12. Investigation of Cause
12.2 Goal and Expectations of Investigation of Cause
12.3 Developing Hypotheses and Study in IOC
12.4 Using Response Patterns and Population Dynamics
- 12.4.1 Overview of Metal Mining EEM Results
- 12.4.2 Fish Response Patterns
- 12.4.3 Possible Effect Profiles for Fish
- 12.4.4 Benthic Community Response Patterns
- 12.4.5 Possible Effect Profiles for Benthos
12.5 Tiered (Elimination) Approach
- 12.5.1 Toxicity Reduction Evaluation and Toxicity Identification Evaluation
- 12.5.2 Investigation of Cause Framework Example of Toxicity Reduction Evaluation and Toxicity Identification Evaluation
12.6 Integrated (Weight-of-Evidence) Approach
- 12.6.1 Using Weight of Evidence
- 12.6.2 The Sediment Quality Triad, as an Example of Weight of Evidence
12.7 Metal Toxicology and Bioaccumulation
- 12.7.1 Ecotoxicity of Metals
- 12.7.2 Selenium Toxicity
- 12.7.3 Biomarkers (Metallothionein)
- 12.7.4 Using Bioaccumulation Methods
- 12.7.5 Methods for Correlating Bioaccumulation and Toxicity
12.8 Field Study Approaches for Fish and Benthos
- 12.8.1 Field Study Design
- 12.8.2 Caged Organisms
- 12.8.3 Mesocosms (Fish and Invertebrate)
- 12.8.4 Benthic Transplant Devices
- 12.8.5 Macrophytes, Periphyton, Phytoplankton, Chlorophyll
12.9 Laboratory Methods and Toxicity Tests
- 12.9.1 Histology and Physiological Parameters
- 12.9.2 Toxicity Tests
- 12.9.3 Fish Toxicity Testing: Fathead Minnow Lifecycle
- 12.9.4 Whole Sediment Toxicity Testing
- 12.9.5 Metals in Overlying Water in Whole-Sediment Toxicity Tests
- 12.9.6 Sediment Pore Water Toxicity Testing
12.10 Tools for Effluent and Water Quality Analysis
- 12.10.1 Measurement of Dissolved Metals during Investigation of Cause
- 12.10.2 Metal Speciation for Metals of Concern
- 12.10.3 Measurement of Reagents & Reagent By-products used in Processing
- 12.10.4 Monitoring of Flow and Loadings in the Exposure Area
12.11 Tools to be Considered for Sediment Analysis
- 12.11.1 Sediment Monitoring as a Tool for Investigation of Cause
- 12.11.2 Sediment Mass Transport
- 12.11.3 Sediment Depositional Rate & Sediment Dating for Historical Trends
- 12.11.4 Sediment Coring
- 12.11.5 Sediment Chemistry
12.12 Sediment Pore Water Analysis
List of Tables
- Table 12-1: Examples of approaches that may be used to determine possible causes during IOC
- Table 12-2: Formalized set of causal criteria forming part of a weight-of-evidence approach for assessment of mining effluent effects
List of Figures
12.1 Introduction
This chapter is a compilation of all the Investigation of Cause (IOC) sections that were included in various chapters of the 2002 version of the Metal Mining (MM) Environmental Effects Monitoring (EEM) Guidance Document. Several methods and concepts have been updated with new information.
An EEM MM IOC workshop was hosted jointly by the Metal Mining Association of Canada and Environment Canada (EC) in December 2009. The objective of the workshop was to explore various aspects and challenges related to the IOC phase of the Metal Mining EEM program. Background information on IOC, environmental studies, potential causes of effects, various case studies, and tools and approaches for conducting IOC studies were presented. The workshop provided a forum for discussion and development of IOC for metal mines, and allowed research needs to be identified. The proceedings of the workshop were used to further develop this IOC chapter for the Metal Mining EEM Program, and further details can be found within the proceedings, which were published in 2012 and are available on the EEM website.
12.2 Goal and Expectations of Investigation of Cause
The goal of an IOC study is to determine the cause of confirmed effects (i.e., what is responsible for the effects) (see Chapter 1, section 1.3.2.1 for information regarding confirmed effects). The guidance presented in this chapter is intended to allow flexibility in designing IOC studies in order to accommodate site-specific needs, and should be complemented by all available information, including publicly available scientific literature. As with all EEM studies, IOC studies are required to use validated methods and good scientific practice.
Acceptable validated methods include those whose soundness has been established on an authoritative basis, such as standardized methods/procedures prepared by recognized international agencies (for example, EC, the Organisation for Economic Co-operation and Development (OECD), the United States Environmental Protection Agency (US EPA), the American Society for Testing and Materials (ASTM), the International Organization for Standardization (ISO), the European Union (EU) and the World Health Organization (WHO)), as are methods published in peer-reviewed journals within the literature and accepted as scientifically defensible protocols. In addition, non-authoritative methods that have not been peer reviewed may be used provided that evidence is presented to demonstrate their soundness.
Components of good scientific practice include scientific integrity, experimental design, laboratory safety, error analysis, quality assurance and control, critical data interpretation, and accurate record keeping (i.e., method documentation and data collection, documentation, curation and access) (OECD 2007, IARC 2008, Deutsch Forschungsgemeinshaft 1998). EEM studies are expected to be of sound science, i.e. “organized investigations and observations conducted by qualified personnel using documented methods and leading to verifiable results and conclusions” (SETAC 1999). Sound science implies that the data and conclusions are supported by the high standards of the scientific method, which include the development of a readily testable hypothesis or hypotheses, the use of systematic and well-documented experimental or analytical methods (adequate sample sizes, appropriate controls), the use of appropriate data analysis tools (statistics or models), and the articulation of conclusions that address the hypothesis(es) and that are supported by the results (SETAC 1999). The Society of Environmental Toxicology and Chemistry (SETAC) (1999) makes note of important cautions with respect to data interpretation, including statements of certainty vs. uncertainty, causation vs. correlation, absence of evidence vs. evidence of absence, and potential for misinterpretation (overlooking variables, inadequate sample sizes, lack of appropriate controls, bias, anecdotes). In addition, Bosker and Munkittrick (2009) raise caution regarding the following generic quality assurance / quality control (QA/QC) and statistical issues within EEM monitoring studies: insufficient sample size, failure to remove outliers, uncertain exposure, poor reference site selection, incomplete/poor reporting, data entry error, and wrong data reported.
12.3 Developing Hypotheses and Study in IOC
In developing the IOC study design (see Chapter 2, 2.2, and Chapter 1, 1.4.2.2), mines are encouraged to outline potential hypotheses that could explain the causes of the confirmed effects, taking into account any identifiable response patterns. Multiple effects may have the same cause, and may be captured through establishing an overarching hypothesis. In other cases, multiple hypotheses may be constructed to address multiple confirmed effects. Tools appropriate to address the established hypotheses could then be proposed. The scope of work could range. It may be sufficient to examine and present solid evidence using existing data, alone or in combination with new data (weight-of-evidence approach) and/or a literature review. Alternatively, it may be necessary to conduct full field and/or laboratory studies.
The table below shows examples of different IOC approaches to examine various hypotheses of possible causes. The following sections of this document suggest many ways of investigating the cause of effects.
| Hypotheses (Possible Cause) |
Approach | Example |
|---|---|---|
| Habitat, nutrients | Collection of biological data with supporting measures to characterize habitat, water quality and sediment chemistry that would allow for expanded statistical analyses (e.g., correlative, multivariate approaches). Effluent chemistry samples (extensive if necessary) collected simultaneously may allow for links to be made between conditions in the receiving environment to possible causative components in the mine effluent. Characterization of unusual events or conditions may provide further knowledge. | 1) Standard benthic survey with corresponding water, sediment and effluent chemistry to explore relationships with habitat, nutrient and contaminant measures. 2) Collection of data on temperature, nutrients and primary productivity (chlorophyll a, periphyton) in the receiving environment with corresponding measures of nutrients in effluent to study a nutrient-related cause of effect(s), such as eutrophication, and may include modelling and/or mass balance component. |
| Natural variability | See Chapters 2, 3, 4, 6, 7, 8 and 9. | |
| Contaminants | Analysis of metal body burdens, alone or in combination with other measurements e.g., fish gonad or liver histology, to look for signs of abnormal development (gonads) or signs of pathology (e.g., lesions on livers); measurement of liver enzymes. | 1) Analysis of trace elements in amphipods collected in the reference and exposure area, and comparison of levels to published “critical body concentrations.” 2) Biomarker approach for fish e.g., measure metallothionein concentration and/or other low molecular weight proteins that are known to be induced by exposure to metals. 3) Analysis of trace elements in tissues (e.g., liver, viscera, whole body, and muscle, as appropriate for identified effect and/or contaminant of concern) of fish collected in the reference and exposure area; determines whether contaminants are bioavailable and being accumulated. 4) Use of a tiered approach (toxicity reduction evaluation / toxicity identification evaluation) to determine the source of the contaminants in the waste streams. |
| Food limitation | Literature review on the diet of fish to understand preferences and examination of existing benthic data to assess food availability, alone or in combination with other information, such as stable isotopes. | Measures of stomach contents, relevant prey types (e.g., benthic, planktonic prey), or water quality parameters such as turbidity, temperature. |
12.4 Using Response Patterns and Population Dynamics
12.4.1 Overview of Metal Mining EEM Results
The national analyses of metal mining EEM data (Lowell 2007; Environment Canada 2012) suggest that the fish response patterns (fish were older, thinner (reduced condition), with smaller livers and gonads) may be due to toxicity or habitat alteration. The data also suggest that benthos response patterns (significantly reduced taxon richness, changes in community structure in exposed areas as measured by the increased Bray-Curtis Index and Simpson’s evenness endpoints, and increased density) may be due to toxicity/habitat alteration, or eutrophication for some mines. To date, reported mine effluent effects on fish usability (as measured by mercury in fish tissue) appear to be minimal.
12.4.2 Fish Response Patterns
It is important to understand that the response of the fish population sampled is a snapshot in time, and that the response should not be assumed to represent a step in a progression of responses that may lead from one steady-state condition to a new one (Environment Canada 2010). The response is also a reflection of how the existing fish are performing, and not an indication of the mechanism of impact. For example, with an increase in the quantity or quality of food or habitat, there should be an increase in growth, size, reproductive investment (gonad size), and condition. A faster growth rate typically results in fish reproducing at a younger age, and therefore lowers age-to-maturity (the age at which the fish start reproducing). In combination, these changes in the population normally decrease the average age of the population. When the population adapts to its new carrying capacity, parameters should revert to reference levels, but at a higher density of fish. As well, an acutely lethal accidental discharge may be reflected later in time as an increase in food resources, because there is a lower density of fish and the same amount of food is available. Thus, the effects may not result in longer-term changes in the fish community, because the population is maintaining equilibrium, and corrective actions may not be ecologically effective or cost effective.
Not all species will respond directly to stress, but some may respond indirectly due to changes in predation pressure or food availability. The observed response pattern can, however, be used to interpret results and design studies for the next phase. It is important to look at supporting data to help with interpretation and study design. Gibbons and Munkittrick (1994) and Munkittrick et al. (2000) grouped fish characteristics according to age structure (mean age or age distribution), energy expenditure (growth rate, reproductive rate) and energy storage (condition, liver weight). They assigned an increase, decrease or no change to each characteristic, to come up with a generalized response pattern that could be used to provide direction for research into causal factors. Moreover, the nature of the response pattern over successive monitoring phases enables characterization of the status of the system in question, eventually enabling management decisions to be made regarding the effectiveness of current regulations (Environment Canada 2010). The IOC/IOS chapter of the Pulp and Paper EEM Technical Guidance Document (Environment Canada 2010) and Munkittrick et al. (2000) give detailed information regarding effect profiles and interpretable response patterns. Possible interpretable response patterns that mines could consider, for example, are nutrient limitation, toxicity, eutrophication, and metabolic disruption. Other response patterns exist and may also be applicable.
12.4.3 Possible effect profiles for fish
Three effect profiles are described below; others exist and may be applicable.
12.4.3.1 Nutrient Limitation/Toxicity
Nutrient limitations affecting fish health (which may be characterized by decreased condition factor, liver size and gonad size) can result initially in a decrease in fish growth and reproduction. Over time this can lead to an increase in age of the population, because fewer young are being produced (Gibbons and Munkittrick 1994). A prolonged problem with food availability and performance will eventually lead to a reduction in population size below the carrying capacity of the system, and the performance parameters in fish (growth, condition) may begin to recover as the population continues to grow older and the population grows smaller.
Chemical toxicity may cause an increase in liver weight, with a decrease in condition and gonad weight. It has been suggested that the increased liver size is associated with increased activity of detoxification processes. It is important to note, however, that chemical toxicity can result in increased liver detoxification enzymes without the presentation of enlarged livers, and vice versa. Enlarged livers may be an indicator of altered energy storage due to toxicity but not directly related to an increase in the detoxification enzymes (Munkittrick et al. 1994, 2000).
12.4.3.2 Metabolic Disruption
As noted in Hewitt and Servos (2001), the Government of Canada (in the Canadian Environmental Protection Act, 1999) has defined endocrine-disrupting substances as substances that have the ability to affect the synthesis, secretion, transport, binding, action or elimination of hormones, with effects on the maintenance of homeostasis, reproduction, development or behaviour of an organism. Effects on reproduction may be characterized by reduced gonad size and increased indicators of energy use (growth, liver size, condition), indicating that there is energy available but fish are not directing this energy to reproduction (Munkittrick et al. 1991). Heavy metals in particular are known to be metabolic or endocrine disrupters, including cadmium, copper, lead, iron, mercury, and selenium (Hewitt and Servos 2001; Fajreaus-Van Ree 2004). Such heavy metals have been associated with impaired stress responses and adrenal function in fish in the laboratory environment and in the wild (Brodeur et al. 1998; Brodeur et al. 1997). Hontela et al. (1992) showed that fish exhibit an exposure-related decrease in condition factor and growth efficiency, and a reduced capacity to elevate blood cortisol.
12.4.3.3 Eutrophication
The eutrophication pattern (characterized by increases in gonad weight, liver weight and condition) can be a result of either a decrease in population size or an increase in available habitat and food resources. Decreased population size may be associated with increased predation because of an increased abundance of predators, or an increase in mortality due to the aging population. However, with an increased reproductive rate, the long-term results could be an increase in younger fish, which could eventually lead to limitation of food resources (Gibbons and Munkittrick 1994), if the younger fish are not kept in check by predation.
12.4.4 Benthic Community Response Patterns
The EEM benthic community survey effect indicators (and effect endpoints) are density (number of animals per unit area), taxa richness (number of taxa), similarity index (Bray-Curtis Index) (measure of community structure differences between 2 assemblages) and evenness index (Simpson’s Evenness Index) (how evenly individuals are distributed among the taxa). By comparing these effect endpoints between an exposure area and a reference area, or along a gradient, it is possible to detect structural differences in the benthic community. This information can be used to determine the amount of energy available for the fish, and thus is a measure of fish habitat health. The IOC/IOS chapter of the Pulp and Paper EEM Technical Guidance Document (Environment Canada 2010) and Munkittrick et al. (2000) give detailed information regarding certain effect profiles and some interpretable response patterns. Possible interpretable response patterns that mines could consider, for example, are nutrient limitation, toxicity, and eutrophication. Other response patterns exist that may also be applicable.
12.4.5 Possible Effect Profiles for Benthos
Two effect profiles are described below; others exist that may also be applicable.
12.4.5.1 Eutrophication/Smothering
Eutrophication, or nutrient enrichment, is a process of over-fertilization of a water body by nutrients, resulting in the production of more organic matter than the self-purification reactions of that water body can overcome (Chambers et al. 2001). The degree of eutrophication affecting benthic invertebrates can range from mild to moderate or pronounced. In the case of mild to moderate eutrophication, the typical response is an increase in the abundance1 and number of benthic invertebrate taxa (taxon richness) relative to reference conditions. Pronounced eutrophication (decreased taxon richness, increased density) will begin to shift the composition of the benthic community. Hyper- or severe eutrophication may be observed when the abundance and taxon richness of benthic organisms decline; at this stage, negative impacts on fish stocks and plant life are usually observed as oxygen is depleted by decomposing organic matter (Environment Canada 2007; Lowell et al. 2000).
Some studies in the literature suggest that phosphorus complexes with metals in the water column as suspended solids; these complexes then deposit in the sediment (Ledo et al. 2004; Pereira et al. 2008). Under certain conditions (reducing environment, seasonality (fall/winter) and daylight), the phosphorus is released, causing eutrophication (Ledo et al. 2004; Pereira et al. 2008). Ammonia/nitrates are commonly elevated due to blasting materials, cyanide breakdown, or sewage inputs (CCME 2009); these can act as toxicants but could also contribute to enrichment.
Pronounced eutrophication is commonly associated with an increase in the number and type of pollution-tolerant (benthic) taxa (e.g., oligochaetes, chironomids or nematodes) and a decrease in the number and type of sensitive species (e.g., mayflies, stoneflies or caddisflies). Severe eutrophication frequently masks toxicity effects that may otherwise have been measured (Lowell et al. 2000).
12.4.5.2 Toxicity/Smothering
Trace metals are potentially toxic to organisms and can impact individual and population performance. Decreases in both taxon richness and abundance are typically a sign of overall inhibitory effects, such as toxicity or smothering (Lowell et al. 2000). Such population-level changes are reflected in the benthic community structure, with shifts towards an increasingly simple, and often predicable, species composition as sensitive taxa become rare or disappear and more metal-tolerant taxa dominate (Clements 2004; Pollard and Yuan 2006; Courtney and Clements 2002; Canfield et al. 1994).
Hypoxic or anoxic water conditions can develop when oxygen is consumed by decomposing organic matter. If currents are weak and the organic matter is not being flushed from the area, these conditions may generate potentially toxic reduced compounds such as methane, ammonia and hydrogen sulphide (Pearson and Rosenberg 1978). Toxic and anoxic conditions can lower invertebrate feeding rates, which potentially can lower invertebrate growth and biomass (which in turn will affect the fish community) (Lowell et al. 2000).
12.4.5.3 Meromictic/Hypoxic Conditions
Mine effluent can produce stratification in some deep water lakes, resulting in meromictic conditions in these lakes. Normal seasonal mixing does not occur, causing vertical and temporal differentials of dissolved metals, and meromictic cycling of some metals. Dissolved oxygen at depths below the chemocline of the lake is not replenished, resulting in oxygen depletion (Sánchez et al. 2008; Szarek-Gwiazda and Żurek 2006; Campbell and Torgersen 1980). This ultimately leads to toxic conditions and the loss of habitat.
Abundance is defined as the number of benthic invertebrate individuals. The term density is used when abundance is expressed per unit area sampled.
12.5 Tiered (Elimination) Approach
12.5.1 Toxicity Reduction Evaluation and Toxicity Identification Evaluation
12.5.1.1 Toxicity Reduction Evaluation
A toxicity reduction evaluation is a site-specific step-wise diagnostic approach to resolving toxicity issues (Novak and Holtze 2012). Protocols to investigate the probable causes of toxicity have been developed by the US EPA (1989) and are known as Toxicity Reduction Evaluation (TRE) and Toxicity Identification Evaluation (TIE) studies.
The general objectives of TRE/TIE processes (US EPA 1989) are to determine those actions necessary to reduce the effluent’s toxicity to acceptable levels. A six-tier approach was developed, based on US EPA (1989), (1991a), (1991b), (1993a) and (1993b), and Novak and Holtze (2012), directed toward the reduction of toxicity of the whole effluent rather than specific components within the effluent. The six tiers are:
- Information and data acquisition
- Evaluation of remedial actions in operation/process to reduce effluent toxicity
- TIE of the effluent:
- Phase I: Characterization of toxicity through various treatments
- Phase II: Identification of suspected toxicants
- Phase III: Confirmation of toxicants
- Source(s) investigation/identification of the toxicity in the facility
- Toxicity Treatability Evaluation for reduction of toxicity in the final effluent
- Confirmation and removal of toxicity
12.5.1.2 Toxicity Identification Evaluation)
The TIE approach uses the responses of organisms within an appropriate bioassay format to detect the presence of active agents. This approach characterizes the active substances of interest in a complex matrix comprising three phases: characterization, identification, and confirmation of toxicants.
- Phase I aquatic TIE methods were originally developed for use with acute lethality tests using fathead minnows or Ceriodaphnia dubia (US EPA 1991a), but have been adapted for sublethal testing (US EPA 1991b).
- Phase II aquatic toxicity identification is described in US EPA document (1993a).
- Phase III aquatic toxicity confirmation is described in US EPA document (1993b).
TIE protocols also exist for sediment and pore water toxicity (US EPA 1991c and 1997) and marine toxicity (phase I only) (US EPA 1996).
The standard US EPA Phase I effluent characterization treatments involve subjecting the effluent or water to various treatments that remove or separate specific chemical classes (for example, filtration, aeration, extraction, chelation). This process of elimination is used to determine how its chemistry and toxicity changes after such treatment. A significant portion of toxicity observed in industrial effluents is often attributed to pH effects. Therefore, pH adjustment is used throughout Phase I to provide more information on the nature of the toxicants. In all cases, the toxicity of treated samples is compared to non-treated samples to determine which approach, if any, reduced toxicity.
One of the most important benefits of the TRE/TIE process is that it incorporates the responses of organisms into the assessment of complex mixtures to determine the identity of the substance(s) responsible for toxicity. Attempts to use chemical screening alone to identify substances responsible for toxicity are typically unsuccessful.
Determining the cause of transient or non-persistent toxicity can be difficult and may require the testing and analysis of a large number of samples.
Case studies using TRE/TIE were presented at the 2009 IOC Workshop by Novak and Holtze (2012).
12.5.2 Investigation of Cause Framework Example of Toxicity Reduction Evaluation and Toxicity Identification Evaluation
The Investigation of Cause Framework is an application of the TRE/TIE approach in the IOC context. It was developed by Hewitt et al. (2003), and is described in further detail in the Pulp and Paper EEM Technical Guidance Document IOC Chapter (Environment Canada 2010). The framework consists of step-by-step questions that follow a tiered approach (Figure 12-1). The questions are defined by a continuum of investigative phases, each providing more information regarding the cause of the effect with concomitant investments of time and resources. A review of relevant information concerning mine history, process type, process or operational changes, extent and magnitude of effects, and response patterns observed in EEM phases is critical before decisions can be made regarding the initial steps and direction of the IOC. The guidance for addressing these questions has evolved through review of the published literature and the ongoing results of the Pulp and Paper EEM program (Hewitt et al. 2003).
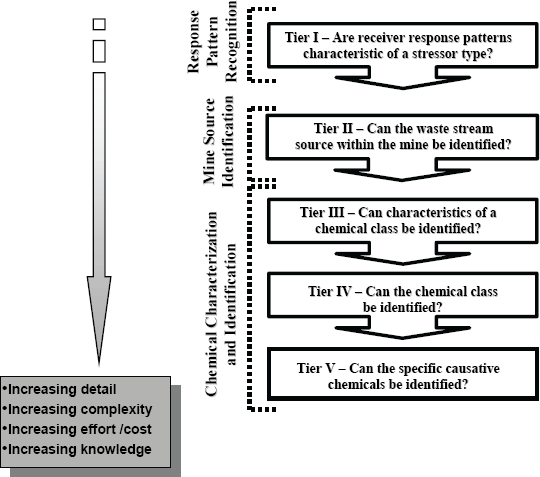
Figure 12-1: Tiered framework for investigation of cause in environmental effects monitoring (Hewitt et al. 2003). (text description)
12.5.2.1 Defining Response Patterns
The first step is to define the response patterns. Characterizing the type of stressor(s) based on response patterns will greatly benefit any IOC at the outset, by narrowing the focus and making the best utilization of potentially limited resources. Once the response pattern has been defined, the framework (Figure 12-1) is tiered to identify the stressor for that pattern type.
12.5.2.2 Source Identification & Selective Operation of In-plant Processes
The purpose of source identification is to attempt to specify or isolate specific waste streams within the manufacturing or treatment process that are responsible for the observed effects measured in the receiving environment. Studies should begin with a systematic investigation of individual process streams when looking for the source of effects within the mine.
Determining the source(s) of the effect has several potentially important outcomes, including 1) focusing further investigations to a particular area of the mine for a more detailed inventory of process stream sources, quantities, and waste stream qualities and toxicities; 2) identifying an area of the mine where operations can be reviewed to ensure that “normal operations” are occurring and to eliminate anomalies; 3) evaluating the potential for source treatment and the consequences in terms of final effluent quality; and 4) focusing subsequent detailed investigations of the waste stream source(s), including identification of chemicals.
Generally, acute toxicity tests are performed on different waste streams within the plant. Using the acute toxicity data, it is possible to isolate the waste streams that could be used for longer-term sublethal testing. The focus should be on those effluent constituents that would be carried through primary treatment and affect final effluent quality. Further information can be found in Environment Canada (2010), Rickwood et al. (2006) and Martel et al. (1997). Longer-term testing can occur using a variety of approaches, including mesocosms, laboratory bioassays, partial to full life-cycle studies, etc.
12.5.2.3 Chemical Isolation and Characterization
This step represents a TIE. If identification of waste streams has not provided sufficient information to characterize the effect, the procedures below can be used for isolating and identifying the responsible substances.
Chemical characterization and identification can be very complex. Compound characteristics, compound classes or the compounds themselves can be identified; however, each step becomes more costly and complex. The approaches described in this section are designed to identify specific characteristics of the chemical(s) that are responsible for effects under investigation. Information from field studies, source identification, laboratory studies, and the exposure profile may provide indications of chemical class.
Phase I of the TIE involves characterizing the chemical class: 1) determining the characteristics of the active agents and 2) establishing whether the effect is caused by the same substances. This step utilizes specific methods for isolating active chemicals and proposing structures for their identification. The physicochemical properties of the active substances can be described using effluent manipulations coupled to a bioassay that either duplicates the field effects or is mechanistically linked to them (see section 1.2.6.2.6 of the Pulp and Paper EEM Guidance Document, IOC Chapter) (Environment Canada 2010). Each test is designed to alter the substances themselves or change their bioavailability so that information on the nature of the substances can be obtained.
Phase II of the TIE involves identifying the chemical class. Active components are further isolated or separated from inactive substances for their identification and confirmation. These methods are specific to the classes of chemicals and utilize bioassay responses (see section 1.2.6.2.6 of the Pulp and Paper EEM Guidance Document, IOC Chapter) (Environment Canada 2010) to evaluate the success or failure of extraction, separation and concentration of bioactive substances. Chemical isolation steps proceed in an iterative fashion, directed by bioassay responses until further isolations are not possible or candidate chemicals are identified. Once there is strong evidence that one or more candidate chemicals are associated with the response, the third phase of the TIE (below) can be initiated.
Phase III of the TIE involves identifying the specific causative chemical. This step involves techniques for confirming that the proposed substances are in fact responsible for the observed toxicity. This is usually accomplished through a weight-of-evidence assemblage of information that collectively establishes the identity of the active compounds, and establishing cause of the effect consistently over time so that amelioration efforts can adequately address the effect. Confirmatory approaches include the following:
- Correlation approach: A strong, consistent relationship between the concentrations of the suspected agents and the bioassay response can be established.
- Symptom approach: Different active substances often produce different symptoms in response. By comparing exposures of the effluent sample to those of pure, suspected active substances, one can obtain further evidence on whether the suspected agents are responsible. Examples of symptoms include species sensitivities, shapes of dose-response curves, and time for the effect to occur.
- Spiking approach: Suspected agents are added to the effluent to determine if a proportional response in the bioassay is obtained.
12.6 Integrated (Weight-of-Evidence) Approach
12.6.1 Using Weight of Evidence
Distinguishing among the cumulative impacts of multiple stressors (which sometimes have confounding effects) requires establishing a definitive causality link to the mine effluent that is being evaluated. Environmental monitoring of aquatic ecosystems is particularly prone to these impediments because these ecosystems often receive multiple interacting effluent discharges. Assessments of monitoring results often rely, in large part, on field monitoring data that can only show correlation (rather than clear cause and effect) between the mine effluent and the observed effect. Establishing a strong causal link, however, can benefit from a weight-of-evidence approach, which is the process of combining information from a variety of sources, i.e., multiple lines of evidence. The strength of the causal link can be evaluated by using a formalized set of criteria originally developed in the field of epidemiology. These criteria are outlined in Table 12.2, which is based on Fox (1991), Suter (1993), Gilbertson (1997), Beyers (1998), Culp (1999), Culp et al. (2000), and Lowell et al. (2000). In many cases, not all of the criteria may be satisfied or the findings for some criteria may conflict with those for other criteria. In these cases, it can be useful to assign weight to each criterion in terms of its relative importance for a given assessment. In particular, assessments that include evidence from field and laboratory experiments will be stronger than those based on field monitoring alone. It is often not necessary to satisfy all of the criteria to provide a strong causal argument (e.g., see Beyers 1998). The advantages to applying formalized criteria to assessments of the data generated by an EEM program include: 1) helping to tie together diverse assemblages of data on the effects of multiple stressors; 2) more clearly assigning causality; and 3) identifying important informational gaps (i.e., criteria that have not yet been addressed). This kind of approach can help to make assessments of mine effluent effects more rigorous and robust by combining alternative approaches and investigations of cause methods.
Table 12-2: Formalized set of causal criteria forming part of a weight-of-evidence approach for assessment of mining effluent effects. (text description)
Causal criteria for weight-of-evidence approach
Spatial correlation of stressor and effect along gradient from more to less exposed areas
Temporal correlation of stressor and effect relative to time course of exposure
Plausible mechanism linking stressor and effect
Experimental verification of stressor effects under controlled conditions and concordance of experimental results with field data
Strength: steep exposure/response curve
Specificity: effect diagnostic of exposure to a particular stressor
Evidence of exposure (contaminants or other indicators) in body of affected organisms
Consistency of stressor/effect association among different studies within the region being studied
Coherence with existing knowledge from other regions where the same or analogous stressors and effects have been studied
12.6.2 The Sediment Quality Triad, as an Example of Weight of Evidence
The use of the sediment quality triad (SQT) approach is recommended during investigation of cause of effects on benthic invertebrate communities (Parker and Dumaresq 2002). The AETE (Aquatic Effects Technology Evaluation Program) endorsed the use of the SQT for use on a site-specific basis, but not for “routine” monitoring (ESG 1999).
The SQT originally integrated three components (lines of evidence): sediment chemistry to determine chemical contamination, sediment toxicity bioassays, and benthic community structure to determine the status of resident biota (Chapman 1992). Using the different lines of evidence, statistical correlations can be made and integrated in a weight- of-evidence analysis (qualitative and quantitative), making the data more powerful than if each component were interpreted individually (see Alden 1992; Chapman 1992; Warwick and Clarke 1991).
The traditional SQT, which is based on correlation, can provide definitive conclusions regarding the pollution status of contaminated sediments (e.g., exposure and effects). It thus provides the minimum level of information necessary, i.e., a screening-level risk assessment upon which hypotheses regarding cause can be proposed. In the risk assessment paradigm provided by Chapman and Hollert (2006), causation is addressed after SQT, in which initial studies are relatively simple and serve to either provide definitive conclusions or to indicate gaps where more information is needed. Definitive conclusions regarding causation usually cannot be determined without further studies. The investigation of cause is examined at a higher tier, i.e., a more detailed assessment. Chapman and Hollert suggest four categories of additional lines of evidence (LOE) that can be added to the SQT:
- Direct replacements for or additions to the existing alteration LOE: bottom fish histopathology, crab exposure and health.
- Variations on existing LOE: in situ or sediment contact toxicity tests (toxicity LOE); colonization experiments (alteration LOE).
- Additional LOE: crab exposure and health; surface water; bacterial community structure; biomagnification / secondary poisoning; biomarkers.
- LOE to determine causation: habitat morphology (TIEs); effect-directed analyses (EDAs); critical body residues (CBRs); in situ sediment contact assays and laboratory toxicity tests; biomarkers; and mechanism-specific endpoints.
Overview of the Sediment Quality Triad Study Approach
- sample collection
- sediment chemistry
- laboratory toxicity testing of sediment
- identification and enumeration of benthic invertebrates in sediment samples
- collection of fish for tissue analysis
- additional analysis
- data analysis and report preparation
1) Sample Collection
Samples are collected from reference and exposure areas or over an exposure gradient, to help delineate the geographic extent of the effect. The total number of sampling locations should be at least ten, which should provide enough data for a robust statistical analysis. Samples at each location should be collected for chemistry, benthos and sediment toxicity.
2) Sediment Chemistry
Chemical analysis of the sediments should include physical characteristics (particle size and total organic content), nutrients (e.g., nitrate, nitrite, phosphorous, ammonia, Total Kjehldahl nitrogen (TKN), general chemistry (e.g., potassium, chloride, sulphate), and metals (total and partially extractable) (ESG 1999). Chemical parameters relevant to the site should be analyzed based on previous information from the site, particularly from water and effluent chemistry. Analyses of redox potential should also be considered.
3) Laboratory Toxicity Testing of Sediment
A battery of three tests is ideal for the triad approach, involving three different test species (MOEE 1997; ESG 1999) (formerly MOEE Ontario Ministry of the Environment and Energy, now MOE Ontario Ministry of the Environment). This provides a safeguard that a biological effect is not missed if one test species is not sensitive to the chemicals present in the sediments. It also provides test organisms from different trophic levels in the ecosystem so that the major relevant trophic groups (and their respective levels of complexity with differing capabilities for metabolizing and depurating chemicals) are represented. The recommended tests use benthic invertebrates and fish, and incorporate different biological parameters (i.e., survival and growth). Any tests with organisms that can incorporate reproduction should also be considered (e.g., Ceriodaphnia dubia).
The three recommended tests are:
- Hyalella azteca 14-day growth and survival (Environment Canada 1997a)
- C. dilutus (formerly Chironomus tentans (Shobanov et al. 1999)) 14-day growth and survival (Environment Canada 1997b)
- Fathead minnow 28-day toxicity (Bedard 1992; Van Geest et al. 2011a, 2011b) and bioaccumulation (Van Geest et al. 2011a; 2011b)
4) Identification and Enumeration of Benthic Invertebrates in Sediment Samples
The identification and enumeration of benthic invertebrates should be conducted as detailed in Chapter 4 of this guidance document.
5) Collection of Fish for Tissue Analysis
This step is optional and is useful, in particular in areas where there is a concern about the usability of fish for human consumption. The collection of fish tissue for metals analyses would provide information on whether or not chemicals in resident biota may affect the usability of fish for human consumption. Tissues can be frozen for future analysis (MOEE 1997).
6) Additional Analysis
In order to obtain enough lines of evidence to determine causation, Chapman and Hollert recommend (2006) additional studies from their four categories (discussed in 12.6.2 above). Choices of species to use in toxicity tests and lines of evidence to investigate should be made based on contaminants, receptors and stressors of potential concern.
In the model proposed by Chapman and Hollert (2006), causation can be determined using the following lines of evidence: in situ sediment chemistry, benthos colonization, TIE, effect-directed analysis or bioassay-directed fractionation (to identify organometallic contaminants), bioaccumulation / critical body residues, habitat morphology, and sediment dynamics. Choices regarding these lines of evidence will depend on findings of the standard screening level SQT (chemistry, toxicity, and benthic invertebrate analysis).
7) Data Analysis and Report Preparation
The last step in the SQT approach is to statistically analyze the data obtained from the benthic survey, chemical analysis and toxicity tests and integrate this in a weight-of-evidence approach.
12.7 Metal Toxicology and Bioaccumulation
At the 2009 Metal Mining IOC Workshop, two particular publications on metal toxicity (US EPA 2007 and Adams et al. 2011, and the references contained within them) were discussed and should be referred to for further information. The Framework for Metals Risk Assessment (US EPA 2007) provides guidance regarding properties of metals, such as environmental chemistry, bioavailability, and bioaccumulation. As part of this framework, a series of papers related to metals were developed, one of which was McGeer et al. (2004), which discusses bioavailability and bioaccumulation of metals. The definition of bioaccumulation, as defined in McGeer et al. (2004) and US EPA (2007), is the net accumulation of a metal in a tissue or whole organism that results from environmental exposure media such as air, water, soil and sediment, as well as diet. Bioavailability is defined as the extent to which bioaccessible metals absorb onto or into and across biological membranes of organisms (McGeer et al. 2004; US EPA 2007). Bioaccumulation measures are usually an effective indicator of metal bioavailability. Excess accumulation of some metal species is potentially toxic to organisms. However, it is important to note that different types of organisms will accumulate metals to varying degrees (Adams et al. 2011).
12.7.1 Ecotoxicity of Metals
In the 2002 version of the Metal Mining EEM Guidance Document, it was assumed that bioaccumulation could be used to directly infer the cause of toxicity, based upon invertebrate research (Borgmann and Norwood 1997, 1997a, 1997b) and organic chemical models (Campbell 2012). There have been many advances on the topic of metal bioaccumulation in relation to toxicity, and the current state of the science indicates that the relationship between bioaccumulation and toxicity is confounded by physiology (Adams et al. 2011; US EPA 2007). It is now known that not all metals that accumulate in an organism interact at the site(s) (e.g., biochemical receptors, organelles, cells, organs, tissues) of toxic action (McGeer et al. 2010). Correlations between toxicity and metal bioaccumulation do not always indicate a cause and effect relationship, particularly when the relationship is confounded, as is the case for metal bioaccumulation and toxicity. In addition, extrapolating correlations from single metal exposures in laboratory conditions to complex effluents with a mix of potential contaminants would require extensive validation. Direct correlation between accumulation and toxicity has been observed for a few metals (non-essential, non-regulated metals such as cadmium and thallium) and organometallic forms, and in invertebrates only (McGeer et al. 2012; Borgmann and Norwood 1997a, 1997b, 1999; Borgmann 2000). Bioaccumulation of copper and zinc in invertebrate tissues is less useful because these elements are regulated to varying degrees, although their background concentrations can be high (McGeer et al. 2012).
Metals must first interact with the cell membrane in order to elicit a biological response or accumulate within an organism. Unlike organic contaminants, metals in the aquatic environment (with the exception of organometallics and neutral metal complexes) are unable to cross the cell membrane by simple diffusion; rather, they are transported (facilitated) across the cell membrane (Campbell 1995, 2012). Organism physiology sequesters or detoxifies significant amounts of toxic metals. The biologically or metabolically active portion of metal that is available to contribute to toxicity must do so at the site of action and, further, that total metal is at best a surrogate for the fraction of metabolically active metal at the site of action (McGeer et al. 2010).
Because metals are detoxified once they enter the intracellular environment (Vijver et al. 2004; Luoma and Rainbow 2008), simple predictions of metal-induced toxicity on the basis of metal quotas or burdens (tissue residue approach), as is often done with organic contaminants, are rarely applicable (Campbell 2012). Metals bound to inducible metal-binding proteins such as metallothionein, or precipitated into insoluble concretions consisting of metal-rich granules, can be considered to be a biologically detoxified metal, as compared to metals in metal-sensitive fractions such as organelles and heat-sensitive proteins (Campbell 2012). A corollary to this model of metal accumulation is that metal tolerance or resistance will be related to the ability of an organism to prevent "inappropriate" metals from binding to sensitive sites. The binding of an "inappropriate" metal to a metal-sensitive site, often termed "spillover" or incomplete detoxification, could be the precursor to the onset of metal-induced stress (Campbell and Hare 2009).
12.7.2 Selenium Toxicity
Selenium is a trace metal that has a high potential risk as a toxicant. Selenium, an essential dietary element, can accumulate in the tissues of oviparous fish through dietary exposure (Palace 2012). Although not toxic to adult fish, at slightly elevated concentrations the element is deposited in the egg, and can induce teratogenicity (Holm et al. 2005). Because the biogeochemistry of selenium is complex, tissue-based criteria (as opposed to aqueous concentration) are generally accepted as the most reliable indicator in potential selenium toxicity in fish (US EPA 2004). The most reliable indicator of potential selenium toxicity is measurement in eggs or ovary, although species-specific toxicity curves are required to achieve accurate risk assessment (Holm et al. 2005). Muscle can be used as a surrogate for egg and ovary; however, correlations between these tissues depend on the partitioning of selenium between muscle and egg, which varies with the reproductive cycle and between species (NAMC 2008). Because of the uncertainty of residency of some highly mobile fish and because of the shorter reproductive cycle of some fish species, reproductive toxicity may not be adequately detected between EEM phases (Palace 2012). Therefore, selenium toxicity may be an important factor to investigate as a source of toxicity in IOC. Much information on selenium toxicity was presented in the Toxicity of Metals and General Aquatic Toxicity streams at the Aquatic Toxicity Workshop (ATW) in 2011.
12.7.3 Biomarkers (Metallothionein)
Biomarkers are tools used for assessing metal-specific exposure. Significant increases in biomarker responses (either over time or relative to reference sites) could indicate that metals are bioavailable in the receiving environment. Metallothionein (MT), a low-molecular-weight metal-binding protein (US EPA 2007), is considered as a biomarker of heavy metal pollution in aquatic environments and has received much attention. In the domain of biomarkers for metals, MT conforms to most of the criteria defined for such tools. For example, MT responds specifically in a dose-dependent manner to changes in ambient levels of a trace metal or of a group of trace metals (Cd, Cu, Zn, Ag). In addition, high levels of MT may be associated with deleterious effects on organisms and populations because these high concentrations are indicative of metal detoxification, but if this capacity is exceeded, then there will be non-specific metal binding to cellular targets of toxicity.
There is no field evidence yet that MT concentrations respond to metals like As, Ni, Pb, and Hg. In addition, the MT biomarker will not track exposure to non-metallic chemicals such as ammonia and cyanide. MT constitutes an excellent biomarker of metal-induced effects; however, the extrapolation of lab data is cautioned (US EPA 2007).
Additional information on MT can be found in Couillard 1997, Couillard et al. 1999, Giguère et al. 1999, Gillis et al. 1999, Laflamme et al. 1998, Perceval et al. 1999, Pinel-Alloul et al. 1999, Wang et al. 1999, and US EPA 2007.
12.7.4 Using Bioaccumulation Methods
For assessing metal toxicity, the Framework for Metals Risk Assessment (US EPA 2007) should be referred to, as it addresses questions related to the methods or tools used to reflect metal bioavailability, and scientifically based approaches used to determine metal bioaccumulation (US EPA 2007). In addition, Adams et al. (2011) summarize the work from a SETAC expert subgroup on metal contaminant bioaccumulation. This subgroup evaluated the potential use of metal tissue residues for predicting effects in aquatic organisms (Adams et al. 2011).
The following hierarchical approach for assessing whether metals are responsible for confirmed effects in metal mining receiving environments was presented by Peter Campbell at the 2009 Metal Mining IOC Workshop (Campbell 2012) (the approach considers metal-organism interactions previously discussed, and would be applicable for non-essential metals):
- Identify appropriate biomonitor organisms that live in the exposure and reference areas. Criteria for selecting biomonitor organisms are well established (Phillips and Rainbow 1993). If appropriate indigenous organisms are unavailable, caged organisms may be considered.
- For each biomonitor species, choose the appropriate target organ, (e.g., gill or liver, or, for smaller species, whole-body concentrations) (subcellular fractionation approach).
- Compare the levels of bioaccumulated metals in specimens collected in the reference and exposure areas. The observation of markedly increased metal levels in the exposed specimens is evidence that one or more of these metals may be responsible for the confirmed effects. Note that by relying on metal bioaccumulation (rather than on the determination of metal concentrations and metal speciation in the receiving environment), one can circumvent the need to estimate the bioavailability of the metals found in the water body receiving the mining effluent.
- To refine the interpretation of the bioaccumulated metal levels, determine the subcellular partitioning (subcellular fractionation) of the metals in the target organisms (Campbell and Hare 2009). However, this distinction between biologically detoxified metals and metals that have “spilled over” onto metal-sensitive sites would only be justified for biomonitoring species that demonstrate a clear threshold response to increasing metal exposure (see discussion in Campbell et al. (2008) and Campbell and Hare (2009)).
Campbell (2012) also described a method to separate the relative importance of individual metals within a mixture. This method compares gene transcription between exposed and reference organisms. It shows some promise as a discriminatory biomonitoring tool to detect and differentiate among metal contaminants (Pierron et al. 2009; Walker et al. 2008).
12.7.5 Methods for Correlating Bioaccumulation and Toxicity
Three methods for correlating bioaccumulation and toxicity are briefly discussed below.
12.7.5.1 Tissue Residue Approach
The use of bioaccumulation as an indicator of toxicity is known as the tissue residue approach (TRA) and it relies on establishing a dose-response relationship to link tissue residues with toxic effects. Its premise is that tissue concentrations are a better surrogate for characterizing toxicity than external exposure concentrations (water, sediment/soil or diet). Meador et al. (2011) indicate that five exposure-based metrics can be used in the TRA, all of which have proportional relationships to each other: external exposure concentration, whole-body concentration, organ concentration, target concentration, and receptor concentration. TRAs, relating accumulation to toxicity, have been developed for some metals (e.g., selenium, copper, methylmercury, cadmium, zinc, arsenic, cobalt, chromium, manganese) in some fish and invertebrates (Borgmann 2000; Tessier et al. 1993), including in Hyalella (Adams et al. 2011; Borgmann et al. 2001; Norwood et al. 2007; Borgmann et al. 1991; Borgmann and Norwood 1997a, 1997b; Borgmann and Norwood 1999; Holm et al. 2005; Hodson 1990; Ridal et al. 2010; Meador et al. 2011). The use of the TRA for metals, however, can be problematic (Meador et al. 2011). Studies have shown that bioaccumulation relationships with Hyalella may be unique (Adams et al. 2011) and the interpretation of data derived from Hyalella studies may not be straightforward (Wang et al. 2004). Adams et al. (2011) concluded that the TRAs for metals other than organometals are not currently supported as they have not led to the development of a generalized approach to estimate effects, and under the circumstances a determination of applicability may require field validation. Sappington et al. (2011) discuss the benefits and limitations of incorporating a TRA into an ecological assessment. Therefore, caution is advised when considering the use of TRAs for IOC, and US EPA (2007), Adams et al. (2011), Sappington et al. (2011) and references herein should be considered for further information. Even though TRAs are a complex means for determining toxicity relationships, there is still a potential use for understanding causes of adverse effects in certain scenarios (e.g., indication of metal exposure to determine which metals are being taken up by organisms).
12.7.5.2 Biotic Ligand Model
Metals in the aquatic environment (with the exception of species such as organometallics and neutral metal complexes) are transported across respiratory and digestive membranes by facilitated transport, involving either membrane carriers or channels (ionic binding to cell ligands, assimilable ligand such as thiosulfate). Such transport is normally a function not of the total dissolved metal but rather of the free metal ion concentration. This is the foundation for the Biotic Ligand Model (BLM) (Campbell 2005, 2012). The BLM is a tool that can be used to quantitatively evaluate how several water chemistry parameters affect the speciation and bioavailability of metals in the aquatic environment (Niyogi and Wood 2004; CCME 2007). The BLM is one example of when metal bioaccumulation can be applied successfully as an indicator of impact. The BLM is based on predictions of the binding of metal at the site of toxic action, and the approach integrates metal interactions along the exposure–uptake–accumulation–toxicity pathway (Di Toro et al. 2000, 2001; McGeer et al. 2010; McGeer et al. 2012). Metal uptake within the BLM is based on estimates of the relative bioavailability of different dissolved forms (species) of metal. These interactions between metal species and the biotic ligand are incorporated into the equilibrium modelling framework. The strength of BLMs is that they simultaneously account for the geochemical speciation as well as the relative binding of metal species (or not) to the site of toxicity. More information on the BLM can be found in Paquin et al. (1999, 2002a, 2002b), CCME (2007), US EPA (2003), Santore et al. (2001, 2002), Niyogi and Wood (2004) and McGeer et al. (2010).
12.7.5.3 Subcellular Fractionation
Subcellular fractionation methods characterize bioaccumulation in a tissue or a whole organism. Using the following processes, homogenization, centrifugation and heat treatment, a distinction can be made between metal in detoxified and metabolically active forms within a cell (Campbell et al. 2008; Campbell and Hare 2009). Subcellular partitioning of bioaccumulated metal has the potential to provide valuable information on metal toxicity. Partitions include metal-rich granules, cellular debris, organelles (e.g., mitochondria, microsomes and lysosomes), heat-sensitive proteins, and heat-insensitive proteins.
Studies have grouped subcellular compartments into metal-sensitive pools such as mitochondria and heat-sensitive proteins (where metal toxicity may occur) and metabolically inactive pools such as granules and heat-insensitive proteins (where metal accumulation is benign).
12.8 Field Study Approaches for Fish and Benthos
Several IOC study tools can be used in the field for fish and benthos studies. Cost effectiveness and availability from consulting, academic or government laboratories should be considered.
12.8.1 Field Study Design
Gradient designs can be used to associate stressor level and geographic distance between outfall. Co-occurrence of contamination with metal effects is one line of evidence in establishing metal effects. Gradients of contamination offer the opportunity to determine co-occurrence of metals with predicted patterns of response (Luoma and Rainbow 2008). Field study designs such as gradient designs and control impact designs are presented in other chapters. Gradient designs are discussed in greater detail in chapters 2, 3, 4 and 9. Other field study designs such as control impact designs are presented in chapters 2 and 4.
12.8.2 Caged Organisms
Caged bivalves are discussed in Chapter 9 (Alternative Methods) of this Technical Guidance Document.
IOC studies can make use of non-mobile fish and invertebrates or caging techniques. Cage bioassays are often limited to the use of older (larger) and less sensitive individuals, because the cage mesh size must be large enough to allow adequate water exchange to prevent cage effects such as fouling, sedimentation and low dissolved oxygen concentration.
Fish have been used in in situ caging (Muir et al. 1991). Some debate has existed regarding the use of caged fish, due to concerns regarding interactive effects of stress in captive fish (Courtenay 2002). It was suggested that small-bodied fish with potentially smaller home ranges may be less susceptible to stress and more appropriate for caging (Palace et al. 2005; Bandler et al. 2012). This method may be useful in IOC, particularly where effects of discharges need to be resolved at a small spatial scale, if optimized to reduce stress (verification of caging effect on fish weight).
The caging of amphipods for in situ assessments of biological responses to environmental conditions is an established methodology (Grapentine 2012; Borgmann et al. 2007; Couillard et al. 2008; Mulliss et al. 1996; Muir et al. 1991; VanWingaarden et al. 1996; Henry et al. 1994). Experiments can be designed to (a) test for direct effects of effluents or contaminated sediment, (b) link particular metals to toxicity and benthic impairment, (c) identify important contaminant exposure and uptake pathways, (d) quantify stressor-response relationships, and (e) examine effects of natural factors and other anthropogenic stressors.
The method is well developed, not technically difficult, addresses multiple contaminant exposure pathways, is amenable to high experimental replication, incorporates multiple biological endpoints, and is low to moderate in cost. Amphipods are held in screened plastic cages in pre-selected locations in the receiving environment, followed by recovery and analysis for survivorship, growth and/or concentrations of metals in tissues. Cages can be designed for exposure in the water column, sediment and water column, or pore water, and are deployable over most substrates. Each cage can hold up to 15 amphipods without showing significant mortality after deployment. Food can be added at the start or during the experiment if it is not an experimental factor. Amphipods placed in cages can be held in buckets for several hours or days without mortality, allowing for easy transport. The following endpoints can be measured: the number of survivors, mean individual growth over the exposure period, estimation of reproduction, and whole-body concentrations of relevant elements. Analysis of these endpoints can determine: effects of location or stressor level on amphipod health and populations; metal bioaccumulation and bioavailability; and whether the metal concentrations in tissues are associated with adverse effects for the test organism or species that feed on the amphipods. The strength of this method is to distinguish between sediment and water effects. Standardization may be required for tests involving amphipods other than Hyalella azteca.
Other aquatic invertebrates that have been used in field cages include midges, leeches, snails (Henry et al. 1994), and the water flea (Daphnia magna) (van Wingaarden et al. 1996).
12.8.3 Mesocosms (Fish and Invertebrate)
Mesocosms are helpful for identifying specific effluent contributing factors and are elaborated in depth in Chapter 9.
12.8.4 Benthic Transplant Devices
The Benthic Transplant Device evolved from spatial and gradient designs in mining and pulp and paper EEM studies conducted in rivers, creeks, reservoirs and complex marine environments over many EEM studies (Thomas 2012). This method employs the use and relocation (transplant) of indigenous benthos populations and associated substrate/habitats, and has been effective in elucidating comparative trends in benthos communities and substrate quality with space and time (Thomas 2012). A key feature of this approach is the ability to tease out subtle biophysical, chemical or benthic differences between sites, such as: changes with exposure/distance measured; benthos abundance (density) /diversity; sediment quality (chemistry/physical measures/appearance); biomass changes; bacterial presence and composition; and benthic deformities/symmetry. Furthermore, this method may contribute to separate historical and current effluent effects from other natural and human-created confounding sources and variability, and in determining the effluent exposure scenario.
12.8.5 Macrophytes, periphyton, phytoplankton, chlorophyll
In addition to benthic invertebrates, phytoplankton, macrophytes and periphyton can be evaluated in IOC. AETE (1997) evaluated each of these biotic categories on the basis of sensitivity, ecological relevance, validity and repeatability, site specificity, and applicability.
12.8.5.1 Macrophytes
AETE (1997) reviewed many studies that have demonstrated a reduction in species diversity and abundance in macrophytes exposed to metal-polluted waters. The absence of macrophytes from impact areas where they would normally be expected can be regarded as an indication of pollution (AETE 1997). Impacts on macrophyte density and biomass could substantially affect the other members of the freshwater community, as they may provide habitat for higher trophic levels within the community. Macrophytes are sedentary and provide a measure of the impacts of the bioavailable fraction of metals over a time-integrated picture (Whitton et al. 1981). Macrophyte community composition appears to be more of a long-term indicator of environmental stress rather than a tool for early detection of potential impacts. Kelly (1988), Haslam (1982), Small et al. (1996), and Sortkjaer (1984) have developed methods of monitoring macrophytes for assessment of pollution. There are several frequently encountered macrophyte species recommended as potential biomonitoring candidates in Canadian environments: Leptodictyum riparium, Potomogeton epihydrus v. nuttallii, Potomogeton sp., Fontinalis spp., Potomogeton richardsonii, Ericaulon esotangulare, Elocharis acicularis (AETE 1997; Whitton et al. 1981; Crawford and Luoma 1993). At the same time, it should be appreciated that macrophyte populations vary seasonally in most rivers and lakes, with marked seasonal variations in biomass, and are really only available as a biomonitoring species during a relatively short growing season (Hellawell 1986). The use of macrophytes for the marine environment is only feasible in intertidal hard-substrate areas. If this is deemed to be a major habitat that is potentially affected by mining effluents, shoreline quadrat studies of percent coverage and biomass per unit area, major species, and possibly associated epifauna such as amphipods may be appropriate as supportive indicators (AETE 1997).
12.8.5.2 Periphyton
Periphyton is generally non-mobile, and integrates effects of environmental variables in a relatively short time frame. The microalgal component of periphyton is important as both a primary producer in rivers and littoral zones in lakes (Cattaneo and Kalff 1980), and as a food source for invertebrates (Whitton 1984). As a food source for higher trophic levels, periphyton potentially plays an important role in contaminant transfer between trophic levels. Also due to their sedentary nature, periphyton are a good indicator of local conditions, and play a key role at the interface between substratum and surrounding water, perhaps by influencing biogeochemical pathways and dynamics. Organisms within the periphyton mat have relatively high turnover rates. Hence, they are among the first organisms to respond to environmental stress, and among the first to recover. Sensitive species are often replaced by more tolerant species (Austin 1983; Austin et al. 1985). As a result, periphyton communities often reflect the current environmental conditions (Lowe and Pan 1996) and could be used as an early warning indicator.
Periphyton has been used as an indicator for water quality assessments in different systems by a large number of investigators (Chessman 1985; Clements and Kiffney 1994). Various sampling methods have been used, including scraping, brushing or aspiration. Structural measurements of periphyton generally involve counts of organisms, providing information on abundance, species richness and community structure, whereas functional measures study changes in primary production, respiration and detrital processing (Clements 1991). Periphyton communities are often species-rich and spatially compact relative to other aquatic groups, and representative samples can be collected from a few square centimetres of substratum (Lowe and Pan 1996). However, some authors have suggested that there is difficulty obtaining representative and uniform samples because of spatial heterogeneity and difficulty sampling organisms (Weitzel et al. 1979; Kutka and Richards 1986; St-Cyr 2000). Regardless of potential sampling problems, many studies have characterized algal communities in streams and have related abundance of specific taxa to the presence of metals (Clements 1991; Whitton 1984). Studies in lakes have similarly identified shifts in species composition with loss of metal- sensitive species (Austin and Deniseger 1985; Austin et al. 1985; Roch et al. 1985). Lowe and Pan (1996) concluded that benthic algal monitoring data would be most valuable when combined with a suite of additional monitoring data, including physical and chemical measurements and the analysis of other biota of aquatic communities such as invertebrates.
12.8.5.3 Phytoplankton
The structure of phytoplankton communities can be altered due to environmental stressors that affect certain sensitive species and not others. This approach has been used widely to examine the effects of metal pollution and has been applied to long-term monitoring progress at mine sites. Standing crop and biomass (usually measured as chlorophyll a) of phytoplankton communities are typically assessed in any study in which primary producers are considered, and have been used as indicators in long-term environmental programs at mine sites (AETE 1997). Consistent patterns of tolerance or intolerance to metal pollution are found in some species, and they may serve as an early warning of metal pollution (AETE 1997). On a long-term basis, biomass does not appear to be a useful measure of the effects of metal contamination on primary producers, since long-term shifts in species composition or reduction in grazing pressure can counteract any short-term depression in algal biomass (Yan 1979). Further, biomass disperses, and density and biomass, tend to be similar between impact and reference areas (AETE 1997).
12.8.5.4 Indicators for Phytoplankton and Periphyton
The measurement of chlorophyll a, chlorophyll b, and phytoplankton periphyton biomass may be helpful in cases where upstream discharges of nutrients represent confounding influences, or where nutrient enrichment from mine effluent is suspected. The concentration of photosynthetic pigments is often used to estimate phytoplankton biomass.
Phytoplankton and periphyton biomass tends to be patchy in its distribution, and varies with time as blooms develop and waters stratify. It is more suitable for lake than for river settings, although evidence for significant phytoplankton biomass in riverine samples is nevertheless an indicator that nutrients reflecting trophic status upstream may be, and probably are, relevant in assessing nutrient (as opposed to contaminant) influences on the downstream structure of benthic communities, zooplankton and presumably fish as well. Criteria have been established to classify eutrophication based on periphyton and phytoplankton (Dodds and Welch 2000; Dodds 2006). Nutrients and many other factors such as light, temperature, velocity, suspended solids, and physical disturbances may affect periphyton community composition and biomass (Culp et al. 1996; Chambers et al. 2006; Azim and Asaeda 2005; Biggs and Kilroy 2000). Relationships between nutrients and periphyton biomass have been investigated (Dodds and Welch 2000; Chambers et al. 2001).
Frequently, two standard measures of biomass are used: chlorophyll a, which is used as an indicator of the total amount of autotrophic organisms in the sample, and ash-free dry mass (AFDM), which is a measure of the total amount of organic material in the sample. The AFDM includes living autotrophic and heterotrophic microorganisms, plus dead periphyton, micro-invertebrates, and possibly terrestrial debris. It is recommended to analyze both of these parameters, because they provide complementary information and they can be combined to form a ratio called the “autotrophic index” (Weber 1973). More information on the use of chlorophyll a as an indicator of biomass can be found in Weitzel (1979). Methods for determining AFDM are described in Ridley Thomas et al. (1989) and Aloi (1990).
Chlorophyll a can be measured using spectrophotometry, fluorometry, or liquid chromatography, and taking into account chlorophyll b and c, chlorophyll degradation products (e.g., chlorophyllides, pheophorbides and pheophytins), and turbidity. More information can be found on the analytical methods in Standard Methods for the Examination of Water and Wastewater (method 10200 H) (American Public Health Association (APHA), American Water Works Association (AWWA), and the Water Environment Federation (WEF), 2001).
12.9 Laboratory Methods and Toxicity Tests
12.9.1 Histology and Physiological Parameters
The impacts of contamination can be seen not only at the ecosystem, community, population and individual levels, but also at cellular, subcellular and molecular levels (Peplow and Edwards 2005). At the 2009 Metal Mining IOC workshop (Environment Canada 2012), work was presented in regards to studying histological and physiological parameters to determine the cause of increased weight and size of livers and gonads in fish (Sharpe et al. 2012). Gonad histology was used to assess fish sex and state of maturity (due to small fish size), and to observe asynchronous or abnormal development. Liver histology was used to assess gross pathology (deformities, abnormalities and parasites), liver mitotic index (indicator of cell proliferation due to tumours or arsenic for example), lipid vacuolation and glycogen content. In addition, physiological parameters such as liver glycogen and triglycerides were measured. These indices taken together gave an indication of gonad and liver function. Some indicators and methods include: diagnostic approaches to hepatic toxicity (Wolf and Wolfe 2005), histopathological approaches (Hinton et al. 1990, 2005; Costa et al. 2009; Werner et al. 2003), and physiological and biochemical indicators (Taylor 2004; Weber et al. 2003; Werner et al. 2003).
12.9.2 Toxicity Tests
Laboratory lethal and sublethal toxicity tests using fish, benthic species as test organisms, and whole sediment, sediment pore water, effluent, and water as test media, can provide a direct determination of toxicity. Toxicity tests can be used in conjunction with many of the monitoring tools and other field tests described elsewhere in this chapter to help interpret results in situations of confounding factors, multiple discharges, or habitat modifications from historical effluent discharges. These toxicity testing methods may also be useful in identifying the cause for the absence of taxa at exposure sites.
There is an array of laboratory test methods available; this chapter refers to some relevant methods, but many more are available from Canada and other international bodies, such as Environment Canada’s Biological Methods Division, as well as the US EPA, OECD, EU, WHO and so forth. It is recommended that toxicity testing be conducted by accredited laboratories that follow recognized standard protocols.
Lethal and sublethal toxicity tests have a number of limitations that must be considered. Because laboratory toxicity test methods do not determine toxicity in situ, they might not account for toxicity due to overlying water, or for the effects of overlying water chemistry on contaminant toxicity in the testing of sediments or sediment pore water. These problems will be most severe when the chemistry of the overlying water used in the toxicity test differs substantially from in situ water, or when conditions in the field or during toxicity tests are such that contaminant concentrations in overlying water are far from equilibrium with the sediment (Parker and Dumaresq 2002).
12.9.3 Fish Toxicity Testing: Fathead Minnow Lifecycle
The fathead minnow whole lifecycle laboratory test can be used for assessing long-term exposure effects of effluents on fish (Parrott et al. 2012; Parrott 2005; Parrott and Bennie 2009). This lifecycle test encompasses all critical windows of exposure during the life cycle of the fish: egg, larvae, developing and mature juvenile, reproduction of adult fish, and survival of the F1 generation.
Lifecycle assays are expensive and lengthy; however, they mimic the effects of real environmental exposures, and provide data that are impossible to obtain with shorter fish exposure bioassays. The method could provide valuable data in cases where capture of wild fish is difficult (Parrott et al. 2012).
12.9.4 Whole Sediment Toxicity Testing
Sediment toxicity tests may be used to evaluate potential contamination in aquatic environments (Parker and Dumaresq 2002). These tests provide a direct method to determine chemical availability of contaminants in sediment, and can be used as an evaluation tool in conjunction with chemical data and other contaminant analysis, benthic community analysis, and other sediment monitoring tools such as the Sediment Quality Triad (see section 12.6.2). Whole sediment toxicity tests provide direct measurement of toxicity by evaluating the effects of exposure-area sediments on test organisms relative to effects due to control or reference-site sediments. Test results are expressed in terms of survival, growth and reproduction in comparison with a reference site. Effects can also be expressed as the difference (in %) with response in control or reference sediments.
Whole sediment toxicity testing can be used to corroborate that changes in benthic invertebrate communities are due to toxicity of sediments and not other physical or biological factors. Adverse effects on benthic invertebrate community structure could be due to sediment toxicity, but could also be due to other factors (predation, habitat differences, etc.). Concurrent impairment of benthic invertebrate community structure and sediment toxicity implicates the sediment itself as the cause of effects on the benthic invertebrate community. These tests also provide important information for interpretation of field effects in situations where benthic invertebrate community data are inconclusive, or when only pollution-tolerant species are present in both exposure and reference areas.
Batteries of tests can be used to demonstrate sediment toxicity in more than a single species. The use of a battery of tests helps to demonstrate the significance and universality of the sediment toxicity response in different levels of organism complexity (i.e., number of test species responding). The relative responses observed in sediment tests with different species can aid in the identification of the cause of sediment toxicity if information is available in the literature on the relative sensitivity of different species to different metals. Multi-species testing can also aid in explaining the relationship between benthic invertebrate community structure and sediment toxicity. Use of multiple species in toxicity tests allows for more direct comparisons of abundance/absence of benthic invertebrate species with toxicity test results for the same or closely related species. Standard test procedures are available (Bedard et al. 1992; ASTM 1997, 2010; Environment Canada 1997a, 1997b).
The limitation of whole-sediment toxicity tests is that they are laboratory tests that do not determine in situ toxicity directly. Furthermore, if a standard overlying water is used (as is commonly done), instead of water collected from the study site, the effects of differences in overlying water quality on sediment toxicity, if present, are not taken into account.
12.9.4.1 Survival and Growth of Hyalella azteca
Hyalella azteca is a sediment-dwelling amphipodthat has beenroutinely used in both field and laboratory studies to investigate the source and cause of sediment toxicity (Shuhaimi-Othman et al. 2006; Borgmann et al. 2004; Borgmann et al. 2005a, 2005b; Couillard et al. 2008; Ingersoll et al. 2000; Borgmann and Norwood 2002; Nowierski et al. 2005). Environment Canada has an existing standard method (EPS 1/RM/33: Test for Survival and Growth in Sediment Using the Freshwater Amphipod H. azteca) (Environment Canada 1997a). The endpoints for the EPS 1/RM/33 method are survival (lethal toxicity) and growth (by dry weight) at the end of a 14-day toxicity test for sediments and effluent/water. The test may be run as a single or multi-concentration assay (Environment Canada 1997a).
Taylor et al. (2012) modified the EPS 1/RM/33 method to employ both a sediment toxicity test and an aqueous-only toxicity test to separate the effects of current effluent discharge from that of historical accumulation of metals and other toxic substances in the sediment. The sediment toxicity test involves preparing various sediment/water overlay combinations: three sediment types (exposure site, field reference, lab reference) are each overlaid with one of three water types (standard lab dilution water, reference water or receiving water) (nine maximum combinations in total). Using the above combinations, it is possible to determine the toxicity of the effluent or the sediment independently. By using a combination of field and lab sediment and water samples, the study design also takes into account the influence that site-specific water chemistry (e.g., dissolved organic carbon (DOC) in receiver) may have on toxicity (Taylor et al. 2012) (Borgmann et al. 2005c; Borgmann 2002; Environment Canada 1997a, Ingersoll et al. 2000). The aqueous-only test is based on the EPS 1/RM/33 method and a draft method for an aqueous-only method developed by Borgmann et al. (2005). The test design is based on a 14-day static-renewal exposure examining both survival and growth of the test organisms. Tests on field reference water, field sample, and lab dilution water sample are conducted in the absence of sediment, but with an artificial substrate for the test organisms.
Combined results from the study presented at the Metal Mining IOC Workshop (Taylor et al. 2012) indicate the H. azteca test method (EPS 1/RM/33) is a quick, cost-effective, readily available screening tool when conducted with benthic surveys and chemical analysis (Sediment Quality Triad approach). It can be used to isolate the biological effects of historical contamination in sediment from current effluent quality, taking into account temporal variability, and toxicity of metals in water and sediment. However, temporal variability in effluent constituents should be taken into consideration when deciding if current effluent quality may impact benthic invertebrate communities.
12.9.4.2 Survival and Growth of the Freshwater Midges Chironomus dilutus or Chironomus riparius
Chironomids have been used extensively in sediment toxicity tests in the United States and Canada. The standardized test method by Environment Canada is described in EPS 1/RM/32 (Environment Canada 1997b): Test for Growth and Survival in Sediment Using Larvae of Freshwater Midges (Chironomus tentans or Chironomus riparius). A summary and additional references pertaining to chironomid test procedures is available in Environment Canada (1997b). The EPS 1/RM/32 method exposes ten-second (C. dilutus formerly C. tentans) or first (C. riparius) instar organisms to a sediment sample and bioassay water. At the end of the test, the sediment is sieved and the organisms (dead and surviving) are recovered, and the surviving animals are counted, dried and weighted. The two endpoints calculated are the mean percentage of organisms that survived the exposure and the mean dry weight per surviving animal (Environment Canada 1997b).
12.9.4.3 Survival and Reproduction of the Oligochaete Tubifex tubifex
This test is described in ASTM (2010).
Tubifex individuals are exposed to equal amounts of sediment and bioassay water. Sexually mature individuals (aged 8 weeks) are introduced and incubated for 28 days. The production of cocoons indicates reproduction of the organisms. At the end of the test, the sample is sieved and the number of surviving adults, the number of full and empty cocoons, the number of young less than 500 mm and the number of young greater than 500 mm are counted as measurements of survival and reproduction.
12.9.4.4 Survival and Growth of the Mayfly Hexagenia limbata
This test is described in the Ontario Ministry of the Environment “Laboratory Sediment Biological Testing Protocol” (Bedard et al. 1992) and in ASTM (2010).
The test uses early instar Hexagenia limbata mayfly nymphs that are 3–4 months old and that are laboratory-reared from field-collected eggs. Ten to fifteen H. limbata individuals are exposed for 21 days to bioassay water and sediment. Animals are not fed during this time. At the end of the test, the number of surviving animals is counted and the animals are dried and weighed to determine dry weight and to give an indication of growth. Survival and growth in the test sediment are compared statistically to survival and growth in the control.
12.9.5 Metals in Overlying Water in Whole-Sediment Toxicity Tests
Measurement of metals in overlying water of whole-sediment toxicity tests can be used for the quantification of bioavailable metal or the identification of cause of sediment toxicity. Measurement of dissolved metals in the overlying water can provide information on the relative bioavailability, and hence potential toxicity, of metals in sediments. However, the toxicity of metals in sediments is not proportional to total metal concentrations in sediment, and bioavailability can vary greatly from one sediment to another. Metal bioavailability, especially to H. azteca, often appears to be primarily due to dissolved metals in the overlying water (Deaver and Rodgers 1996; Warren et al. 1998; Borgmann 2000; Borgmann and Norwood 1999a). If data on metal toxicity in water are available, and if toxicity is known to be primarily due to dissolved metals for the benthic invertebrate species in question, water concentrations can sometimes be used to infer the cause of toxicity. The use of metal concentrations in overlying water is restricted to static toxicity tests in which the overlying water is not renewed.
The main limitation of this type of measurement is that the toxicity of metals in water can be reduced substantially by complexation with DOC leaching from sediments. Water concentrations resulting in a given level of toxicity may, therefore, be higher in sediment toxicity tests than in water-only tests (Borgmann 2000). This effect can be reduced by using a large water-to-sediment ratio in the toxicity test (Borgmann and Norwood 1999b). Water chemistry may also affect metal toxicity, and metal concentrations in overlying water should be compared to metal concentrations causing toxicity in water-only tests only if the major ion composition of the water used in these tests is similar. This technique will not help identify the cause of toxicity if the solid phase of the sediment contributes significantly to toxicity.
12.9.6 Sediment Pore Water Toxicity Testing
The concentrations of contaminants in pore water may be more highly correlated with toxicity to aquatic organisms (Liber et al. 2011; Cairns et al. 1984; Nebeker et al. 1984; Schuytema et al. 1984; Knezovich and Harrison 1988; Giesy and Hoke 1990) than those in bulk sediments (Patrick et al. 1977; Adams et al. 1985; Shaner and Knight 1985; van de Guchte and Mass-Diepeveen 1988; Di Toro 1989; Ankley et al. 1991; Carr and Chapman 1992). Therefore, toxicity testing using sediment pore water can be an important complement to whole-sediment toxicity testing. The relative importance of pore water and whole sediments as sources of toxic contaminants to aquatic organisms appears to depend on the species of test organism and the type of contaminant (Knezovich and Harrison 1987; Giesy and Hoke 1990; Harkey et al. 1994).
There is a relatively large amount of literature describing toxicity testing with pore water (Burton 1992, 1998). However, it is not as extensive as whole sediments, and there are few standardized methods for toxicity testing of freshwater organisms with pore water. Environment Canada (1992) has two standard methods using pore water for Echinoids (Sea Urchins and Sand Dollars) and luminescent bacteria.
Tests that may be used for pore water toxicity testing are briefly described below. Note that these are standard tests for toxicity testing of water and effluent, and are not unique to pore water testing.
12.9.6.1 Sea Urchin Fertilization Bioassay
The sea urchin fertilization test is described in the sublethal toxicity chapter (Chapter 6, Table 6.2).
12.9.6.2 Microtox Acute Test
The Microtox acute test is used to evaluate the effect of effluent exposure on light production by the naturally luminescent marine bacteria Vibrio fischeri. The result is expressed as the concentration where light output is reduced by 25% or 50% (IC25, IC50).
The Microtox test is frequently used on site by many industries. The test is described in the manufacturers’ handbook Microtox Manual: A Toxicity Testing Handbook (Microbics Corp.). The Microtox test is a rapid-screening bioassay kit, which measures toxic effects on the light output of a standardized luminescent bacterial culture. The main limitation of this method is that the diluent (dilution water) is a saline solution and the test organism is a marine bacterium, with little relevance to Canadian mining environments, as most mine effluents are deposited in freshwater environments.
12.9.6.3 Acute Lethality with Daphnia magna
The purpose of this test is to determine the concentration of test water that causes 50% mortality to Daphnia magna during a 48-hour exposure period. This standard test uses groups of less than 24-hour-old D. magna neonates in a range of test water concentrations. The D. magna lethality test is described in detail in the EPS 1/RM/11 (Environment Canada 1990, 1996).
12.9.6.4 Reproduction and Survival Using Ceriodaphnia dubia
This test is described in the Sublethal Toxicity Chapter (Chapter 6, Table 6.2).
12.9.6.5 Growth Inhibition of the Alga Pseudokirchneriella subcapitata
This test is described in the Sublethal Toxicity Chapter (Chapter 6, Table 6.2).
12.9.6.6 Sediment Pore Water Toxicity Testing Using Hyalella azteca and Chironomus dilutus
The standard tests for Hyalella azteca (Environment Canada 1997) and Chironomus dilutus (Environment Canada 1997a), described in sections 12.9.4.1 and section 12.9.4.2, respectively, have recently been used for sediment pore water toxicity testing. Liber et al. (2011) evaluated the hypothesis that pore water metal concentrations are better correlated with sediment toxicity to benthic organisms than whole-sediment metal concentrations. Using H. azteca and C. dilutus they found that, in some cases for the specific metals examined, their approach has potential to predict sediment toxicity using sediment pore water metals data (Liber et al. 2011).
12.10 Tools for Effluent and Water Quality Analysis
At sites where there are effects on fish or the benthic invertebrate community, there are a number of effluent characterization and water quality monitoring techniques available that may help in understanding the nature and cause of effects. In addition, effluent characterization of additional mine-related contaminants from other sources, particularly non-point sources, may be appropriate at some sites as part of IOC. In some cases, these techniques could also provide valuable information during investigation of cause monitoring, as supporting environmental data, when determining the extent and magnitude of effects observed in fish or the benthic invertebrate community. The techniques described below are recommended, and mines may use individual techniques or combinations of techniques, as appropriate, to address site-specific questions. More information on effluent characterization and water quality monitoring can be found in Chapter 5.
12.10.1 Measurement of Dissolved Metals during Investigation of Cause
The measurement of total and dissolved metals in effluent and exposure-area water quality samples in IOC would assist in determining which metals might be causing or contributing to the observed effects (Parker and Dumaresq 2002). The rationale for measuring dissolved metals during IOC is based on the theory that it is the metals in the dissolved fraction, particularly the free metal ions, that are the most bioavailable to aquatic organisms. There is qualitative evidence to support this theory, especially in defined synthetic media (i.e., laboratory bioassays). However, this relationship appears to break down in natural waters, particularly in the presence of natural dissolved organic matter (ESG 1999).
The normal procedure for measuring dissolved metal concentrations involves the immediate filtration of the raw water sample through a 0.45-micron filter and then preserving the filtered sample with nitric acid to a pH of less than 2.0 to keep the dissolved metals in solution until the analysis. There are differences in the filter size used, but the 0.45-micron filter is the most commonly used. There are concerns that certain amounts of colloid-bound metals, which are not really dissolved, can pass through this size of filter membrane, and some researchers recommend filter-pore sizes as small as 0.1 microns to minimize this concern (EVS 1997).
In a report by Hall (1998), three filter systems were evaluated (syringe, in-line, and vacuum filters). The recommendations from this study are based upon the contamination contributed by these filters and their ease of use. Before a system is chosen, it is important to evaluate its propensity to become clogged with sample water. Anyone planning on completing dissolved metals analyses is strongly encouraged to consult Hall (1998) prior to sample collection.
Peepers, a dialysis device, and diffusive gradient in thin films can be used for measuring bioavailable metal in overlying water (Liber and Doig 2000). Bioavailable metals can be compared to dissolved metal concentrations, which gives a better prediction of toxicity.
12.10.2 Metal Speciation for Metals of Concern
Metals are neither created nor destroyed by biological or chemical processes. However, these processes are capable of transforming metals from one species to another (US EPA 2007). The form in which a metal (or metalloid) occurs (i.e., the speciation of the metal) can have a significant effect on the bioavailability, bioaccessibility and toxicity of that metal to aquatic organisms (Tessier and Turner 1995; Stumm and Morgan 1996; US EPA 2007). As a result, understanding the speciation of contaminants of concern can be important to understanding the nature and causes of effects on aquatic ecosystems (Parker and Dumaresq 2002). Langmuir et al. (2004) discuss the environmental chemistry of metals and provide examples of how speciation impacts behaviour and effects.
Metal speciation can be measured through direct analysis, or estimated with modelling tools. Two of the most important factors affecting metal speciation are pH and oxidation state. Thus, no matter which method is to be used to assess metal speciation, it is important to take accurate field measurements of pH and dissolved oxygen. In addition, if samples are to be analyzed for metal speciation, it is important to ensure that proper sample collection, preservation and storage procedures are followed.
The speciation, or at least valency determination, of some metals in water can be determined analytically, although such a service may be limited. Analytical methods include methods using ion-exchange, electrochemistry, size exclusion chromatography, voltammetry, X-ray absorption fine structure spectroscopy, inductively coupled plasma mass spectrometry, diffusive gradient thin film, ion-pair reversed phase high performance liquid chromatography, gel integrated microelectrodes, hollow fibre permeation liquid membrane, as well as several methods outlined in section 12.11.5.1 (Partial Metal Concentrations in Sediment). Measurement techniques for the speciation of metals in aqueous solutions have been comprehensively reviewed by Tessier and Turner (1995), Unsworth et al. (2006) and Ekberg et al. (2011). If samples are to be submitted for analysis for speciation, extra care should be taken in sample collection, handling and storage. Such care should be taken because changes in factors such as the dissolved oxygen content of the sample could result in speciation of the sample changing significantly between the time of collection and the time of analysis. It is strongly recommended that the laboratory that will be completing the analyses be contacted in advance of sample collection to identify appropriate procedures.
A number of computer programs are available for the modelling of chemical speciation of metals in water, such as the following:
- MINTEQA2 (Allison et al. 1991)
- MINEQL+ (Schecher and McAvoy 1992, 1994)
- Windermere Humic Aqueous Model (WHAM) (Tipping 1994, 1998)
- VMINTEQ (Gustafson 2004)
- Chemical Equilibria in Soils and Solutions (CHESS) Model (Santore et al. 1998; Meyer et al. 1999)
- MINEQL+ in the BLM by McGeer et al. (2000) (Paquin et al. 2002a, 2002b)
- BIOCHEM ORCHESTRA (Vink and Meeussen 2007)
- TRANSPEC (Bhavsar et al. 2008)
- PHREEQCI (Parkhurst and Appelo 2000)
- FITEQL (Herbelin and Westall 1999)
- TICKET (Farley et al. 2008)
Since metal speciation is dependent on a number of factors, all of these programs require data on: pH, alkalinity, hardness, major cations and anions, ionic strength, total dissolved solids, DOC, total dissolved metal, sulphide, unique anthropogenic inputs such as EDTA, and concentrations of aluminum and iron (III) (EVS 1997). These programs can be used to predict the forms and concentrations of metals in effluent or water, and to predict toxicity (EVS 1997). Potential sources of error are described in EVS 1997.
12.10.3 Measurement of Reagents & Reagent By-products used in Processing
A wide range of chemical reagents are used in ore processing, and these reagents, or the by-products of the decomposition of these reagents, may occur in mine effluent. As a result, reagents and reagent by-products may occur in receiving environments. Analyses for reagents and reagent by-products may be helpful in cases where observed effects cannot be attributed to metals and other parameters monitored regularly as part of the EEM program at a site. Several particular reagents and by-products that were discussed during the 2009 Investigation of Cause Workshop for Metal Mining (Environment Canada 2012), are discussed briefly below.
Cyanide species may be present in effluents or as contaminated solid tailings. The toxicity of cyanide is related to its speciation. The free cyanide form (HCN, CN-) is classified as the most toxic because of its high metabolic inhibition potential, whereas the metal-cyanide complexes (e.g., [Fe(CN)6]3-, [Fe(CN)6]4-) are considered less toxic (Shifrin et al. 1996). Zagury (2012) showed that the more reactive cyanide species initially associated with solid tailings degrade primarily due to volatilization, leaching and bacterial degradation. The higher proportion of stable cyanide species observed in aged tailings probably results from early dissociation of weak to moderately strong complexes, possibly during weathering (e.g., breakdown in the presence of UV light).
Xanthates are commonly used as collectors of sulphide ores through flotation. Xanthates were reported in effluents and their receiving waters in concentrations up to 4.0 mg/L. These concentrations are sufficient to cause potential toxicity, given that xanthates exhibited toxicity (as measured by IC25) ranging from 0.5 mg/L to 3 mg/L (Vigneault et al. 2012).
Thiosalts (thiosulphate, trithionate, tetrathionate, and other polythionates) are generated as a result of the flotation of sulphidic ore. Vigneault et al. (2012) reported that thiosulphate was generally more toxic than tetrathionate. Sensitivity to thiosalts ranged from no responses for rainbow trout to an IC25 of 59.4 mg S2O3 / L for C. dubia (reproduction). The acute toxicity of thiosulphate to D. magna (lethality) was also greater than tetrathionate (EC50 ~ 300 and 750 mg/L, respectively) (Vigneault et al. 2012). Thiosulphate has been reported in mine effluent concentrations of 700 mg/L, thus in sufficient quantity to cause toxicity (Vigneault et al. 2012).
Selenium is mobilized to nearby watersheds during the mining and smelting of copper, lead, mercury, silver, uranium, and zinc ores. Selenium is a teratogen at elevated levels. Determining the aqueous concentration of selenium in effluents, wastewater and receiving water may determine whether selenium is present at elevated concentrations. Selenium biogeochemistry is complex, however, and more in-depth studies of selenium toxicity in fish tissue may yield more valuable results (Palace 2012).
Other process reagents and wastewater treatments likely to be discharged in concentrations sufficient to cause toxicity include Magnafloc, Nalmet, and lime (Vigneault et al. 2012).
12.10.4 Monitoring of Flow and Loadings in the Exposure Area
During IOC study, measuring water flow in the exposure area could help with data interpretation and understanding of the dilution ratio, mixing, mass balance, fate and effects of contaminants and causal relationships. It may be useful to evaluate the degree of exposure of communities to the mining effluent and its contaminants over a longer period of time and in a variety of conditions.
Assessments of loading rates are essential to mass balance calculations. Assuming complete mixing, the principal statement for mass balance at an industrial outfall is:
Mass or loading rate of substance upstream + Mass rate added by outfall
= Mass rate of substance immediately downstream of the outfall
Since the loading rate is the product of flow and concentration, the mass balance is given by:
Q0s0 + Qese = Qs
Where Q0 , Qe and Q = flow rates upstream, effluent discharge rate, and downstream, respectively; and
s0 , se and s = concentration upstream, in effluent, and downstream, respectively.
A similar statement can be made for the balance of flows:
Q0 + Qe = Q
Upstream conditions of flow and concentrations are often known or can be readily measured. Effluent characteristics are usually known in greater detail. Solving for the downstream concentration, s, yields:
![]()
The downstream concentration is therefore dependent on the upstream and downstream flows and the concentration of upstream and effluent chemical inputs. If the upstream concentration is zero, the downstream concentration, s, will equal the effluent concentration reduced by the ratio of effluent flow to total river flow. This is commonly known as a dilution effect.
The above equations apply to the simplest scenario immediately downstream of the outfall. Mass balance computations become more complex as one moves further downstream of the outfall and contributing factors of tributaries, multiple point sources, distributed sources, as well as dispersion, chemical settling, and chemical decay effects are considered. Useful references for formulating appropriate mass balance equations include Thomann and Mueller (1987), McCutcheon and French (1989), and Henderson-Sellers and French (1991).
12.11 Tools to be Considered for Sediment Analysis
12.11.1 Sediment Monitoring as a Tool for Investigation of Cause
At sites where there are effects in the benthic invertebrate community, there are a number of sediment monitoring techniques that may help in understanding the nature and cause of effects (Parker and Dumaresq 2002). The techniques described below are recommended. Not all of these techniques need to be used; possibly one or few techniques should be applied to investigate the cause of an effect.
12.11.2 Sediment Mass Transport
In certain stream systems, an understanding of sediment mass transport characteristics can be important, particularly to understanding the origin and fate of sediments. The morphology of alluvial stream and river channels with mobile beds is believed to be determined by storm events. Thus, the pool-and-riffle sequence characteristics of such riverbeds, and the corresponding sediment particle size distributions within the channels, may have resident-times that are short relative to the EEM program. In these circumstances, appropriate characterization of stream channel morphology, and in particular of its more dynamic elements, may be important information in determining the cause of effects on benthic invertebrate communities. It may be important to know, for example, whether annual deposition and subsequent removal of fine-grained material is a regular feature of the study area, and whether ecologically significant movement of relatively coarse bed material (gravel and cobbles) is taking place on time scales of a few years or less.
Much of the evidence relating channel morphology to sediment transport and basin hydrology has been gathered in the United States from river basins that yield relatively large amounts of suspended matter. In glaciated regions typical of much of Canada, alluvial sediments in stream channels dominated by material of glacial origin may be more stable in relation to storm events. Further, in the Canadian Shield in particular, the abundance of lakes and wetlands, particularly in first- and second-order catchments, may help to buffer the effects of storm events. Thus, comparison of results with results of studies in the United States may be of limited value.
Direct observations of suspended sediment transport and bedload movement are difficult, because the key events are of short duration and occur at times of stream flows that prevent direct measurement due to hazardous conditions. However, there are a number of qualitative indicators of the dynamic nature of river channels that can be employed. Photographic records of peak run-off events that involve bankfull discharge (e.g., with a return period of the order of a year and greater) can be used to provide qualitative evidence of the importance of suspended sediment transport and deposition. Another method is to identify boulders or cobbles with colour codes, and periodically observing the extent of their displacement. It is suggested that the mine operator use a combination of methods to describe the physical setting of such dynamic river systems.
12.11.3 Sediment Depositional Rate & Sediment Dating for Historical Trends
An understanding of the sediment depositional rate can be important in understanding influences on sediment chemistry, and in particular the relative influence of a mining operation.
Depositional rates may be estimated quantitatively using sediment traps, but representative sampling may be difficult, and it may not be possible, using this method, to account for the impact of storms or other significant hydrological events. Because of these difficulties, direct measurement of sediment deposition rates should be reserved for exceptional circumstances. Long-term depositional rates can also be estimated quantitatively using sediment coring.
The relative influence of the mining operation can also be estimated in a more qualitative manner in gravel-bed streams or in lake sediments dominated by soft organic matter. In such cases, the presence of fine mineral-derived sediments would likely indicate some influence from the mine. In other environment types, this method may not be effective, since the deposition of natural mineral-derived fine sediments would mask any influence from the mine. See Håkanson and Jansson (1983) for guidance on lake sedimentology.
Precise dating of sediments, combined with an inventory of the remains of certain organisms and plant material (e.g., diatoms, zooplankton, insects), provide a chronology of changes that often can be linked to the period of anthropogenic influence (Frey 1998). Isotope dating of sediment cores has been used to assess geochronology of year over year (Weech et al. 2012) (and multiple years to decades) sediment deposition rates to tease out confounding factors, such as historical effects. Isotopes such as carbon-14, uranium-234/238, lead-210, cesium-137 and beryllium-10 can be assessed (Cohen 2003; Ritchie and McHenry 1990; Zapata 2003; Bierman and Nicols 2004; Mabit et al. 2008). See Frey (1998) and Cohen (2003) for detailed information on paleolimnology.
12.11.4 Sediment Coring
Core samplers are often used to collect sediment profiles for the determination of the vertical distribution of sediment characteristics (Parker and Dumaresq 2002). Corers are also generally preferred where maintaining the integrity of the sediment profile is essential, because they are considered to be the least disruptive. Core samplers should be used where it is important to maintain an oxygen-free environment below the surficial sediment, to minimize oxidation (EPS 1/RM/29: Environment Canada 1994). A range of sediment coring devices is available. Although core samplers have the advantage of collecting minimally disturbed, intact sediment samples from surficial sediments (upper 15–30 cm) and deep sediments (> 30 cm deep), there are few that function efficiently in substrates with large amounts of sand, gravel, clay or till. Note that the Environment Canada document Guidance Document on Collection and Preparation of Sediments for Physicochemical Characterization and Biological Testing (EPS 1/RM/29: Environment Canada 1994), and ASTM (1992), contain extensive guidance on sediment coring, including sediment coring procedures, advantages and disadvantages of sediment corers, and transport and manipulation of collected samples.
12.11.5 Sediment Chemistry
12.11.5.1 Partial Metal Concentrations in Sediment (Partial Extraction), Sequentially Extracted Metals, SEM/AVS Ratios
Although total metal concentrations may not be directly related to biological availability and toxicity, many sediment quality guidelines are currently based on total metal concentrations (Parker and Dumaresq 2002). A variety of methods have been used to predict the biological effects of metals from metal-contaminated sediments. These include the normalization of sediments for particle size, organic content, or extractable fraction of metals using AVS (acid volatile sulphides) and SEM (simultaneously extracted metals) (Parker and Dumaresq 2002).
It is generally thought that a particular chemical form of an element determines its behaviour, biological availability and potential toxicity, rather than the total concentration in sediments. Specific chemical forms can be measured in these ways:
- by direct instrument techniques;
- directly by sequential digestion of sediments; or
- by predicting levels through thermodynamic modelling.
Direct instrument techniques include: X-ray photoelectron spectrometry; scanning electron microscopy / X-ray microanalysis; secondary ion mass spectrometry; and Auger electron spectrometry (Parker and Dumaresq 2002). These methods have been applied to geochemical studies and for mineral exploration.
The relative strength of association between metals and particles can be assessed by single or sequential extraction or sediment-digestion methods. Weak acids or chelating agents (e.g., EDTA) and reducing agents may be used to differentiate between different chemical forms. Sediment fractions can be operationally defined (e.g., ferromanganese oxyhydrides) depending on the digestion method used. The recent AVS concept assumes that metal concentrations in pore water of anoxic sediments are controlled by sulphides. AVS are extracted by the cold-acid purge and trap technique. SEMs represent the portion of total metals released during AVS dissolution. The SEM/AVS ratio is sometimes used to characterize metal availability. When the SEM fraction exceeds the AVS fraction (e.g., SEM/AVS ratio > 1), the free metal may be present in the pore water at levels adequate to cause acute toxicity. However, toxicity cannot be predicted; only non-toxicity can be predicted (Parker and Dumaresq 2002).
The limitation of these analyses is that anoxic sediment samples must be carefully collected and stored to prevent oxidation. A description of sediment collection methods can be found in Warren et al. (1998).
12.11.5.2 C/N Ratio for Marine Sediment
Effects on the benthic invertebrate community may occur as a result of organic enrichment in sediments. To determine if organic enrichment is contributing to effects, a combination of measurement techniques should be used in the marine environment. The measurement of TOC provides an indication of organic enrichment. Measuring sediment Eh (redox) provides an indication of the oxygen conditions in marine sediments. Measuring sulphides in the sediment provides an indication as to whether the breakdown of organic sediment material is occurring (Environment Canada 1994).
Measuring the carbon to nitrogen ratio (C/N ratio) in marine sediments should provide an indication of the source of the organic enrichment. If the organic enrichment is a result of land-based sources (e.g., municipal sewage, and pulp and paper effluent), the C/N ratio will be higher (Hargrave et al. 1995). If the organic enrichment is a result of the natural source such as the breakdown of marine aquatic plants, the C/N ratio will be much lower. Therefore, if a benthic invertebrate community study indicates an effect on the benthic invertebrate community, and there is evidence that the effect could be due to organic enrichment (elevated TOC, elevated Eh), determining the marine sediment C/N ratios can help identify the source of the organic loading to that ecosystem.
12.12 Sediment Pore Water Analysis
Fine-grained surface sediments in lakes typically contain 90–95% water (Adams 1991). Some of this water is bound to the crystalline lattice of minerals in the sediments, but most of the water simply occupies the space between sediment particles. This water is referred to as pore or interstitial water. The intimate association of this water with the surface of sediment particles results in reactions between the particles and the water that approach equilibrium. The partitioning of contaminants in sediments between the particulate and water phases depends to a large extent on the amount of organic carbon, sediment particle size, the chemical form of the contaminants, and the physiochemical environment (e.g., pH, temperature, redox potential, sorption/desorption properties of sediments, or the equilibrium between the solid and liquid phases) (Parker and Dumaresq 2002). The dynamics of these processes are not well understood; however, it is generally assumed that concentrations of most substances in the pore water approach equilibrium with the solid phase and its associated contaminants, and that metals in pore water largely represent the biologically available fraction in sediments (Parker and Dumaresq 2002). Consequently, pore water has been collected for toxicity testing to approximate the relative toxicity of contaminated sediment, and/or to assess contaminant levels.
The nature of sediments at the study site can largely influence the usefulness of pore water measurements. Sediments that are either very coarse-grained, or hard, compacted clays, will not likely have pore waters that are significantly contaminated (Burton and Pitt 2002). Therefore, sampling of pore waters should be restricted to sediments ranging from sandy to non-compacted clays.
If sediments are anoxic (most depositional sediments are below 2 cm in depth), all steps involved in sample collection and processing should be conducted in an inert atmosphere or with limited exposure to prevent oxidation and subsequent sorption/precipitation of reduced metal species if metal speciation is of interest. When anoxic sediments are exposed to air, volatile sulphides may also be lost, which may increase the availability (and toxicity) of sulphide-bound metals. Finally, pore water samples undergo rapid chemical changes, giving a storage life of only hours to days. A common device for sampling sediment pore water is the dialysis cell, also known as a peeper (Doig and Liber 2000). Field collection using peepers or suction devices are the most accurate methods to obtain representative samples, because it is less likely to alter the in situ chemistry of the pore water and is recommended for geochemical investigations (Burton and Pitt 2002; US EPA 2001). Laboratory methods that allow for extraction of greater volumes of water are preferred when samples are being collected for toxicity testing, including centrifugation, pressurization or suction. The use and advantages of minipeepers for laboratory sediment toxicity tests are discussed in Doig and Liber (2002).
For information on field (in situ) and laboratory methods for collection of pore water, see Environment Canada’s Guidance Document on Collection and Preparation of Sediments for Physiochemical Characterization and Biological Testing (EPS 1/RM/29), Environment Canada (1994), US EPA (2001), Doig and Liber (2002), and Burton and Pitt (2002).
For toxicity tests on pore water, see section 12.9.6.
12.13 References
Adams DD. 1991. Sampling Sediment Pore Water, pp. 203-202, in: CRC Handbook of Techniques for Aquatic Sediments Sampling, Mudroch A and MacKnight SD (eds.) CRC Press, Inc. Boca Raton, FL, 210 p.
Adams WU, Kimerle RA and Mosher RG. 1985. Aquatic Safety Assessment of Chemicals Sorbed to Sediments, pp. 429-453, in: Aquatic Toxicology and Hazard Assessment, Cardwell RD, Purdy R and Bahner RC (eds.), American Society for Testing and Materials, Philadelphia, PA.
Adams WJ, Blust R, Borgmann U, Brix KV, DeForest DK, Green AS, Meyer JS, McGeer JC, Paquin PR, Rainbow PS and Wood CM. 2011. Utility of tissue residues for predicting effects of metals on aquatic organisms. Integr. Environ. Assess. Manag. 2011: 7(1):75-98.
Alden III RW. 1992. Uncertainty and Sediment Quality Assessments: I. Confidence limits for the triad. Env. Tox. Chem. 11: 637-644.
Allison JD, Brown DS and Novo-Gradac KJ. 1991. MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: version 3.0 user’s manual. U.S. Environmental Protection Agency, Athens, GA. EPA/600/3-91/021.
Aloi JE. 1990. A critical review of freshwater periphyton field methods. Canadian Journal of Fisheries and Aquatic Sciences 47(3): pp. 656-670.
American Public Health Association (APHA), American Water Works Association (AWWA), and the Water Environment Federation (WEF). 2001. Standard Methods for the Examination of Water and Wastewater, Method 10200H (Chlorophyll), 2001.
Ankley GT, Schubauer-Berigan MK and Dierkes JR. 1991. Predicting the Toxicity of Bulk Sediments to Aquatic Organisms with Aqueous Test Fractions: Pore Water Versus Elutriate, Environ. Toxicol. Chem. 10: 1359-1366.
Aquatic Effects Technology Evaluation (AETE) Program. 1997. Technical Evaluation of Monitoring Methods using Macrophytes, Phytoplankton, and Periphyton to Assess the Impacts of Mine Effluents on the Aquatic Environment. AETE Project 2.3.2.
Aquatic Toxicity Workshop (ATW). October 2-5, 2011, Winnipeg, Manitoba, Canada.
Austin A. 1983. Evaluation of changes in a large oligotrophic wilderness park lake exposed to mine tailings effluent for 14 years: The periphyton. Natur. Can. 110:119-134.
ASTM (American Society for Testing and Materials). 1992. E 1391-90, Standard Guide for Collection, Storage, Characterization and Manipulation of Sediments for Toxicological Testing, pp. 1134-1153, in: 1992 Annual Book of ASTM Standards, Vol. II.04, Section 11, Philadelphia, PA.
ASTM (American Society for Testing and Materials). 1997. Standard test methods for measuring the toxicity of sediment-associated contaminants with fresh water invertebrates (Annex 3, Hexagenia spp., and Annex 4, Tubifex tubifex). Volume 11.05, Standard E1706-95b.
ASTM (American Society for Testing and Materials). 2010. Standard Test Method for Measuring the Toxicity of Sediment-Associated Contaminants with Freshwater Invertebrates. ASTM Method E 1706-05(2010).
Austin A. 1993. Evaluation of changes in a large oligotrophic wilderness park lake exposed to mine tailing effluent for 14 years: The periphyton. Natur. Can. 110: 119-134.
Austin A and Deniseger J. 1985. Periphyton community changes along a heavy metal gradient in a long narrow lake. Environ. Exp. Bot. 25: 41-52.
Austin A, Deniseger J and Clark MJR. 1985. Lake algal populations and physico-chemical changes after 14 years input of metallic mining wastes. Water Res. 19: 299-308.
Azim ME, Verdegem MCJ, van Dam AA and Beveridge MCM. 2005. Periphyton. Ecology, Exploitation and Management. CABI Publishing, Cambridge, MA.
Bandler C, Baron CL and Palace VP. 2012. An update on the use of caged fish for Environmental Effects Monitoring, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Bedard D, Hayton A and Persaud D. 1992. Ontario Ministry of Environment laboratory sediment biological testing protocol. Water Resources Branch, Ontario Ministry of Environment, Toronto, ON, 26 pp.
Beyers DW. 1998. Causal inference in environmental impact studies. J. N. Am. Benthol. Soc. 17: 367-373.
Bhavsar SP, Gandhi N and Diamond ML. 2008. Extension of coupled multispecies metal transport and speciation (TRANSPEC) model to soil. Chemosphere: 914-924.
Bierman PR and Nichols KK. 2004. Rock to sediment - slope to sea with 10Be - rates of landscape change. Annual Review of Earth and Planetary Sciences; 32 Palo Alto: Annual Reviews Inc., 2004, 215-255.
Biggs BJF and Kilroy C. 2000. Stream Periphyton Monitoring Manual. Prepared for the New Zealand Ministry of Environment. National Institute of Water and Atmospheric Research, Christchurch, NZ.
Borgmann U, Norwood WP and Babirad IM. 1991. Relationship between chronic toxicity and bioaccumulation of cadmium in Hyalella azteca. Can. J. Fish. Aquat. Sci. 48: 1055–1060.
Borgmann U and Norwood WP. 1997a. Toxicity and accumulation of zinc and copper in Hyalella azteca exposed to metal-spiked sediments. Can. J. Fish. Aquat. Sci. 54: 1046-1054.
Borgmann U and Norwood WP. 1997b. Identification of the toxic agent in metal-contaminated sediments from Manitouwadge Lake, Ontario, using toxicity-accumulation relationships in Hyalella azteca. Can. J. Fish. Aquat. Sci. 54: 1055-1063.
Borgmann U and Norwood WP. 1999. Assessing the toxicity of lead in sediments to Hyalella azteca: the significance of bioaccumulation and dissolved metal. Can. J. Fish. Aquat. Sci. 56: 1494–1503.
Borgmann U. 2000. Methods for assessing the toxicological significance of metals in aquatic ecosystems: Bioaccumulation-toxicity relationships, water concentrations, and sediment spiking approaches. Aquat. Ecosyst. Health Management 3(3): 277-289.
Borgmann U and Norwood WP. 1999a. Predicting the toxicity of lead in sediments to Hyalella azteca: the significance of bioaccumulation and dissolved metal. Can. J. Fish. Aquat. Sci. 56: 1494-1503.
Borgmann U and Norwood WP. 1999b. Sediment toxicity testing using large water-sediment ratios: an alternative to water renewal. Environ. Poll. 106: 333-339.
Borgmann U. 2002. Toxicity test methods and observations using the freshwater amphipod, Hyalella. Environment Canada, National Water Research Institute, Burlington/Saskatoon, NWRI Contribution No. 02-332.
Borgmann U, Norwood WP, Reynoldson TB and Rosa F. 2001. Identifying cause in sediment assessments: Bioavailability and the sediment quality triad. Can. J. Fish. Aquat. Sci. 58: 950–960.
Borgmann U, Norwood WP and Dixon DG. 2004. Re-evaluation of metal bioaccumulation and chronic toxicity in Hyalella azteca using saturation curves and the biotic ligand model. Environ. Pollut. 131(3): 469-484.
Borgmann U, Grapentine L, Norwood WP, Bird G, Dixon DG and Lindeman D. 2005a. Sediment toxicity testing with the freshwater amphipod Hyalella azteca: relevance and application. Chemosphere 61 (11): 1740-1743.
Borgmann U, Ingersoll CG, Mathyk S and Lennie-Misgeld P. 2005b. Draft biological test method: Test for survival in 10-day water–only exposures using the freshwater amphipod, H. azteca, with emphasis on detection of toxicity due to ammonia.
Borgmann U, Ingersoll CG, Mathyk S and Lennie-Misgeld P. 2005c. Draft biological test method: Test for survival in 10-day water–only exposures using the freshwater amphipod, H. azteca, with emphasis on detection of toxicity due to ammonia.
Borgmann U, Couillard Y and Grapentine LC. 2007. Relative contribution of food and water to 27 metals and metalloids accumulated by caged Hyalella azteca in two rivers affected by metal mining. Environmental Pollution 145: 753-765.
Bosker T and Munkittrick K. 2009. Often overlooked: Biological QA/QC. Integrated Environmental Assessment and Management 5(3): 489-91.
Brodeur JC, Sherwood G and Hontela A. 1997. Impaired cortisol secretion in yellow perch (Perca flavescens) from lakes contaminated by heavy metals: in vivo and in vitro assessment. Can. J. Fish. Aquat. Sci. 54: 2752–2758.
Brodeur JC, Daniel C, Ricard AC and Hontela A.1998. In vitro response to ACTH of the interrenal tissue of rainbow trout (Oncorhynchus mykiss) exposed to cadmium. Aquat. Toxicol. 42: 103–113.
Burton GA Jr. (ed.). 1992. Sediment Toxicity Assessment, Lewis Publishers Inc. Chelsea, MI, 457 pp.
Burton GA. 1998. Assessing aquatic ecosystems using pore water and sediment chemistry. Report prepared for AETE program, CANMET, Natural Resources Canada, Ottawa, Ontario.
Burton GA and Pitt RE. 2002. Stormwater Effects Handbook. A Toolbox for Watershed Managers, Scientists, and Engineers. CRC Press, Boca Raton, Florida. 875 pp.
Cairns MA, Nebeker AV, Gakstatter JH and Griffis WL. 1984. Toxicity of Copper-spiked Sediments to Freshwater Invertebrates, Environ. Toxicol. Chem. 3: 435-445.
Campbell P and Torgerson T. 1980. Maintenance of iron meromixis by iron redeposition in a rapidly flushed monimolimnion. Can. J. Fish. Aquat. Sci. 37(8): 1303-1313.
Campbell PGC. 1995. Interactions between trace metals and organisms: a critique of the free-ion activity model. In Metal Speciation and Bioavailability in Aquatic Systems. Edited by Tessier A and Turner DJ. Wiley & Sons, Chichester, UK, pp. 45-102.
Campbell PGC. 2012. Ecotoxicology of trace metals in the aquatic environment, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Campbell PGC, Kraemer LD, Giguère A, Hare L and Hontela A. 2008. Subcellular distribution of cadmium and nickel in chronically exposed wild fish: inferences regarding metal detoxification strategies and implications for setting water quality guidelines for dissolved metals. Human Ecol. Risk Assess. 14: 290-316.
Campbell PGC and Hare L. 2009. Metal detoxification in freshwater animals. Roles of metallothioneins. In Metallothioneins and Related Chelators. Edited by Sigel A, Sigel H and Sigel RKO. Royal Society of Chemistry, Cambridge, UK, pp. 239-277.
Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33.
Canfield TJ, Kemble NE, Brumbaugh WG, Dwyer FJ, Ingersoll CG and Fairchild JF. 1994. Use of Benthic Invertebrate Community Structure and the Sediment Quality Triad to Evaluate Metal-Contaminated Sediment in the Upper Clark Fork River, Montana. Environmental Toxicology and Chemistry 13(12): 1999-2012.
Carr RS and Chapman DC. 1992. Comparison of Solid-phase and Pore-water Approaches for Assessing the Quality of Marine and Estuarine Sediments. Chem. Ecol. 7: 19-30.
Cattaneo A and Kalff J. 1980. The relative contribution of aquatic macrophytes and their epiphytes to the production of macrophyte beds. Limnol. Oceanogr. 25: 280-289.
[CCME] Canadian Council of Ministers of the Environment. 1999. Canadian environmental quality guidelines. Chapter 4: Canadian water quality guidelines for the protection of aquatic life. Hull (QC): Canadian Council of Ministers of the Environment. Available from: http://ceqg-rcqe.ccme.ca/
[CCME] Canadian Council of Ministers of the Environment. 2007. A protocol for the derivation of water quality guidelines for the protection of aquatic life. Canadian Council of Ministers of the Environment, Winnipeg, MN.
Chambers PA, Guy MA, Roberts ES, Charlton MN, Kent R, Gagnon C, Grove G and Foster N. 2001. Nutrients and their impact on the Canadian environment. Ottawa (ON): Agriculture and Agri-Food Canada, Environment Canada, Fisheries and Oceans Canada, Health Canada and Natural Resources Canada. 241 p.
Chambers PA, Culp JM, Glozier NE, Cash KJ, Wrona FJ, Norton L. 2006. Northern Rivers Ecosystem Initiative: Nutrients and Dissolved Oxygen--issues and impacts. Environmental Monitoring and Assessment 113(1-3): 117-142.
Chapman PM. 1992. Sediment Quality Triad Approach. US EPA Sediment Classification Methods Compendium. Office of Science and Technology, Washington, DC. 10-1-10-18.
Chapman PM and Hollert H. 2006. Should the Sediment Quality Triad become a tetrad, a pentad, or possibly even a hexad? Journal of Soils and Sediments 6(1): 4-8.
Chessman BC. 1985. Artificial-substratum periphyton and water quality in the lower La Trobe River, Victoria. Aust. J. Mar. Freshwater. Res. 36:855-871.
Clements WH. 1991. Community responses of stream organisms to heavy metals: a review of observational and experimental approaches, in: Metal Ecotoxicology. Concepts and applications. Edited by MC Newman, AW McIntosh, Lewis Publ. Chelsea, Michigan, pp. 363-391.
Clements WH. 2004. Small-scale experiments support causal relationships between metal contamination and macroinvertebrate community responses. Ecological Applications 14: 954-957.
Clements WH and Kiffney DM. 1994. Integrated laboratory and field approach for assessing impacts of heavy metals at the Arkansas River, Colorado. Environ. Toxicol. Chem. 13: 397-404.
Cohen AS. Paleolimnology: The history and Evolution of Lake Systems. 2003. Oxford University Press. New York, NY, 509 pp.
Costa PM, Diniz MS, Caeiro S, Lobo J, Martins M, Ferreira AM, Caetano M, Vale C, DelValls TA and Costa MH. 2009. Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: a weighted indices approach. Aquatic Toxicology 92 (3): 202-212.
Couillard Y. 1997. With the collaboration of L. St-Cyr. Technical evaluation of metallothionein as a biomarker for the mining industry. Technical report for the Aquatic Effects Technology Evaluation (AETE) program, AETE Project 2.2.1, prepared for Natural Resources Canada, Canada Centre for Mineral and Energy Technology (CANMET). 191 pp.
Couillard Y, Giguère A, Campbell PGC, Perceval O, Pinel-Alloul B and Hare L. 1999. Field evaluation of the use of metallothionein as a biomarker for metal contamination and toxic effects in the freshwater bivalve Pyganodon grandis: Subcellular metal partitioning. SETAC 20th Annual Meeting, 14-18 November 1999, Philadelphia, PA.
Couillard Y, Grapentine LC, Borgmann U, Doyle P and Masson S. 2008. The amphipod Hyalella azteca as a biomonitor in field deployment studies for metal mining. Environmental Pollution 156: 1314-1324.
Courtenay LA. 2002. Quantifying impacts of pulp mill effluent on fish in Canadian marine and estuarine environments: problems and progress. Water Qual. Res. J. Can. 37: 79-99.
Courtney LA and Clements WH. 2002. Assessing the influence of water and substratum quality on benthic macroinvertebrate communities in a metal-polluted stream: an experimental approach. Freshwater Biology 47(9): 1766-1779.
Crawford JK, Luoma SN. 1993. Guidelines for studies of contaminants in biological tissues for the National Water– Quality Assessment Program. U.S. Geological Service Open-File Rep. No. 92-494. Denver, CO.
Culp JM. 1999. Weight-of-Evidence Assessments for Large Rivers: Linking Community Bioassays with Field and Laboratory Studies. Workshop on Integrated Approaches for Interpreting Environmental Effects Monitoring Data. DOE EEM/1999/1.
Culp JM, Podemski CL, Cash KJ and Lowell RL. 1996. Utility of field-based artificial streams for assessing effluent effects on riverine ecosystems. J. Aquat. Ecosystem Health 5: 117-124.
Culp JM, Lowell RB and Cash KJ. 2000. Integrating in situ community experiments with field studies to generate weight-of-evidence risk assessments for large rivers. Environ. Toxicol. Chem. 19(4): 1167-1173.
Deaver E and Rodgers JH Jr. 1996. Measuring bioavailable copper using anodic stripping voltammetry. Environ. Toxicol. Chem. 15: 1925-1996.
Deutsch Forschungsgemeinshaft (German Research Council). 1998. Proposals for Safeguarding Good Scientific Practice. Wiley-VCH, Verlag GmbH, D-69469Weinheim (Federal Republic of Germany). 85 pp.
Di Toro DM. 1989. A Review of the Data Supporting the Equilibrium Partitioning Approach to Establishing Sediment Quality Criteria, report to the National Research Council (as cited in Baudo 1990).
Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR and Santore RC. 2000. The Biotic Ligand Model: A Computational Approach for Assessing the Ecological Effects of Metals in Aquatic Systems, published by the International Copper Association, Ltd., Environmental Program, as part of its series Copper in the Environment and Health.
Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR and Santore RC. 2001. A Biotic Ligand Model of the Acute Toxicity of Metals. I. Technical Basis, Environmental Toxicology and Chemistry 20(10): 2383-2396.
Dodds WK. 2006. Eutrophication and trophic state in rivers and streams. Limnology and Oceanography 51 (1, part 2): 671-680.
Dodds WK and Welch EB. 2000. Establishing nutrient criteria in streams. J. N. Am. Benthol. Soc. 19(1): 186-196.
Doig L and Liber K. 2000. Dialysis minipeeper for measuring pore-water metal concentrations in laboratory sediment toxicity and bioavailability tests. Environmental Toxicology and Chemistry 19 (12): 2882-2889.
Ekberg C and Ödegaard-Jensen A. 2011. Uncertainties in chemical modelling of solubility, speciation and sorption. Accreditation and Quality Assurance 16 (4/5), Heidelberg: Springer Berlin, 2011, 207-214.
Environment Canada. 1990 (amended 1996). Biological Test Method: Acute Lethality Test Using Daphnia spp., Environmental Protection Series Report, EPS 1/RM/11, Ottawa, Ontario, 57 pp.
Environment Canada. 1992. Biological Test Method: Fertilization Assay Using
Echinoids (Sea Urchins and Sand Dollars), Environmental Protection Series Report, EPS 1/RM/27, Ottawa, Ontario, 97 pp.
Environment Canada. 1994. Guidance Document on Collection and Preparation of Sediments for Physiochemical Characterization and Biological Testing, Environmental Protection Series Report EPS 1/RM/29. Ottawa.
Environment Canada. 1996. Biological Test Method: Acute Lethality Test Using Daphnia spp., Environmental Protection Series Report, EPS 1/RM/11, July 1990 with May 1996 amendments. Ottawa, Ontario, 75 pp.
Environment Canada. 1997a. Test for Growth and Survival in Sediment Using the Freshwater Amphipod Hyalella azteca, Environmental Protection Series Report, EPS 1/RM/33, Ottawa, Ontario, 123 pp.
Environment Canada. 1997b. Test for Growth and Survival in Sediment Using Larvae of Freshwater Midges (Chironomus tentans, Chironomus riparius), Environmental Protection Series Report, EPS 1/RM/32, Ottawa, Ontario, 131 pp.
Environment Canada. 2007. Criteria and Guidance for Determination of Pronounced Eutrophication. National Environmental Effects Monitoring Office, Environment Canada.
Environment Canada. 2010. Pulp and Paper Environmental Effects Monitoring Technical Guidance Document--Chapter 11 Investigation of Cause and Investigation of Solutions. Environment Canada, 40 pp.
Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, Canada, ISBN: 978-1-100-19968-9.
Environment Canada. 2012. Second National Assessment of Environmental Effects Monitoring Data From Metal Mines subjected to the Metal Mining Effluent Regulations. Environment Canada, 66 pp.
ESG International Inc. (ESG). 1999. AETE Synthesis Report of Selected Technologies for Cost-Effective Environmental Monitoring of Mine Effluent Impacts in Canada, AETE Project 4.1.4, March 1999, CANMET, Natural Resources Canada, Ottawa, Ontario.
EVS Environmental Consultants. 1997. Technical evaluation: water quality and biological effects. Report for AETE program, CANMET, Natural Resources Canada, Ottawa, Ontario.
Fåhræus-van Ree GE and Payne JF. 2005. Endocrine disruption in the pituitary of white sucker (Catostomus commersoni) caged in a lake contaminated with iron-ore mine tailings. Hydrobiologia 532: 221-224.
Farley KJ, Rader KJ and Miller BE. 2008. Tableau Input Coupled Kinetic Equilibrium Transport (TICKET) Model. Environmental Science & Technology 42(3): 838-847.
Fox GA. 1991. Practical causal inference for ecoepidemiologists. J. Toxicol. Environ. Health 33: 359-379.
Frey DG. 1998. What is paleolimnology? J. Paleolimnology 1: 5-8.
Gibbons WN and Munkittrick KR. 1994. A sentinel monitoring framework for identifying fish population responses to industrial discharges. J. Aquat. Eco. Health 3: 227-237.
Giesy JP and Hoke RA. 1990. Freshwater Sediment Quality Criteria: Toxicity Bioassessment, pp. 265-348, in: Sediments: Chemistry and Toxicity of In-place Pollutants, Baudo R, Giesy JP and Muntau H (eds.), Lewis Publishers, Inc. Chelsea, MI, 405 p.
Giguère A, Couillard Y, Perceval O, Campbell PGC, Pellerin-Massicotte J and Pinel-Alloul B. 1999. Field evaluation of the use of metallothionein as a biomarker for metal contamination and toxic effects in the freshwater bivalve Pyganodon grandis: Responses at the organism level. SETAC 20th Annual Meeting, 14-18 November 1999, Philadelphia. PA.
Gilbertson M. 1997. Advances in forensic toxicology for establishing causality between Great Lakes epizootics and specific persistent toxic chemicals. Environ. Toxicol. Chem. 16: 1771-1778.
Gillis PL, Dixon DG, Reynoldson TB and Diener LC. 1999. Metallothionein as a biomarker for trace metal exposure and effects in Tubifex tubifex and Chironomus riparius. SETAC 20th Annual Meeting, 14-18 November 1999, Philadelphia, PA.
Grapentine LC. 2012. In situ measurements of toxicity and contaminant bioaccumulation with caged amphipods exposed to water and sediment for Investigation of Cause in metal mining Environmental Effects Monitoring studies in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Gustafson JP. 2004. Visual MINTEQ, version 2.30; a Windows version of MINTEQA2, version 4.0.
Håkanson L and Jansson M. 1983. Principles of Lake Sedimentology, Springer-Verlag, Berlin, GD, 316 pp.
Hall GEM. 1998. Cost-effective protocols for the collection, filtration and preservation of surface waters for detection of metals and metalloids at ppb ad ppt levels. Report for the AETE program, CANMET, Natural Resources Canada, Ottawa, Ontario.
Hargrave BT, Phillips GA, Doucette LI, White MJ, Milligan TG, Wildish DJ and Cranston RE. 1995. Biogeochemical observations to assess benthic impacts of organic enrichment from marine aquaculture in the Western Isles region of the Bay of Fundy, Can. Tech. Rep. Fish. Aquat. Sci. No. 2062, 159 pp.
Harkey GA, Landrum PF and Kaine SJ. 1994. Comparison of Whole-sediment, Elutriate and Pore-water Exposures for Use in Assessing Sediment-associated Organic Contaminants in Bioassays, Environ. Toxicol. Chem. 13(8): 1315-1329.
Haslam SM. 1982. A proposed method for monitoring river pollution using macrophytes. Environ. Technol. Letters 3: 19-34.
Hellawell JM. 1986. Biological indicators of freshwater pollution and environmental management. Elsevier Applied Science Publishers, London, 546 pp.
Henderson-Sellers B and French RH. 1991. Water Quality Modelling Volume IV:
Decision Support Techniques for Lakes and Reservoirs. CRC Press, Boca Raton,
Florida.
Henry CJ, Higgins KF and Buhl KJ. 1994. Acute toxicity and hazard assessment of Rodeo®, X-77 Spreader®, and Chem-Trol® to aquatic invertebrates. Arch. Environ. Contam. Toxicol. 27: 392-399.
Herbelin AL and Westall JC. 1999. FITEQL, A Computer Program for Determination of Chemical Equilibrium Constants from Experimental Data. Report 99-01, Version 4.0. Oregon State University, Corvallis, OR.
Hewitt ML and Servos MR. 2001. An Overview of Substances Present in Canadian Aquatic Environments Associated with Endocrine Disruption. Water Qual. Res. J. Canada 36(2): 191–213.
Hewitt ML, Dube MG, Culp JM, MacLatchy DL and Munkittrick KR. 2003. A proposed framework for investigation of cause for environmental effects monitoring. Human and Ecological Risk Assessment 9(1): 195-211.
Hinton DE, David E, Darrel JL and Adams SM. 1990. Integrative histopathological approaches to detecting effects of environmental stressors on fishes. American Fisheries Society. Symposium: Biological indicators of stress in fish No. 8, pp. 51-66. 1990. FR 36(1).
Hinton DE, Kullman SW, Bencic DC, Hardman RC, Chen PJ, Carney M and Volz DC. 2005. Resolving mechanisms of toxicity while pursuing ecotoxicological relevance. Marine Pollution Bull. 51: 635-648.
Hodson PV. 1990. Indicators of ecosystem health at the species level and the example of selenium affects on fish. Environmental Monitoring and Assessment 15(3): 241-255.
Holm J, Palace VP, Siwik P, Sterling G, Evans RE, Baron CL, Werner J and Wautier K. 2005. Developmental effects of bioaccumulated selenium in eggs and larvae of two salmonid species. Environ. Toxicol. Chem. 24: 2373-2381.
Hontela A, Ramussen JB, Audet C and Chevalier G. 1992. Impaired cortisol stress response in fish from environments polluted by PAHs, PCB, and mercury, Arch. Environ. Contam. Toxicol. 22: 278–283.
Ingersoll CG, Ivey CD, Brunson EL, Hardesty DK and Kemble NE. 2000. Evaluation of toxicity: Whole-sediment versus overlying-water exposures with amphipod H. azteca. Environmental Toxicology and Chemistry 19: 2906-2910.
International Agency for Research on Cancer. 2008. Code of Good Scientific Practice. World Health Organization, Geneva, Switzerland, 11 pp.
Kelly M. 1988. Mining and the freshwater environment. Elsevier Applied Science, London, UK, 231 pp.
Knezovich JP and Harrison FL. 1987. The Bioavailability of Sediment-sorbed Organic Chemicals: A Review, Water Air Soil Pollut. 32: 233-245.
Knezovich JP and Harrison FL. 1988. The Bioavailability of Sediment-sorbed Chlorobenzenes to Larvae of the Midge, Chironomous decorus, Ecotoxicol. Environ. Safety 15: 226-241.
Kutka FJ and Richards C. 1996. Relating diatom assemblage structure to stream habitat quality. J.N. Am. Benthol. Soc. 15: 469-480.
Laflamme JS, Couillard Y, Campbell PGC and Hontela A. 1998. Impaired physiological response to stress and high levels of metallothionein in perch (Perca flavescens) from lakes contaminated by heavy metals. 25th Annual Aquatic Toxicity Workshop, 18- 21 October 1998, Quebec City, QC.
Langmuir D, Chrostowski P, Vigneault B and Chaney R. Issue Paper on the Environmental Chemistry of Metals. Submitted to: U.S. Environmental Protection Agency Risk Assessment Forum. Submitted by: ERG 110 Hartwell Avenue Lexington, MA.
Ledo H, Rivas Z, Gutierrez J, Gutierrez E, Ojeda J and Avila H. 2004. Baseline of Ca, Mg, Fe, Mn, and Al concentrations in Catatumbo River Surficial sediments. Water, Air, and Soil Pollution 155: 117–135.
Liber D and Liber K. 2000. Dialysis minipeeper for measuring pore-water metal concentrations in laboratory sediment toxicity and bioavailability tests. Environ. Toxicol. Chem. 19 (1): 2882-2889.
Liber K, Doig LE and White-Sobey SL. 2011. Toxicity of uranium, molybdenum, nickel, and arsenic to Hyalella azteca and Chrionomus dilutus in water-only and spiked-sediment toxicity tests. Ecotox. Environ. Saf. 74: 1171-1179.
Lowe RL and Pank Y. 1996. Benthic algal communities as biological monitors, in: Algal ecology. Freshwater benthic ecosystem. Edited by Stevenson RJ, Bothwell ML and Lowe RL. Academic Press, San Diego, CA.
Lowell RB, Culp JM and Dube MG. 2000. A weight-of evidence approach for northern river risk assessment: integrating the effects of multiple stressors. Environ. Toxicol. Chem. 19(4): 1182-1190.
Lowell RB, Tessier C, Walker SL, Willsie A, Bowerman M and Gautron D. 2007. National Assessment of Phase 1 Data from the Metal Mining Environmental Effects Monitoring Program. National Environmental Effects Monitoring Office, Environment Canada, Gatineau, QC, 45 pp.
Luoma SN and Rainbow PS. 2008. Metal contamination in aquatic environments: science and lateral management. Cambridge University Press, Cambridge, UK.
Mabit L, Benmansour M and Walling DE. 2008. Comparative advantages and limitations of the fallout radionuclides 137Cs, 210Pbex and 7Be for assessing soil erosion and sedimentation. Journal of Environmental Radioactivity 99 (12): 1799-1807.
Martel PH, Kovacs TG, O’Connor BL and Voss RH. 1997. Source and identity of compounds in a thermomechanical pulp mill effluent inducing hepatic mixed-function oxidase activity in fish. Environ Toxicol. Chem. 16: 2375-83.
McCutcheon SC and French RH. 1989. Water Quality Modelling Volume I: Transport
and Surface Exchange in Rivers. CRC Press, Boca Raton, Florida.
McGeer JC, Playle RC, Wood CM and Galvez F. 2000. A Physiologically Based Biotic Ligand Model for Predicting the Acute Toxicity of Waterborne Silver to Rainbow Trout in Freshwaters. Environmental Science and Technology 34: 4199-4207.
McGeer J, Henningsen G, Lanno R, Fisher N, Sappington K and Drexler J. 2004. Issue paper on the bioavailability and bioaccumulation of metals.
McGeer JC, Clifford M, Janssen CR and De Schamphelaere KAC. 2010. Modeling the toxicity of metals to aquatic biota using the biotic ligand approach, pp. 205-231 in: Essential Reviews in Experimental Biology Vol. 2. Surface Chemistry, Bioavailability and Metal Homeostasis in Aquatic Organisms: an Integrated Approach. Edited by Bury NR and Handy RD. SEB Press, London, UK.
McGeer J, Clifford M, Ng T and Wood C. 2012. Using bioaccumulation models for predicting dissolved metal toxicity, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Meador J, Parkerton T and McElroy A. 2011. Tissue residue approach to toxicity assessment: A step forward. Society of Environmental Toxicology and Chemistry.
Meyer JS, Santore RC, Bobbitt JP, DeBrey LD, Boese CJ, Paquin PR, Allen HE, Bergman HL and Di Toro DM. 1999. Binding of nickel and copper to fish gills predicts toxicity but free ion activity does not. Environ. Sci. Tech. 33: 913-916.
[MOEE] Ontario Ministry of Environment and Energy. 1997. Sediment and biological assessment of Canagaigue Creek at the Uniroyal Chemical Ltd. Plant, Elmira, Ontario. 1995-1996. Ministry of Environment and Energy, 37 pp. + appendices.
Muir DCG, Kenny DF, Grift N, Robinson RD, Titman RD and Murkin HR. 1991. Fate and acute toxicity of bromoxynil esters in an experimental prairie wetland. Environ. Toxicol. Chem. 10: 395-406.
Mulliss RM, Revitt DM and Shutes RBE. 1996. A statistical approach for the assessment of the toxic influences on Gammarus pulex (Amphipoda) and Asellus aquaticus (Isopoda) exposed to urban discharges. Wat. Res. 30: 1237-1243.
Munkittrick KR, Portt CB, Van Der Kraak GJ, Smith IR and Rokosh DA. 1991. Impact of bleached kraft mill effluent on population characteristics, liver MFO activity, and serum steroid levels of a Lake Superior white sucker (Catostomus commersoni) population. Can. J. Fish Aquat. Sci. 48: 1371-1380.
Munkittrick KR, Van Der Kraak GJ, McMaster ME, Portt CB, van den Heuvel MR and Servos MR. 1994. Survey of receiving-water environmental impacts associated with discharges from pulp mills. 2. Gonad size, liver size, hepatic EROD activity and plasma sex steroid levels in white sucker. Environ. Toxicol. Chem. 13: 1089-1101.
Munkittrick KR, McMaster ME, Van Der Kraak G, Portt C, Gibbons WN, Farwell A and Gray M. 2000. Development of Methods for Effects-Driven Cumulative Effects Assessment Using Fish Populations: Moose River Project. The Society of Environmental Toxicology and Chemistry, Pensacola, FL, 236 pp.
Nebeker AV, Cairns MA, Gakstatter JH, Malueg KW, Schuytema GS and Krawczyk DR. 1984. Biological Methods for Determining Toxicity of Contaminated Freshwater Sediments to Invertebrates, Environ. Toxicol. Chem. 3: 617-630.
Niyogi S and Wood CM. 2004. The Biotic Ligand Model, a flexible tool for developing site-specific water quality guidelines for metals. Environ. Sci. Technol.: 6177-6192.
North America Metals Council. 2008. Selenium Tissue Thresholds: Tissue Selection Criteria, Threshold Development Endpoints, and Potential to Predict Population or Community Effects in the Field. 178 pp.
Norwood WP, Borgmann U and Dixon DG. 2007. Chronic toxicity of arsenic, cobalt, chromium and manganese to Hyalella azteca in relation to exposure and bioaccumulation. Environmental Pollution 147: 262-272.
Novak LJ and Holtze LE. 2012. Toxicity Reduction Evaluations (TREs) as a Tool for Investigation of Cause--Mining Case Studies, in: Environment Canada. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Nowierski M, Dixon DG and Borgmann U. 2005. Effects of water chemistry on the bioavailability of metals in sediment to Hyalella azteca: Implications for sediment quality guidelines. Arch. Environ. Contam. Toxicol. 49: 322–332.
[OECD] Organisation for Economic Co-operation and Development, Global Science Forum. 2007. Best Practices for Ensuring Scientific Integrity and Preventing Misconduct. Organisation for Economic Co-operation and Development, Paris, France, 13 pp.
Palace VP, Doebel C, Baron CL, Evans RE, Wauiter KG, Klaverkamp JF, Werner J and Kollar S. 2005. Caging small bodied fish as an alternative method for environmental effects monitoring (EEM). Can. Wat. Qual. Res. J. 40: 328-333.
Palace VP. 2012. Investigating Selenium Toxicity Using an Environmental Effects Monitoring Approach, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Paquin PR, Di Toro DM, Santore RC, Trivedi D and Wu KB. 1999. A Biotic Ligand Model of the Acute Toxicity of Metals. III. Application to Fish and Daphnia Exposure to Silver, Section 3 in Integrated Approach to Assessing the Bioavailability and Toxicity of Metals in Surface Waters and Sediments, a submission to the EPA Science Advisory Board, Office of Water, Office of Research and Development, Washington, DC, pp. 3-59 to 3-102. US EPA-822-E-99-001.
Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PGC, Delos CG, Di Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hogstrand C, Janssen CR, McGeer JC, Naddy RB, Playle RC, Santore RC, Schneider U, Stubblefield WA, Wood CM, Wu KB. 2002a. The Biotic Ligand Model: A Historical Overview, Special Issue: The Biotic Ligand Model for Metals–Current Research, Future Directions, Regulatory Implications, Comparative Biochemistry and Physiology, Part C 133(1-2): 3-35.
Paquin PR, Zoltay V, Winfield RP, Wu KB, Mathew R, Santore R, Di Toro DM. 2002b. Extension of the Biotic Ligand Model of Acute Toxicity to a Physiologically-based Model of the Survival Time of Rainbow Trout (Oncorhynchus mykiss) Exposed to Silver, Special Issue: The Biotic Ligand Model for Metals–Current Research, Future Directions, Regulatory Implications, Comparative Biochemistry and Physiology, Part C 133(1-2): 305-343.
Parker R and Dumaresq C. 2002. Effluent characterization, water quality monitoring, and sediment monitoring in the metal mining EEM program. Water Qual. Res. J. Canada 37(1): 219-228.
Parkhurst DL and Appelo CA. 2000: User's guide to PHREEQC (Version 2)--A computer program for speciation. hatch-reaction, one-dimensional transport, and inverse geochemical calculations. U.S. Geological Survey, Water-Resources Investigations Report 99-4259, 112 pp.
Parrott JL. 2005. Overview of methodology and endpoints in fathead minnow lifecycle tests assessing pulp mill effluents. Water Qual. Res. J. Can. 40(3): 334-346.
Parrott JL, Bennie DT. 2009. Lifecycle exposure of Fathead minnows to a mixture of six common pharmaceuticals and triclosan. J. Toxicol. Environ. Health, Part B, 9: 297-317.
Parrott JP, Hewitt LM and McMaster ME. 2012. Fathead Minnow Lifecycle Assays for Assessment of Complex Effluents, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Patrick WH Jr, Gambrell RP and Khalid RA. 1977. Physicochemical Factors Regulating Solubility and Bioavailability of Toxic Heavy Metals in Contaminated Dredged Sediment, J. Environ. Sci. Health A12: 475-492.
Pearson TH, Rosenberg R. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology Annual Review 163: 229-311.
Peplow D, Edmonds R. 2005. The effects of mine waste contamination at multiple levels of biological organization. Ecological Engineering 24 (1/2): 101-119.
Perceval O, Couillard Y, Giguere A, Pinel-Alloul B and Campbell PGC. 1999. Field evaluation of the use of metallothionein as a biomarker for metal contamination and toxic effects in the freshwater bivalve Pyganodon grandis: Responses at the population level. SETAC 20th Annual Meeting, 14-18 November 1999, Philadelphia, PA.
Pereira P, de Pablo H, Vale C, Rosa-Santos F and Cesário R. 2009. Metal and nutrient dynamics in a eutrophic coastal lagoon (Óbidos, Portugal): the importance of observations at different time scales. Environ. Monit. Assess. 158: 405–418.
Phillips DJH and Rainbow PS. 1993. Biomonitoring of trace aquatic contaminants. Elsevier Applied Science Publishers, London, UK.
Pierron F, Bourret V, St-Cyr J, Campbell PGC, Bernatchez L and Couture P. 2009. Transcriptional responses to environmental metal exposure in wild yellow perch (Perca flavescens) collected in lakes with differing environmental metal concentrations (Cd, Cu, Ni). Ecotoxicol. 18: 620-631.
Pinel-Alloul B, Campbell PGC, Hare L, Couillard Y, Giguere A and Perceval O. 1999. Field evaluation of the use of metallothionein as a biomarker for metal contamination and toxic effects in the freshwater bivalve Pyganodon grandis: Overview. SETAC 20th Annual Meeting, 14-18 November 1999, Philadelphia, PA.
Pollard AI and Yuan L. 2006. Community response patterns: evaluating benthic invertebrate composition in metal-polluted streams. Ecological Applications 16 (2): 645-655.
Rickwood CJ, Dubé MG, Hewitt M, Kovacs TG and MacLatchy DL. 2006. Use of paired fathead minnow (Pimephales promelas) reproductive test. Part 2: Source identification of biological effects at a bleached kraft pulp mill. Env. Toxicol. Chem. 25: 1847-1856.
Ridal JJ, Yanch LE, Fowlie AR, Razavi NR, Delongchamp TM, Choy ES, Fathi M, Hodson PV, Campbell L, Blais JM, Hickey MBC, Yumvihoze E and Lean DRS. 2010. Potential causes of enhanced transfer of mercury to St. Lawrence River Biota: implications for sediment management strategies at Cornwall, Ontario, Canada. Hydrobiologia, 2010: 81-98.
Ridley-Thomas CI, Austin A, Lucey WP and Clark MJR. 1989. Variability in the determination of ash free dry weight for periphyton communities: a call for a standard method. Water Research 23(6): 667-670.
Ritchie JC and McHenry JR. 1990. Application of radioactive fallout cesium-137 for measuring soil erosion and sediment accumulation rates and patterns: a review. Journal of Environmental Quality 19 (2): 215-233.
Roch M, Nordin RN, Austin A, McKean CJP, Deniseger J, Kathman RD, McCarter JA and Clark MJR. 1985. The effects of heavy metal contamination on the aquatic biota of Buttle Lake and the Cambpell River drainage (Canada). Arch. Environ. Contam. Toxicol. 14: 347-362.
Sánchez España J, López Pamo E, Santofimia Pastor E and Diez Ercilla M. 2008. The acidic mine pit lakes of the Iberian Pyrite Belt: an approach to their physical limnology and hydrogeochemistry. Applied Geochemistry 23 (5): 1260-1287.
Santore RC, McGrath J, Brix K, Paquin PR and Di Toro DM. 1998. Use of a biotic ligand model to calculate site-specific water effect ratios for metals. SETAC 1998 Annual Conference, Charlotte, NC.
Santore RC, Di Toro DM, Paquin PR, Allen HE and Meyer JS. 2001. A Biotic Ligand Model of the Acute Toxicity of Metals. II. Application to Acute Copper Toxicity in Freshwater Fish and Daphnia, Environmental Toxicology and Chemistry 20(10): 2397-2402.
Santore RC, Mathew R, Paquin PR and Di Toro DM. 2002. Development of a Biotic Ligand Model of Acute Toxicity for Zinc, Special Issue: The Biotic Ligand Model for Metals–Current Research, Future Directions, Regulatory Implications, Comparative Biochemistry and Physiology, Part C 133(1-2): 271-285.
Sappington KG, Bridges TS, Bradbury SP, Erickson RJ, Hendriks AJ, Lanno RP, Meador JP, Mount DR, Salazar MH and Spry DJ. 2011. Application of the tissue residue approach in ecological risk assessment, Integrated Environmental Assessment and Management Special Issue: Tissue Residue Approach Special Series 7(1): 116–140.
Schecher WD and McAvoy DC. 1992. MINEQL+: a software environment for chemical equilibrium modelling. Comput. Environ. Urban Systems 16: 65-76.
Schecher WD and McAvoy DC. 1994. MINEQL+: A Chemical Equilibrium Program for Personal Computers (Version 3.01), Hallowell, Maine: Environmental Research Software, 107 pp.
Schuytema GS, Nelson PO, Malueg KW, Nebeker AV, Krawczyk DF, Ratcliff AK and Gakstatter JH. 1984. Toxicity of Cadmium in Water and Sediment to Daphnia magna. Environ. Toxicol. Chem. 3: 293-308.
Shaner SW and Knight AW. 1985. The Role of Alkalinity in the Mortality of Daphnia magna in Bioassays of Sediment-bound Copper, Comp. Biochem. Physiol. 82C: 273-277.
Sharpe RL, Machtans H, Crowe J, Smith P, Patrick H, Chapman PM, Connell R and Daniels E. 2012. Con Mine: Investigation of Cause Study on fish livers – Challenges to designing a new Investigation of Cause study, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Shifrin NS, Beck BD, Gauthier TD, Chapnick SD and Goodman G. (1996) Chemistry, toxicology and human health risk of cyanide compounds in soils at former manufactured gas plant sites. Regul. Toxicol. Pharmacol. 23: 106-116.
Shobanov NA, Kiknadze II and Butler MG. 1999. Palearctic and Nearctic Chironomus (Camptochironomus) tentans Fabricius are different species (Diptera, Chironomidae). Ent. Scand. 30: 311-322.
Shuhaimi-Othman M, Pascoe D, Borgmann U and Norwood WP. 2006. Reduced metals concentrations of water, sediment and Hyalella azteca from lakes in the vicinity of the Sudbury metal smelters, Ontario, Canada. Environmental Monitoring and Assessment 117 (1/3): 27-44.
Small AM, Adey WH, Lutz SM, Reese EG and Roberts DL. 1996. A macrophyte-based rapid biosurvey of stream water quality: restoration at the watershed scale. Restoration Ecol. 4: 124-145.
[SETAC] Society of Environmental Toxicology and Chemistry. SETAC Technical Issue Paper. Sound Science. SETAC, Pensacola, FL, 1999.
Sortkjaer O. 1984. Macrophytes and macrophyte communities as test systems in ecotoxicological studies of aquatic systems. Ecol. Bull. 36: 75-80.
St. Cyr L and Campbell PGC. 2000. Bioavailability of sediment-bound metals for Vallisneria americana Michx, a submerged aquatic plant, in the St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 57: 1330-1341.
Stumm W and Morgan JJ. 1996. Aquatic chemistry: chemical equilibria and rates in natural waters (3rd ed.) Wiley Interscience, New York, NY.
Suter GW II. 1993. Ecological Risk Assessment. Lewis Publishers, Boca Raton, FL.
Szarek-Gwiazda E and Zurik R. 2006. Distribution of Trace Elements in Meromictic Pit Lake. Water, Air & Soil Pollution 174(1-4): 181-196.
Taylor LN, McFarlane WJ, Pyle GG, Couture P and McDonald DG. 2004. Use of performance indicators in evaluating chronic metal exposure in wild yellow perch (Perca flavescens). Aquatic Toxicology 67: 371–385.
Taylor LN, Novak K, Holtze K, Ali N and Scroggins R. 2012. Separating current effluent quality from historical contamination using a laboratory based monitoring tool for investigation of cause, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Tessier A, Couillard Y, Cambpell PGC and Auclair JCA. 1993. Modeling of Cd partitioning in oxic lake sediments and Cd concentrations in the freshwater bivalve Anodonta grandis. Limnol. Oceanogr. 38: 1-17.
Tessier A and Turner DR (ed.) 1995. Metal speciation and bioavailability in aquatic systems. John Wiley and Sons Ltd., Chichester, UK.
Thomann RT and Mueller JA. 1987. Principles of Surface Water Quality Modelling and
Control. Harper Collins Publishers Inc., New York, NY.
Thomas GP. 2012. Use and application of Benthic Transplant Devices (BTDs) in EEM investigations, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Tipping E. 1998. Humic ion-binding Model VI: an improved description of the interactions of protons and metal ions with humic substances. Aq. Geochem. 4: 3-48.
Unsworth ER, Warnken KW, Zhang H, Davison W, Black F, Buffle J, Cao J, Cleven R, Galceran J, Gunkel P, Kalis E, Kistler D, Herman P, van Leeuwen HP, Martin M, Noël S, Nur Y, Odzak N, Puy J, van Riemsdijk W, Sigg L, Temminghoff E, Tercier-Waeber ML, Toepperwien S, Town RM, Weng L and Xue H). 2006. Model predictions of metal speciation in freshwaters compared to measurements by in situ techniques. Environmental Science & Technology 40(6): 1942-1949.
[US EPA] United States Environmental Protection Agency. 1989. Generalized methodology for conducting industrial toxicity reduction evaluations. EPA-600/2-88/070.
[US EPA] United States Environmental Protection Agency. 1991a. Methods for aquatic toxicity identification evaluations: Phase I toxicity characterization procedures. EPA-600/6-91/003.
[US EPA] United States Environmental Protection Agency. 1991b. Toxicity identification evaluation: characterization of chronically toxic effluents, Phase I. EPA-600/6-91/005.
[US EPA] United States Environmental Protection Agency. 1991c. Sediment toxicity identification evaluation: Phase I (Characterization), Phase II (Identification) and Phase III (Confirmation) modifications of effluent procedures. EPA-600/6-91/007.
[US EPA] United States Environmental Protection Agency. 1993a. Methods for aquatic toxicity identification evaluations: Phase II toxicity identification procedures for samples exhibiting acute and chronic toxicity. EPA-600/R-92/080.
[US EPA] United States Environmental Protection Agency. 1993b. Methods for aquatic toxicity identification evaluations: Phase III toxicity confirmation procedures for samples exhibiting acute and chronic toxicity. EPA-600/R-92/081.
[US EPA] United States Environmental Protection Agency. 1996. Marine toxicity identification evaluation (TIE): Phase I guidance document. Duluth, MN: Environmental Research Laboratory. EPA/600/R-96/054.
[US EPA] United States Environmental Protection Agency. 1997. Sediment Toxicity Evaluation (TIE) Phases I, II, and III, Guidance Document. EPA/600/R-07/080.
[US EPA] United States Environmental Protection Agency. 2001. Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual. EPA-823-B-01-002.
[US EPA] United States Environmental Protection Agency. 2003. Biotic Ligand Model: Technical Support Document for its Application to the Evaluation of Water Quality Criteria for Copper Office of Science and Technology. Health and Ecological Criteria Division, Washington, DC. EPA Number 822R03027.
[US EPA] United States Environmental Protection Agency. 2004. Draft Aquatic Life Water Quality Criteria for Selenium--2004. EPA-822-D-04-001. Office of Water, Washington, DC.
[US EPA] United States Environmental Protection Agency. 2007. Framework for Metals Risk Assessment. Office of the Science Advisor, Risk Assessment Forum. Washington, DC: EPA/120/R-07/001.
van de Guchte C, Maas-Diepeveen JL. 1988. Screening Sediments for Toxicity: A Water-concentration Related Problem, in: Proceedings of the 14th Annual Aquatic Toxicity Workshop, November 1-4, 1987, Toronto, ON, Can. Tech. Rep. Fish. Aquat. Sci. 1607: 81-91.
Van Geest JL, Poirier DG, Solomon KR and Sibley PK. 2011. A comparison of the bioaccumulation potential of three freshwater organisms exposed to sediment-associated contaminants under laboratory conditions. Environ. Toxicol. Chem. 30: 939–949.
Van Geest JL, Poirier DG, Sibley PK and Solomon KR. 2011. Validation of Ontario’s new laboratory-based bioaccumulation methods with in situ field data. Environ. Toxicol. Chem. 30: 950–958.
van Wijngaarden RPA, van den Brink PJ, Crum SJH, Voshaar JHO, Brock TCM, Leeuwangh P. 1996. Effects of the insecticide Dursban® 4E (active ingredient chlorpyrifos) in outdoor experimental ditches: I. Comparison of short-term toxicity between the laboratory and the field. Environ. Toxicol. Chem. 15: 1133-1142.
Vigneault B, Desforges M, McGeer, J. 2012. Mining Reagents and By-Products (e.g. Thiosalts) As Potential Toxicants in Mine Effluents, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Vijver MG, Van Gestel CAM, Lanno RP, Van Straalen NM and Peijnenburg WJGM. 2004. Internal metal sequestration and its ecotoxicological relevance: a review. Environ. Sci. Technol. 38: 4705-4712.
Vink JPM and Meeussen JCL. 2007. BIOCHEM-ORCHESTRA: a tool for evaluating chemical speciation and ecotoxicological impacts of heavy metals on river flood plain systems. Environmental Pollution 148 (3): 833-841.
Walker PA, Kille P, Hurley A, Bury NR and Hogstrand C. 2008. An in vitro method to assess toxicity of waterborne metals to fish. Toxicol. Appl. Pharmacol. 230: 67-77.
Wang D, Couillard Y, Campbell PGC and Jolicoeur P. 1999. Changes in subcellular metal partitioning in the gills of freshwater bivalves (Pyganodon grandis) living along an environmental cadmium gradient. Can. J. Fish. Aquat. Sci. 56: 774-784.
Wang F, Goulet RR and Chapman PM. 2004. Testing sediment biological effects with the freshwater amphipod Hyalella azteca: the gap between laboratory and nature. Chemosphere 57: 1713-1724.
Warren LA, Tessier A and Hare L. 1998. Modelling cadmium accumulation by benthic invertebrates in situ: The relative contributions of sediment and overlying water reservoirs to organism cadmium concentrations. Limnol. Oceanogr. 43: 1442-1454.
Warwick RM and Clarke KR. 1991. A Comparison of Some Methods for Analysing Changes in Benthic Community. J. Mar. Biol. Ass. U.K. 71: 225-244.
Weber CI. 1973. Biological field and laboratory methods for measuring the quality of surface waters and effluents. U.S. Environmental Protection Agency Report 670/4/73/001.
Weber LP, Higgins PS, Carlson RI and Janz DM. 2003. Development and validation of methods for measuring multiple biochemical indices of condition in juvenile fishes. Journal of Fish Biology 63: 637–658.
Weech S, Orr P, Russel C, Fedat L and Yaschyshyn D. 2012. Investigation of Cause of Effects on Benthic Invertebrates at the Kidd Metallurgical Site, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Weitzel RL, Sanocki SL and Holecek H. 1979. Sample replication of periphyton collected from artificial substrates, in: Methods and Measurements of Periphyton Communities: a Review. Edited by RL Weitzel. ASTM STP 690, Philadelphia, PA, pp. 90-115.
Werner I, Clar SL and Hinton DE. 2003. Biomarkers aid understanding of aquatic organism responses to environmental stressors. California Agriculture 57 (4): 110-115.
Whitton BA. 1984. Algae as monitors of heavy metals in freshwaters, in: Algae as Ecological Indicators. Edited by LE Shubert, Academic Press, London, UK, pp. 257-280.
Whitton BA, Say PJ and Wher JD. 1981. Use of plants to monitor heavy metals in rivers, in: Heavy metals in Northern England: environmental and biological aspects. Edited by PJ Say and BA Whitton. Department of Botany, University of Durham, Durham, UK, pp. 135-145.
Wolf JC and Wolfe MJ. 2005. A Brief Overview of nonneoplastic hepatic toxicity in fish. Toxicologic Pathology 33: 75–85.
Yan ND. 1979. Phytoplankton community of an acidified, heavy metal contaminated lake near Sudbury, Ontario: 1973-1977. Water, Air, and Soil Poll. 11:43-45.
Zagury GJ. 2012. Cyanide speciation and fate in gold mine tailings: A case study, in: Environment Canada. 2012. Environmental Effects Monitoring Investigation of Cause Workshop for Metal Mining: Proceedings, Gatineau, QC.
Zapata F. 2003. The use of environmental radionuclides as tracers in soil erosion and sedimentation investigations: recent advances and future developments. Soil & Tillage Research 69 (1/2):3-13.
Tables and Figures
Table 12-1 outlines the possible causes that may be examined during IOC for confirmed effects below CES. Possible causes include habitat differences, elevated nutrients, effluent contaminant response, natural variation, historical sediment contamination, food limitation. A suggested approach to the study of each cause is also provided. Further, examples are included to illustrate each suggested approach.
Table 12-2 outlines a formalized set of causal criteria forming part of a weigh-of-evidence approach for the assessment of mining effluent effects. Primary causal criteria include spatial correlation, temporal correlation, plausible mechanism, experimental verification, strength, specificity, evidence of exposure, consistency, and coherence.
Figure 12-1 is a conceptual diagram that outlines the tiered framework for investigation of cause in environmental effects monitoring. Tiers I through V are divided into three categories: response pattern recognition; mine source identification; and chemical characterization and identification. Passing through each investigation tier, the results increase in detail, complexity, effort and cost.
Footnotes
- Footnote 13
Abundance is defined as the number of benthic invertebrate individuals. The term density is used when abundance is expressed per unit area sampled.
Page details
- Date modified: