmer, S.R. Colla and N. O’Toole (2012) Significant expansion of the distribution of the Bumble Bee Bombus moderatus (Hymenoptera:Apidae) over twenty years. Canadian Journal of Zoology 90:133-138.
- Packer, L., pers. comm. 2011 Email correspondence to S. Colla. 2011. Professor at York University, Toronto, Ontario
- Patanaude, A. 2007. Diversity, composition and seasonality of wild bees (Hymenoptera: Apoidea) in a northern mixed-grass prairie preserve. M.Sc. Thesis, University of Manitoba, 235 pp.
- Pest Management Regulatory Agency (PMRA). 2001. Imidacloprid. Regulatory Note. REG2001-11. Ottawa: Health Canada, Pest Management Regulatory Agency [accessed January 22, 2013].
- Phillips, S.J., M. Dudik, and R.E. Shapire. 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190:231-259.
- Plath, O.E. 1934. Bumble Bees and their ways, Macmillan, New York, US, 201 pp.
- Power, A.G., and C.E. Mitchell. 2004. Pathogen Spillover in Disease Epidemics. American Naturalist 164:S79-S89.
- Ratti, C.M. 2006. Bee abundance and diversity in berry agriculture. M.Sc. thesis, Simon Fraser University
- Salafsky, N., D. Salzer, A.J. Stattersfield, C. Hilton-Taylor, R. Neugarten, S.H.M. Butchart, B. Collen, N. Cox, L.L. Master, S. O’Connor, and D. Wilkie. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology 22:897–911.
- Savard, M. 2012. Données inédites sur la présence historique du Bombus (Psithyrus) ashtoni au sud du Québec, au Saguenay-Lac-Saint-Jean et en Minganie. Déposé au ministère des Ressources naturelles et de la Faune du Québec, 17 mai 2012. 8 pp.
- Sheffield, C.S., pers. comm. 2011 Email correspondence to S. Colla. 2011. Research Associate, at York University, Toronto, Ontario
- Stotyn, S. 2012. Wild Bumble Bees of the Nahanni River Region, NT. Final Report, Environment Canada, 10 pp.
- Stout, J.C and D. Goulson. 2000. Bumble Bees in Tasmania: their distribution and potential impact on Australian flora and fauna. Bee World 81: 80-86.
- Sur, R. and A. Stork. 2003. Uptake, translocation and metabolism of imidacloprid in plants. Bulletin of Insectology 1:35-40.
- Szabo, N., S.R. Colla, D.Wagner, L.F. Gall, and J.T. Kerr (2012) Is pathogen spillover from commercial bumble bees responsible for North American wild Bumble Bee declines? Conservation Letters 5: 232-239.
- Tasei, J. N., G. Ripault, and E. Rivault. 2001. Hazards of Imidacloprid seed coating to Bombus terrestris (Hymenoptera: Apidae) when applied to Sunflower. Journal of Economic Entomology 94:623-627.
- Thorp R.W., Shepherd M.D. 2005/ Profile: subgenus Bombus. In: Shepherd M.D., Vaughan D.M., Black S.H.(eds) Red list of pollinator insects of North America. The Xerces Society for Invertebrate Conservation, Portland, OR.
- Turnock, W.J. P.G. Kevan, T.M. Laverty, and L. Dumouchel. 2007. Abundance and species of Bumble Bees in fields of canola, Brassica rapa, in Manitoba: an 8 year study. Journal of the Entomological Society of Ontario 137:31-41
- Williams, P. H. 1991. The bumble bees of the Kashmir Himalaya (Hymenoptera: Apidae, Bombinae). Bulletin of the British Museum of Natural History (Entomology) 60:1-204 (page 46).
- Williams, P.H. 2011. Bombus of the World [accessed January 22, 2013].
- Williams, P.H., Brown, M.J.F., Carolan, J.C., Goulson, D., An, Jiandong, Aytekin, A.M., Best, L.R., Byvaltsev, A.M., Cederberg, B., Dawson, R., Huang, J., Ito, M., Monfared, A., Raina, R.H., Schmid-Hempel, P., Sheffield, C.S., Šima, P., Xie, Z. 2012. Assessing cryptic species of the bumblebee subgenus Bombus s. str. world-wide with COI barcodes (Hymenoptera: Apidae). Systematics and Biodiversity 10: 21-56.
- Williams, P.H. 2013. Bombus: Species world-wide listed by old and new subgenera Natural History Museum, London, UK. [accessed January 22, 2013].
- Williams, P.H., R.W. Thorp, L.L. Richardson, and S.R. Colla. 2014. The Bumble Bees of North America: an identification guide. Princeton University Press. NY, USA. 208 pp.
- Williams, P.H., and J.L. Osborne. 2009. Bumble Bee vulnerability and conservation worldwide. Apidologie 40:367-387.
- Williams, P.H., S.R. Colla and Z. Xie. 2009. Bumble Bee vulnerability: common correlates of winners and losers across three continents. Conservation Biology 23: 931-940
- Williams, P.H. 1989. Bumble Bees - and their decline in Britain. Ilford: Central Association of Bee-Keepers. 15 pp. [accessed January 22, 2013].
- Zayed, A. and L. Packer. 2005. Complementary sex determination substantially increases extinction proneness of haplodiploid populations. Proceedings of the National Academy of Sciences 102:10742-10746.
- Zimma, B.O., M. Ayasse, J. Tengo, F. Ibarra, C. Schulz and W. Francke. 2003. Do social parasitic Bumble Bees use chemical weapons? (Hymenoptera, Apidae). Journal of Comparative Physiology 189:769-775.
Sheila R. Colla has been studying native North American Bumble Bees since 2003 during her undergraduate degree at the University of Toronto. She received her Ph.D. and was the recipient of the National Science and Engineering Research Council Alexander Graham Bell Canadian Graduate Scholarship at York University, Toronto, ON under the supervision of Dr. Laurence Packer. Her dissertation examined changes in Bumble Bee communities over the past century and looked into the causes for observed declines. She is the North American Co-Coordinator of the IUCN Species Survival Commission Bumble Bee Specialist Group and her research has been featured in The Washington Post, Canadian Gardening, The Toronto Star, BioScience, CBC’s Quirks and Quarks, and The Daily Planet for Discovery Channel Canada.
Leif L. Richardson is a PhD candidate at Dartmouth College, Hanover, NH, where he examines the effects of floral nectar chemistry on bees and their parasites. Additionally, he combines bee surveys with museum collections data to study changes in the distribution of North American bumble bees. With Sheila R. Colla and other co-authors he produced a Guide to the Bumble Bees of North America, to be published by Princeton University Press in 2014.
Cory S. Sheffield has been studying bees and pollination since 1993, as part of undergraduate honours studies at Acadia University, Wolfville, NS. He continued graduate studies (MSc) of insect-plant interactions at Acadia, and at Agriculture and Agri-Food Canada (AAFC), Kentville, NS from 1994 - 2006. Cory did graduate studies (PhD) at the University of Guelph, ON while continuing to work at the AAFC. These studies focused on the bee fauna of Nova Scotia, including their diversity and contributions to crop pollination. During this time, Cory and several co-authors published on the re-discovery of Epeoloides pilosulus in Nova Scotia, which was thought extinct. Cory then worked on post-doctoral studies at York University, ON in bee taxonomy and DNA barcoding, followed by a research associate position in bee taxonomy with the Canadian Pollination Initiative (CANPOLIN). He is now research scientist and curator of invertebrate zoology at the Royal Saskatchewan Museum in Regina, SK. His research continues to focus on bees: he has published on the taxonomy of Canadian/North American bees, the utility of DNA barcoding for bees, bee physiology, pollination contributions and diversity of the Canadian bee fauna.
- University of Guelph Insect Collection, Guelph, Ontario
- Canadian National Collection, Ottawa, Ontario
- Lyman Museum, McGill University, Ste. Anne-de-Bellevue, Québec
- The Royal Ontario Museum, Toronto, Ontario
- Packer Collection, York University, Toronto, Ontario
- Royal British Columbia Museum, Victoria, British Columbia
- Royal Saskatchewan Museum, Regina, Saskatchewan
- Illinois Natural History Survey, Champaign, Illinois
- Kansas State University Insect Collection, Manhattan, Kansas
- Nova Scotia Museum, Halifax, Nova Scotia
- Ohio State University Columbus Collection, Columbus, Ohio
- American Museum of Natural History, New York, New York
- University of Massachusetts, Amherst, Massachusetts
- University of New Hampshire, Durham, New Hampshire
- New York State Museum, Albany, New York
- Connecticut Agricultural Experiment Station, New Haven, Connecticut
- University of Prince Edward Island, Charlottetown, Prince Edward Island
- Collection André Francoeur (Université du Québec), Chicoutimi, Québec
- Collection Michel Savard, Saguenay, Québec
- Collection Christophe Buidin-Yann Rochepault (Association Le Balbuzard de la Minganie), Rivière-Saint-Jean, Québec
- Canadian Museum of Nature, Ottawa, Ontario
- Cape Breton University, Sydney, Nova Scotia
| Province | Survey Area/Site | Day | Month | Year |
|---|---|---|---|---|
| Nova Scotia | Colchester County, DEBERT, N.S. | 27 | 6 | 1995 |
| Nova Scotia | Colchester County, DEBERT, N.S. | 5 | 6 | 1995 |
| Nova Scotia | Colchester County, DEBERT, N.S. | 6 | 6 | 1995 |
| Nova Scotia | Digby County, DELAPS COVE, N.S. | 19 | 6 | 1995 |
| Nova Scotia | Colchester County, DEBERT, N.S. | 19 | 7 | 1996 |
| Nova Scotia | Colchester County, DEBERT, N.S. | 6 | 6 | 1996 |
| Nova Scotia | Halifax County, WEST DOVER, N.S. | 6 | 8 | 1998 |
| Nova Scotia | Colchester County, SHUBENACADIE, N.S. | 20 | 5 | 1998 |
| Nova Scotia | Halifax County, ARMDALE, N.S. | 11 | 8 | 1999 |
| Nova Scotia | Colchester County, GREENFIELD, N.S. | 5 | 8 | 1999 |
| Nova Scotia | Hants County, MOUNT UNIACKE, N.S. | 11 | 8 | 1999 |
| Nova Scotia | Hants County, MOUNT UNIACKE, N.S. | 11 | 8 | 1999 |
| Nova Scotia | West Black Rock, Kings County | 16 | 8 | 2001 |
| Nova Scotia | Whycocomagh, Inverness County | 3 | 8 | 2001 |
| Nova Scotia | 3 | 8 | 2001 | |
| Nova Scotia | 16 | 8 | 2001 | |
| Nova Scotia | Middleton, Annapolis County | 12 | 6 | 2002 |
| Nova Scotia | 12 | 6 | 2002 | |
| Ontario | Flesherton | 12 | 5 | 1991 |
| Ontario | Blue twp. | 29 | 5 | 1992 |
| Ontario | Worthington Twp. | 11 | 6 | 1992 |
| Ontario | Worthington Twp. | 9 | 6 | 1992 |
| Ontario | Chapple twp. | 17 | 8 | 1992 |
| Ontario | Chapple twp. | 4 | 8 | 1992 |
| Ontario | Atwood twp. | 15 | 6 | 1992 |
| Ontario | Atwood twp. | 22 | 6 | 1992 |
| Ontario | Atwood twp. | 26 | 7 | 1992 |
| Ontario | Atwood twp. | 26 | 7 | 1992 |
| Ontario | Atwood twp. | 27 | 7 | 1992 |
| Ontario | Atwood twp. | 18 | 8 | 1992 |
| Ontario | Blue twp. | 14 | 8 | 1992 |
| Ontario | Chapple twp. | 22 | 7 | 1992 |
| Ontario | Chapple twp. | 17 | 8 | 1992 |
| Ontario | Morley township | 15 | 6 | 1992 |
| Ontario | Pinery Provincial Park, Lambton County | 8 | 6 | 1992 |
| Ontario | Worthington twp. | 8 | 6 | 1992 |
| Ontario | Macgregor Point Provincial Park | 21 | 5 | 1993 |
| Ontario | Shallow Lake | 15 | 7 | 1993 |
| Ontario | Spencer Gorge, Niagara escarpment | 18 | 8 | 1993 |
| Ontario | Hepworth, Grey County | 16 | 7 | 1994 |
| Ontario | Luther Marsh, Wellington County | 20 | 8 | 1994 |
| Ontario | Pinery Prov pk, Lambton County | 1 | 6 | 1994 |
| Ontario | Pinery Prov pk, Lambton County | 15 | 6 | 1994 |
| Ontario | Pinery Prov pk, Lambton County | 24 | 6 | 1994 |
| Ontario | Spencer Gorge, Niagara escarpment | 31 | 7 | 1994 |
| Ontario | St.Williams tract, Norfolk County | 5 | 6 | 1994 |
| Ontario | St.Williams tract, Norfolk County | 5 | 6 | 1994 |
| Ontario | Lac Jean-Venne | 5 | 6 | 1995 |
| Ontario | Pinery Prov pk, Lambton County |
27 | 6 | 1995 |
| Ontario | Pinery Prov pk, Lambton County |
17 | 6 | 1995 |
| Ontario | Pinery Prov pk, Lambton County |
17 | 6 | 1995 |
| Ontario | Lake Matchedash, Simcoe County |
12 | 8 | 1996 |
| Ontario | Port Franks, Lambton County |
15 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
20 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
27 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
9 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
9 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
9 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
12 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
14 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
14 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
14 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
16 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
16 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
16 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
15 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
15 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
23 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
23 | 6 | 1996 |
| Ontario | Port Franks, Lambton County |
14 | 8 | 1996 |
| Ontario | Port Franks, Lambton County |
29 | 8 | 1996 |
| Ontario | Elmira, Salem Creek | 18 | 5 | 1997 |
| Ontario | Crane River, Bruce County | 27 | 9 | 1997 |
| Ontario | Hope Bay, Bruce County | 1 | 6 | 1997 |
| Ontario | Huntsville | 18 | 8 | 1997 |
| Ontario | Orillia, Simcoe County | 13 | 8 | 1998 |
| Ontario | Cameron Lake, Bruce County | 16 | 6 | 1999 |
| Ontario | Dorcas Bay, Bruce County | 19 | 6 | 1999 |
| Ontario | Dorcas bay, Bruce County | 19 | 6 | 1999 |
| Ontario | Toronto, Humber River near Old Mill, | 9 | 5 | 1999 |
| Ontario | Dunks Bay, Bruce County | 5 | 7 | 2000 |
| Ontario | Oliphant Fen, Bruce County | 5 | 7 | 2000 |
| Ontario | Presqu'ile Provincial Park, | 28 | 4 | 2000 |
| Ontario | Presqu'ile Provincial Park, | 17 | 6 | 2000 |
| Ontario | Pinery, | 6 | 8 | 2008 |
| Quebec | Lac Jean-Venne | 24 | 8 | 1995 |
| Quebec | Lac Jean-Venne | 4 | 6 | 1995 |
| Quebec | Magpie | 17 | 6 | 2000 |
| Quebec | Aguanish | 16 | 6 | 2001 |
| Quebec | Baie-Johan-Beetz | 16 | 6 | 2001 |
| Quebec | Rivière-Saint-Jean | 12 | 8 | 2001 |
| Quebec | Rivière-Saint-Jean | 15 | 7 | 2001 |
| Quebec | Rivière-Saint-Jean | 13 | 7 | 2001 |
| Quebec | Rivière-Saint-Jean | 16 | 7 | 2001 |
| Quebec | Rivière-Saint-Jean | 9 | 6 | 2001 |
| Quebec | Rivière-Saint-Jean | 17 | 6 | 2001 |
| Quebec | Rivière-Saint-Jean | 16 | 6 | 2001 |
| Quebec | Rivière-Saint-Jean | 28 | 5 | 2001 |
| Quebec | Havre-Saint-Pierre | 22 | 6 | 2001 |
| Quebec | Chemin du Grand-Ruisseau ou ch. du Grand-Ruisseau | 22 | 5 | 2001 |
| Quebec | Chemin du Grand-Ruisseau ou ch. du Grand-Ruisseau | 28 | 5 | 2001 |
| Quebec | Chemin du Grand-Ruisseau ou ch. du Grand-Ruisseau | 9 | 6 | 2001 |
| Quebec | Sentier Val-Menaud, Saint-Charles-de-Bourget | 9 | 6 | 2001 |
| Quebec | Baie-à-Forest, Saint-Gédéon | 10 | 6 | 2001 |
| Quebec | Chemin du Grand-Ruisseau ou ch. du Grand-Ruisseau | 16 | 6 | 2001 |
| Quebec | Chemin du Grand-Ruisseau ou ch. du Grand-Ruisseau | 17 | 6 | 2001 |
| Quebec | Havre-Saint-Pierre | 19 | 6 | 2001 |
| Quebec | Baie-à-Forest, Saint-Gédéon | 24 | 6 | 2001 |
| Quebec | Camp Patmos, L'Ascension | 25 | 6 | 2001 |
| Quebec | Havre-Saint-Pierre | 26 | 6 | 2001 |
| Quebec | Bout-du-Banc, space Rivière-Saint-Jean | 13 | 7 | 2001 |
| Quebec | Berge de la riviere Saint-Jean, Longue-Pointe-de-Mingan | 15 | 7 | 2001 |
| Quebec | Chemin du Grand-Ruisseau ou ch. du Grand-Ruisseau | 16 | 7 | 2001 |
| Quebec | Bout-du-Banc, Rivière-Saint-Jean | 18 | 7 | 2001 |
| Quebec | Parc Rivière-du-Moulin, Chicoutimi | 6 | 6 | 2002 |
| Quebec | Parc Rivière-du-Moulin, Chicoutimi | 8 | 6 | 2002 |
| Quebec | Chicoutimi (réservoir d'eau au quartier des Oiseaux), Chicoutimi | 13 | 6 | 2002 |
| Quebec | Baie-à-Forest, Saint-Gédéon | 1 | 7 | 2002 |
| Quebec | Route Fillion, Saint-Honoré | 7 | 6 | 2003 |
| Quebec | Camp Patmos, L'Ascension-de-Notre-Seigneur | 28 | 6 | 2003 |
| Quebec | Baie-à-Forest, Saint-Gédéon | 1 | 7 | 2003 |
| Quebec | Pied-du-Mont, Parc national du Mont-Orford | 29 | 7 | 2007 |
| Quebec | Anticosti Island | 2007 | ||
| Quebec | Chalet La Courtepointe, Parc national du Mont-Orford | 4 | 7 | 2008 |
Page details
- Date modified: