Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Toluene Diisocyanate
Benzene, 2,4,-diisocyanato-1-methyl- (2,4-TDI)
CAS RN 584-84-9
Benzene, 1,3,-diisocyanato-2-methyl- (2,6-TDI)
CAS RN 91-08-7
Benzene, 1,3,-diisocyanatomethyl- (TDI mixed isomers)
CAS RN 26471-62-5
Environment Canada
Health Canada
July 2008
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Sources
- Uses
- Releases to the Environment
- Persistence and Bioaccumulation Potential
- Environmental Fate
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusions
- References
- Appendix 1: Upper-bounding estimates of daily intake of 2,4-toluene diisocyanate and 2,6- toluene diisocyanate by the general population in Canada
- Appendix 2: Exposure estimates from the use of consumer products
- Appendix 3: Summary of health effects information for toluene diisocyanates
The Ministers of the Environment and of Health have conducted a screening assessment of mixed isomers of toluene diisocyanate (TDI), Chemical Abstracts Service Registry Number (CAS RN) 26471-62-5; Benzene, 2,4-diisocyanato-1-methyl- (2,4-toluene diisocyanate = 2,4-TDI), CAS RN 584-84-9; and Benzene, 1,3-diisocyanato-2-methyl- (2,6 toluene diisocyanate = 2,6-TDI) CAS RN 91-08-7. These substances were identified in the categorization of the Domestic Substances List as high priorities for action under the Ministerial Challenge. They were considered to pose the greatest potential for exposure to individuals in Canada (GPE) in the case of CAS RN 26471-62-5, or an intermediate potential for exposure to individuals in Canada (IPE) in the case of CAS RN 91-08-7 and CAS RN 584-84-9, and have been classified by other agencies on the basis of carcinogenicity. As these substances were determined not to meet the ecological categorization criteria for persistence, bioaccumulation potential, or inherently toxic to aquatic organisms, the focus of this screening assessment relates to human health aspects.
The screening assessments for 2,4TDI, 2,6TDI and the mixed isomers have been combined because most toxicological data are available for the mixed isomers of TDI. The terms “TDI” and toluene diisocyanate in this assessment refer to either 2,4TDI, 2,6TDI, or the mixed isomers of TDI.
Toluene diisocyanate is an industrial chemical which was not manufactured by any company in Canada in a quantity above the reporting threshold of 100 kg in 2006, but which is imported into the country. In Canada, more than 85% of toluene diisocyanate was used in the manufacture of flexible polyurethane foam in 2006.
Industrial releases of TDI in Canada are mainly to air and it is expected that most TDI released to air will remain in the vapour state and react chiefly with photolytically produced hydroxyl radicals, resulting in a half-life of less than two days. However, exposure to TDI may be elevated during use of consumer products containing the substance.
Based principally on a weight of evidence for carcinogenicity in assessments by several international and national agencies, the available human epidemiological data and the experimental animal data are equivocal and thus inadequate to determine the carcinogenic risk to TDI in humans, via the inhalation route. TDI is considered to be carcinogenic, since oral dosing in animals was associated with appearance of tumours at multiple sites. Mixed results have been obtained for TDI in in vivo and in vitro genotoxicity assays. Therefore, although the mode of induction of tumours has not been fully elucidated, it cannot be precluded that the tumours observed in experimental animals resulted from direct interaction with genetic material.
The upper-bounding estimate of exposure via inhalation to the general population or during use of consumer products containing TDI may approach or exceed the critical effect levels for non-cancer effects in the respiratory system, including respiratory hypersensitivity. Additionally, TDI has been classified as a dermal and respiratory sensitizer by the European Union.
On the basis of the carcinogenicity of TDI mixed isomers, 2,4-TDI and 2,6-TDI, for which there may be a probability of harm at any level of exposure, as well as the potential inadequacy of the margins between estimated exposure to the general population or exposure from products and critical effect levels for non-cancer effects, it is concluded that they are substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of ecological hazard and reported releases of TDI mixed isomers, 2,4TDI and 2,6-TDI, it is concluded that these substances are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends. Additionally, TDI mixed isomers, 2,4-TDI and 2,6-TDI do not meet criteria for persistence and bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations.
These substances will be included in the Domestic Substances List inventory update initiative, to be launched in 2009. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment and, where appropriate, the performance of potential control measures identified during the risk management phase.
Based on the information available, TDI mixed isomers, 2,4-TDI and 2,6-TDI meet one or more of the criteria set out in Section 64 of the Canadian Environmental Protection Act, 1999.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that:
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherently toxic to aquatic organisms (iT), and were believed to be in commerce; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as the highest priorities.
Isomers of toluene diisocyanate were identified as high priorities for assessment of human health risk because they were considered to present IPE or GPE and had been classified by other agencies on the basis of carcinogenicity. The Challenge for isomers of toluene diisocyanate was published in the Canada Gazette on February 3, 2007 (Canada 2007a). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information were received.
Although isomers of toluene diisocyanate were determined to be a high priority for assessment with respect to human health, they did not meet the criteria for potential for persistence, bioaccumulation or inherent toxicity for aquatic organisms. Therefore, this assessment focuses principally on information relevant to the evaluation of risks to human health.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to September 2007. Key studies were critically evaluated; modelling results may have been used to reach conclusions. Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
The screening assessments for 2,4-TDI, 2,6-TDI and the mixed isomers have been combined because most toxicological data are available for the mixed isomers of TDI. The term "TDI" in this assessment refers to either 2,4TDI, 2,6TDI, or the mixed isomers of TDI.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. This assessment has undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from Meridian Environmental Inc. and Starodub & Associates. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. The critical information and considerations upon which the assessment is based are summarized below.
| CAS RN and name | CAS RN 584-84-9: benzene, 2,4,-diisocyanato-1-methyl- (2,4-TDI) CAS RN 91-08-7: benzene, 1,3,-diisocyanato-2-methyl- (2,6-TDI) CAS RN 26471-62-5: benzene, 1,3,-diisocyanatomethyl- (TDI mixed isomers) |
|---|---|
| TDI is most commonly sold as an 80:20 mixture of 2,4- and 2,6- isomers. A mixture that is 65:35 is also in commerce and a crude TDI mixture with an undefined isomer ratio is also sold commercially. Pure 2,4- TDI is used for some specialty purposes. Pure 2,6-TDI has no industrial use. | |
Synonyms CAS RN 91-08-7 CAS RN 26471-62-5 |
1,3-Diisocyanato-4-methylbenzene; 2,4-Toluene diisocyanate (2,4-TDI); Isocyanic acid, 4-methyl-m-phenylene ester 1,3-Diisocyanato-2-methylbenzene; 2,6- Toluene diisocyanate (2,6-TDI); Isocyanic acid, 2-methyl-m-phenylene ester Crude tolylene diisocyanate; 1,3-Diisocyantomethylbenzene ; Toluene diisocyanate (80% 2,4-TDI; 20% 2,6-TDI) |
| Chemical group | Organic |
| Chemical subgroup | Cyanates and derivatives |
| Chemical formula | C9H6N2O2 |
| Chemical structure | 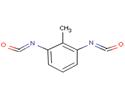 2,6- toluene diisocyanate 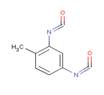 2,4- toluene diisocyanate |
| SMILES Notation | 91-08-7: O=C=Nc(c(c(N=C=O)cc1)C)c1 584-84-9: O=C=Nc(c(ccc1N=C=O)C)c1 |
| Molecular mass | 174.16 |
| Property | Value | Type | Reference |
|---|---|---|---|
| Exp = experimental data | |||
| Melting point 2,4-TDI 2,6- TDI Mixed 2,4- and 2,6- isomers of TDI |
22°C 7.2°C < 15°C (80:20 mix) < 8°C (65:35 mix) |
Exp Exp Exp Exp |
IARC 1986 IARC 1986 IARC 1986 IARC 1986 |
| Boiling point 2,4-TDI 2,6- TDI Mixed 2,4- and 2,6- isomers of TDI |
252.5–254°C 247–248.5°C 252–254°C |
Exp at 1 atm Exp at .98 atm Exp at 1 atm |
IUCLID 2000 IUCLID 2000 IUCLID 2000 |
| Density | 1.22 g/cm3 | Exp at 20°C | IUCLID 2000 |
| Vapour pressure 2,4-TDI 2,6- TDI Mixed 2,4- and 2,6- isomers of TDI |
0.013–0.021 hPa 0.016 hPa 0.014–0.015 hPa |
Exp all at 20°C | IUCLID 2000 IUCLID 2000 IUCLID 2000 |
| Henry's Law constant (H) | TDI reacts with water therefore no estimation of H is made. | ||
| Water solubility | TDI reacts with water and solubility in water cannot be determined. | IUCLID 2000 | |
| Log Kow | TDI reacts with water and with octanol, therefore no estimation of Kow is made | IUCLID 2000 | |
| Log Koc | TDI reacts with water and with octanol, therefore no estimation of Koc is made | ||
There are no known natural sources of TDI.
Toluene diisocyanate is an industrial chemical which was not manufactured by any company in Canada in a quantity above the reporting threshold of 100 kg in 2006, but which is imported into the country. The industrial demand in Canada for TDI in 2006 was estimated to be 33.6 million kilograms (IAL Consultants 2007). From responses to a notice published under Section 71 of CEPA 1999, the quantity of TDI imported into Canada in 2006 was determined to be greater than10 million kilograms (Canada 2007a). This is the total for toluene diisocyanate isomers represented by the three CAS numbers identified in this report. It includes TDI imported in mixtures with other chemical substances and in consumer products if the quantity of TDI imported was equal to or greater than 100 kg in 2006.
Toluene diisocyanate may be released to the environment from industrial processes that use TDI as a reactive intermediate. An example of this would be the manufacture of flexible polyurethane foam. Emissions from industrial activities are principally to air. Another potential source of TDI emissions to the environment is the incineration of polyurethane waste. Because TDI is a reactive chemical, long-range transport of TDI is not expected to contribute significantly to human exposure in Canada.
Toluene diisocyanate is present in some consumer products, which may emit TDI when used. Some examples of such products are specialty polyurethane-based paints, varnishes and coatings, polyurethane adhesives, sealants and mastic (IARC 1986; NTP 2005). It has been reported that mixtures of isocyanates, including TDI may be released when isocyanate-based paint, which is used extensively for the factory-applied primer coat on automobiles is heated during grinding or sanding of the surface (Karlsson et al. 2000). Potential sources of TDI in food arise from the use of polyurethanes in inks and adhesives applied to food packaging, and polyurethane elastomers in food handling equipment such as conveyor belts. If there is no functional barrier between inks or adhesives and food, the residual levels of TDI isomers and the potential migration of TDI into food are estimated based on professional judgement by Health Canada (as per e-mail from Food Directorate, Health Canada, May 2008, unreferenced). Polyurethane foam liners which may be made with TDI have been approved for restricted use as food contact material in Canada (Damant et al. 1995; Ellendt et al. 2003; CFIA 2007).
Some products used in construction such as sealants, coatings, and adhesives which contain measurable amounts of free isocyanate are intended for use only by trained contractors. The degree to which untrained individuals may have access to such products is unknown.
Mixtures of 2,4- and 2,6- toluene diisocyanate isomers dominate the industrial use of TDI. Pure 2,4- isomer is used in some specialty elastomers applications. Pure 2,6- isomer is not a commercial industrial chemical (Allport et al. 2003).
The use patterns reported in 2006 for TDI in Canada are shown in Tables 3 and 4.
In Canada, about 86% of toluene diisocyanate was used in the manufacture of flexible polyurethane foam (PUF) in 2006. TDI reacts with a polyol in the presence of a surfactant, catalyst, blowing agent and other process chemicals to form PUF. Flexible PUF is used extensively in household furniture and automotive upholstery, in mattresses, pillows, packaging and carpet underlay. Use of TDI to bond foam scrap to make carpet underlay consumes about one percent of all TDI in Canada (IAL Consultants 2007). This use of TDI is being phased out in other places in favour of the use of methylenediphenyl diisocyanate, MDI (Meeting with Isocyanates Panel, American Chemistry Council – ACC, Ottawa, 2007/08/08). Semi-flexible and semi-rigid PUF are used in automotive panels, padding and bumpers. Use of TDI to produce rigid PUF for insulation in applications such as refrigeration and foam-in-place caulking has largely been replaced by MDI (Meeting with Isocyanates Panel, ACC, Ottawa, 2007/08/08).
The market demands for TDI used in coatings and adhesives in 2006 were about seven and five percent respectively of the total demand. TDI or a TDI derivative is used in polyurethane-modified alkyd paints and coatings including automotive and marine paint, wood varnish, floor treatment, wire and powder coating. TDI or a TDI derivative is used in sealants, adhesives and elastomers. Poly(urethane urea) cast elastomers based on TDI may be used in wheels, rollers, conveyors and similar products. TDI is used in the production of coatings for textiles for durability and waterproofing and in other textile applications (Ulrich 1996; IARC 1986; NTP 2005). TDI-based coatings are also used in paper production (Canada 2007a). Several biomedical applications of polyurethanes based on TDI have been reported elsewhere. In Canada, no products made with TDI-based polyurethane are licensed for use in biomedical applications. Two historical uses are the Today contraceptive sponge, made of polyurethane foam and the Même breast implant, no longer licensed for use in Canada, which consisted of a silicone core enclosed in a TDI-based polyurethane envelope (Benoit 1993).
| Product type | TDI (millions of kg) |
|---|---|
| Adapted from 2006 End-Use Market Survey on the Polyurethanes Industry in the United States, Canada and Mexico, October 2007, by IAL Consultants for the Centre for the Polyurethanes Industry | |
| Flexible foam slabstock | 19.64 |
| Flexible foam moulded | 9.25 |
| Coatings | 2.31 |
| Binders | 0.36 |
| Elastomers | 0.45 |
| Adhesives | 1.59 |
| Total | 33.6 |
| End-use application | TDI (millions of kg) |
|---|---|
| Adapted from 2006 End-Use Market Survey on the Polyurethanes Industry in the United States, Canada and Mexico, October 2007, by IAL Consultants for the Centre for the Polyurethanes Industry | |
| Construction | 2.90 |
| Transportation | 12.11 |
| Furniture | 9.53 |
| Foam Scrap | 2.45 |
| Appliance | 0.14 |
| Packaging | 0.41 |
| Bedding | 2.04 |
| Textiles and fibre | 0.36 |
| Industrial | 0.32 |
| Foundry | - |
| Marine | 0.09 |
| Machinery | 0.18 |
| Electronics | - |
| Wheels and tires | - |
| Footwear | - |
| Decorative | - |
| Other | 3.13 |
| Total | 33.6 |
Submissions made under Section 71 of CEPA 1999 indicate that in 2006, more than 10 million kilograms of toluene diisocyanate isomers were imported into Canada (Canada 2007a). No further detail on the quantity of TDI represented by each CAS number is given here because of confidentiality requirements.
Industrial facilities in Canada that annually manufacture, process or otherwise use more than 10 tonnes of TDI, either as the mixture of isomers or as a specified isomer must report to the National Pollutant Release Inventory (NPRI) all releases to the environment and other transfers of the substance. The NPRI summary of releases of TDI from industrial facilities in Canada for 2002 to 2006 is shown in Table 5. No releases to surface waters or land were reported during that period; however, transfers to waste disposal facilities were reported (NPRI 2008).
| Year | 2,4 - TDI 584-84-9 |
2,6 - TDI 91-08-7 |
Mixed isomers of TDI 26471-62-5 |
Total Releases of TDI isomers |
|---|---|---|---|---|
| Tonnes | ||||
| 2006 | 0.005 | 0.0 | 2.022 | 2.027 |
| 2005 | 0.002 | 0.0 | 1.479 | 1.481 |
| 2004 | 0.003 | 0.0 | 1.096 | 1.099 |
| 2003 | 0.011 | 0.001 | 1.586 | 1.598 |
| 2002 | 0.106 | 0.001 | 1.232 | 1.339 |
Persistence
In air, indirect photolytic degradation as a result of photogenerated hydroxyl radicals has been demonstrated to be a significant degradation pathway for TDI (Pemberton and Tury 2004). Although TDI reacts rapidly with water, Tury et al. (2003) observed that reaction (i.e., hydrolysis) with atmospheric water was not significant. Tury et al. (2003) reported a half-life of 1.4 days for TDI based on experimental reaction rates, which compares well with the predicted and calculated values of 1.7 and 1.5 days (see Table 6). TDI is not expected to react with other photo-oxidative species in the atmosphere, such as ozone (Tury et al. 2003). Based on the evidence available, TDI is not considered persistent in air.
| Medium | Fate process | Degradation value/range | Degradation endpoint | Reference |
|---|---|---|---|---|
| [*] Based on hydroxyl radical concentration of 1.5 × 106 OH radicals/cm3 (12-h day) | ||||
| Air | Atm-oxidation by OH radicals[*] | 1.7 | half-life (days) | AOPWIN (2000) |
| Air | Atm-oxidation by OH radicals | 1.5 | half-life (days) | Atkinson R (1989), calculated value |
| Air | Ozone reaction | negligible | half-life (days) | AOPWIN (2000) |
| Water/soil/ sediment | Hydrolysis | < 10 minutes | half-life (days) | HYDROWIN (2000) |
| Water/soil/ sediment |
Biodegradation | 15-60 | half-life (days) | BIOWIN (2000) |
| Water/Soil/ Sediment |
Biodegradation | 0.002-0.2186 | Probability of rapid biodegradation | TOPKAT (2004) |
| Water/soil/ sediment |
Biodegradation (BOD) | > 15 | half-life (days) | ASTER (1999) |
The Transport and Persistence Level III Model (TaPL3) (TaPL3 2000) is not considered appropriate in estimating characteristic travel distance (CTD) for TDI since the applicability domain of the model does not cover substances that react in water or soil. The CTD is defined as the maximum distance travelled in air by 63% of a substance; or the distance that 37% of the substance may travel beyond. Nevertheless, the three TDI substances are expected to have a CTD under 700 km based on two considerations. The first consideration is that a large number of non-reactive substances with water or soil modelled by TaPL3 have a CTD < 700 km if their atmospheric half lives are less than 2 days, while the three TDI substances all have an atmospheric half life of less than 2 days (1.4 days reported by Tury et al., 2003; 1.5 days calculated according to Atkinson, 1989; and 1.7 days predicted by AOPWIN, 2000). The second consideration is that when a substance like TDI transforms into other forms in water and soil, the chance for the substance to re-enter the atmosphere from water and soil is reduced and this translates into a reduced potential for long-range transport. According to the criteria proposed by Beyer et al. (2000) for CTDs of > 2000 km as representing high long-range atmospheric transport potential (LRATP), 700-2000 km as moderate LRATP, and < 700 km as low LRATP, the long-range atmospheric transport potential of TDI is considered to be low (<700 km). This means that TDI is not expected to be able to reach areas far from its emission sources
In media where water is present (e.g. water, moist soils, sediment), TDI is considered transient, with a half-life of under a minute (Yakabe et al. 1999). The reaction of an amine with isocyanate is faster than the hydrolysis reaction of water with isocyanate, which, in the case of diisocyanates like TDI, leads primarily to reactions forming polyureas (Yakabe et al. 1999; Pemberton and Tury 2004).
It is well documented in numerous studies that the aromatic diisocyanates will hydrolyze and react rapidly in both water and soil (Pemberton and Tury 2004; Heimbach et al. 1996; Yakabe et al. 1999; Tury et al. 2003).
The weight of evidence based on the above-described data indicates that TDI does not meet the persistence criteria for air (half-life in air ≥ 2 days) or water or soil (half-life in soil and water ≥ 182 days) or sediments (half-life in soil and water ≥ 365 days), as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Bioaccumulation
Modelled data suggest that TDI does not have the potential to bioaccumulate in the environment (see Table 7). Given the highly reactive properties of diisocyanates, TDI compounds are likely metabolized/degraded at rates fast enough to counter any potential for bioaccumulation or biomagnification. In addition, they are likely hydrolyzed in the gastrointestinal tract, thereby reducing the potential for uptake of the chemical from the gut (Yakabe et al. 1999; Pemberton and Tury 2004; IPCS 1987; IPCS1997).
| Test Organism | Endpoint | Value wet weight (L/kg) | Reference |
|---|---|---|---|
| Fish | BAF | 380 | Gobas BAF T2MTL (Arnot and Gobas 2003) |
| Fish | BCF | 151–1183 | Gobas BCF T2LTL (Arnot and Gobas 2003); OASIS Forecast (2005); BCFWIN (2000) |
For toluene diamine, a product of hydrolysis, experimental bioconcentration factors (BCFs) for 2,4-toluene diamine of < 5 to < 50 (MITI 1992) suggest they are also not likely to accumulate in organisms.
The weight of evidence indicates that TDI does not meet the bioaccumulation criterion (BCF, BAF ≥ 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Industrial releases of TDI are expected to be mainly to air, where photolytic degradation is expected to yield a half-life of less than 2 days (see above). TDI has a transient existence in water, moist soils and sediments due to rapid hydrolysis. Environmental fate modelling to estimate partitioning into environmental compartments is therefore not considered relevant for TDI.
As indicated earlier, TDI does not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Due to the anticipated transient existence of TDI in aqueous media, including moist soils, it is unlikely that ecological effects will be elicited by the parent TDI compounds. Experimental ecotoxicological data for TDI and its degradation products (LC50’s and EC50 for water flea, fish, shrimp and algae > 1 mg/L; Tadokoro et al 1997; Curtis et al 1978; Danish EPA 1998; MITI 1992) indicate low to moderate toxicity to aquatic organisms. Other toxicity data suggest low likelihood of effects to terrestrial biota such as plants, earthworms (NOEC’s, EC50 and LC50 > 1000 mg/L; Van der Hoeven et al. 1992a,b) and birds (oral LC50’s of ≥ 100 mg/kg body weight for Agelaius phoenicens exposed to 2,4-TDI and 2,6-TDI; as cited in IUCLID 2000).
The National Pollutant Release Inventory reported 2 200 kg of the isomer mixture was released (92% to air, 8% media unspecified) in 2006 (NPRI 2008). Given the dispersive nature of these releases, they are unlikely to result in significant exposure of organisms in the environment.
Based on the information available, TDI is unlikely to cause ecological harm in Canada.
Exposure Assessment
Environmental Media and Diet
Measured concentrations of toluene diisocyanate in environmental media and dietary sources in Canada were not located. Reports of the concentration of TDI in air outside a manufacturing facility in North Carolina, USA that was subsequently closed (Levine et al. 2001) were not considered representative of exposures in Canada.
The partitioning of TDI into environmental media was not estimated by modelling, but an estimate of the maximum concentration of TDI in air downwind of a manufacturing plant in Canada was made. The International Isocyanate Institute has estimated that in the European industry, 25 grams of TDI are released to the atmosphere per tonne of TDI used in producing flexible polyurethane foam (Chapman 1994). Modelling the ambient air concentration at 100 metres from a low building with no stack using a model within EUSES (European Union System for the Evaluation of Substances) gave the prediction of an annual air concentration of 6.7 E-3 µg/m3 for a plant with a continuous release of TDI of 8.8 kg/yr (Tury et al. 2003). A Canadian plant reported a release of 1,400 kg of mixed isomers of TDI to air in 2006 (NPRI 2008). Extrapolating from the result by Tury et al., the estimated annual average concentration of TDI in air at 100 metres from the plant is 1.06 µg/m3. It is expected that short-term air concentrations will vary from this average because of production cycles, plant stack height, wind, local topography and other factors. Furthermore, this concentration of TDI in air is a conservative estimate applicable only to the population in the vicinity of the plant. Industrial releases of TDI in Canada are mainly to air and it is expected that most TDI released to air will remain in the vapour state and react chiefly with photolytically produced hydroxyl radicals, resulting in a half-life of less than two days (Tury et al. 2003; Atkinson 1989).
The upper-bounding estimate of exposure to TDI via environmental media ranged from 0.21 µg/kg-bw (kilogram of body weight) per day for persons aged 60 years and more to 0.64 µg/kg-bw per day for children aged 6 months to four years (see Appendix 1).
Consumer Products
The use of consumer products may contribute to the exposure of individuals to TDI. Free isocyanate has been detected on the surface of cured polyurethane foam aged 1-30 years using a solvent-coated colour indicating pad, but this test was not specific for TDI. Samples of PUF were shredded, extracted with solvent, and the extract was analysed. Both TDI and total reactive isocyanate groups were quantified in the extracts (Krone et al. 2003). In another experiment, the 2,4- and 2,6- isomers of TDI were extracted by solvent from polyurethane foam and positively identified and measured at levels of nanograms per gram of foam (Gagné et al. 2003). The amines 2,4- and 2,6- toluene diamine, which may be present as the result of hydrolysis of 2,4- and 2,6- toluene diisocyanate have also been extracted from polyurethane foam (Marand et al. 2004). These experiments indicate that TDI and the corresponding amines may migrate from foam in consumer products and thus be available for dermal or oral uptake. The degree to which the use of solvents may be responsible for enhancing extraction of TDI from PUF is unquantifiable.
An experiment in which humidified air was drawn through pieces of foam loaded with 1 ppm of TDI and aged for three days showed that 99.9% of the TDI was retained in the foam (Hugo et al. 2000). There is further experimental evidence that TDI which is present in polyurethane foam is not volatilized in measurable quantities after an initial curing period of several days (Kelly et al. 1999; Katsuyama et al. 2003). Kelly et al. (1999) investigated a number of polyurethane consumer products that are made with TDI including carpet padding, furniture cushions, sheet foam, varnishes and sealants and found no detectable emissions of isocyanates at an emission rate higher than 0.96 µg/m2/hour for coatings applied to surfaces or for polyurethane foam. Kelly et al. (1999) further estimated an equilibrium concentration in household air based on the detection limit of the experimental method in this study and concluded that the highest steady-state residential air concentration would be not more than 0.19 µg/m3 for application of a polyurethane coating.
In a study by Katsuyama et al. (2003) of consumer products, no emissions of TDI above the detection limits were measured when urethane adhesive, paint and varnish were tested, but TDI was found when rigid polyurethane foam was placed in an atmosphere with acetone vapour. The investigators estimated a flux rate of TDI and, based on average Japanese home size and ventilation rate, calculated an equilibrium concentration of 2,4-TDI of 4.28 x 10-2 ppb (305 µg/m3) in Japanese houses. Rigid polyurethane foam slab is not a building material typically used in Canadian residential construction and the concentration of acetone in air used in the experiment does not represent normal residential conditions; therefore, the exposure described by Katsuyama et al. (2003) is not considered pertinent.
It has been shown experimentally that isocyanate-based paint for concrete floors and deck sealer emit measurable concentrations of 2,6-TDI to air following application of the coating (Kelly et al. 1999; Jarand et al. 2002). Although the concentrations of TDI in air that were measured in these experiments in small environmental chambers cannot be extrapolated to residential exposures, the concentration of TDI in coatings and the percentage of TDI released to air can be used to model exposure.
Using data from Jarand et al. (2002) to estimate the maximum concentration of 2,6-TDI in a room during and five hours after painting a concrete floor with a two part polyurethane sealer, an estimate of the concentration of TDI in air of 24 µg/m3was obtained. The dose received by an adult doing light work for an hour was estimated to be 0.44 µg/kg-bw per day. A second scenario using data on 2,6-TDI emissions from a concrete sealer measured by Kelly et al. (1999) yielded a much higher estimate of daily intake over a one hour period; however, the authors stated that the product tested by Kelly et al. (1999) was sold for application solely by licensed contractors. Given uncertainties regarding availability of this TDI-based sealer to Canadian retail consumers, only the scenario based on the findings by Jarand et al. (2002) was retained. It was determined that the product that was tested by Jarand et al. (2002) is available at the retail level in Canada in 2008. The dermal exposure that might arise from use of concrete sealer was not estimated in these scenarios. Dermal exposure would be an additional contribution to exposure.
It should be noted that the estimate of dose is for a one hour exposure. It should be further noted that use of such a concrete sealer is a relatively infrequent activity, probably less than once every 3 to 5 years.
Elaboration of a scenario for exposure to infants mouthing polyurethane foam from pillows or other objects made of polyurethane foam using the figure of 7.8 ng TDI released/g foam (Gagné et al. 2003) resulted in an estimated intake of 0.1 ng/kg-bw per day. This is an overestimate of the exposure because the extraction conditions in mouthing are much milder than those used by Gagné et al. (2003). Note that this estimate of exposure does not account for the reactivity of TDI with saliva. It is anticipated that a much smaller quantity of TDI will be available for ingestion than the 0.1 ng/kg-bw per day estimated for this scenario. The estimated intake of 0.1 ng/kg-bw per day from the foam mouthing scenario is less than 0.1% of the estimated intake from ambient and indoor air for the age group 6 months to 4 years and is therefore considered to be negligible.
Exposure scenarios are presented in Appendix 2.
The confidence in the exposure database is considered to be very low. No data on the concentration of toluene diisocyanate in any environmental medium used to estimate human exposure were located. Confidence in the estimate of exposure from the use of consumer products is low because of uncertainty about consumer access to urethane surface coatings containing TDI.
Health Effects Assessment
Appendix III contains a summary of the available health effects information for toluene diisocyanate. The majority of the available data for TDI is on the mixture of isomers.
The International Agency for Research on Cancer (IARC) classified toluene diisocyanate as "possibly carcinogenic to humans" (Carcinogenicity Group 2B), based on inadequate evidence for the carcinogenicity of toluene diisocyanates in humans and sufficient evidence for the carcinogenicity of toluene diisocyanates in experimental animals (IARC 1986; IARC 1999). TDI has also been classified by the European Commission (EC) as Category 3 for carcinogenicity (“causes concerns for humans owing to possible carcinogenic effects”) (European Commission 1997, 2004; ESIS v.4.50 2006); and by the United States National Toxicology Program (NTP) as “reasonably anticipated to be a human carcinogen” (NTP 2005). These classifications were based on significantly increased tumour incidences at multiple sites in mice and rats exposed orally by gavage for 2 years. Female mice exposed orally to 60 or 120 mg/kg-bw/day TDI had significantly increased hepatocellular adenomas and combined hemangiomas (spleen, subcutaneous) and hemangiosarcomas (liver, ovaries, peritoneum) (NTP 1983). Female rats exposed orally to 60 or 120 mg/kg-bw/day TDI had significantly increased mammary gland fibroadenomas and pancreatic islet cell adenomas; male rats exposed to 30 or 60 mg/kg-bw/day TDI had increased subcutaneous fibromas and fibrosarcomas (significant when combined), and pancreatic acinar cell adenomas (significant at the high dose) (NTP 1983). In each study, the increased tumours were observed at the lowest exposure level tested. Male mice exposed to up to 240 mg/kg-bw/day TDI did not develop exposure-related tumours (NTP 1983).
Exposure-related tumours were not observed in mice and rats exposed via inhalation to 0.36 and 1.07 mg/m3 TDI for 2 years (Loeser 1983). The International Programme on Chemical Safety (IPCS 1987) noted that the animals in this study were probably not given the maximum tolerated dose and therefore the highest dose may not have been sufficient to detect a response. However, this was based on body weight changes, not on other effects. There was also an unexplained high mortality rate in control and treated animals, reducing the sensitivity of the bioassay. Although Loeser (1983) reported a significant increase in mortality of female mice at both doses, the United States Environmental Protection Agency (US EPA 1995) concluded that there were no exposure-related effects observed on mortality, organ weights or clinical chemistry parameters in rats or mice. Histopathological findings from the rat nasal passages from this study were reported by Owen (1984). The lowest-observed-adverse-effect concentration (LOAEC) was 0.36 mg/m3 in rats based on a dose-related increase in the incidence and severity of chronic and necrotic rhinitis in female rats. Similar effects were reported in mice but there was insufficient detail to establish effect levels.
IARC (1999) concluded that in humans, there was no strong association or consistent pattern regarding the carcinogenicity of inhalation exposure to TDI from three industrial cohort studies and a population-based case-control study (Hagmar et al 1993a and 1993b; Sorahan and Pope 1993; Schnorr et al.1996). These studies may be of limited value due to the small sample sizes, relatively short follow-up, young cohorts and lack of consideration of smoking (Bolognesi et al 2001). In the review by Bolognesi et al (2001), it was concluded that “…the available human evidence and the experimental data are inadequate to evaluate the carcinogenic risk of TDI for humans."
Although more recent epidemiological studies are available (Kerr et al. 2000; Mikoczy et al. 2004; Sorahan and Nichols 2002; Clark et al 2003), no international assessments are yet available on these updated data and full weight of evidence analyses of the overall epidemiological data are not practicable within the context of this screening assessment.
Based on the oral carcinogenicity study in male rats outlined above (NTP 1983, 1986), the California EPA has calculated an oral cancer slope factor for TDI, and a related inhalation cancer unit risk factor for TDI using cross-route extrapolation and California EPA’s Expedited Proposition 65 methodology (California EPA 1999).
Mixed results have been obtained for TDI in in vivo and in vitro genotoxicity assays (Appendix 3). In vivo, TDI induced sex-linked mutations in Drosophila(oral exposure) but did not induce micronuclei in mouse or rat erythrocytes (inhalation exposure). In one study of human exposure, workers exposed to a mixture of TDI isomers in air had a statistically significant increase in structural chromosome aberrations, sister chromatid exchange, and micronuclei in peripheral blood lymphocytes relative to controls (Bilban 2004). However, this study had a small sample size, subjects may have been occupationally exposed to numerous chemicals in addition to TDI, and there was no consideration for smoking. Studies with smaller sample sizes indicated negative results for DNA strand breaks in human lymphocytes of workers (Marczynski et al. 2003, 2005). In vitro, TDI induced mutations, DNA damage, chromosomal aberrations and sister chromatid exchanges in some studies but not in others. Bolognesi et al. (2001) indicated that the stability of the applied diisocyanate was not studied in the in vitrostudies, and that the reacting species may not be the same between the in vitro and in vivo studies. However, the authors concluded that TDI was “probably genotoxic” due to TDI’s potential to react with DNA.
The primary target organs for non-neoplastic effects of exposure to TDI by the inhalation, dermal, and oral routes were the lung and respiratory tract. Inhalation and dermal exposure to TDI lead to respiratory hypersensitivity. In a review on dermal exposure to isocyanates, it is concluded that multiple lines of evidence from animal, human, and biomarker studies indicate that in certain exposure settings, human skin is likely an important route of isocyanate exposure and can contribute to the development of isocyanate asthma (Bello et al 2007).. TDI has been classified for dermal and inhalation sensitization (“may cause sensitization by inhalation or skin contact”) by the European Union (European Commission 1997, 2004; ESIS 2006). Exact exposure conditions and concentrations leading to sensitization have not been determined. However, in experimental animals, single challenge exposures at levels as low as 0.03 mg/m3 induced respiratory hypersensitivity in previously sensitized guinea pigs (Karol et al. 1981), and in humans, challenge at 1 ppb (0.007 mg/m3) induced an asthmatic response in previously sensitized humans (Lemière et al. 2002; Ott et al. 2003). This was observed in humans who had previously been exposed occupationally to TDI, whereas in humans with non-occupational asthma, sensitization to TDI in controlled experiments occurred as low as 10 ppb (0.07 mg/m3;) (Ott et al. 2003). Thus, based on both human and experimental animal data, values in the range of 0.007 to 0.07 mg/m3; are considered to be the critical effect levels for acute exposure in this assessment.
Note that occupational asthma has been reported from excessive exposure to diisocyanates in polyurethane production facilities (Cragg 2001). Also, there is evidence to show that asthmatic symptoms may continue in one-half of patients with TDI-asthma who avoided occupational exposure for more than 2 yr and had received anti-asthma medications (Krone and Klingner 2005).
The lowest observed effect concentration (LOEC) for repeated inhalation exposure to TDI was 1.9 ppb (0.014 mg/m3Human Equivalent Concentration derived by the US EPA (1995)) based on significantly decreased lung function in humans in a 5-year prospective occupational study (Diem et al. 1982). This was the critical study used for determination of the chronic inhalation reference concentration (RfC) by the US EPA (1995). The value of 0.014 mg/m3 is considered to be a critical effect level for chronic exposure in this assessment. Several other long-term epidemiological studies were reviewed by the US EPA. However, the study by Diem et al. (1982) was selected as the only study from which a no-observed-effect concentration (NOEC) (0.006 mg/m3) and a LOEC could be determined. The EPA noted difficulties and uncertainties associated with quantitation of TDI exposure in epidemiological studies. Effects on the respiratory system were also observed in experimental animals exposed via inhalation to TDI levels 5- to 26-fold higher than 0.014 mg/m3 (LOAEC = 0.36 mg/m3 in chronic rat study; LOEC = 0.07 mg/m3 in 5-day guinea pig study).
The lowest-observed-effect level (LOEL) for oral exposure to TDI was 30 mg/kg-bw/day based on bronchopneumonia, decreased body weight gain and mortality in rats exposed by gavage for 2 years (NTP 1986). Microscopic changes in the lungs and trachea were also observed at this exposure level in a 28-day rat study (Japanese MHLW 2005).
The confidence in the toxicity database is moderate as data for acute toxicity, repeated dose toxicity, carcinogenicity, genetic toxicity and reproductive and developmental toxicity are available, although limited in nature.
Although a thorough analysis of the mode of action of TDI is beyond the scope of this screening level assessment, it is recognized that the route of exposure to TDI may influence tumourigenesis. By oral exposure, TDI undergoes hydrolysis in the stomach to form toluene diamine (TDA). By inhalation exposure, TDI is absorbed in the upper respiratory tract and results in the formation of acid-labile conjugates with little diamine formed. The differential formation of TDA via the two routes of exposure may contribute to the mechanism by which TDI was carcinogenic in mice and rats by oral but not inhalation exposure (Doe and Hoffman 1995; IARC 1999; Collins 2002). However, the potential occurrence of neoplasms from inhalation exposure cannot be discounted, as it is uncertain whether maximum tolerable doses were attained in the chronic inhalation studies.
Characterization of Risk to Human Health
Based principally on weight of evidence based assessments by several international and national agencies (IARC, EU and US NTP), as well as an independent review by a multinational group of experts (Bolognesi et al, 2001), the available human epidemiological data and the experimental inhalation animal data are equivocal and thus inadequate to determine the carcinogenic risk of inhalation exposure to TDI in humans. Applying a precautionary approach, TDI is considered to be carcinogenic, since oral dosing in animals was associated with appearance of tumours at multiple sites. Mixed results have been obtained for TDI in in vivo and in vitro genotoxicity assays. Therefore, although the mode of induction of tumours has not been fully elucidated, it cannot be precluded that the tumours observed in experimental animals resulted from direct interaction with genetic material. Note that a similar approach was taken by the California EPA in its assessment in that they extrapolated the oral rat carcinogenicity study data to calculate a TDI inhalation cancer unit risk factor (California EPA 1999).
For non-cancer effects, TDI has been classified as a dermal and respiratory sensitizer by the European Union. With respect to non-cancer effects, comparison of the critical effect level for chronic non-neoplastic effects via inhalation in humans (i.e. 14 µg/m3) and the conservative upper-bounding exposure estimate via inhalation for TDI (1.06 µg/m3), in the vicinity of a plant using TDI, results in a margin of exposure of approximately 13.
In this assessment, values in the range of 0.007 to 0.07 mg/m3; are considered to be the critical effect levels for acute exposure. Also, the lowest LOEC determined for short-term to subchronic non-neoplastic effects is 0.07-0.14 mg/m3; based effects on the respiratory system in a 5-day guinea pig, a 6-month rat, and a 10-month dog study, respectively. This range of 0.07-0.14 mg/m3; will be used for the general human population. Note that these levels are 2.5-150 fold below the concentrations used in the long term inhalation studies in rats and mice (0.36 and 1.07 mg/m3).
Comparison of the critical effect levels for acute non-neoplastic effects via inhalation in sensitized humans (i.e. 7-70 µg/m3 ) and the conservative upper-bounding exposure estimate via inhalation for TDI from consumer products (i.e. 24 µg/m3 ) results in margins of exposure of <1 to 2.9.
Comparison of the critical effect levels for short term to subchronic non-neoplastic effects via inhalation in the general population (i.e. 70-140 µg/m3) and the conservative upper-bounding exposure estimate via inhalation for TDI from consumer products (i.e. 24 µg/m3 ) results in margins of exposure of 2.9-5.8.
Thus, the margins of exposure for non-neoplastic effects and exposure via inhalation to the general population and for consumer product exposure scenarios (although conservative in nature), are not adequate to account for uncertainties in the databases on exposure and effects
Uncertainties in Evaluation of Risk to Human Health
The estimate of human exposure to isomers of toluene diisocyanate is highly uncertain. No data on the concentration of TDI in environmental media representative of daily exposure were located for Canada or elsewhere. Modelling of environmental partitioning of TDI in Canada was not estimated because of the high degree of reactivity of the substance in water, sediments and moist soils; results of fugacity modelling would not be meaningful. Modelling of the concentration of TDI 100 metres from a plant producing polyurethane foam was used to give an upper-bounding estimate of the concentration of TDI in both ambient and indoor air for an exposed sub-population. The concentration of TDI in indoor air may be less than in ambient air in these circumstances. These simplifications may overestimate daily exposure.
Estimates of exposure arising from the use of consumer products are uncertain. The concentration of TDI in a range of consumer products is not known with certainty, but availability to Canadian consumers of a surface coating containing up to 3% TDI was established.
The scope of this screening assessment does not take into consideration a full analysis of the mechanism of action of TDI. There is uncertainty surrounding the mechanism of tumourigenesis of TDI following exposure. With regards to effects reported for previously sensitized humans, they may occur at a lower level of exposure than the effects reported for non-sensitized humans. However, effects do occur in short-term studies of non-sensitized experimental animals at exposures only one order of magnitude higher than those reported for sensitized humans.
Conclusion
Based on the available information, it is concluded that TDI is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the carcinogenicity of TDI, for which there may be a possibility of harm at any level of exposure, as well as the potential inadequacy of the margins of exposure for non-cancer effects, and applying a precautionary approach, it is concluded that TDI be considered as a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed that TDI does not meet the criteria in Paragraphs 64(a) and 64(b) of CEPA 1999, but it does meet the criteria in Paragraph 64(c) of CEPA 1999. Additionally, TDI does not meet criteria for persistence and bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations.
ACC (American Chemistry Council), Diisocyanates Panel. 2005. An Assessment of Persistence, Bioaccumulation and Inherent Ecotoxicity of Aromatic Diisocyanate Substances on the Canadian Domestic Substances List (DSL). In cooperation with: Gilbert International, Ltd. Arlington, VA. Jan. 10 2005.
Allport DC, Gilbert DS, Outterside SM, editors. 2003. MDI and TDI: Safety, health and the environment: a source book and practical guide. Chichester (GB).
Andersen M, Binderup M-L, Kiel P, Larsen H, Maxild J. 1980. Mutagenic action of isocyanates used in the production of polyurethanes. Scand J Work Environ Health 6:221-226.
Anderson D, Styles JA. 1978. The bacterial mutation test. Six tests for carcinogenicity. Br J Cancer 37(6):924-930.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337-345.
[ASTER] Assessment Tools for the Evaluation of Risk. 1999. Duluth (MN): US Environmental Protection Agency, Mid-Continent Ecology Division.
Atkinson R. 1989. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds. J Phys Chem Ref Data Monograph No 1. p.1-246.
Ban M, Morel G, Longonne I, Huguet N, Pepin E, Binet S. 2006. TDI can induce respiratory allergy with Th2-dominated response in mice. Toxicology 218: 39-47.
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Benoit, F. 2004. Degradation of polyurethane foams used in the Même breast implant. J Biomedical Materials Res 27:1341-1348.
Bello D, Herrick CA, Smith TH, Woskie SR, Streichner RP, Cullen MR, Liu YL, Redlich CA. 2006. Skin exposure to isocyanates: Reasons for concern. Environmental Health Perspectives 115: 328-335.
Beyer A, Mackay D, Matthies M, Wania, F, Webster, E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environ Sci Technol 34(4): 699-703.
Bilban M. 2004. Mutagenic testing of workers exposed to toluene-diisocyanates during plastics production process. Am J Ind Med 45(5):468-474.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Bolognesi C, Baur x , Marczynski B, Norppa H, Sepai O, Sabbioni G. 2001. Carcinogenic risk of toluene diisocyanate and 4,4'-methylenediphenyl diisocyanate: epidemiological and experimental evidence. Crit Rev Toxicol 31: 737-772.
Canada, Dept. of the Environment, Dept. of Health. 2007a.Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List (DSL). Canada Gazette Part I, Vol. 141, no. 3.
Canada, Dept. of the Environment, Dept. of Health. 2007b. Substance profiles for The Challenge: toluene diisocyanates; CAS RN 91-08-7, 2,6-diisocyanato-1-methyl-benzene (2,6 toluene diisocyanate); CAS RN 584-84-9, 2,4-diisocyanato-1-methyl-benzene (2,4-toluene diisocyanate); CAS RN 26471-62-5, 1,3-diisocyanatomethyl-benzene (mixed isomers of toluene diisocyanate).
Canada, Dept. of the Environment, Dept. of Health. 2006.Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette Part I, Vol. 140, no. 49.
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette Part II, Vol. 134, no. 7.
Canada. 1999. Canadian Environmental Protection Act, 1999 = Loi canadienne sur la protection de l’environnement, 1999. Statutes of Canada = Statuts du Canada, Chapter 33, part III. Canada Gazette Part III, Vol. 22, no. 3.
[CalEPA] California Environmental Protection Agency. 1999. Air Toxics Hot Spots Pr ogram Risk Assessment Guidelines: Part II. Technical Support Document for Describing Available Cancer Potency Factors. Berkley (CA): Office of Environmental Health Hazard Assessment.
[CFIA] Canadian Food Inspection Agency. 2007. Reference listing of accepted construction materials, packaging materials and non-food chemical products. Accessed July 2007.
Chapman JF. 1994. European Emissions Project, Phase 1(A) - Gas Emissions Study - A rationale. Manchester (GB): Gilbert International Isocyanates. Report No.: 11157 (cited in Tury et al. 2003).
Clark RL, Bugler J, Paddle GM, Chamberlain JD, Allport DC. 2003. A 17-year epidemiological study on changes in lung function in toluene diisocyanate foam workers. Int Arch Occup Environ Health 76: 295-301.
Collins MA. 2002. Toxicology of toluene diisocyanate. Appl Occup Envir Hyg 17: 846-855.
Cragg, ST. 2001. Polyurethanes, miscellaneous organic polymers, and silicones. In Allan, RE et al, editors & contributors. Patty's industrial hygiene and toxicology [book on the internet]. John Wiley and Sons.
Curtis, M.W., Copeland, T.L. and C.H. Ward. 1978. Aquatic toxicity of substances proposed for spill prevention regulation. In: Proc. Natl. Conf. Control of Hazardous Material Spills, Miami Beach, FL: 99-103.
Damant AP, Jickells SM, Castle L. 1995. Liquid chromatographic determination of residual isocyanate monomers in plastics intended for food contact use. J of AOAC Int 78(3): 711-719.
Danish Environmental Protection Agency. 1998. Immobilization test of aniline compounds with the crustacean Daphnia magna. Project no. 303587, Danish EPA, Copenhagen, Denmark. [Cited in ACC 2005].
Descotes, J. 1988. Identification of contact allergens: the mouse ear sensitization assay. J Toxicol-Cut Ocular Toxicol 7:263-272.
Diem JE, Jones RN, Hendrick DJ, Glindmeyer HW, Dharmarajan V, Butcher BT, Salvaggio JE, Weill H. 1982. Five-year longitudinal study of workers employed in a new toluene diisocyanate manufacturing plant. Am Rev Respir Dis 126(3):420-428.
Doe JE, Hoffman HD. 1995. Toluene diisocyanate: An assessment of carcinogenic risk following oral and inhalation exposure. Toxicol Ind Health 11:13-32.
Duff PB. 1983. The fate of TDI in the environment. Proceedings of the Society of the Plastic Industry's 6th International Technical Conference, San Diego, California. New York (NY): Society of Plastics Industry. pp. 408-412.
Ebino K, Ueda H, Kawakatsu H, Shutoh Y, Kosaka T, Nagayoshi E, Lemus R, Karol MH. 2001. Isolated airway exposure to toluene diisocyanate results in skin sensitization. Toxicol Lett 121: 79-85.
[ECB] European Chemicals Bureau. 2004. Risk assessment report for Toluene-2,4-diamine (CAS #95-80-7). Ispra (IT): European Chemicals Bureau.
Ellendt K, Gutsche B, Steiner G. 2003. Analysis of Laminates - Determination of Isocyanate Residues and Primary Aromatic Amine Migration. Deutsches Lebensmittel-Rundschau 99(4):131-136.
Environ Corporation International. 2003. VCCEP Report - Tier 1 Assessment of the potential health risks to children associated with exposure to the commercial pentabromodiphenyl ether product: prepared for Great Lakes Chemical, USA.
[ESIS] European Chemical Substances Information System [database on the Internet]. 2006. CAS No. 91-08-7. 2-methyl-m-phenylene diisocyanate. CAS No. 584-84-9. 4-methyl-m-phenylene diisocyanate. CAS No. 26471-62-5. M-tolylidene diisocyanate. Version 4.50. European Chemical Bureau (ECB).
Estlander T, Keskinen H, Jolanki R, Kanerva L. 1992. Occupational dermatitis from exposure to polyurethane chemicals. Am J Contact Dermat 27:161-165.
European Commission. 1997. Summary Record - Toluene di-isocyanates. Commission Working Group on the Classification and Labelling of Dangerous Substances. Meeting at ECB Ispra, 15-21 October 1997. Report No.:ECBI/51/97 - Rev. 3.
European Commission. 2004. 2-methyl-m-phenylene diisocyanate. 4-methyl-m-phenylene diisocyanate. M-tolylidene diisocyanate. Commission Directive 2004/73/EC of April 29, 2004. Annex 1A. Official Journal of the European Union. 16.6.2004. L 216/49. European Commission. 29th ATP.
Foureman P, Mason JM, Valencia R, Zimmering S. 1994. Chemical mutagenesis testing in Drosophila. X. Results of 70 coded chemicals tested for the National Toxicology Program. Environ Mol Mutagen 23(3):208-227.
Gad,SC, Dunn BJ, Dobbs DW, Reilly C, Walsh RD. 1986. Development and validation of an alternative dermal sensitization test: the mouse ear swelling test (MEST). Toxic appl Pharmac 84:93-114.
Gagnaire F, Ban M, Cour C, Micillino JC, Bonnet P, Hettich D. 1997. Role of tachykinins and neutral endopeptidase in toluene diisocyanate-induced bronchial hyperresponsiveness in guinea pigs. Toxicology 116(1-3):17-26.
Gagné S, Lesage J, Ostiguy C, Van T H. 2003. Determination of unreacted 2,4-toluene diisocyanate (2,4TDI) and 2,6-toluene diisocyanate (2,6TDI) in foams at ultratrace level by using HPLC-CIS-MS-MS. Analyst 128:1447-51.
Goossens A, Detienne T, Bruze M. 2002. Occupational allergic contact dermatitis caused by isocyanates. Am J Contact Dermat 47:304-308.
Gulati DK, Witt K, Anderson B, Zeiger E, Shelby MD. 1989. Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. III. Results with 27 chemicals. Environ Mol Mutagen 13(2):133-193.
Hagmar L, Welinder H, Mikoczy Z. 1993a. Cancer incidence and mortality in the Swedish polyurethane foam manufacturing industry. Br J Ind Med 50(6):537-543.
Hagmar L, Stromberg U, Welinder H, Mikoczy Z. 1993b. Incidence of cancer and exposure to toluene diisocyanate and methylene diphenyldiisocyanate: a cohort based case-referent study in the polyurethane foam manufacturing industry. Br J Ind Med 50(11):1003-1007.
Harton EEJr, Rawl RR. 1976. Toxicological and skin corrosion testing of selected hazardous materials. Springfield (VA): US Department of Commerce.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Environmental Health Directorate, Health Canada.
Heimbach F, Jaeger K, Sporenberg W. 1996. Fate and Biological Effects of Polymeric MDI (4,4-diphenylmethane diisocyanate and homologues) in small artificial ponds. Ecotox Environ Safety 33:143-153.
Henschler D, Assmann W, Meyer K-O. 1962. Toxicology of toluene diisocyanate. Arch Toxikol 19:364-387.
Holdren MW, Spicer CW, Riggin RM. 1984. Gas Phase Reaction of Toluene Diisocyanate with Water Vapour. Am Ind Hyg Assoc J 45(9):626-633
Holness DL, Nethercott JR. 1997. Results of patch testing with a specialized collection of plastic and glue allergens. Am J Contact Derm 8:121-124.
Huang J, Wang XP, Ueda A, Aoyama K, Chen BM, Matsushita T. 1991. Allergologic evaluation for workers exposed to toluene diisocyanate. Ind Hlth 29:85-92.
Hugo JM, Spence MW, Lickly TD. 2000. The Determination of the Ability of Polyurethane Foam to Release Toluene Diisocyanate into Air. Appl. Occ Env Hygiene 15(6): 512-519.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
IAL Consultants. 2007. End-use market survey on the polyurethane industry in the US, Canada and Mexico. Alliance for the Polyurethanes Industry.
[IARC] Working Group on the Evaluation of Carcinogenic Risks to Humans. 1986. Some Chemicals Used in Plastics and Elastomers. IARC Monogr Eval Carcinog Risks Hum. 39: 287-332.
[IARC] Working Group on the Evaluation of Carcinogenic Risks to Humans. 1999. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. IARC Monogr Eval Carcinog Risks Hum. 71 Pt 2: 865-879.
[IPCS] International Programme on Chemical Safety. 1987. Toluene Diisocyanates. Geneva (CH): World Health Organization. (Environmental Health Criteria 75). Jointly sponsered by the United Nations Environment Programme, the International Labour Organization, and the World Health Organization.
[IPCS] International Programme on Chemical Safety. 1997.Poison Information Monograph : Toluene-2,4-diisocyanate.
[IUCLID] International Uniform Chemical Information Database. 2000. CAS RN. 26471-62-5. m-tolyidene diisocyanate. European Commission. European Chemicals Bureau.. Creation date: 2000 February 18.
Jarand CW, Akapo SO, Swenson LJ, Kelman BJ. 2002. Diisocyanate emission from a paint product: a preliminary analysis. Appl Occup Environ Hyg 17(7):491-494.
Jeong YC, Kim DH, Kim BR, Park M. 1998. In vitro and in vivo reactions of 2,4-toluene diisocyanate with DNA and blood proteins. J Toxicol Sci 23 Suppl 4:660-661.
Kanerva L, Jolanki R, Alanko K, Estlander T. 1999. Patch-test reactions to plastic and glue allergens. Acta derm.-vener Stockh 79:296-300.
Kanerva L, Estlander T, Jolanki R, Keskinen H. 2001. Asthma from diisocyanates is not mediated through a Type IV, patch-test-positive mechanism. Am J Contact Dermat 44:247-248.
Karlsson D, Spanne N, Dalene M, Skarping G. 2000. Airborne thermal degradation products of polyurethane coatings in car repair shops. J Environ Monit 2:462-469.
Karol MH, Hauth BA, Riley EJ, Magreni CM. 1981. Dermal contact with toluene diisocyanate (TDI) produces respiratory tract hypersensitivity in guinea-pigs. Toxicol appl Pharmacol 58: 221-230 [cited in IPCS 1987].
Katsuyama Y, Shinohara N, Kumagai K, Fujii M, Yanagisawa Y. 2003. Emission of diisocyanates in indoor air. Proceedings of ISIAQ 7th International Conference, December 2003.
Kelly TJ, Myers JD, Holdren MW. 1999. Testing of household products and materials for emission of toluene diisocyanate. Indoor Air 9:117-124.
Kerr MA, Nascav PC, Mundt KA, Michalek AM, Baptiste, Mahoney MC. 2000. Parental occupational exposures and risk of neuroblastoma: a case-control study (United States). Cancer Causes Control 11:635-643.
Krone CA, Ely JTA, Klingner T, Rando RJ. 2003. Isocyanates in flexible polyurethane foams. Bull Environ Contam Toxicol 70(2):328-335.
Krone CA, Klingner T. 2005. Isocyanates, polyurethane and childhood asthma (review article). Pediatr Allergy Immunol 16: 368-379.
Le Coz, C-J, El Aboubi S, Ball C. 1999. Active sensitization to toluene di-isocyanate. Contact Dermatitis 41:104-105.
Lemière C, Romeo P, Chaboillez S, Tremblay C, Malo JL 2002. Airway inflammation and functional changes after exposure to different concentrations of isocyanates. J Allergy Clin Immunol. 110(4):641-646.
Levine SP, Redinger CF, Robert WP. 2001. Community exposure assessment and intervention effectiveness at Trinity American Corporation, Glenola, North Carolina. Amer Ind Hyg Assoc J 62: 649-657.
Loeser E. 1983. Long-term toxicity and carcinogenicity studies with 2,4/2,6-toluene-diisocyanate (80/20) in rats and mice. Toxicol Lett 15(1):71-81.
Maestrelli P, Del Prete GF, De Carli M, D'Elios MM, Saetta M, Di Stefano A, Mapp CE, Romagnani S, Fabbri LM. 1994. CD8 T-cell clones producing interleukin-5 and interferon-gamma in bronchial mucosa of patients with asthma induced by toluene diisocyanate. Scand J Work Envir Hlth. 20:376-381.
Maki-Paakkanen J, Norppa H. 1987. Chromosome aberrations and sister-chromatid exchanges induced by technical grade toluene diisocyanate and methylenediphenyl diisocyanate in cultured human lymphocytes. Toxicol Lett 36(1):37-43.
Marand Å, Karlsson D, Dalene M, Skarping G. 2004. Extractable organic compounds in polyurethane foam with special reference to aromatic amines and derivatives thereof. Anal Chim Acta 510(1):109-119.
Marczynski B, Czuppon AB, Marek W, Baur X. 1992. Indication of DNA strand breaks in human white blood cells after in vitro exposure to toluene diisocyanate (TDI). Toxicol Ind Health 8(3):157-69.
Marczynski B, Merget R, Teschner B, Korn M, Rabstein S Bruning T. 2003. Changes in low molecular weight DNA fragmentation in white blood cells after diisocyanate exposure of workers. Archs Toxicol 77: 470-476.
Marczynski B, Merget R, Mensing T, Rabstein S, Kappler M, Bracht A, Haufs MG, Kafferlein HU, Bruning T. 2005. DNA strand breaks in the lymphocytes of workers exposed to diisocyanates: indications of individual differences in susceptibility after low-dose and short-term exposure. Archs Toxicol 79:355-362.
Marek W, Potthast J, Marczynski B, Mensing T, Baur X. 1999. Subchronic exposure to diisocyanates increases guinea pig tracheal smooth muscle responses to acetylcholine. Respiration 66(2):156-161.
McGregor DB, Brown AG, Howgate S, McBride D, Riach C, Caspary WJ. 1991. Responses of the L5178Y mouse Lymphoma cell forward mutation assay. V: 27 coded chemicals. Environ Mol Mutagen 17(3):196-219.
Meredith SK, Bugler J, Clark RL. 2000. Isocyanate exposure and occupational asthma: a case-referent study. Occup envir Med 57:830-836.
Mikoczy Z, Welinder H, Tinnerberg H, Hagmar L. 2004. Cancer incidence and mortality of isocyanate exposed workers from the Swedish polyurethane foam industry: updated findings 1959-98. Occup envir Med 61:432-437.
Militello G, Sasseville D, Ditre C, Brod BA. 2004. Allergic contact dermatitis from isocyanates among sculptors. Dermatitis 15:150-153.
[MHLW] Ministry of Health Labour and Welfare (JP). 2005. Safety examination of existing chemicals and safety programmes in Japan (in Japanese with English summary).
[MITI] Ministry of International Trade & Industry (Jpn). 1992. Biodegradation and bioaccumulation: data of existing chemicals based on the CSCL Japan. Edited by Chemicals Inspection & Testing Institute (JP): The Ministry, Chemical Products Safety Division Basic Industries Bureau..
Moscato G, A. Dellabianca A, Corsico G, Biscaldi G, Gherson, Vinci G. 1993. Bronchial responsiveness to ultrasonic fog in occupational asthma due to toluene diisocyanate. Chest 104:1127-1132.
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2007. Gatineau (QC): Environment Canada [cited 2007].
[NTP] National Toxicology Program. 1983. NTP technical report on the toxicology and carcinogenesis studies of commercial grade 2,4 (80%)- and 2,6 (20%)-toluene diisocyanate (CAS No. 26471) in F344/N rats and B6C3F1 mice (gavage studies). Technical Report No. 251 (draft).
[NTP] National Toxicology Program. 1986. NTP technical report on the toxicology and carcinogenesis studies of commercial grade 2,4 (80%)- and 2,6 (20%)-toluene diisocyanate (CAS No. 26471) in F344/N rats and B6C3F1 mice (gavage studies). Technical Report No. 251.
[NTP] National Toxicology Program. 2005. 11th report on carcinogens. Substance profile: toluene diisocyanate.
[OASIS Forecast] Optimized Approach Based on Structural Indices Set [Internet] 2005. Version 1.20. Bourgas, Bulgaria: Laboratory of Mathematical Chemistry.
Ott MG, Diller WF, Jolly AT. 2003, Respiratory effects of toluene diisocyanate in the workplace: A discussion of exposure-response relationships. Critical Reviews in Toxicology 33:1–59.
Owen PE. 1984. The toxicity and carcinogenicity of toluene isocyanate vapour administered by inhalation for a period of 113 weeks. Addendum Report: Volume 2. Hazelton Laboratories Europe [cited in US EPA 1995].
Paggiaro, PL, Vagaggini B, Dente FL, Bacci E, Bancalari L, Carrara M, Di Franco A, Giannini D, Giuntini C. 1993. Bronchial hyperresponsiveness and toluene diisocyanate. Long-term change in sensitized asthmatic subjects. Chest 103:123-1128.
Park H.-S, Kim HY, Nahm DH, Son JW, Kim YY. 1999. Specific IgG, but not specific IgE, antibodies to toluene diisocyanate-human serum albumin conjugate are associated with toluene diisocyanate bronchoprovocation test results. J Allergy clin Immun 104:847-851.
Patterson R, Zeiss CR, Harris KE. 1983. Immunologic and respiratory responses to airway challenges of dogs with toluene diisocyanate. J Allergy Clin Immunol 71(6):604-611.
Peel M, Marczynski B, Baur X. 1997. Comparison of the binding potential of various diisocyanates on DNA in vitro. J Toxicol Environ Health 52(6):517-526.
Pemberton D, Tury, B. 2004. TDI industry risk assessment: sections on physico-chemical properties, environmental exposures, and environmental effects. Report 2004/E. Manchester (UK): Gilbert International Ltd.
Pisati F, Baruffini A, Zedda S. 1993. Toluene diisocyanate induced asthma: outcome according to persistence or cessation of exposure. Br J ind Med 50:60-64.
Schnorr TM, Steenland K, Egeland GM, Boeniger M, Egilman D. 1996. Mortality of workers exposed to toluene diisocyanate in the polyurethane foam industry. Occup Environ Med 53(10):703-707.
Seel K, Walber U, Herbold B, Kopp R. 1999. Chemical behaviour of seven aromatic diisocyanates (toluenediisocyanates and diphenylmethanediisocyanates) under in vitro conditions in relationship to their results in the Salmonella/microsome test. Mutat Res 438(2):109-123.
Shaddock JG, Robinson BY, Casciano DA. 1990. Effect of pretreatment with hepatic mixed-function oxidase inducers on the genotoxicity of four rat carcinogens in the hepatocyte/DNA repair assay. Mutagenesis 5(4):387-391.
Shiotsuka RN, Warren DL, Halliburton AT, Sturdivant DW. 2000. A comparative respiratory sensitization study of 2,4- and 2,6-toluene diisocyanate using guinea pigs. Inhal Toxicol 12: 605-615.
Sorahan T, Pope D. 1993. Mortality and cancer morbidity of production workers in the United Kingdom flexible polyurethane foam industry. Br J Ind Med 50(6):528-536.
Tadokora H, et al. 1997. Ecotoxicities of TDI and TDA to fish, algae and aquatic invertebrates. IN: Chemicals Inspection and Research Institute, Japan. III Report No. 11217
[TaPL3] Long Range Transport and Persistence Level III model[Internet]. 2000. Version 2.10. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre.
Thorne, PS, Hillebrand JA, Lewis GR, Karol MH. 1987. Contact sensitivity by diisocyanates: potencies and cross-reactivities. Toxic Appl Pharmac 87:155-165.
[TOPKAT] Toxicity Prediction Program [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc.
Tury B, Pemberton D, Bailey RE. 2003. Fate and potential environmental effects of methylenediphenyl diisocyanate and toluene diisocyanate released into the atmosphere. J Air Waste Manage Assoc 53:61-66.
Tyl RW. 1988. Developmental toxicity study of inhaled toluene diisocyanate vapor in CD (Sprague-Dawley) rats. Union Carbide, Bushy Run Research Center. Revised Project No. 50-592.
Tyl RW, Fisher LC, Dodd DE, Pritts IM, Kubena MF, Losco PE, Troup CM, Lyon JP, Landry TD. 1999a. Developmental toxicity evaluation of inhaled toluene diisocyanate vapor in CD rats. Toxicol Sci 52(2):248-257.
Tyl RW, Neeper-Bradley TL. 1989. Two-generation reproductive study of inhaled toluene diisocyanate in CD (Sprague-Dawley) rats. Union Carbide, Bushy Run Research Center. Project No. 86-33-90704.
Tyl RW, Neeper-Bradley TL, Fisher LC, Dodd DE, Pritts IM, Losco PE, Lyon JP, Landry TD. 1999b. Two-generation reproductive toxicity study of inhaled toluene diisocyanate vapor in CD rats. Toxicol Sci 52(2):258-268.
Ulrich H. 1996. Chemistry and Technology of Isocyanates. Chichester (GB): John Wiley & Sons, Ltd.
[US EPA] US Environmental Protection Agency. 1995. 2,4-/2,6-Toluene Diisocyanate mixture (TDI). Washington (DC): US Environmental Protection Agency, Integrated Risk Information System (IRIS).
[US EPA] US Environmental Protection Agency. 2002. Child-specific exposure factors handbook. Interim report. Washington (DC): US Environmental Protection Agency, National Center for Environmental Assessment, Office of Research and Development.
Van der Hoeven N, Roza P, Henzen L. 1992a. Determination of the effect of TDI, TDA, MDI, and MDA on the emergence and growth of the plant species Avena sativa and Lactuca sativaaccording to OECD Guideline no. 208. TNO. III Report no. 11024. [Cited in Pemberton and Tury 2004].
Van der Hoeven N, Roza P, Henzen L. 1992b. Determination of the LC50 (14 days) of TDI, TDA, MDI, and MDA to the earthworm Eisenia fetida according to OECD guideline no. 207. TNO. III Report no. 11025. [Cited in Pemberton and Tury 2004].
Vanoirbeek, JA, Tarkowski JM, Ceuppens JL, Verbeken EK, Nemery B, Hoet PH. 2004. Respiratory response to toluene diisocyanate depends on prior frequency and concentration of dermal sensitization in mice. Toxic Sci 80:310-321.
Yakabe Y, Henderson KM, Thompson WC, Pemberton D, Tury B, Bailey RE. 1999. Fate of methylenediphenyl diisocyanate and toluene diisocyanate in the aquatic environment. Environ Sci Technol 33(15):2579-2583.
Zapp JA Jr. 1957. Hazards of isocyanates in polyurethane foam plastic production. Arch Ind Hlth 15(4):324-330.
Zissu D, Binet S, Limasset JC. 1998. Cutaneous sensitization to some polyisocyanate prepolymers in guinea pigs. Contact Dermatitis 39:248-251.
| Route of exposure | Estimated intake (µg/kg-bw per day) of TDI by various age groups | ||||||
|---|---|---|---|---|---|---|---|
| 0-6 months [1] | 0.5-4 years [3] | 5-11 years [4] | 12-19 years [5] | 20-59 years [6] | 60+ years [7] | ||
| formula fed [2] | not formula fed | ||||||
| [1] Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed) and to ingest 30 mg of soil per day (Health Canada, 1998). [2] For formula-fed infants, intake from water is synonymous with intake from food. No data on concentrations of TDI in formula were identified for Canada. [3] Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day and to ingest 100 mg of soil per day (Health Canada 1998). [4] Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day and to ingest 65 mg of soil per day (Health Canada 1998). [5] Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998). [6] Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998). [7] Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998). [8] An annual average concentration 100 metres from a polyurethane foam plant was calculated to be 1.06 µg/m3 (see Exposure Assessment, this report). Ambient air was assumed to be representative of exposure to indoor air, although use of certain consumer products may give rise to the release of toluene diisocyanate in indoor air. [9] No reports of measurement of toluene diisocyanate in drinking water were located. The half-life of toluene diisocyanate in water with a moderate degree of mixing is estimated to be 1–3 hours (Yakabe et al. 1999). It is therefore assumed that toluene diisocyanate is not present in drinking water. [10] No reports of measurement of toluene diisocyanate in food or beverages were located. Reports of the migration of toluene diisocyanate from food packaging were located, but they are not indicative of the concentration of TDI in food and beverages. [11] No reports of measurement of toluene diisocyanate in soil were located. The half-life of toluene diisocyanate in moist soil has been experimentally determined to be less than one hour (Duff, 1983). TDI is not considered to be persistent and except at the site of an industrial spill, is not expected to be present on soil. |
|||||||
| Air [8] | 0.30 | 0.64 | 0.50 | 0.28 | 0.24 | 0.21 | |
| Drinking water [9] | _ | _ | _ | _ | _ | _ | _ |
| Food and beverages [10] | _ | _ | _ | _ | _ | _ | |
| Soil [11] | _ | _ | _ | _ | _ | _ | |
| Total intake | 0.30 | 0.30 | 0.64 | 0.50 | 0.28 | 0.24 | 0.21 |
| Endpoint | Lowest effect levels / Results |
|---|---|
| [*] Data is for the mixture of isomers unless otherwise stated. | |
| Laboratory animals and in vitro | |
| Acute toxicity | Lowest oral LD50 (rat): 3060 mg/kg (Harton and Rawl 1976, cited in IPCS 1987) Additional studies: Woolrich 1982, cited in IPCS 1987 Lowest inhalation LC50 (rat) = 57 mg/m3 (Harton and Rawl 1976, cited in IPCS 1987) Additional studies: Duncan et al 1962, Bunge et al 1977, both cited in IPCS 1987. Lowest dermal LD50 (rabbit) = 10 000 mg/kg (Harton and Rawl 1976, cited in IPCS 1987) |
| Short-term repeated-dose toxicity | Lowest oral LOEL (rat, 28 days): 30mg/kg-bw/day based on microscopic changes in the lungs and trachea (Japanese MHLW 2005 - study summary only). Additional studies: Zapp 1957, cited in IPCS 1987; NTP 1986. Lowest inhalation LOEC (guinea pig, 5 days): 0.07mg/m3 based on increased responsiveness of tracheal smooth muscle (Marek et al. 1999) Numerous additional studies |
| Subchronic toxicity | Lowest oral LOEL (rat, mouse, 13 weeks) : 120mg/kg-bw/day based on bronchopneumonia and decreased body weight in rat; liver inflammation and necrosis in mouse (NTP 1986) Lowest inhalation LOEC (rat, 27 weeks; dog, 10 months): 0.14mg/m3 based on histological changes in nasal cavity and upper respiratory tract of F1 generation rats (Tyl and Neeper-Bradley 1989, cited in US EPA 1995; published as Tyl et al 1999b); elevated antibody titres, decreased pulmonary function and immediate skin reaction in dogs (Patterson et al 1983, cited in IPCS 1987) Additional studies: Gagnaire et al. 1997; Henschler et al. 1962, cited in IPCS 1987; Friebel 1955, cited in IPCS 1987; Zapp 1957, cited in IPCS 1987 |
| Chronic toxicity/ carcinogenicity | Oral cancer bioassay in rats: Oral cancer bioassay in mice: Inhalation cancer bioassay in mice and rats: Rats were exposed for 6 hours/day, 5 days/week for 104 weeks, at 0, 0.36 or 1.07 mg/m3 for 108 weeks (males) or 110 weeks (females). There was no significant difference in tumour incidences and types between dosed and control groups (Loeser 1983, cited in IARC 1986). There were dose-related increases in the incidence and severity of chronic and necrotic rhinitis in female rats. Lowest non-neoplastic oral (gavage) LOEL (rat, 105–106 weeks): 30 mg/kg-bw/day (lowest dose tested) based on acute bronchopneumonia, decreased body weight gain and mortality (NTP 1986, cited in IPCS 1987). Lowest non-neoplastic inhalation LOAEC: 0.36 mg/m3 based on significantly increased chronic and necrotic rhinitis in female rats. |
| Developmental toxicity | Lowest inhalation LOEC : 0.5ppm (3.57mg/m3) based on a single skeletal variation, which was noted to be a common variation and possibly not significant relative to historical controls. In this study, pregnant rats were exposed to TDI vapour for 6 h/day during gestation days 6–15. (Tyl 1988, cited in US EPA 1995; published as Tyl et al. 1999a) |
| Reproductive toxicity | Lowest inhalation NOEC : No effect of exposure on any reproductive organs or parameters at the highest dose level of 0.3 ppm (2.14mg/m3). In this study, male and female rats were exposed to TDI vapour for 6 h/day, 5 days/wk, then 7 days/wk during a 3-wk mating period, gestation and lactation in both generations of a 2-generation reproduction study (Tyl and Neeper-Bradley 1989, cited in US EPA 1995; published as Tyl et al. 1999b). |
| Genotoxicity and related endpoints: in vivo | Sex-linked recessive lethal assay Micronucleus assay |
| Genotoxicity and related endpoints: in vitro | Mutagenicity in Salmonella typhimurium: Mutagenicity in rodent cell lines : Chromosomal aberrations: Unscheduled DNA synthesis Sister chromatid exchange DNA strand breaks: DNA binding |
| Sensitization | Numerous qualitative studies (e.g., patch tests in humans, Buehler tests in guinea pigs, ear-swelling tests in mice) indicate that TDI is a dermal sensitizer. In addition, dermal exposure to TDI can induce respiratory sensitivity (Descotes 1988; Estlander et al. 1992; Gad et al. 1986; Goossens et al. 2002; Holness and Nethercott 1997; Huang et al. 1991; Kanerva et al. 1999, 2001; Le Coz et al. 1999; Maestrelli et al. 1994; Mandervelt et al. 1997; Meredith et al. 2000; Militello et al. 2004; Moscato et al. 1993; Paggiaro et al. 1993; Park et al. 1999; Pisati et al. 1993; Shiotsuka et al. 2000; Thorne et al. 1987; Vandenplas et al. 1993; Vanoirbeek et al. 2004; Zissu et al. 1998). One study suggests that inhalation exposure may also result in skin sensitization (Ebino et al. 2001). A single exposure may also cause an immediate or delayed asthmatic reaction in previously exposed individuals. Single challenge exposures at levels as low as 0.03mg/m3induced respiratory hypersensitivity in previously sensitized guinea pigs (Karol et al. 1981). |
| Epidemiological studies | |
| Chronic toxicity/ carcinogenicity | In humans, no strong association or consistent pattern (cancer) from 3 industrial cohort studies and a population-based case-control study (Sorahan and Pope 1993, Hagmar et al. 1993(a and b), Schnorr et al. 1996; all cited in IARC 1999) LOEC–non-neoplastic inhalation (human, 5 years): 1.9 ppb (0.014 mg/m3) based on decreased lung function in a prospective occupational study (Diem et al. 1982, cited in US EPA 1995). The decreased lung function was expressed as significant annual declines in forced expiratory volume over one second (FEV1) and forced expiratory flow at 25-75% (FEF 25–75). Occupational epidemiology studies Sensitization studies |
Page details
- Date modified: