Screening Assessment for the Challenge Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- (Disperse Orange 30)
Chemical Abstracts Service Registry Number 5261-31-4
Environment Canada
Health Canada
August 2009
Table of Contents
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- (Disperse Orange 30), Chemical Abstracts Service Registry Number 5261-31-4. This substance was identified as a high priority for screening assessment and included in the Challenge because it had been found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
The substance Disperse Orange 30 was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List. Therefore, this assessment focuses on information relevant to the evaluation of ecological risks.
Disperse Orange 30 is an organic substance that is used in Canada and elsewhere as a orange colorant dye mainly in textiles and fabric. The substance is not naturally produced in the environment. Between 1 000 and 10 000 kg of Disperse Orange 30 were imported into Canada in 2006 for use mainly as a colorant in the chemical product manufacturing, textile and fabric finishing and fabric coating mills industry. In 2005, fewer than four companies imported Disperse Orange 30 into Canada in the100-100 000 kg/year range. The quantity of Disperse Orange 30 imported into Canada, along with the potentially dispersive uses of this substance, indicate that it could potentially be released into the Canadian environment.
Based on reported use patterns and certain assumptions, most of the substance is expected to end up in solid waste disposal sites and a significant proportion is estimated to be released to sewer water (14.8%). Disperse Orange 30 is not expected to be soluble in water or to be volatile; it is expected to partition to particles because of its hydrophobic nature. For these reasons, after release to water, Disperse Orange 30 will likely end up mostly in sediments, and to a lesser extent, in agricultural soil that has been amended with sewage sludge. It is not expected to be significantly present in other media. It is also not expected to be subject to long-range atmospheric transport.
Based on its physical and chemical properties, Disperse Orange 30 is expected to be persistent in the environment (in water, sediment and soil). However, new experimental data relating to its bioaccumulation potential suggest that this dye has a low potential to accumulate in the lipid tissues of organisms. The substance therefore meets the persistence criteria but does not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. In addition, experimental toxicity data for Disperse Orange 30 as well as for chemical analogues suggest that the substance does not harm aquatic organisms exposed at low concentrations.
For this screening assessment, two conservative exposure scenarios were selected in which an industrial operation (user of the dye) and consumer use of products containing this substance resulted in discharge of Disperse Orange 30 into the aquatic environment. The predicted environmental concentrations in water were below the predicted no-effect concentration calculated for sensitive aquatic organisms.
This substance will be included in the upcoming Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, it is concluded that Disperse Orange 30 does not meet any of the criteria set out in section 64 of CEPA 1999.
Introduction
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006a), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of these substances identified as high priorities.
The substance Disperse Orange 30 was identified as a high priority for assessment of ecological risk as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on February 16, 2008 (Canada 2008). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the bioaccumulation, aquatic toxicity and uses of the substance were received.
Although Disperse Orange 30 was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE, and was not identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Therefore, this assessment focuses principally on information relevant to the evaluation of ecological risks.
Screening assessments under CEPA 1999 focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by applying a weight of evidence approach and precaution.
This screening assessment considers any new information on chemical properties, hazards, uses and exposure submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review documents, stakeholder research reports and from recent literature searches up to October 2008. Key studies were critically evaluated and generally only results from studies of good quality were used to reach conclusions, although other studies and modelling results may have been considered as part of the weight of evidence. When available and relevant, information presented in hazard assessments from other jurisdictions was also used. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Program at Health Canada and Environment Canada and it incorporates input from other programs within these departments. The assessment has undergone external written peer review. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. The critical information and considerations upon which the assessment is based are summarized below.
Substance Identity
For the purposes of this document, the substance propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- will be referred to as Disperse Orange 30 (which is its common name – CCI 2002). Information on substance identity is included in Table 1.
| Chemical Abstracts Service Registry Number (CAS RN) | 5261-31-4 |
|---|---|
| Domestic Substances List (DSL) name | Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- |
| National Chemical Inventories (NCI) namesTable note a | Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- (TSCA, DSL, AICS, PICCS, ASIA-PAC) 2-[N-(2-cyanoethyl)-4-[(2,6-dichloro-4-nitrophenyl)azo]anilino]ethyl acetate (EINECS, PICCS) Disperse Orange 30 (ENCS) C.I. disperse orange 030 (ECL) PROPANENITRILE, 3-[[2-(ACETYLOXY)ETHYL][4-[(2,6- DICHLORO-4-NITROPHENYL)AZO]PHENYLAMINO]- (PICCS) C.I. DISPERSE ORANGE 30, Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- (PICCS) |
| Other names | 2-[N-(2-cyanoethyl)-4-[(2,6-dichloro-4-nitrophenyl)azo]anilino]ethyl acetate; 3-[4-[(2,6-Dichloro-4-nitrophenyl)azo]-N-(2-hydroxyethyl)anilino]propionitrile, acetate (ester); 4-(2,6-Dichloro-4-nitrophenylazo)-N-(b-acetoxyethyl)-N-(b-cyanoethyl)aniline; 4-[(2,6-Dichloro-4-nitrophenyl)azo]-N-(cyanoethyl)-N-(acetoxyethyl)aniline; 4-[N-((b-Cyanoethyl)-N-b-acetoxyethyl)amino]-2',6'-dichloro-4'-nitroazobenzene; Benzenamine, N-(2-acetoxy)ethyl-N-(2-cyano)ethyl-4-[[(2,6-dichloro-4-nitro)phenyl]azo]-; C.I. disperse orange 030 ; C.I. Disperse Orange 30; Dianix Yellow Brown 2R-FS; Disperse Brown Yellow 2RFL; Disperse Orange 30; Disperse Orange S 4RL; Disperse Yellow Brown 2AE; Disperse Yellow Brown S 2RFL Dispersol Orange C-R; Fantagen Brown 4GL; Foron Yellow Brown S 2RFL; Foron Yellow Brown S 2RFLI; Golden-Brown Synten P 2RL; Kayalon Polyester Yellow Brown 2RL-S; Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4nitrophenyl)azo]phenyl]amino]- Propionitrile, 3-[p-[(2,6-dichloro-4-nitrophenyl)azo]-N-(2-hydroxyethyl)anilino]-, acetate ; Propionitrile, 3-[p-[(2,6-dichloro-4-nitrophenyl)azo]-N-(2-hydroxyethyl)anilino]-, acetate (ester) Serene Disperse Orange 20; Serilene Yellow Brown R-LS Sumikaron Yellow Brown S 2RL; Synten Gold Brown P 2RL; Synten Golden Brown P 2R; Synten Golden Brown P 2RL; Terasil Yellow Brown 2RFL; Tersetile Brown GRL; Tersetile Yellow Brown GRL; Tulasteron Fast Yellow Brown GR-C |
| Chemical group | Discrete organics |
| Chemical sub-group | Monoazo dye |
| Chemical formula | C19H17Cl2N5O4 |
| Chemical structure | 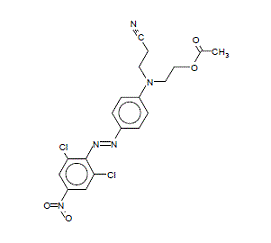 |
| Simplified Molecular Line Input Entry System (SMILES) | O=C(OCCN(c(ccc(N=Nc(c(cc(N(=O)(=O))c1)Cl)c1Cl)c2)c2)CCC(#N))C |
| Molecular mass | 450.28 g/mol |
Physical and Chemical Properties
Few experimental data are available for Disperse Orange 30. At the Environment Canada-sponsored Quantitative Structure-Activity Relationship (QSAR) Workshop in 1999 (Environment Canada 2000), invited modelling experts identified many structural classes of pigment and dyes as "difficult to model" using QSARs. The physical and chemical properties of many of the structural classes of dyes and pigments (including acid and disperse dyes) are not amenable to model prediction because they are considered "out of the model domain of applicability" (e.g., structural and/or property parameter domains). Therefore, to determine potential utility, the domains of applicability of QSAR models to dyes and pigments are evaluated on a case-by-case basis. It is generally considered inappropriate to use QSAR models to predict the physical and chemical properties of Disperse Orange 30. Consequently, a number of analogues were identified and "read-across" data has been used to determine the approximate physical and chemical properties in Table 2. These properties were subsequently used for further modeling and lines of evidence in this assessment.
An analogue is a chemical which is structurally similar to the substance under assessment and is therefore expected to have similar physical-chemical properties, behaviour in the environment and/or toxicity. Where there are experimental data for a given parameter for an analogue substance, these can be used directly or with adjustment as an estimate of that parameter value for the substance under assessment.
To find acceptable analogues, a review of data for several disperse azo dyes was performed (Anliker et al. 1981, Anliker and Moser 1987, Baughman and Perenich 1988, ETAD 1995, Brown 1992, Yen et al. 1989, Sijm et al. 1999). These compounds have structural similarities to Disperse Orange 30 but also share other important attributes that contribute to their suitability as analogues. This includes properties affecting their fate in the environment such as high molecular weights (generally greater than 300 g/mol), similar cross sectional diameters (1.31-2.05nm), solid particulate structures, decomposition at greater than 74 oC (to 240 oC), and "dispersibility" in water (i.e. not truly soluble). The presence of the ethanolamine grouping on the azo dye is meant to increase the dispersibility in water (Bomberger and Boughton 1984). In addition, they have limited solubility in n-octanol, a negligible vapour pressure and are stable under environmental conditions as they are designed to be so.
Table 2 contains experimental and modelled physical-chemical properties for Disperse Orange 30 and structural analogues that are relevant to its environmental fate.
| TypeTable note b | Value | Temperature (°C) | Reference | |
|---|---|---|---|---|
| Physical state | powder | Canada 2008 | ||
| Melting pointTable note c (°C) | Experimental | 126.9-128.5 | ETAD 2005 | |
| Melting pointTable note c (°C) | Read-across for disperse azo dyes | 117-175 | Anliker and Moser 1987 | |
| Melting pointTable note c (°C) | Read-across for disperse azo dyes | 74-236 | Baughman and Perenich 1988 | |
| Melting pointTable note c (°C) | Analogue Disperse Blue 79 | 157 | PhysProp 2006 | |
| Melting pointTable note c (°C) | Analogue Disperse Blue 79:1 | ≥138-153 | Sandoz Chemicals 1989, Yen et al. 1989 | |
| Boiling pointTable note d (°C) | Not Applicable | Not Applicable | Not Applicable | Not Applicable |
| Density (kg/m3) | Not Available | Not Available | Not Available | Not Available |
| Vapour pressure (Pa) | Analogue Disperse Blue 79 | 4.53×10-7 | Clariant 1996 | |
| Vapour pressure (Pa) | Read-across for disperse azo dyes | 5.33×(10-12 to 10-5) (4×10-14 to 4 × 10-7 mm Hg) |
25 | Baughman and Perenich 1988 |
| Henry's Law constant (Pa·m3/mol) | Read-acrossTable note e | 10-8 to 10-1 (10-13 to 10-6 atm·m3/mol) |
Baughman and Perenich 1988 | |
| Log Kow (octanol-water partition coefficient) (dimensionless) | Experimental | 4.2 | Brown 1992 | |
| Log Kow (dimensionless) | Analogue Disperse Blue 79:1 | 4.44, 4.8 | Sijm et al. 1999, Yen et al. 1989 | |
| Log Kow (dimensionless) | Analogue Disperse Blue 79 | 4.1, 4.3 | Clariant 1996, Brown 1992 | |
| Log Kow (dimensionless) | Read-across for disperse azo dyes | 1.79-5.1 | Baughman and Perenich 1988 | |
| Log Kow (dimensionless) | Read-across for disperse azo dyes | greater than 2 -5.1 | Anliker et al. 1981; Anliker and Moser 1987 | |
| Log Koc (organic carbon-water partition coefficient) (dimensionless) | Read-across, calculatedTable note f | 3.4 to 4.2 | Baughman and Perenich 1988 | |
| Water solubility (mg/L) | Experimental | 0.07 | Brown 1992 | |
| Water solubility (mg/L) | Read-across for disperse azo dyes | less than 0.01 | Anliker and Moser 1987 | |
| Water solubility (mg/L) | Read-across for disperse azo dyes | 1.2 × 10-5 to 35.5 (4 × 10-11 to 1.8 × 10-4 mol/L) |
Baughman and Perenich 1988 | |
| Water solubility (mg/L) | Analogue Disperse Blue 79 | 0.02, 0.000938, 0.0054 | 15-25 | Brown 1992, Baughman and Perenich 1988, Clariant 1996 |
| Water solubility (mg/L) | Analogue Disperse Blue 79:1 | 0.0052, 0.022 | 25 | Baughman and Perenich 1988, Sijm et al. 1999 |
| n-octanol solubility (mg/L) | Experimental | 576 | ETAD 2005 | |
| n-octanol solubility (mg/L) | Read-across for disperse azo dyes | 81-2100 | 20 | Anliker and Moser 1987 |
| pKa (acid dissociation constant) (dimensionless) | Modelled | 0.42 (base form) | ACD/pKaDB 2005 |
Structural disperse azo analogues to Disperse Orange 30 are presented in Tables 3a and 3b below. Certain physical and chemical properties (see Table 2), empirical bioaccumulation data (Table 6) and empirical toxicity data (see Table 7) of these analogues were used in support of the weight of evidence and decisions in this SAR. Specifically, data were obtained for the structural analogues: Disperse Blue 79, Disperse Blue 79:1, Disperse Red 17, Disperse Red 73, Disperse Orange 25 and Disperse Yellow 3 (Table 3a).
| CAS RN | Common Name | DSL name | Structure of analogue | Available empirical data |
|---|---|---|---|---|
| 12239-34-8 | Disperse Blue 79 | Acetamide, N-[5-[bis[2-(acetyloxy)ethyl]amino]-2-[(2-bromo-4,6-dinitrophenyl)azo]-4-ethoxyphenyl]- |  |
Melting point, vapour pressure, log Kow, water solubility, aquatic toxicity, |
| 3618-72-2 | Disperse Blue 79:1 | Acetamide, N-[5-[bis[2-(acetyloxy)ethyl] amino]-2-[(2-bromo-4,6-dinitrophenyl)azo]-4-methoxyphenyl ]- | 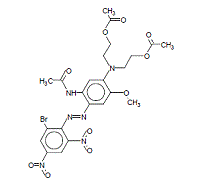 |
Melting point, log Kow, water solubility, , aquatic toxicity |
| 31482-56-1 | Disperse Orange 25 | 3-(Ethyl(4-((4-nitrophenyl)azo)phenyl)amino)propanenitrile |  |
Aquatic toxicity |
| 3179-89-3 | Disperse Red 17 | Ethanol, 2,2'-((3-methyl-4-(2-(4- nitrophenyl)diazenyl)phenyl)imino)bis- |
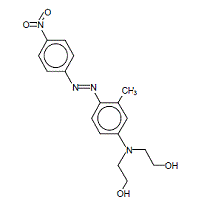 |
Aquatic toxicity |
| 16889-10-4 | Disperse Red 73 | 2-((4-((2-Cyanoethyl)ethylamino)phenyl)azo)-5- nitrobenzonitrile |
 |
Aquatic toxicity |
| 2832-40-8 | Disperse Yellow 3 | 4-(2-Hydroxy-5-methylphenylazo)acetanilide | 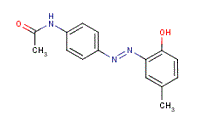 |
Aquatic toxicity |
It should be noted that there are several uncertainties associated with the use of physical and chemical-toxicological and bioaccumulation data available for the substances presented in Table 3a. All these substances belong to the same chemical class (disperse azo dyes with their characteristic azo bond) and are used for similar industrial purposes. However, there are structural differences between these substances associated with their unique functional groups (see Table 3b below) and for some their molecular size (especially for Disperse Orange 25, Disperse Red 17 and 73). As a result, these analogues have empirical water solubilities that range over four orders of magnitude from 10-5 to 0.07 mg/L. Due to this variability, caution should be exercised when applying analogue values to Disperse Orange 30. Clearly it would be preferable to utilise only empirical data – which are available from the Brown (1992) study for water solubility and logKow - specific to the substance. However the analogue data presented can be considered as part of the weight of evidence for the evaluation of this substance.
| CAS RN | Common Name | Molecular mass (g/mol) | Structure similarityTable note g (%) | Minimum-maximum cross-sectional diameterTable note h (nm) |
|---|---|---|---|---|
| 12239-34-8 | Disperse Blue 79 | 639.42 | 74.83 | 1.69-2.045 |
| 3618-72-2 | Disperse Blue 79:1 | 625.39 | 73.24 | 1.43-2.03 |
| 31482-56-1 | Disperse Orange 25 | 323.35 | - | 1.37-1.95 |
| 3179-89-3 | Disperse Red 17 | 344.36 | 60.2 | 1.41-1.86 |
| 16889-10-4 | Disperse Red 73 | 348.36 | 70.37 | 1.31-1.93 |
| 2832-40-8 | Disperse Yellow 3 | 269.31 | NA | 1.59-1.70 |
Sources
Disperse Orange 30 is not naturally produced in the environment.
Recent information was collected through industry surveys conducted for the years 2005 and 2006 under Canada Gazette Notices issued pursuant to section 71 of CEPA 1999 (Canada 2006b and 2008). These Notices required submission of data on the Canadian manufacture and import of the substance. For 2006, data were also required on use quantities of Disperse Orange 30.
Less than four companies reported importing Disperse Orange 30 into Canada in 2006 for a collective total of between 1 000 and 10 000 kg (Canada 2008). The same companies reported using a collective total of between 100 and 100 000 kg of Disperse Orange 30 in 2005 (Canada 2006b). No manufacture of Disperse Orange 30 in Canada was reported above the threshold of 100 kg/year for 2006 (Canada 2008). In the Declaration of Stakeholder-Interest form associated with the section 71 survey for 2006, one company reported a stakeholder interest in this substance despite not meeting mandatory reporting requirements (Canada 2008).
Three companies reported importing Disperse Orange 30 into Canada in 2005 with two companies in the 100-1 000 kg/year range, and one company in the 1,001 – 100 000 kg/year range (Canada 2006b). The upper bound of that range is high, and adds uncertainty to the estimation of current uses in Canada, however where exact quantities are reported they are within the same order of magnitude for both years. No manufacture of Disperse Orange 30 was reported above the 100 kg/year threshold, for the 2005 calendar year.
The quantity reported during development of the Domestic Substances List (DSL) to be manufactured, imported or in commerce in Canada during the 1986 calendar year was 11,000 kg (Environment Canada 1988).
Disperse Orange 30 has been identified as a Low Production Volume (LPV) chemical by the European Union (EU), indicating that production within the EU is estimated to be between 10 and 1000 tonnes per year (ESIS 2008). Production of Disperse Orange 30 within the United States has been estimated to be between 500,000 and 1,000,000 pounds in each of the following years: 1986, 1990, 1994, 1998 and 2002 (USEPA 2007). The Substances in Preparation in Nordic Countries (SPIN) database states that this substance was in use in Finland from 2001 – 2006, Denmark from 2000 – 2006 and Sweden from 1999 – 2006 (SPIN 2008). Reported use quantities range from one to over 4,500 tonnes/year (SPIN 2008).
Uses
Information on uses for the 2005 and 2006 calendar years was gathered in response to the CEPA 1999 section 71 Notices (Canada 2006b and 2008).
In 2006, a company importing and using Disperse Orange 30 indicated that its business activity was chemical product manufacturing (Canada 2008). Companies identified their business activities in 2005 as: "Textile and Fabric Finishing and Fabric Coating Mills Chemical (except Agricultural)" and "Allied Product Wholesaler-Distributors".
The following DSL use codes have been identified for the substance during the DSL nomination (1984-1986): "Colourant - pigment/stain/dye/ink", "Pigment, Dye and Printing Ink", "Textile, Primary Manufacture" and "Textile, Product"(Environment Canada 1988).
Review of the available scientific and technical information indicates that Disperse Orange 30 is used primarily in the textile industry (SPIN 2008). Disperse Orange 30 may be used for dyeing polyester, polyester/natural fiber blends, nylon and acetate for clothing and home textile uses (Clariant 2000, CII 2002).
Releases to the Environment
Mass Flow Tool
To estimate potential releases of the substance to the environment at different stages of its life cycle, a Mass Flow Tool was developed (Environment Canada 2008a). Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of release to the different environmental media are estimated, as are the proportion of the substance chemically transformed or sent for waste disposal. Unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the Mass Flow Tool does not quantitatively account for releases to the environment from disposal.
Assumptions and input parameters used in making the release estimates are based on information obtained from a variety of sources including responses to regulatory surveys, Statistics Canada, manufacturers' websites and technical databases and documents. Of particular relevance are emission factors, which are generally expressed as the fraction of a substance released to the environment, particularly during its manufacture, processing, and use associated with industrial processes. Sources of such information include emission scenario documents, often developed under the auspices of the Organization for Economic Cooperation and Development (OECD), and default assumptions used by different international chemical regulatory agencies. It is noted that the level of uncertainty in the mass of substance and quantity released to the environment generally increases towards the end of the life-cycle.
Based on Statistics Canada information and an analysis by Industry Canada (2008), it is proposed that Disperse Orange 30 may be imported in manufactured articles (principally textiles). A ratio of textiles manufactured in Canada / textiles imported into Canada of 30/70 has been used to estimate the amount of dye imported in textiles (Environment Canada 2008b). This import quantity was included in the Mass Flow Tool calculations.
| Fate | Proportion of the mass (%)Table note i | Major life cycle stage involvedTable note j |
|---|---|---|
| Releases to soil | 0.0 | Not applicable |
| Releases to air | 0.0 | Not applicable |
| Releases to sewerTable note k | 14.8 | Formulation, consumer use |
| Chemically transformed | 0.0 | Not applicable |
| Transferred to waste disposal sites (e.g., landfill, incineration) | 85.2 | Formulation, waste disposal |
Results indicate that Disperse Orange 30 can be expected to be found largely in waste management sites (85.2%), due to the eventual disposal of manufactured items containing it. Mass Flow Tool calculations do not quantitatively account for releases of the substance to the environment from waste disposal sites (such as landfills, incinerators) unless specific information on the rate or potential for release is available. No such information has been identified for Disperse Orange 30. A small fraction of solid waste is incinerated which is expected to result in transformation of the substance. Based largely on information contained in OECD emission scenario documents for processing and uses associated with this type of substance, it is estimated that 14.8% of Disperse Orange 30 may be released to sewers.
Based on the above, sewer water is the medium receiving the greatest proportion of Disperse Orange 30 emitted during product processing. It is anticipated that the majority of the substance bound in products will be sent to landfills for disposal.
Environmental Fate
As indicated by the results of the Mass Flow Tool (Table 4), the substance Disperse Orange 30 is expected to be released to waste water effluents during industrial processing and use. The moderate log Kow (4.2) and high log Koc (read-across; 3.4 to 4.2) values (see Table 2) indicate that this substance may have affinity for solids. However, the log Koc is a calculated value (see footnote 3 below Table 2) and the adsorption potential of solid particulate dye structures is generally not well understood, therefore the degree of adsorption of Disperse Orange 30 is uncertain.
Disperse Orange 30 is expected to be mostly found in sediment or soil. It is not expected to be subject to long-range atmospheric transport.
Disperse Orange 30 does not biodegrade fast (see Table 5 below). It may inadvertently be applied to agricultural soils and pasture lands in Canada as a component of biosludge which is commonly used for soil enrichment. Moreover, it may also be released from coloured textiles deposited in landfills.
Disperse Orange 30 is a powder with a limited water solubility (see Table 2). In solution, Disperse Orange 30 behaves as a base with an estimated pKa that is very low (0.42; see Table 2). Consequently, dissolved forms of Disperse Orange 30 are not expected to ionize in water at environmentally relevant pHs. Because of its low solubility, when released into water, this substance is expected to behave as a colloidal dispersion (Yen et al. 1991). Thus, this substance will be mostly present as a solid or adsorbed to suspended particles and to sink eventually to bed sediments where it is expected to remain in a relatively biologically unavailable form. It has been concluded by Yen at al. (1989) that disperse dyes tend to accumulate extensively in sediments and biota unless they are degraded at rates comparable to uptake. Razo-Flores et al. (1997) have stated that due to the recalcitrant nature of azo dyes in the aerobic environment, they eventually end up in anaerobic sediments due to sediment burial, shallow aquifers and in groundwater. Yen et al. (1991) observed that some azobenzene dye analogues were transformed under anaerobic conditions in sediment via hydrolysis and reduction, and concluded that most azo dyes would likely not persist in anaerobic sediment systems. In buried sediment, Disperse Orange 30 may undergo anaerobic degradation, as described in the following section on Persistence.
The rate of volatilization from the surface of water is proportional to the Henry's law constant (Baughman and Perenich 1988). Baughman and Perenich (1988) also state that volatilization from aquatic systems will not be an important loss process for disperse dyes which agrees with the low to negligible Henry's Law constant (10-8 to 10-1 Pa·m3/mol, read-across data in Table 2) as well as the low vapour pressure (5.33×(10-12 to 10-5) Pa, read-across data in Table 2). Transfer to or transport in air due to the loss of this substance from moist and dry soil surfaces is not likely to be important for this substance as indicated by very low vapour pressure of Disperse Orange 30. This behaviour is consistent with the physical state (solid particle) of Disperse Orange 30 which does not make it a likely candidate for volatilization.
Persistence and Bioaccumulation Potential
Persistence
No experimental biological degradation data for Disperse Orange 30 have been identified. According to the Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers, with some exceptions, dyes are considered essentially non-biodegradable under aerobic conditions (ETAD 1995). Repeated evaluation of ready and inherent biodegradability using accepted screening tests (see OECD Guidelines for Testing Chemicals) have generally confirmed this expectation (Pagga and Brown 1986; ETAD 1992). Based on the chemical structure of Disperse Orange 30, there is no reason to suspect that biodegradation will be other than that described for dyes generally (ETAD 1995).
Some disperse azo dyes have been shown to undergo relatively rapid anaerobic degradation in sediment at depth where anoxic conditions persist (Yen et al. 1991, Baughman and Weber 1994, Weber and Adams 1995). Disperse dyes enter the aquatic system mostly as a dispersion of fine suspended particles, eventually settling to the aerobic layers of surface sediment where they will persist until sediment burial creates reducing conditions. The rate of sediment deposition and the extent of bioturbation varies from site to site and thus it is very difficult to ascertain the residence time of dyes in aerobic sediment layers. It is likely however, that in many cases this is greater than 365 days. Once under anaerobic or reducing conditions, azo dyes may undergo rapid degradation to substituted aromatic amine constituents as demonstrated by Yen et al. (1991) who measured reduction half-life values in compacted sediments at room temperature of 2.9 hours to 2.0 days for azobenzene dyes. However, in deep anoxic sediment, these biodegradation transformation products are not expected to present a high degree of exposure potential to most aquatic organisms, and therefore they are not likely to present an ecological concern.
Since no experimental biodegradation data are available for Disperse Orange 30, a QSAR-based weight-of-evidence approach (Environment Canada 2007) was applied using the degradation models shown in Table 5 below. Although the expected release of Disperse Orange 30 will be to wastewater, its residence time in the water column may be short before finally sinking to the sediment bed due to its low solubility and behaviour as a colloidal dispersion. However, given the lack of data regarding this issue, persistence was primarily examined using predictive QSAR models for biodegradation in water. The following analysis applies primarily to the portion of this substance that is present in the environment in the dissolved form, recognizing that a significant proportion would also likely exist in dispersed form as solid particles. Disperse Orange 30 does not contain functional groups expected to undergo hydrolysis in aerobic environments (dyes are designed to be stable in aqueous conditions). Table 5 summarizes the results of available QSAR models for biodegradation in water.
| Model | Model Basis | Medium | Value | Interpretation | Extrapolated half-life (days) | Extrapolation Reference and/or Source |
|---|---|---|---|---|---|---|
| BIOWIN1Table note l v4.1 (2000) | Linear probability | water (aerobic) |
-0.103 | Does not biodegrade fast | n/a | |
| BIOWIN2Table note l v4.1 (2000) | Non-linear probability | water (aerobic) |
0.00 | Does not biodegrade fast | n/a | |
| BIOWIN3Table note l v4.1 (2000) | Expert Survey (ultimate biodegradation) | water (aerobic) |
1.12 | Recalcitrant | 180 | US EPA 2002 |
| BIOWIN4Table note l v4.1 (2000) | Expert Survey (primary biodegradation) | water (aerobic) |
2.58 | Weeks-months | 37.5 | US EPA 2002 |
| BIOWIN5Table note l v4.1 (2000) | MITI linear probability | water (aerobic) |
-0.27 | Does not biodegrade fast | n/a | |
| BIOWIN6Table note l v4.1 (2000) | MITI non-linear probability | water (aerobic) |
0.00 | Does not biodegrade fast | n/a | |
| BIOWIN Overall ConclusionTable note m | BIOWIN 3 + BIOWIN 5 | water (aerobic) |
no | Not readily biodegradable | n/a | |
| TOPKAT 2004 | MITI 1 probability | water (aerobic) |
0 | Persistent (less than 20%) | greater than 182 | TOPKAT 2004 |
| CATABOL v. 5.10.2 | % BOD (OECD 301C) | water (aerobic) |
12.4 | Persistent (less than 20%) | greater than 182 | Aronson et al. 2006 |
The results from Table 5 show that the majority of the probability models (BIOWIN 1, 2, 5, 6) suggest this substance does not biodegrade rapidly. In fact, all probability results are less than 0.3, the cut-off suggested by Aronson et al. (2006) identifying substances as having a half-life greater than 60 days (based on the Japanese Ministry of International Trade and Industry (MITI) probability models). The half-life from the primary survey model (BIOWIN 4) result of weeks-months is suggested to mean approximately 37.5 days (US EPA 2002, Aronson et al 2006); however, the nature of the degradation products is unknown. The ultimate survey model (BIOWIN 3) result of recalcitrant is suggested to mean 180 days by the US EPA 2002, Aronson et al 2006). The overall conclusion from BIOWIN (2000) is that this substance is not readily biodegradable in water.
Other ultimate degradation models (CATABOL and TOPKAT) predict that Disperse Orange 30 does not undergo mineralization in a 28 day timeframe with probability or extent of biodegradation in the range of very persistent chemicals. TOPKAT, which simulates the Japanese MITI I 28 day biodegradation test, produced a probability of 0 which is less than the suggested cut-off for persistent substances in this model (less than 0.3) (note: 0.7 is suggested for non-persistence chemicals) (TOPKAT 2004). CATABOL predicted only 12.4% biodegradation based on the OECD 301 readily biodegradation test (%BOD) which has been suggested as meaning likely persistent (Aronson and Howard 1999) and having a half-life in water of greater than 182 days.
When the results of the probability models, the overall BIOWIN conclusion and ultimate degradation models are considered, there is model consensus suggesting that the half-life in water is greater than 182 days, which is consistent with what would be expected for a chemical used as a disperse dye (i.e., manufactured to be relatively insoluble and durable). Using a ratio of 1:1:4 for a water:soil:sediment half-life extrapolation (Boethling et al. 1995), the half-life in soil should be greater than 182 days and the half-life in aerobic sediments should be greater than 365 days.
Based on the results of predictive modelling (principally for ultimate degradation) and expert judgement (ETAD 1995), Disperse Orange 30 meets the persistence criteria for water and soil (half life in soil and water ≥ 182 days) as well as sediments (half life in sediments ≥ 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential for Bioaccumulation
A recent empirical bioconcentration test of Disperse Orange 30 in fish was submitted to Environment Canada (Shen and Hu 2008). This test was performed according to OECD Guidelines for Testing of Chemicals, Test No. 305B-1996, Bioconcentration: Semi-Static Fish Test. The bioconcentration of Disperse Orange 30 in zebra fish (Brachydanio rerio) was determined in a 28-day semi-static test with test medium renewal every two days. An exposure test at a nominal concentration of 20 mg/L (mean measured concentration 0.028 - 0.28 mg/L) was performed in accordance with the result of the fish acute toxicity test to check the bioconcentration potential of the test substance. Samples from both test solutions and test organisms were taken daily from the 26th day to the last day during the 28-day exposure test period. Samples were prepared by extracting the lipid component from the test fish. The measured concentration of test substance, fish lipid content and bioconcentration factor (BCF) calculation are reported in Table 6.
| Treatments (20 mg/L) | Sampling on 26th day | Sampling on 27th day | Sampling on 28th day |
|---|---|---|---|
| Measured concentration of the test substance in extracted solutions (mg/L) | less than 0.028 | less than 0.028 | less than 0.028 |
| Content of the test substance in the fish lipids (mg) | less than 1.68 | less than 1.68 | less than 1.68 |
| Fish total weight (g) | 2.07 | 2.13 | 2.53 |
| Concentration of the test substance in the fish Cf (mg/kg) | less than 0.81 | less than 0.79 | less than 0.66 |
| Measured concentration of the test substance in the water Cw (mg/L) | 0.028 ~ 0.28 | 0.028 ~ 0.28 | 0.028 ~ 0.28 |
| Fish lipid content (%) | 0.81 | 0.57 | 1.25 |
| BCF | less than 100 | less than 100 | less than 100 |
| Average BCF | less than 100 | less than 100 | less than 100 |
The Shen and Hu (2008) study has been reviewed and considered acceptable (see Appendix 1). Lack of detection in fish extracts (less than 0.028 mg/L) suggests a limited solubility in lipids and/or limited potential to partition into fish tissue from aqueous systems - more likely both. However, there is some uncertainty associated with limit bounded values in any study because the absolute value is not known.
But given the structure of the substance and the likely behaviour of this class of disperse dye in aqueous systems, a low BCF result would be expected. Most disperse dyes, as their name would suggest, exist as fine dispersible particles with limited truly soluble fractions. Solubility, however, can be increased by adding polar functional groups to the molecule. Disperse Orange 30 contains some of these solubilizing groups (nitro, acetate), thus some degree of water solubility would be expected. Therefore, given a melting point of 128.5oC (highest experimental data in Table 2) and an experimental log Kow of 4.2 (Table 2), the predicted water solubility (WSKOWIN 2000) corrected for melting point and log Kow is 0.176 mg/L which is within the aqueous detection limit in the study and is in general agreement with the experimental value of 0.07 mg/L reported by Brown (1992). Using a water solubility of 0.176 mg/L and using the fish concentration of 0.81 mg/kg, the BCF may be calculated to be less than 100.
While the above study serves as primary evidence to support Disperse Orange 30's lack of bioaccumulation potential, other research corroborates this conclusion. Anliker et al. (1981) reported experimental fish bioaccumulation values for 18 disperse monoazo dyes, performed according to test methods specified by the MITI. Expressed on the basis of wet body weight of the fishes, these log bioaccumulation factors ranged from 0.00 to 1.76 (Anliker et al. 1981). A lack of reporting of chemical registry numbers and chemical structures limited the utility of this study for read-across purposes to Disperse Orange 30. However, follow-up studies, which provided the chemical structures for the disperse dyes tested, confirmed low bioaccumulation potential for ten nitroazo dyes, with reported log bioaccumulation factors ranging from 0.3 to 1.76 (Anliker and Moser 1987; Anliker et al. 1988). Studies available from MITI also support low bioaccumulation potential for disperse azo dyes. Reported BCFs for three disperse azo dyes (CAS# 40690-89-9, 61968-52-3 and 71767-67-4) tested at a concentration of 0.01 mg/L were in the range of less than 0.3 to 47 (MITI 1992). An accumulation study by Brown (1987) also showed that none of the twelve disperse dyes tested accumulated during an eight week study with carp.
A high log Kow value of 4.2 for Disperse Orange 30 (Table 2) is the only line of evidence that suggests Disperse Orange 30 may have a high potential for bioaccumulation. In spite of the high Kow values for Disperse Orange 30 and other disperse azo dyes, evidence for bioaccumulation of such dyes is lacking (Anliker et al. 1981, Anliker and Moser 1987, MITI 1992). Authors who have measured high log Kows and concomitant low bioaccumulation factors for disperse azo dyes suggest the low accumulation factors may be due in some cases to their low absolute fat solubility (Brown 1987) or to their relatively high molecular weight (typically 450-550) which may make transport across fish membranes difficult (Anliker et al. 1981, Anliker and Moser 1987). It is also likely that the lack of bioavailability and limited capacity to partition under BCF test conditions limits accumulation in fish lipids.
It has been stated by ETAD (1995) that the molecular characteristics indicating the absence bioaccumulation are a molecular weight of greater than 450 g/mol and a cross-sectional diameter of greater than 1.05 nm. Recent investigation by Dimitrov et al. (2002), Dimitrov et al. (2005) and the BBM (2008) suggests that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum cross-sectional diameter (Dmax). The probability of passive diffusion lowers appreciably when cross-sectional diameter is greater than ~1.5 nm and more significantly for molecules having a cross-sectional diameter of greater than 1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion from a test set of about 1200 new and existing chemicals, also observing that substances not having a very high bioconcentration potential often have a Dmax greater than 2.0 nm and an effective diameter (Deff) greater than 1.1 nm.
Disperse Orange 30 has a molecular weight of 450.28 g/mol (see Table 1) and its molecular structure is relatively uncomplicated; both these characteristics indicate a potential bioaccumulation capability of this substance. In addition, an Environment Canada (2007) report points out that there is no clear evidence for establishing strict molecular size cut-offs for assessing bioaccumulation potential. However, the report does not dispute the notion that a reduction in uptake rate can be associated with increasing cross-sectional diameter as demonstrated by Dimitrov et al. (2002, 2005). The maximum diameter of Disperse Orange 30 and its conformers ranges from 1.75 to 1.98 nm (BBM 2008) suggesting that a potential for a significantly reduced uptake rate from water and in vivo bioavailability exists with this dye.
Results of bioaccumulation modelling were not used in this assessment of Disperse Orange 30. Many higher molecular weight pigments and non-soluble dye classes, including disperse azo dyes are considered difficult to model and thus the results are generally unreliable. Predicted and/or empirical properties of disperse dyes relating to bioaccumulation (e.g., log Kow) can be of uncertain relevance, or associated with a high degree of error, which would limit the utility of calculated BCF and BAF values. In addition, disperse azo dyes fall outside of the domains of applicability of the available bioaccumulation models.
Based on a lack of accumulation in bioconcentration tests of Disperse Orange 30 and other related disperse azo dyes, and Disperse Orange 30's large molecular size, which likely limits its partitioning behavior, Disperse Orange 30 is expected to have a low potential for bioaccumulation. Therefore, considering the BCF test data for disperse Orange 30, analogue BCF evidence, and structural and bioavailability considerations, Disperse Orange 30 does not meet the bioaccumulation criteria (BCF or BAF greater than 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential to Cause Ecological Harm
Ecological Effects Assessment
A - In the Aquatic Compartment
Few empirical ecotoxicity data were identified for Disperse Orange 30. According to a study submitted to Environment Canada on behalf of ETAD (Brown 1992), a 96-hour LC50 of 710 mg/L for zebra fish, a 48-hour EC50 of 5.8 mg/L for Daphnia magna, a 72-hour EC50(for growth) of 6.7 mg/L for Scenedesmus subspicatus have been obtained experimentally for a toxicity study using disperse Orange 30 (Table 7a). However, the original studies have not been provided to allow verification of their reliability.
Another result was submitted to Environment Canada under the voluntary data submission. A LC50 for rainbow trout (Oncorhynchus mykiss) was established as greater than 700 mg/L (Sandoz 1975). Reliability of the study could also not be assessed directly due to the fact that it was written in German. However attached to the submission were answers to robust study summary questions. Therefore an evaluation was conducted based on the robust study summary provided in the submission (Appendix 1). The Sandoz (1975) study was, after review, considered to be unacceptable (see Appendix 1).
Another experimental study on the toxicity of effluent containing Disperse Orange 30 reported an LC50 (48 hours) for mysid shrimp of 46% (Reife 1989). However this study did not present the LC50 as a concentration of Disperse Orange 30 and therefore these results could not be included in Table 7a or used in the risk quotient analysis.
An additional acute toxicity study, using rainbow trout, was submitted to Environment Canada (Table 7a) in August 2008. An assessment of the reliability of the study using a robust study summary was conducted and as a result the study was deemed to be of "low confidence" due to lack of details (Appendix 1).
| Test organism | Type of test | Duration (hours) | End point | Reliability of the study | Value (mg/L) | Reference |
|---|---|---|---|---|---|---|
| Rainbow trout | Acute | 48 | The median concentration of a substance that is estimated to be lethal to 50% of the test organisms (LC50) | unacceptable | greater than 700 | Sandoz 1975 |
| Rainbow trout | Acute | 96 | LC50 | Low confidence | greater than 100 | SafePharm laboratories Ltd 1990 |
| Zebra fish | Acute | 96 | LC50 | Not Available | 710 | Brown 1992 |
| Daphnia magna | Acute | 48 | The median concentration of a substance that is estimated to cause some toxic sublethal effect on 50% of the test organisms (EC50) | Not Available | 5.8 | Brown 1992 |
| Scenedesmus subspicatus | Acute | 72 | EC50 | Not Available | 6.7 | Brown 1992 |
| Bacteria | Acute | n/a | The median concentration of a substance that is estimated to cause inhibition to growth 50% of the test organisms (IC50) | Not Available | greater than 100 | Brown 1992 |
Environment Canada received ecotoxicological data for a structurally similar substance through the New Substance Notification Regulations (Environment Canada 1995). The molecular weight of this notified substance was 471.46 which was similar to Disperse Orange 30. Ecotoxicological data were provided with this notification. The results for the 96-hr static toxicity test with rainbow trout revealed that the LC50 for this species is 505 mg/L (Table 7b). The test was conducted according to OECD guideline No. 203. The Material Safety Data Sheets (MSDS) of the notified substance also contained information on bacterial toxic effects. The results indicate an activated sludge respiration inhibition EC50 of greater than 100 mg/L (Table 7b). Based on the available ecotoxicity information, the notified substance is expected to be of low concern for toxic effects to aquatic organisms. Reliability of the study was assessed using a robust study summary and is considered to be satisfactory (Appendix 1).
In another study, a summary of which was submitted to Environment Canada on behalf of ETAD (Brown 1992), 11 disperse dyes were tested on the following organisms: zebra fish, Daphnia magna, algae and bacteria. One of the disperse dyes tested was Disperse Orange 30 (Table 7a). Of the remainder of the disperse dyes tested by Brown (1992), four are azo analogues of Disperse Orange 30 (Brown 1992). These are Disperse Red 73, Disperse Blue 79, Disperse Orange 25, and Disperse Red 17 (Table 7b). Disperse Blue 79 tested showed moderate toxicity to D. magna (48 hr EC50=4.5 mg/L) and the remaining four analogues showed moderate to low toxicity to zebra fish (96 hr LC50=17 to 340 mg/L) (see table 7b). Moderate toxicity was also presented for algae growth (EC50 for growth = 7 to 54 mg/L) and no toxicity detected for bacteria (IC50 greater than 100 mg/L). The experimental details for the dyes tested were not provided, greatly limiting evaluation of these studies (Brown 1992). However, these data were considered usable and are included in this Screening Assessment as part of the weight of evidence as they provide further empirical information to establish the range of ecotoxicity values for these structures. Lastly, an analogue, Disperse Blue 79:1, had a no effect concentration (NOEC) for rainbow trout of greater than 0.0048 mg/L (Table 7b). Reliability of this study was assessed as high (Appendix 1). However, this value was not used to calculate the predicted no effect concentration because the value is a hypothesis-based unbounded result. These values would also therefore suggest that Disperse Orange 30 is not hazardous to aquatic organisms (i.e., acute LC50 are greater than 1 mg/L).
| Common Name or CAS RN | Test Organism | End point | Value (mg/L) | Reference |
|---|---|---|---|---|
| Analogous disperse azo dye | Rainbow Ttout | LC50 | 505 | Environment Canada 1995 |
| Analogous disperse azo dye | Bacteria | EC50 | greater than 100 | Environment Canada 1995 |
| Disperse Red 73 | Zebra fish | LC50 | 17 | Brown 1992 |
| Disperse Red 73 | Daphnia magna | EC50 | 23 | Brown 1992 |
| Disperse Red 73 | Scenedesmus subspicatus | EC50 | greater than 10 | Brown 1992 |
| Disperse Red 73 | Bacteria | IC50 | greater than 100 | Brown 1992 |
| Disperse Red 73 | Zebra fish | LC50 | 17 | Brown 1992 |
| Disperse Red 73 | Daphnia magna | EC50 | 23 | Brown 1992 |
| Disperse Red 73 | Scenedesmus subspicatus | EC50 | greater than 10 | Brown 1992 |
| Disperse Red 73 | Bacteria | IC50 | greater than 100 | Brown 1992 |
| Disperse Red 17 | Zebra fish | IC50 | 103 | Brown 1992 |
| Disperse Red 17 | Daphnia magna | LC50 | 98 | Brown 1992 |
| Disperse Red 17 | Scenedesmus subspicatus | EC50 | 7 | Brown 1992 |
| Disperse Red 17 | Bacteria | EC50 | greater than 100 | Brown 1992 |
| Disperse Orange 25 | Zebra fish | IC50 | 268 | Brown 1992 |
| Disperse Orange 25 | Daphnia magna | LC50 | 110 | Brown 1992 |
| Disperse Orange 25 | Scenedesmus subspicatus | EC50 | 54 | Brown 1992 |
| Disperse Orange 25 | Bacteria | EC50 | greater than 100 | Brown 1992 |
| Disperse Blue 79:1 | Rainbow trout | NOEC (122 days) | greater than 0.0048 | Cohle and Mihalik 1991 |
| Disperse Yellow 3 | Fathead minnow | LC50 | greater than 180 | Little and Lamb 1973 |
In general, due to their low solubility (less than 1 mg/L), disperse dyes are expected to have a low acute ecological impact (Hunger 2003). The results of empirical toxicity studies with both Disperse Orange 30 and several analogues are consistent with this expectation, indicating LC50s in the 5 to 710 mg/L range, with Daphnia being the most sensitive organisms tested (EC50/LC50s from 4.5 to greater than 100 mg/L). Although interpretation of results from these tests is complicated by the fact that these effect values are based on nominal concentrations sometimes more than 100 fold greater than the estimated solubility of the substance (i.e., approximately 0.07 mg/L Table 2), they do represent possible worst-case environmental loadings. The data available indicate that the toxicity of Disperse Orange 30 to pelagic organisms is low and is not likely to result in adverse effects at water saturated concentrations.
A range of aquatic toxicity predictions were also obtained from the various QSAR models considered for Disperse Orange 30 and its analogues. However, as with bioaccumulation, these QSAR ecotoxicity predictions for Disperse Orange 30 are not considered reliable because of the potential error associated with input parameters and the unique nature of disperse dyes, such as specifically structural and/or physico-chemical properties which fall outside of the models' domain of applicability.
The available empirical ecotoxicity information for Disperse Orange 30 and several analogues of Disperse Orange 30 thus indicates that it is not likely to be highly hazardous to aquatic organisms.
B - In Other Environmental Compartments
Since Disperse Orange 30 may potentially be discharged to soil from the disposal of products that degrade and release Disperse Orange 30, it would be desirable to obtain toxicity data for soil organisms. This is of particular importance since it has been shown that dyes are strongly absorbed and stick to wastewater treatment plant sludge (Tincher 1988). Although no suitable ecological effects studies were found for this compound in soil, considering the toxicity data for aquatic organisms as well as the lack of bioaccumulation potential and its low bioavailability, potential for toxicity to soil-dwelling organisms is likely to be low. For the same reasons the toxicity potential is also likely to be low for sediment dwelling species, although this cannot be substantiated due to lack of whole organism sediment toxicity data for Disperse Orange 30 or suitable analogues.
Ecological Exposure Assessment
No data concerning concentrations of this substance in water in Canada have been identified. Environmental concentrations are, therefore, estimated from available information, including substance quantities in commerce, estimated release rates, and characteristics of receiving water bodies.
The Mass Flow Tool predicted releases to the water (sewers) from formulation use and from consumer use of products containing this substance (Table 4). To address releases from industrial activities, Environment Canada's Industrial Generic Exposure Tool – Aquatic (IGETA) was employed to estimate the substance concentration (worst-case) in a generic water course receiving industrial effluents (Environment Canada 2008c). The generic scenario is designed to provide these estimates based on conservative assumptions regarding the amount of chemical processed and released, the number of processing days, sewage treatment plant removal rate, and the size of the receiving watercourse. The tool models an industrial-release scenario based on loading data from sources such as industrial surveys and knowledge of the distribution of industrial discharges in the country, and calculates a predicted environmental concentration (PEC). The equation and inputs used to calculate the PEC in the receiving water course are described in the Environment Canada (2008d). The mass of Disperse Orange 30 input into the IGETA model was 3 566 kg. This value is the greatest quantity of this substance imported by one facility in Canada in 2006. As indicated previously, the upper bound of the range reported in 2005 was much higher than this amount (i.e. 100 000 kg), however for those companies that reported exact quantities in both surveys, their reported amounts were within the same order of magnitude for both years. As a result, it's unlikely that the company who reported the largest amount in 2006 (i.e. 3 566 kg) and on which the exposure assessment is based would have imported 100 000 kg in 2005.
The release to water (sewers) from industrial activities only from the Mass Flow Tool was conservatively estimated at 16%. The chemical is assumed to be released to a very small river which is considered to be a realistic worst-case scenario. A removal rate in a sewage treatment plant was estimated using ASTreat 1.0 to be 68.5% (ASTreat 2006). This is of similar range to the removal rate of 78% presented for 5 disperse dyes in ETAD (1992). The ASTreat removal rate was included in the IGETA model since it was the most conservative (lowest) value. Two other models of removal rate from sewage treatment plant yielded removal rate values between 90 and 96.2%. The conservative PEC for water was calculated to be 0.0201 mg/L (Environment Canada 2008d).
To address down-the-drain releases from consumer uses, Environment Canada's spreadsheet model (Mega Flush) was used. Using Mega Flush, potential substance concentrations are estimated in multiple water bodies receiving sewage treatment plant (STP) effluents to which consumer products containing the substance may have been released (Environment Canada 2008e). The spreadsheet model is designed to provide these estimates based on conservative assumptions regarding the amount of chemical used and released by consumers. By default, primary and secondary STP removal rates are set at 0%, fraction released during use of 100%, the consumer use of the substance is assumed to extend over 365 days/year, and the flow rate used for receiving water bodies at all sites is a low-end (10th percentile) value. These estimates are made for approximately 1 000 release sites across Canada, which account for most of the major STPs in Canada. These parameter values are considered to result in a very conservative scenario.
The equation and inputs used in Mega Flush to calculate the PEC of Disperse Orange 30 in the receiving water bodies are described in Environment Canada (2008f). A scenario was run assuming a total consumer use quantity of 10 770 kg/year (Environment Canada 2008b). This consumer use quantity was estimated conservatively using the total mass of substance reportedly imported into Canada by fewer than four companies based on information from the s. 71 survey, and applying the 30/70 ratio for dyes in textiles manufactured / imported in Canada. A 10% loss of dye was then assumed for the total amount of the substance being used by consumers (Øllgaard et al. 1998). That is, 1077 kg of Disperse Orange 30 were predicted to be released to waters nation-wide, as a result of loss to sewers during the laundering of manufactured articles that contain this dye (articles either imported or manufactured in Canada). Primary and secondary STP removal rates of 0% were used. These assumptions result in a very conservative scenario. Using this scenario, the Mega Flush tool estimates that the PEC in the receiving water bodies ranges from 0.00013 to 0.0016 mg/L.
Characterization of Ecological Risk
A predicted no-effect concentration (PNEC) was estimated based on the EC50 to the aquatic invertebrate (Daphnia magna). The 96-hour EC50 for Disperse Orange 30 was 5.8 mg/L (Table 7a) based on nominal concentrations. A factor of 100 was then applied to account for extrapolating from acute to chronic (long-term) toxicity and from laboratory results for one species to other potentially sensitive species in the field. The resulting PNEC is 0.058 mg/L.
When compared to the conservative PEC calculated above using IGETA, the resulting risk quotient for industrial discharges (PEC/PNEC) is 0.0201/0.058 = 0.35. Therefore, concentrations of Disperse Orange 30 in surface waters in Canada appear unlikely to cause adverse effects to aquatic organisms. Given that IGETA provides a conservative estimate of exposure, the results indicate a low potential for ecological harm resulting from local exposure to point source industrial releases to the aquatic environment.
For exposure resulting from down-the-drain releases through consumer uses (conservative scenario), MegaFlush results estimate that the PEC will not exceed the PNEC at any site (i.e., all risk quotients less than 1). This indicates that down-the-drain consumer releases of Disperse Orange 30 are not expected to harm aquatic organisms.
Based on the available information, Disperse Orange 30 is expected to be persistent in water, soil and sediment; it is however expected to have a low bioaccumulation potential. The low importation quantities of Disperse Orange 30 into Canada, along with information on physical and chemical properties and its uses, indicate a low to moderate potential for releases into the Canadian environment. If released into the environment, it is expected that Disperse Orange 30 will be mainly discharged to surface waters where ultimately it is expected to be transferred to sediment. It is also expected to have only a low to moderate potential for toxicity to aquatic organisms. Risk quotients for aquatic exposures indicate that Disperse Orange 30 concentrations likely do not exceed concentrations associated with effects, even when using conservative scenarios and assumptions. Therefore Disperse Orange 30 is unlikely to be causing harm to populations of aquatic organisms in Canada.
Uncertainties in Evaluation of Ecological Risk
An area of uncertainty for Disperse Orange 30 is associated with the use of read-across data for physical and chemical properties, as well as toxicity data from analogues. While the chemicals identified (Disperse Blue 79, Disperse Blue 79:1, Disperse Orange 25, Disperse Red 17, Disperse Red 73 and Disperse Yellow 3), share many similarities with Disperse Orange 30, including being azo dyes with high molecular weights, similar cross sectional diameters, having solid particulate structures that decompose at greater than 74 °C (to 240 °C), and being "dispersible" in water (i.e., not truly soluble), they do have some differences in functional groups. These differences in chemical structure add uncertainty because the properties and toxicity of Disperse Orange 30 may be somewhat different. However, it was reasoned that the similarities were sufficient to include the data from analogues to contribute to the weight of evidence in the assessment of Disperse Orange 30.
The persistence assessment is limited by the absence of biodegradation data, which necessitated generation of model predictions. Although all model prediction has some degree of error, the aerobic biodegradation model outputs confirmed the expected persistence of Disperse Orange 30 given its uses and structural characteristics. In addition, the persistence assessment is limited by the uncertainty about the rate and extent to which degradation occurs in anaerobic sediments and whether the degradation products (e.g., amines) would be biologically available. Nevertheless, it is clear that anaerobic degradation of the bioavailable portion azo dyes in sediments to constitutive amines is much faster (half-lives in the order of days) than aerobic biodegradation. Although the amine degradation products are not expected to be biologically available because they form only in relatively deep anoxic sediment and can be irreversibly bound to sediment through nucleophilic addition and oxidative radical coupling (Colon et al. 2002, Weber et al. 2001), this issue is a source of uncertainty in the assessment of Disperse Orange 30.
Uncertainties are also present due to the lack of information on environmental concentrations in Canada for Disperse Orange 30. However, the lack of manufacturing and the quantity of this substance imported into Canada suggests a low to moderate potential for release of this chemical into the Canadian environment.
The experimental concentrations associated with toxicity for aquatic organisms may have an additional source of uncertainty when these concentrations exceed the solubility of the chemical in water (either experimental or predicted). Despite this, the available data indicate that Disperse Orange 30 is not highly hazardous to aquatic organisms.
Uncertainties are also associated with the fraction of the substance that is released during use and with the fraction that is removed in sewage treatment plants. These uncertainties were addressed by making conservative assumptions using best model estimates.
Regarding ecotoxicity,based on the predicted partitioning behaviour of this chemical the significance of soil and sediment as important media of exposure is not well addressed by the effects data available. Indeed, the only effects data identified apply primarily to pelagic aquatic exposures.
Conclusion
Based on the information presented in this screening assessment, it is concluded that Disperse Orange 30 is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
It is therefore concluded that Disperse Orange 30 does not meet the definition of toxic as set out in section 64 of CEPA 1999. Additionally, Disperse Orange 30 meets the criteria for persistence but does not meet the criteria for bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
References
ACD/pKaDB [Prediction Module]. 2005. Version 9.04. Toronto (ON): Advanced Chemistry Development. http://www.acdlabs.com/products/phys_chem_lab/pka/
Anliker R, Clarke EA, Moser P. 1981. Use of the partition coefficient as an indicator of bioaccumulation tendency of dyestuffs in fish. Chemosphere 10(3): 263-274.
Anliker R, Moser P. 1987. The limits of bioaccumulation of organic pigments in fish: their relation to the partition coefficient and the solubility in water and octanol. Ecotoxicol Environ Safety 13:43-52.
Anliker R, Moser P, Poppinger D. 1988. Bioaccumulation of dyestuffs and organic pigments in fish. Relationships to hydrophobicity and steric factors. Chemosphere 17(8): 1631-1644.
Aronson D, Howard PH. 1999. Evaluating potential POP/PBT compounds for environmental persistence. North Syracuse (NY): Syracuse Research Corp., Environmental Science Centre. Report No.: SRC-TR-99-020.
Aronson D, Boethling B, Howard P, Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 63: 1953-1960.
ASTreat v.1.0, A software program developed by The Procter & Gamble Company for sewage treatment plant removal predictions, revised and released 2006, available from Dr. Drew C. McAvoy, The Procter & Gamble Company, P.O. Box 538707, Cincinnati, OH 45253-8707, USA, Tel: (513) 634-7603, Email: mcavoy.dc@pg.com
Baughman G.L., Perenich T.A. 1988. Fate of dyes in aquatic systems: I. Solubility and partitioning of some hydrophobic dyes and related compounds. Eviron Toxicol Chem 7(3):183-199.
Baughman GL, Weber EJ. 1994. Transformation of dyes and related compounds in anoxic sediment: kinetics and products. Environ Sci Technol 28(2): 267-276.
[BBM] Baseline Bioaccumulation Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division. [Model based on Dimitrov et al 2005]. [cited 2008-11-21]. Available upon request.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741−752.
Bomberger DC, Boughton RL.1984. Wastes from manufacture of dyes and pigments: Volume 1. Azo dyes and pigments (benzidine and its congeners subsector) (1984), p. 94 EPA-600/2-84-111a .
Brown D. 1987. Effects of colorants in the aquatic environment. Ecotoxicol Environ Safety 13:139-47.
Brown D (ICI Group Environmental Laboratory, Brixham, UK). 1992. Environmental assessment of dyestuffs. Prepared for Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers, Basel, Switzerland. ETAD ecological sub-committee project E3020. Submitted to Environment Canada.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III. vol. 22, no. 3. http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette. Part II, vol. 134, no. 7, p. 607−612. http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006a. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109–4117. http://canadagazette.gc.ca/partI/2006/20061209/pdf/g1-14049.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006b. Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action. Canada Gazette, Part I, vol. 140, no. 9, p. 435–459. http://canadagazette.gc.ca/partI/2006/20060304/pdf/g1-14009.pdf
Canada, Dept. of the Environment, Dept. of Health. 2008. Canadian Environmental Protection Act, 1999: Notice of fifth release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, vol. 147, no. 7. http://canadagazette.gc.ca/partI/2008/20080216/html/notice-e.html#d101
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. http://oasis-lmc.org/?section=software&swid=1
Clariant. 1996. IUCLID dataset for C.I. Disperse Blue 79 (CAS No 12239-34-8).
Clariant. 2000. Foron Dyes on Polyester, Clariant Textile Dyes Technical Information Selection Guide. [Internet] [cited 2008 08 15]. http://www.archroma.com/e2wportal/na/TXShdCrd.nsf/ec2ad7aa5ea2acce852568ba005fa717/88bb6f8235bd13258525687a005a60ab?OpenDocument
ChemID Plus. [Database on the Internet]. 2008. Accessed on October 1, 2008. http://chem.sis.nlm.nih.gov/chemidplus/
[CII] Color Index International [database on the Internet]. 2002. 4th ed. Research Triangle Park (NC): American Association of Textile Chemists and Colorists. [cited August 2008]. http://www.colour-index.org/
Cohle P, Mihalik R. 1991. Early life stage toxicity of C.I. Disperse Blue 79:1 purified preecake to rainbow trout (Oncorhynchus mykiss) in a flow-through system. Final report. ABC Laboratories Inc. Columbia MO.
Colón D, Weber EJ and Baughman GL. 2002. Sediment-associated reactions of aromatic amines. 2. QSAR development. Environ Sci Technol 36:2443-2450.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available upon request.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan OG. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531-554.
Dimitrov SD, Dimitrova NC , Walker JD , Veith GD, and Mekenyan OG. 2002. Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size*. Pure Appl. Chem., 2002, Vol. 74, No. 10, pp. 1823-1830
Environment Canada. 1988. Data relating to the Domestic Substances List
(DSL) 1984-1986, collected under CEPA, 1988, s. 25(1). Based on Reporting for the Domestic Substances List [guide] 1988. Data prepared by: Environment Canada.
Environment Canada. 1995. Acute Fish Toxicity Test Submission in Fulfillment of New Substances Notification Regulations to New Substances Branch, Environment Canada under New Substance Notification Program.
Environment Canada. 2000. Chemicals Evaluation Division. Environmental Categorization for Persistence, Bioaccumulation and Inherent Toxicity of Substances on the Domestic Substances List Using QSARs. Final Report. Environment Canada. July.
Environment Canada. 2007. Review of the limitations and uncertainties associated with use for molecular size information when assessing bioaccumulation potential. Unpublished Final Report. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008a. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mass Flow Tool. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008b. Assumptions, limitations and uncertainties of the mass flow tool for Disperse Orange 30, CAS RN 5261-31-4. Internal draft document. Gatineau (QC): Environment Canada, Existing Substances Division..
Environment Canada. 2008c. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: the Industrial Generic Exposure Tool – Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008d. IGETA report: CAS RN 5261-31-4, 2008-07-21. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008e. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mega Flush consumer release scenario. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008f. Mega Flush report: CAS RN 5261-31-4, 2008-08-12. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
[ESIS] European Substances Information System [database on the Internet]. [date unknown]. Version 5. European Chemical Bureau (ECB). [cited August 2008]. http://ecb.jrc.it/esis
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1992. Draft Guidelines for the Assessment of Environmental Exposure to Dyestuffs.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers Canadian Affiliates, Dayan J, Trebitz H, consultants. 1995. Health and environmental information on dyes used in Canada. Unpublished report submitted to Environment Canada, New Substances Division.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 2005. ETAD data for DSL categorization and screening submitted to Environment Canada on October 27, 2005. Study report by Intertek ASG. File Ref 2005/CC0157-001/REGIS.
Hunger K, editor. 2003. Industrial dyes; chemistry, properties, applications. Weinheim (DE): WILEY-VCH Verlag GmbH & Co. kgaA.
Industry Canada. 2008. Textile and Fabric Finishing [NAICS 31331]: 2004-2007 and Fabrics Coating 2004-2007. [NAICS 31332]: 2004-2007. Prepared by: Apparel and Textiles Directorate, Service Industries and Consumer Products Branch, Industry Canada, Enquiries B John (Jazz) Szabo, 613-957-1242. Email: szabo.john@ic.gc.ca
Little, LW, and Lamb JC, III. 1973. Acute Toxicity of 46 Selected Dyes to the Fathead Minnow, Pimephales promelas. Dyes and the Environment - Reports on Selected Dyes and Their Effects, Vol.1, American Dye Manufacturers Institute, Inc .:130
[MITI] Ministry of International Trade & Industry (Jpn), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (Jpn): Japan Chemical Industry Ecology-Toxicology & Information Centre.
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Columbus (OH): American Chemical Society. [cited 2006 Dec 11]. http://www.cas.org/products/cd/nci/index.html
[OECD] Organisation for Economic Co-operation and Development. 2004. Draft emission scenario on textile manufacturing wool mills [Internet]. Paris (FR): OECD, Environment Directorate. Report No.: ENV/JM/EEA(2004)8/1/REV, JT00175156. [cited 2008 August]. http://www.oecd.org/dataoecd/2/47/34003719.pdf
[OECD] Organisation for Economic Co-operation and Development. 2007. Emission scenario document on adhesive formulation [Internet]. Final report. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents). [Cited 2008 August]. http://ascouncil.org/news/adhesives/docs/EPAFormulation.pdf
Øllgaard H., Frost, L., Galster J. and Hansen OC. 1998. Survey of azo-colorants in Denmark, Consumption, use, health and environmental aspects Miljøprojekt no. 509,Danish Technological Institute, Environment, Ministry of Environment and Energy, Denmark, Danish Environmental Protection Agency, November, 1998.
Pagga, U, Brown, D. 1986. The degradation of dyestuffs: Part II Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere, 15, 4, 479-491.
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2006. Syracuse (NY): Syracuse Research Corporation. [cited 2006 Mar] http://www.syrres.com/esc/physdemo.htm
Razo-Flores E. Luijten M Donlon B. Lettinga G and Field J. 1997. Biodegradation of selected azo dyes under methanogenic conditions. Wat. Sci. Tech 36(6-7): 65-72.
Reife A. 1989. Reduction of toxic wastewaters in disperse azo dye manufacture. American Association of textile chemists and colorists. International Conference and Exhibition.
Safepharm laboratories Ltd. 1990. Acute toxicity to rainbow trout. Project number 47/781. Challenge submission ID#11347
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol. 29(1):89-92
Sandoz. 1975. Voluntary Data Submission under Section 71.
Sandoz Chemicals. 1989. Material Safety Data sheet of Foron Navy S-2GRL Purified presscakes.
Shen, Genxiang and Hu, Shuangqing (Environmental Testing Laboratory, Shanghai Academy of Environmental Sciences, Shanghai, China). 2008. Bioconcentration Test of C.I. Disperse Orange 30 in Fish. Prepared for Dystar in the name of Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers (ETAD) Basel, Switzerland. Report No. S-070-2007. Submitted to Environment Canada in April 2008. Challenge Submission ID#8351
Sijm DTHM., Schuurmann G, deVries PJ and Opperhuizen. A. 1999. Aqueous solubility, octanol solubility, and octanol/water partition coeeficient of nine hydrophobic dyes. Environ Toxicol Chem. 18(6): 1109-1117.
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2008. Copenhagen (DK): Nordic Council of Ministers. [cited 2008] http://195.215.251.229/Dotnetnuke/Home/tabid/58/Default.aspx
Tincher WC. 1988. Dyes in the environment: dyeing wastes in landfill. Study sponsored by the US Operating Committee of ETAD.
[TOPKAT] Toxicity Prediction Program [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. http://www.accelrys.com/products/topkat/index.html
[US EPA] US Environmental Protection Agency. 2002. PBT Profiler Methodology [Internet]. Washington (DC): US EPA, Office of Pollution Prevention and Toxics. [cited 2008 August]. http://www.pbtprofiler.net/methodology.asp
[US EPA] US Environmental Protection Agency. 2007. Inventory Update Reporting, Past IUR Data, Non-confidential Production Volume Information submitted by companies under the 1986,1990,1994,1998, and 2002 Inventory Update Reporting Regulation, CAS RN 52697-38-8 [Internet]. Washington (DC): US EPA; [cited 2007 Feb 28]. http://www.epa.gov/oppt/iur/tools/data/2002-vol.htm
Weber EJ, Colón D,Baughmann GL 2001. Sediment-Associated Reactions of Aromatic Amines. 1. Elucidation of sorption mechanisms. Environ Sci Technol 35:2470-2475.
Weber EJ, Adams RL. 1995. Chemical- and sediment-mediated reduction of the azo dye Disperse Blue 79. Environ Sci Technol 29: 1163-1170.
Yen CC, Perenich TA, Baughman GL. 1989. Fate of dyes in aquatic systems II. Solubility and octanol/water partition coefficients of disperse dyes. Eviron Toxicol Chem 8 (11): 981-986.
Yen C.C., Perenich T.A., Baughman G.L. 1991. Fate of commercial disperse dyes in sediments. Environ Toxicol Chem 10:1009-1017.
Appendix I - Robust Study Summaries for key studies
Table A-1. Robust Study Summaries Form: Aquatic B
Reference: Shen, Genxiang and Hu, Shuangqing. 2008. Bioconcentration Test of C.I. Disperse Orange 30 in Fish. Prepared by Environmental Testing Laboratory, Shanghai Academy of Environmental Sciences, Shanghai, China for Dystar in the name of Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers (ETAD) Basel, Switzerland. Report No. S-070-2007. Submitted to Environment Canada in April 2008. Challenge Submission ID#8351
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | Y | 5261-31-4 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | N | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| 7 | If test material is radiolabelled, were precise position(s) of the labelled atom(s) and the percentage of radioactivity associated with impurities reported? | 2 | n/a |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 8 | Reference | 1 | Y | OECD guidelines for the testing of chemicals No 305B-1996 |
| 9 | OECD, EU, national, or other standard method? | 3 | Y | OECD |
| 10 | Justification of the method/protocol if not a standard method was used | 2 | ||
| 11 | GLP (Good Laboratory Practice) | 3 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 12 | Organism identity: name | n/a | Y | zebra fish, Brachydanio rerio |
| 13 | Latin or both Latin & common names reported? | 1 | Y | both |
| 14 | Life cycle age / stage of test organism | 1 | N | |
| 15 | Length and/or weight | 1 | Y | Mean body length 3.91+/-0.18cm and mean body weight 0.32+/-0.06g |
| 16 | Sex | 1 | N | |
| 17 | Number of organisms per replicate | 1 | Y | 7 |
| 18 | Organism loading rate | 1 | Y | 20 mg/L |
| 19 | Food type and feeding periods during the acclimation period | 1 | Y | Fed a commercial fish diet until one day before start of test |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 28 days |
| 23 | Number of replicates (including controls) | 1 | Y | |
| 24 | Concentrations | 1 | Y | 20 mg/L |
| 25 | Food type/composition and feeding periods during the test | 1 | Y | Fish were fed two hours before water renewal |
| 26 | If BCF/BAF derived as a ratio of chemical concentration in the organism and in water, was experiment duration equal to or longer than the time required for the chemical concentrations to reach steady state? | 3 | Y | 28 days |
| 27 | If BCF/BAF derived as a ratio of chemical concentration in the organism and in water, were measured concentrations in both water and organism reported? | 3 | Y | |
| 28 | Were concentrations in the test water measured periodically? | 1 | Y | On three separate days |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | Yes every second day |
| 30 | Photoperiod and light intensity | 1 | Y | 12:12 |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Analytical monitoring intervals | 1 | Y | Every second day for dissolved oxygen, pH and temperature |
| 33 | Statistical methods used | 1 | Y | |
| 34 | Was solubilizer/emulsifier used, if the chemical was unstable or poorly soluble? | n/a | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 35 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 36 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 37 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | Semi-static |
| 38 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | 7.22-7.84 |
| 39 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | 22-23 |
| 40 | Was lipid content (or lipid-normalized BAF/BCF) reported? | 2 | Y | |
| 41 | Were measured concentrations of a chemical in the test water below the chemical's water solubility? | 3 | N | |
| 42 | If radiolabelled test substance was used, was BCF determination based on the parent compound (i.e. not on total radiolabelled residues)? | 3 | n/a |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 43 | Endpoints (BAF, BCF) and values | n/a | n/a | BCF less than 100 |
| 44 | BAF or BCF determined as: 1) the ratio of chemical concentration in the organism and in water, or 2) the ratio of the chemical uptake and elimination rate constants | n/a | n/a | 1 |
| 45 | Whether BAF/BCF was derived from a 1) tissue sample or 2) whole organism? | n/a | n/a | 2 |
| 46 | Whether 1) average or 2) maximum BAF/BCF was used? | n/a | n/a | 1 |
| No | Item | Specify |
|---|---|---|
| 47 | Score: ... % | 75.0 |
| 48 | EC Reliability code: | 2 |
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence |
| 50 | Comments | The present procedure is based on semi-static conditions (renewal of test solutions every 2 days). Therefore, test chemical with very low water solubility Disperse Orange 30, can also be characterized as to their bioconcentration potential without adding solvents or other auxiliary substances which may affect the results. |
Table A-2. Robust Study Summaries Form: Aquatic iT
Reference: Sandoz. 1975. Acute fish tox (Rainbow trout) 48hr.
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | Y | 5261-31-4 |
| 3 | Substance identity: chemical name(s) | n/a | Y | |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | N | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 7 | Reference | 1 | Y | |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | ||
| 10 | GLP (Good Laboratory Practice) | 3 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 11 | Organism identity: name | n/a | Y | Rainbow trout |
| 12 | Latin or both Latin & common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organis | 1 | N | |
| 14 | Length and/or weight | 1 | Y | |
| 15 | Sex | 1 | N | |
| 16 | Number of organisms per replicate | 1 | N | |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 19 | Test type (acute or chronic | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | ||
| 22 | Exposure duration | n/a | Y | 48 |
| 23 | Negative or positive controls (specify) | 1 | N | |
| 24 | Number of replicates (including controls) | 1 | N | |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | N | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | N | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | N | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||
| 35 | Analytical monitoring intervals | 1 | N | |
| 36 | Statistical methods used | 1 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g. when mortality in the control greater than 10%) or physical effects (e.g. 'shading effect')? | n/a | ||
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | N | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | N | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | N | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 48HR LC50 greater than 700 mg/L |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | ||
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a |
| No | Item | Specify |
|---|---|---|
| 47 | Score: ... % | 28.9 |
| 48 | EC Reliability code: | 4 |
| 49 | Reliability category (high, satisfactory, low): | Not Satisfactory |
| 50 | Comments |
Table A-3. Robust Study Summaries Form: Aquatic iT
Reference: Environment Canada. 1995. Acute fish toxicity test submission in fulfillment of new substances notification regulations to New Substances Branch, Environment Canada under New Substance Notification Program.
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | N | |
| 3 | Substance identity: chemical name(s) | n/a | Y | |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | N | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 7 | Reference | 1 | Y | OECD 203 |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | not applicable | |
| 10 | GLP (Good Laboratory Practice) | 3 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 11 | Organism identity: name | n/a | Y | Rainbow trout |
| 12 | Latin or both Latin & common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organis | 1 | Y | mean length 51mm and mean weight 1.54 |
| 14 | Length and/or weight | 1 | Y | see above |
| 15 | Sex | 1 | not applicable | |
| 16 | Number of organisms per replicate | 1 | Y | 10 |
| 17 | Organism loading rate | 1 | Y | |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 19 | Test type (acute or chronic | n/a | Y | acute |
| 20 | Experiment type (laboratory or field | n/a | y | lab |
| 21 | Exposure pathways (food, water, both) | n/a | y | water |
| 22 | Exposure duration | n/a | y | 96 hrs |
| 23 | Negative or positive controls (specify) | 1 | Y | 3 |
| 24 | Number of replicates (including controls) | 1 | Y | 2 |
| 25 | Nominal concentrations reported? | 1 | Y | 320 to 3200 mg/L |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | not applicable | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | Y | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g. when mortality in the control greater than 10%) or physical effects (e.g. 'shading effect')? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | unknown water solubility |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 96 hr LC50 = 505 mg/L |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | N |
| No | Item | Specify |
|---|---|---|
| 47 | Score: ... % | 77.5 |
| 48 | EC Reliability code: | 2 |
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence |
| 50 | Comments |
Table A-4. Robust Study Summaries Form: Aquatic iT
Reference: Cohle P, R Mihalik R. 1991. Early life stage toxicity of C.I. Disperse Blue 79:1 purified preecake to rainbow trout (Oncorhynchus mykiss) in a flow-through system. Final report. ABC Laboratories Inc. Columbia MO.
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | ||
| 3 | Substance identity: chemical name(s) | n/a | Disperse Blue 79:1 | |
| 4 | Chemical composition of the substance | 2 | n/a | |
| 5 | Chemical purity | 1 | Y | 96.61 |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 7 | Reference | 1 | Y | |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | n/a | |
| 10 | GLP (Good Laboratory Practice) | 3 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 11 | Organism identity: name | n/a | Rainbow trout | |
| 12 | Latin or both Latin & common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organis | 1 | Y | |
| 14 | Length and/or weight | 1 | Y | |
| 15 | Sex | 1 | n/a | |
| 16 | Number of organisms per replicate | 1 | Y | 20 |
| 17 | Organism loading rate | 1 | Y | 0.36 to 4.8ug/L |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 19 | Test type (acute or chronic | n/a | Y | chronic |
| 20 | Experiment type (laboratory or field | n/a | Y | lab |
| 21 | Exposure pathways (food, water, both) | n/a | Y | water |
| 22 | Exposure duration | n/a | Y | 122 days |
| 23 | Negative or positive controls (specify) | 1 | Y | control and carrier blank |
| 24 | Number of replicates (including controls) | 1 | Y | 2 |
| 25 | Nominal concentrations reported? | 1 | Y | 5 |
| 26 | Measured concentrations reported? | 3 | Y | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Y | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | Y | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | Y | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | Y | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | Y | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | Y | no tox value but however solubilizer was used as a control |
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g. when mortality in the control greater than 10%) or physical effects (e.g. 'shading effect')? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | flow through |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | n/a |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | NOEC greater than 5ug/L |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | ||
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a |
| No | Item | Specify |
|---|---|---|
| 47 | Score: ... % | 97.6 |
| 48 | EC Reliability code: | 1 |
| 49 | Reliability category (high, satisfactory, low): | High Confidence |
| 50 | Comments |
Table A-5. Robust Study Summaries Form: Aquatic iT
Reference: Safepharm laboratories Ltd. 1990. Acute toxicity to rainbow trout. Project number 47/781.
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | Y | 5261-31-4 |
| 3 | Substance identity: chemical name(s) | n/a | Y | |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | N | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 7 | Reference | 1 | N | |
| 8 | OECD, EU, national, or other standard method? | 3 | N | |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | N | |
| 10 | GLP (Good Laboratory Practice) | 3 | n/a |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 11 | Organism identity: name | n/a | Rainbow trout | |
| 12 | Latin or both Latin & common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organis | 1 | Y | |
| 14 | Length and/or weight | 1 | Y | |
| 15 | Sex | 1 | n/a | |
| 16 | Number of organisms per replicate | 1 | Y | three to ten |
| 17 | Organism loading rate | 1 | Y | 0.70g bodyweight/L |
| 18 | Food type and feeding periods during the acclimation period | 1 | n/a since acute test |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 19 | Test type (acute or chronic | n/a | acute | |
| 20 | Experiment type (laboratory or field | n/a | lab | |
| 21 | Exposure pathways (food, water, both) | n/a | water | |
| 22 | Exposure duration | n/a | 96 hrs | |
| 23 | Negative or positive controls (specify) | 1 | Y | positive |
| 24 | Number of replicates (including controls) | 1 | Y | two at definitive study |
| 25 | Nominal concentrations reported? | 1 | Y | 3 |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | n/a | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | N | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | n/a | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | n/a | |
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | N |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g. when mortality in the control greater than 10%) or physical effects (e.g. 'shading effect')? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | n/a | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | N | no pH given |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | Water solubility for this substance was 0.07 |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 44 | Toxicity values (specify endpoint and value) | n/a | 96 hrs LC50 greater than 100 mg/L | |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | N |
| No | Item | Specify |
|---|---|---|
| 47 | Score: ... % | 43.6 |
| 48 | EC Reliability code: | 3 |
| 49 | Reliability category (high, satisfactory, low): | Low Confidence |
| 50 | Comments |
Page details
- Date modified: