Appendices of the State of the Science Report
Phthalates Substance Grouping
Long-chain Phthalate Esters
1,2-Benzenedicarboxylic acid, diisodecyl ester
(diisodecyl phthalate; DIDP)
and
1,2-Benzenedicarboxylic acid, diundecyl ester
(diundecyl phthalate; DUP)
Chemical Abstracts Service Registry Numbers
26761-40-0, 68515-49-1;
3648-20-2
Environment Canada
Health Canada
August 2015
Table of Contents
- Appendix A: Information on analogues used for substances in the Long-Chain Phthalates Grouping
- Appendix B: Physical and chemical properties for substances in the Long-Chain Phthalates Grouping
- Appendix C: Estimates of daily intake of DIDP and DUP
- Appendix D: Methodology for biomonitoring intake calculations
- Appendix E: Description and Application of the Downs and Black Scoring and Guidance for Level of Evidence of An Association
- Return to the State of the Science Report
Appendix A: Information on Analogues used for Substances in the Long-Chain Phthalates Grouping
| CAS RN Common name |
Representative chemical structureFootnote Table A-1[a] | Representative molecular formula / molecularweight (g/mol) / chemical propertiesa | Similarity index (%)Footnote Table A-1[b] |
|---|---|---|---|
| Target substance: 26761-40-0 Diisodecyl phthalate (DIDP) |
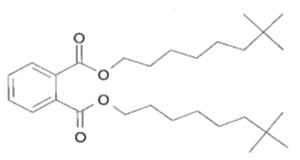 |
C28H46O4 MW: 446.68 Low water solubility (1.7 × 10-4 mg/L) Log Kow: greater than 8 Log Koc: 5.5 - 6.5 Dmax, Deff: 30, 20 nm |
n/a |
| Target substance: 68515-49-1 Diisodecyl phthalate (DIDP) |
 |
C28H46O4 MW: 446.68 Low water solubility (1.7 × 10-4 mg/L) Log Kow: greater than 8 Log Koc: 5.5 - 6.5 Dmax, Deff: 27, 19 nm |
n/a |
| Target substance: 3648-20-2 Diundecyl phthalate (DUP) |
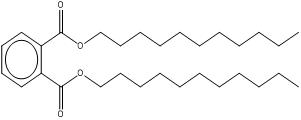 |
C30H50O4 MW: 474.73 Low water solubility (1.73 × 10-6 mg/L) Log Kow: greater than 8 Log Koc: 6.7 - 7.1 Dmax, Deff: 35, 22 nm |
n/a |
| Analogue substance: 28553-12-0 68515-48-0 Diisononyl phthalate (DINP) |
 |
C26H42O4 MW: 418.62 Low water solubility (6.1 × 10-4 mg/L) Log Kow: 8.8 Log Koc: 5.5 - 5.7 Dmax, Deff: 28 - 30, 19 - 20 nm |
with DIDP: 85 - 94 with DUP: 89 - 91 |
| Analogue substance: 85507-79-5 Diisoundecyl phthalate (DIUP) |
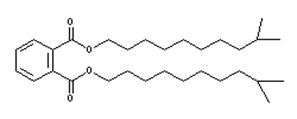 |
C30H50O4 MW: 474.73 Low water solubility (4.4 × 10-6 mg/L) Log Kow: 10.3 Log Koc: 7.1 Dmax, Deff: 31, 20 nm |
with DIDP: 84 - 92 with DUP: 81 |
Appendix B: Physical and Chemical Properties for Substances in the Long-Chain Phthalates Grouping
| CAS RN Acronym |
Physical form | Melting point (°C) | Boiling point (°C) | Density (kg/m3) | Vapour pressure (Pa) |
|---|---|---|---|---|---|
| 26761-40-0 DIDP |
LiquidFootnote Table B-1[a] | -46 - -50* (Exp)Footnote Table B-1[b],Footnote Table B-1[c] 105.95 (Mod)Footnote Table B-1[d] |
450* (Exp)c 463.36 (Mod)d |
966 (Exp)b |
7.0 × 10-5 (Exp, 25°C)Footnote Table B-1[g] 6.7 × 10-5* (Exp, 25°C)c,Footnote Table B-1[f] 1.8 × 10-6 (Cal, 25°C)Footnote Table B-1[h] 6.55 × 10-5 (Mod, 25°C)d |
| 68515-49-1 DIDP |
Liquida | -45 - -50* (Exp)a,Footnote Table B-1[e] 141.06 (Mod)d |
450* - 463 (Exp)c,e 454.16 (Mod)d |
968 – 970 (Exp)a,e |
6.7 × 10-5* (Exp, 25°C)c,f 5.1 × 10-5 (Cal, 25°C)e 1.8 × 10-6 (Cal, 25°C)h 6.55 × 10-5 (Mod, 25°C)d |
| 3648-20-2 DUP |
Liquida | -9* (Exp)f -40 (Exp)e 35.5 (Exp)b 155.88 (Mod)d |
336* (Exp)e 500.56 (Mod)d |
954 (Exp)a 960 (Exp)c |
6.67 × 10-5* (Exp)c 4.97 × 10-7 (Cal)h 0.0377 (Mod, 25°C)d |
| CAS RN | Water solubility (mg/L) | Henry's law constant (Pa·m3/mol) | Log Kow (unitless) |
Log Koc (unitless) |
Log Koa (unitless) |
|---|---|---|---|---|---|
| 26761-40-0 DIDP |
1.7 × 10-4* (Exp, 20°C)Footnote Table B-2[a] 1.19 (Exp, 25°C)Footnote Table B-2[b] less than 0.001 (Exp, 25°C)Footnote Table B-2[c],Footnote Table B-2[d] 3.8 × 10-5 (Cal, 25°C)Footnote Table B-2[e] 9.97 × 10-6 (Mod, 25°C)Footnote Table B-2[f] 5.40 × 10-5 (Mod, 25°C)Footnote Table B-2[g] 0.010 (Mod, 25°C)Footnote Table B-2[h] 0.041 (Mod, 25°C)Footnote Table B-2[i] |
21.6 (Cal, 25°C)e 3.72 (Mod, Bond estimate, 25°C)Footnote Table B-2[j] 4.11 (Mod, Group estimate, 25°C)j 1.75 × 102 (Mod, VP/WS estimate, 25°C)j,Footnote Table B-2[k] |
greater than 8.0 (Exp)c 9.46 (Cal)e 9.78 (Mod)Footnote Table B-2[l] 9.48 (Mod, 25°C)h 9.24 (Mod, 25°C)i |
5.46 (Exp)Footnote Table B-2[m] 6.04 (Mod, MCI estimate)Footnote Table B-2[n] 6.52 (Mod, Log Kow estimate)n |
11.52 (Cal)e 14.70 (Mod)Footnote Table B-2[o] |
| 68515-49-1 DIDP |
1.7 × 10-4* (Exp, 21°C)Footnote Table B-2[p] 3.8 × 10-5 (Cal, 25°C)e less than 0.001 (Exp, 25°C)c,d 1.18 × 10-5 (Mod, 25°C)f 1.16 × 10-4 (Mod, 25°C)g 4.4 × 10-3 (Mod, 25°C)h 0.078 (Mod, 25°C)i |
21.6 (Cal, 25°C)e 3.72 (Mod, Bond estimate, 25°C)j 4.11 (Mod, Group estimate, 25°C)j 1.75 × 102 (Mod, VP/WS estimate, 25°C)j,k |
greater than 8.0 (Exp)c 9.46 (Cal)e 9.71 (Mod)l 9.47 (Mod, 25°C)h 9.12 (Mod, 25°C)i |
5.46 (Exp)m 5.82 (Mod, MCI estimate)n 6.48 (Mod, Log Kow estimate)n |
11.52 (Cal)e 13.10 (Mod)° |
| 3648-20-2 DUP |
1.11 (Exp, 25°C)b less than 0.001 (Exp, 25°C)c 4.41 × 10-6 (Cal, 25°C)e 7.125 × 10-7, (Mod, 25°C)f 1.73 × 10-6* (Mod, 25°C)g 4.6 × 10-6 (Mod, 25°C)h 0.019 (Mod, 25°C)i |
50.5 (Cal, 25°C)e 6.55 (Mod, Bond estimate, 25°C)j 5.68 (Mod, Group estimate, 25°C)j 4.44 × 104 (Mod, VP/WS estimate, 25°C)j,k |
greater than 8 (Exp)c 10.33 (Cal)e 10.91 (Mod)l 12.13 (Mod, 25°C)h 10.50 (Mod, 25°C)i |
6.71 (Mod, MCI estimate)n 7.15 (Mod, Log Kow estimate)n |
12.02 (Cal)e 14.07 (Mod)° |
Appendix C: Estimates of Daily Intake of DIDP and DUP
Appendix C-1. Estimates of daily intake of DIDP
| Route of exposure | 0–0.5 yearFootnote Table C-1a[a]; Breast milk fedFootnote Table C-1a[b] | 0–0.5 yeara; Formula fedFootnote Table C-1a[c] | 0–0.5 yeara; Not formula fed | 0.5–4 yearsFootnote Table C-1a[d] | 5–11 yearsFootnote Table C-1a[e] | 12–19 yearsFootnote Table C-1a[f] | 20–59 yearsFootnote Table C-1a[g] | 60+ yearsFootnote Table C-1a[h] |
|---|---|---|---|---|---|---|---|---|
| Food and beveragesFootnote Table C-1a[i] | - | F | F | 0.12 (1.33) | 0.12 (1.07) | 0.068 (0.70) | 0.062 (0.69) | 0.044 (0.49) |
| DustFootnote Table C-1a[j] | 0.562 (2.199) | 0.562 (2.199) | 0.562 (2.199) | 0.394 (1.540) | 0.186 (0.728) | 0.007 (0.026) | 0.006 (0.025) | 0.006 (0.024) |
| Total oral intake | 0.562 (2.199) | 0.562 (2.231) | 0.562 (2.199) | 0.514 (2.87) | 0.306 (1.798) | 0.075 (0.726) | 0.068 (0.715) | 0.05 (0.514) |
| Gender - Age group | Median | 90th percentile |
|---|---|---|
| under 6 months | 0 | FFootnote Table C-1b[a] |
| 6 months to 1 year | Fa | Fa |
| 1 to 3 years | 0.128 | 1.327 |
| 4 to 8 years | 0.120 | 1.074 |
| M - 9 to 13 years | 0.0865 | 0.758 |
| F - 9 to 13 years | 0.0687 | 0.695 |
| M - 14 to 18 years | 0.0666 | 0.659 |
| F - 14 to 18 years | 0.0523 | 0.550 |
| M - 19 to 30 years | 0.0619 | 0.687 |
| F - 19 to 30 years | 0.0450 | 0.483 |
| M - 31 to 50 years | 0.0529 | 0.551 |
| F - 31 to 50 years | 0.0470 | 0.553 |
| M - 51 to 70 years | 0.0540 | 0.435 |
| F - 51 to 70 years | 0.0444 | 0.492 |
| M - 71 or more | 0.0555 | 0.428 |
| F - 71 or more | 0.0486 | 0.392 |
Appendix C-2. Estimates of daily intake of DUP
| Route of exposure | 0–0.5 yearFootnote Table C-2a[a] | 0.5–4 yearsFootnote Table C-2a[b] | 5–11 yearsFootnote Table C-2a[c] | 12–19 yearsFootnote Table C-2a[d] | 20–59 yearsFootnote Table C-2a[e] | 60+ yearsFootnote Table C-2a[f] |
|---|---|---|---|---|---|---|
| DustFootnote Table C-2a[g] | 0.0198 (0.349) | 0.0138 (0.244) | 0.00654 (0.115) | less than 0.001 (0.00417) | less than 0.001 (0.00397) | less than 0.001 (0.00382) |
Appendix C-3. Derivation of dietary intakes
Occurrence data – DIDP
Phthalate occurrence data for DIDP were available from foods sampled as part of the 2013-2014 Food Safety Action Plan (FSAP) survey conducted by the CFIA; this dataset was determined to be the most recent and comprehensive Canadian survey of the occurrence of these phthalates in foods. Duplicate foods were included in earlier CFIA FSAP surveys (i.e., 2011 to 2012 and 2012 to 2013); therefore, only data from the most recent (i.e., 2013 to 2014) FSAP survey were employed in the exposure assessment. Occurrence data for DIDP in foods not analyzed as part of the CFIA surveys were obtained from an American total diet study (Schecter et al. 2013) and any remaining data gaps were filled using data from a British total diet study (Bradley et al. 2013). Note that these data were only used to fill data gaps. Duplicate occurrence data from these studies for a given food or phthalate were not included if such data were already available from the CFIA's 2013-2014 FSAP survey.
Occurrence data for DIDP in food that was reported as less than the analytical LOD were assigned values of ½ LOD. However, a value of 0 (zero) was assigned to all samples within a broad food category when no phthalates were detected above the LOD in any sample in that category.
Food Consumption Data and Matching to Occurrence Data
The phthalate concentrations in individual foods were matched to consumption figures for these foods from the Canadian Community Health Survey (CCHS) Cycle 2.2 on Nutrition, (Statistics Canada 2004), to generate distributions of phthalates exposure for various age-sex groups. The CCHS included 24-hour dietary recall information for over 35,000 respondents of all ages across Canada.
If a food line item belonged to a recipe that was matched to a set of the assayed foods, then the associated phthalate levels matched to the recipe were assigned to the ingredient. Otherwise, if the food line item itself matched to a set of the assayed foods, then the phthalate levels matched to the food line item were assigned for DIDP; 1003 foods and 153 recipes were matched with the list of assayed foods.
Body Weight Information
For the purpose of determining per kilogram body weight exposure estimates, infant body weights were set to the mean body weights as derived from the body weight data from the United States Department of Agriculture Continuing Survey of Food Intakes by Individuals (CSFII; 1994-96, 1998). For all age groups, body weights reported in the CCHS, whether measured or self-reported, were used and where missing were imputed using the median for the corresponding age-sex group and quintile of energy intake.
Probabilistic Exposure Assessment
For each food consumed by a respondent in the CCHS survey, phthalate concentrations were randomly selected from the matching list of assayed values. For each individual respondent, exposure estimates from each food were summed, generating a distribution of exposure for all respondents. This was repeated 500 times (500 iterations) to model the variability of the distribution of exposures due to the variability of the phthalates levels. For each age-sex group, the median and 90th percentile exposures were derived from the empirical distribution generated by the 500 iterations.
Appendix D: Derivation of daily intakes for DIDP based on biomonitoring
P4 Pregnant Women
Equation 1:
Daily intake (µg/kg bw•day) = [CSum (mole/g Cr) × CER (g/day) × MWparent (g/mole)] / [FUESum× BW (Kg)]
Where,
- C Sum (mole/gCr) =
- sum of molar concentrations of the metabolites
- CER (g/day) =
- Creatinine excretion rate using Mage equation
- MW parent (g/mole) =
- Molecular weight, DINP: 418 g/mol
- FUE Sum =
- Sum of fractional urinary excretion values of the metabolites MHINP and MOINP = 0.18
- BW (Kg) =
- Body weight of the participant
Step 1: Converting the urinary metabolite concentration from µg/g Cr to moles/g Cr
Equation 2:
Cmetabolite (mole/g Cr) = [Cmetabolite(µg/g Cr)] / [MWmetabolite]
DIDP metabolites: MHIDP and MOIDP
For MHIDP,
CMHINP (mole/g Cr) = [CMHINP (µg/g Cr)] / 322 g/mol
For MOIDP,
CMOIDP (mole/g Cr) = [CMOIDP (µg/g Cr)] / 320 g/mol
Step 2: Sum the metabolite concentration (moles/g Cr) from Step 1
CSum (mole/g Cr) = Σ CMHINP + CMOINP
Step 3: Compute CER for individual participants using Mage equation
Step 4: Calculate intake using Equation 1
NHANES
Statistical analysis: The data were analyzed with SAS 9.2 (SAS Institute Inc., USA) and SUDAAN 10.0.1 software (RTI International, USA). Variance estimates were produced using the Taylor Series Linearization approach as recommended by the NHANES analytical guidelines. All analyses were weighted using the NHANES survey weights (environmental subsample) in order to be representative of the U.S. population. Phthalates concentrations that were below LOD were assigned a value of LOD/2.
Estimation of creatinine excretion rate (CER): For each study participant, creatinine excretion rate was calculated using the Mage equations (Huber et al. 2010). The adiposity adjustment (discussed in the supplemental information; Huber et al 2010) was applied for all participants and the body surface area adjustment was applied for children under the age of 18. Median BMIs by age for the adiposity adjustment were computed using the entire NHANES sample. The 2009-2010 and 2011-2012 NHANES phthalates datasets had 58 and 49 children who exceeded the height limits in the Mage equations (186 cm for males and 172 cm for females). The Mage equations were applied directly to the observed heights in order to extrapolate creatinine excretion rates for these participants. The predicted excretion rates for these individuals appeared to be reasonable despite the extrapolation.
Daily intake estimation: The daily intake of each phthalate was estimated for each participant using the following equations and procedure (David et al. 2000; Koch et al. 2007):
Equation 1:
Daily intake (µg/kg bw•day) = [CSUM (mole/g Cr) × CER (g/day) × MWparent (g/mole)] / [FUESUM× BW (Kg)]
Where,
- C SUM(mole/gCr) =
- sum of molar concentrations of the metabolites. In this case, only one metabolite was measured, MCINP = 336.
- CER (g/day) =
- Creatinine excretion rate using Mage equation
- MW parent (g/mole) =
- Molecular weight, DIDP: 447 g/mol
- FUE SUM =
- Sum of fractional urinary excretion values of the metabolites. In this case only one metabolite was measured, MCINP = 0.69
- BW (Kg) =
- Body weight of the participant
Step 1: Converting the urinary metabolite concentration from µg/g Cr to moles/g Cr
Equation 2:
Cmetabolite (mole/g Cr) = [Cmetabolite(µg/g Cr)] / [MWmetabolite]
DIDP metabolite: MCINP
For MCINP,
CMCINP (mole/g Cr) = [CMCINP (µg/g Cr)] / 320 g/mol
Step 2: Sum the metabolite concentration (moles/g Cr) from Step 1(if more than one metabolite was measured).
CSum (mole/g Cr) = Σ CMHINP + CMOINP
Step 3: Compute CER for individual participants using Mage equation
Step 4: Calculate intake using Equation 1
For each selected phthalate diester, the daily intake for each study participant was computed using equation 1. Arithmetic and geometric means and selected percentiles along with their 95% confidence intervals of daily intake were produced for the U.S. population by age group and sex. Descriptive statistics were computed using SUDAAN proc DESCRIPT.
Appendix E: Description and Application of the Downs and Black Scoring System and Guidance for Level of Evidence of An Association
Evaluation of study quality
A number of systematic approaches for assessing the quality of epidemiologic studies were identified and evaluated. The Downs and Black method was selected based on: (1) its applicability to the phthalate database; (2) applicability to multiple study designs; (3) established evidence of its validity and reliability; (4) simplicity; (5) small number of components; and (6) epidemiologic focus. Downs and Black consists of a checklist of 27 questions broken down into the following five dimensions: 1) reporting; 2) external validity; 3) internal validity study bias; 4) internal validity confounding and selection bias; and 5) study power. Overall study quality is based on a numeric scale, summed over the five categories. The range of the scale allows for more variability in rating study quality. The 27 questions are applicable to observational study designs including case-control, cohort, cross-sectional, and randomized controlled trials.
Studies retained for assessment were scored for quality using the Downs and Black tool. As previously mentioned, the Downs and Black allows for a range of scores from 27 questions and each epidemiological study design has a maximum score (the maximum score for cohort studies is 21, case-control studies 18, and cross-sectional studies 17). Studies were divided into quartiles based on the scoring distribution for each study design; the distribution of scores for cohort, case-control and cross-sectional studies appears in Figure E-1. The average scores for cross-sectional and case-control studies were 13.1, whereas cohort studies had higher scores than both other study designs with an average score of 14. 4.
Figure E-1. Distribution of Downs and Black scores by study design

Long description for figure 1
The figure is bar graph describing the range and frequency of Downs and Black scores given to studies of different designs.
The bar graph has the x-axis as the Downs and Black score ranging from 7 to 19 and the y-axis as the frequency of score up to 15. The figure displays the frequency of the following types of studies: cohort, case-control, and cross-sectional.
1) For the cohort studies, 2 studies received a score of 12, 6 studies received a score of 13, 8 studies received a score of 14, 6 studies received a score of 15, 3 studies received a score of 16, 3 studies received a score of 17, and 1 study received a score of 19.
2) For the case-control studies, 1 study received a score of 8, 3 studies received a score of 9, 4 studies received a score of 10, 4 studies received a score of 11, 1 study received a score of 12, 2 studies received a score of 13, 6 studies received a score of 14, 3 studies received a score of 15, and 2 studies received a score of 16.
3) For cross-sectional studies, 1 study received a score of 7, 4 studies received a score of 11, 12 studies received a score of 12, 15 studies received a score of 13, 14 studies received a score of 14, 2 studies received a score of 15, 2 studies received a score of 16, and 1 study received a score of 17.
Guidance for level of evidence of an association
The potential for an association between phthalate exposure and each health outcome was assessed based on strength and consistency as well as the quality of the epidemiology studies as determined by the Downs and Black scores. Descriptions of the levels of evidence of association are as follows:
- Sufficient evidence of an association:Evidence is sufficient to conclude that there is an association. That is, an association between exposure to a phthalate or its metabolite and a health outcome has been observed in which chance, bias and known confounders could be ruled out with reasonable confidence. Determination of a causal association requires a full consideration of the underlying biology/toxicology and is beyond the scope of this document.
- Limited evidence of an association: Evidence is suggestive of an association between exposure to a phthalate or its metabolite and a health outcome; however, chance, bias or confounding could not be ruled out with reasonable confidence.
- Inadequate evidence of an association: The available studies are of insufficient quality, consistency or statistical power to permit a conclusion regarding the presence or absence of an association.
- Evidence suggesting no association: The available studies are mutually consistent in not showing an association between the phthalate of interest and the health outcome measured.
Page details
- Date modified: